1553
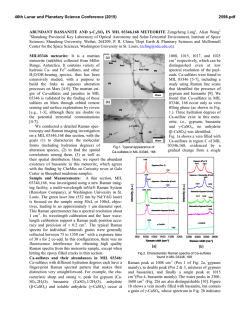
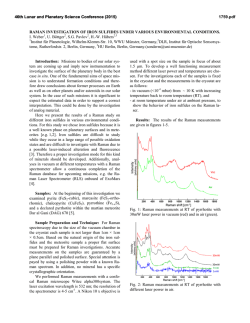
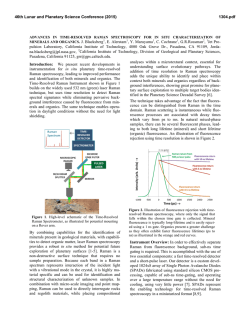
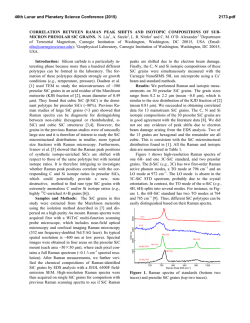
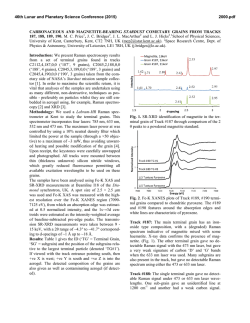
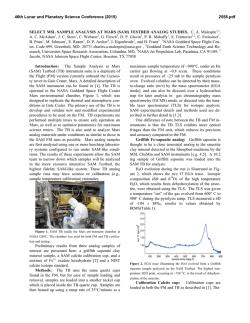
46th Lunar and Planetary Science Conference (2015) 1553.pdf REMOTE RAMAN DETECTION OF FROST ON MINERALS. A. K. Misra1, S. K. Sharma1, T. E. AcostaMaeda1, G. Berlanga1, S. M. Clegg2, R. C. Wiens2, and M. N. Abedin3, 1Hawaii Institute of Geophysics and Planetology, Univ. of Hawaii at Mānoa, Honolulu, HI 96822, USA; 2Los Alamos National Laboratory, Los Alamos, NM 87545, USA; 3NASA Langley Research Center, Hampton, VA 23681, USA. [email protected] Introduction: The search for life on other planets and other Solar System objects is one of the important goals outlined in the NASA Decadal Survey. The detection of biological materials and biomarkers would be evidence in support of life outside our planet and an important step towards meeting the goals of the NASA planetary exploration program. Apart from detecting biological materials, a high priority is given to detecting water, organic compounds, and elements such as C, N, O, S, P, H, Fe, Mn, etc., which are necessary for biological processes as they are currently understood. The University of Hawaii (UH), in collaboration with Los Alamos National Laboratory (LANL) and NASA Langley Research Center, has successfully developed a compact remote Raman+LIBS+Fluorescence system which is capable of measuring Raman, LIBS and fluorescence spectra of minerals under daytime conditions from standoff distances. Under the Mars 2020 mission, UH is collaborating with LANL and French partners (IRAP and CNES), for developing the SuperCam instrument which will be able to perform remote chemical analysis of minerals on Mars using Raman, LIBS and time-resolved fluorescence spectroscopy [1]. Fig. 1: Standoff Raman+LIBS+fluorescence system with 2.5 inch telescope. Among the various techniques to detect water, ice, and H2O/OH bearing minerals, Raman spectroscopy stands out as providing distinctive spectra for their unambiguous identification. The portable remote Raman instrument developed at UH has been shown earlier to detect water, ice, water-bearing minerals, and carbon in carbonate form from a distance of 10 to 50 m under bright day conditions with short integration time [2-5]. Over the years we have demonstrated that a large number of minerals, including dark minerals, can be easily detected using compact remote Raman+LIBS systems [6-9]. Here, we demonstrate the remote Raman capability to distinguish frost on minerals. Mars atmosphere is rich in CO2 and hence is expected to form CO2 frost on minerals under cold conditions. Remote detection of water, water-ice and water bearing minerals from CO2 frosting on minerals would be useful for identifying high value targets for both chemical analysis and sample return missions. Fig 2: Experimetal setup for producing CO2 frost over a mineral (left) and in-situ remote Raman detection (right). Experimental Set-up and Samples: The combined stand-off Raman, LIBS, and Fluorescence system (Fig. 1) uses a 2.5 inch telescope for collecting optical signals. A one inch diameter 532 nm notch filter is used in the back of the telescope to separate out the Rayleigh scattered light. A frequency-doubled mini Nd:YAG pulsed 532 nm laser source is used to excite the target located at a remote distance. The scattered light generated by the target is collected and focused onto the slit of a compact spectrograph of size 10 cm (length) x 8.2 cm (width) x 5.2 cm (height). The spectrograph uses a custom HoloPlex grating and is equipped with a custom gated thermo-electrically cooled mini-ICCD detector. Figure 1 shows the combined stand-off spectroscopy system. All spectra were measured using a 50 micron slit with the intensified CCD in the gated mode under daylight conditions. The rock-forming mineral samples were purchased from Ward’s Natural Science Establishment, Inc., Rochester, New York. For generating a CO2 frost layer on mineral samples, samples were cooled to liquid nitrogen temperature and frost was build up by blowing CO2 gas over the samples. Fig. 2 shows the copper sample holder and shielding which was used to develop the CO2 frost over the minerals. Similarly, a water-ice layer was produced by simply blowing air on cold minerals. The minerals with frost layer were investigated in-situ (Fig. 2, right panel) from a distance of 8 m using 10 mJ/pulse of 532 nm laser power and 5 mm laser spot. Fig. 3 shows an example of CO2 frost and H2O frost formed over a calcite mineral using the above setup. The amount of CO2 frost on calcite is 0.763 g. The estimated amount of CO2 frost detected by the 5 mm laser spot is 0.0044 g. 46th Lunar and Planetary Science Conference (2015)Misra et al. 1553.pdf gases interrogated by the laser (oxygen at 1556 cm-1 and nitrogen at 2331 cm-1). It is also demonstrated that the remote Raman system is capable of detecting a mineral behind the frost layer. Sample at 8 m, 532 nm, 10 mJ/pulse, 10 s 1276 1384 CO2 frost on calcite 3 3114 ice 2331 N2 1556 O2 1748 1 711 2 1434 1085 Mixed frost on calcite 154 282 Intensity (Counts x 106) 4 Water frost on calcite Calcite in air 0 500 1000 1500 2000 2500 3000 3500 4000 Raman Shift (cm -1) Figure 4: Remote Raman detection of various frost on calcite from 8 m distance. In the dry ice spectrum these sharp bands appear at 1278 and 1385 cm-1 [10].The observed Raman peaks of CO2 frost at 1276 and 1384 cm-1 indicate much colder frost temperature than dry ice. Water gives very strong Raman signal in the 3100 – 3600 cm-1 spectral region. Water-ice can be distinguished from liquid water by the presence of a sharper band near 3150 cm-1 [11]. This band shiftes to the observed value of 3114 cm-1 at ~ 173 K. The Raman spectrum of mixed frost shows presence of both CO2 and H2O molecules demonstrating capability of Raman spectroscopy to distinguish mixed chemicals including the atmospheric 1276 1384 1556 O2 CO2 frost on gypsum x5 3113 6 415 2 Water frost on gypsum 3407 3495 4 494 622 671 Results: Fig. 4 shows remote Raman spectra of calcite, CO2 frost on Calcite, H2O frost on calcite and mixed CO2-H2O frost on calcite in the frequency region, 100 to 4500 cm-1, from a distance of 8 m with integration time of 10 s. The spectral region covers the entire Raman spectral range. Calcite Raman fingerprint bands are observed at 154, 282, 711, 1085, 1434 and 1748 cm-1. The strongest Raman band at 1085 cm-1 is the fingerprint of carbonate ions corresponding to the ν1(CO3) symmetric stretching mode of carbonate functional group. In the spectrum of CO2-ice the characteristic doublet due to Fermi resonance of internal modes of vibration of CO2 molecule is clearly visible. In the Raman spectrum of CO2 gas, the characteristic Fermi doublet appears at 1286 and 1388 cm-1. 8 1137 1008 H2O-frost on Calcite Intensity (Counts x 106) CO2-frost on Calcite Calcite Fig. 3: Calcite mineral in air (left), with CO2 frost (middle) and water-frost (right). 2331 N2 Sample at 8 m, 532 nm, 10 mJ/pulse, 10 s Gypsum in air 0 500 1000 1500 2000 2500 3000 3500 4000 Raman Shift (cm-1) Figure 5: Remote Raman detection of various frost on gypsum from 8 m distance. Figure 5 shows standoff Raman spectra of hydrous sulfate mineral gypsum (CaSO4.2H2O) with CO2 and H2O frost layers. The symmetrical stretching mode of sulfate ions (ν1(SO4-2)) in gypsum is observed at 1008 cm-1. The chemically-bonded water molecules in gypsum are observed at 3407 cm-1 and 3495 cm-1. Similar to the data shown in Fig. 4, both CO2 and H2O frost could be easily detected over gypsum. The sharpening of Raman bands corresponding to chemically-bonded water molecules of gypsum indicates a colder sample temperature. Summary: The data presented show the ability of a portable compact remote Raman system to detect frost layer over minerals from a distance of 8 meters in a well illuminated background. Such system would be ideal for identifying minerals and searching for water on a planetary surface during daylight. Acknowledgments: This work has been supported by NASA EPSCoR grant NNX13AM98A and Mars 2020 mission. References: [1] Clegg,S.M., et al., (2015) LPSC, this conf. [2] Sharma, S.K., et al., (2002) Appl. Spectrosc., 56, 699-705. [3] Misra, A.K., et al., (2005) Spectrochim. Acta, A 61, 2281. [4] Misra, A.K., et al., (2006) Appl. Spectrosc., 60, 223-228. [5] Sharma, S.K., et al., (2011) Spectrochim. Acta, A 80, 75-81. [6] Sharma, S.K. et al. (2010), LPSC, 41, abstract#1443. [7] Wiens, R.C., et al., (2005) Spectrochim Acta A, 61, 2324-2334. [8] Sharma, S.K., et al. (2009), Spectrochim. Acta, A 73, 468-476. [9] Misra, A.K., et al., (2011) Proc. SPIE, 8032, 80320Q. [10] Sharma, S.K. et al., (2004), LPSC, 35, abstract#1929. [11] Misra, A.K. et al. (2006), LPSC, 37, abstract#2155.
© Copyright 2026