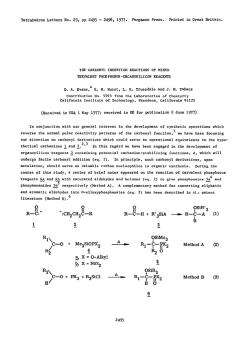
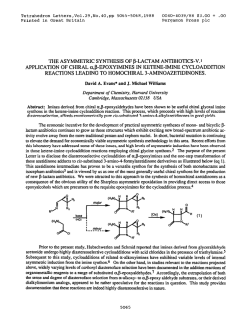
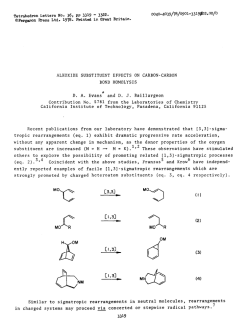
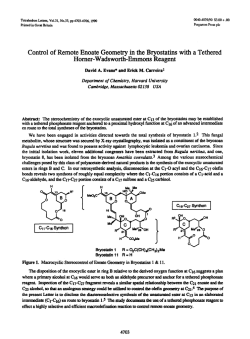
evans.harvard.edu
Tetrahedron Letters,Vol.23,No.3,pp Printed In Great Britain 285-288,1982 0040-4039/82/030285-04$03.00/O 01982 Pergamon Press Ltd. STUDIES DIRECTED TOWARDS THE TOTAL SYNTHESIS OF MORPHINE ALKALOIDS D. A. EVANS* AND C. H. MITCH Contrlbutlon No. 6546from theLaboratorIes of Chemistry Callforrua Institute Pasadena, Abstract: Appkatlon IS reported. A recent morphman which outlmed Scheme have of metallated A formal pubkatlon this laboratory outhned toward the development congeners has been completed. an efflclent The purpose of this commumcatlon been dlrected 91125 enammes to the synthesis of morphme related total synthesis of (+ )-morphme from derlvatlves.1 of Technology Cahforma approach to the constructlon of a morphme total synthesis accordmg m Scheme I. I OMe OH X 2 le 2, x-o a, X = CHe Br H2C H,C Br t br 6 5 285 of simple IS to expand upon the scope of our earher 4 studies to the plan 286 Pursuant In the to executmg followmg cyclopropane the desired fashion. dlcarboxyhc subsequent brommatlon3 8b In 75% yield. plan, the Illustrated dlbromlde acld2 as Illustrated dlbromlde cyclopropylcarbmyl synthesis The requlslte structural 6 was convemently below (eq 1). Borane reduction The reqwslte C02H II FiH, allyhc dlbromlde of 8b (1.0 equlv. ZnBr2, CH2X THF C02H D< HzC -7 cs”, CHzX b, the Acid catalyzed Br E?r r X=OH X=Br [SO%1 80, 96 h) afforded -via Lewis w 2) Per, from SO”C, 36 h) m 80% yleld.fiThesynthesls ZllBr, - steps of 7 (BH3, THF, 25OC, 75%) and 6 was prepared C6H6, were constructed In three of dlol 8a (1.1 molar equlv PBr3, 0.1 equlv 48% HBr, CH2CI2, rearrangement4 D< components synthesized 2 (1) [75%1 OMe _ (2) 9 of the required methyl tetrahydropyrldme 4-plperldone 9 was executed O’C, 45-50%) 1 lO”C, 2 h, 95%) of the resultant alcohol was of carried cannulatlon Scheme out (Et20, by metallatlon analogy to the blcycllc with earher crystallme (mp equlhbrated to the thermodynamlcally 149-ISI’C) 95%) which was readily m anhydrous azlrldmlum salts deserves further process -78’C). lmmomum which, salt ieadmg -lO”c, The resultant 10 m the presence protonatlon (3 equiv. to the blcychc 30 mm) monocyclic to N- TsOH, toluene, enamme enamme 4 (c.f. of sodium lodlde (MeCN, K2CO3 Sot, of 10 (HC104-MeOH, upon dlssolutlon Et20) afforded m MeOH (50°C, three-step Into the a-ammo sulfoxlde attention sequence. the dlastereomerlcally (25°C). aldehyde The reaction 13 m vu-tually This exceptionally as a potentially of 11 (CH2Cl2, pure azlrldmlum the 24 h), generahzable efficient method 25’C) with salt 12 (mp 186-188 C, quantitative “Kornblum yield upon Its oxldatlon” for the constructlon ammo aldehydes. Ar Nu ‘Me CH2Nu 12 10 and subsequent -cls-Isomer II (cls:trans = 95:5) as an amorphous powder In an of the -cls-fused lmmonlum perchlorate 11 to the morphman skeleton afforded transformed dimethyl dehydration preferred of dlazomethane dlssolutlon of 2,3-dlmethoxyphenylhth:um6 9 (n-BuLI,THF, 6 (Et20, enamme m 72% yield by the followmg solution The annelation studies, l kmetlc trans-fused yield of 60% from 9. Conversion was accomplished an ethereal (eq 2). by the addltlon by acid catalyzed tetrahydropyrldme of the anion Into 2 equlv of dlbromlde I) was then cychzed 2 h). In complete overall followed = Cl-, -OH, -O&a), (3) of of o- 287 In the present facile Instance, regloselectlve the oxldatlve substltutlon reactlons converslon of 13 to the morphman BF3 Et20, toluene, derived -IO”c, cleavage of this salt carbmol afforded perchlorate Successive morphman I2 by DMSO by both hydroxide 14 was affected 3 h) In 80% yield. from 14 (LlBEt3H) of azlrldmlum was suggested by the and chloride by Lewis Acid catalyzed methanesulfonatlon 15a m excellent Ions (eq 3). cycllzatlon and reduction The (2.5 equlv of the mesylate yield. Scheme II HCIO., MIOH. A c C&NC CHcCIe ?Me OMSO 25=X I -Ma& clo; -12 [95%1 I) MaCI, TEA 2) LIEt+H 3) 0.0.. NaIO, I OMe OMe 8Me Me0 0 a. -ij,x=o From our earller morphman careful lfia. exammatlon small observations dlazomethane oxldatlon previously that of of II for the direct With a sample of the direct with dlazomethane morphman this rmg system E noted m this could be directly revealed Instance constructed converslon of lmmoruum salt 11 to cycllzatlon product l5a m hand, a the presence of approximately IS In marked from lmmomum contrast 1% of to our earller Ions related to 11 upon treatment.1 completion (0~04, of a formal NaI04) employed total synthesis of 15a under acldlc by Gates m his ploneermg by Its converslon of unsaturated with authentic had been established treatment.1 of the reactlon l5b was accompllshed reduction studies precedent percentage N Me 16 Ce [SO%] c [SO%1 15a upon dlazomethane The The X = CHz c \@ Yl . 17 obtamed ketone total was accomphshed (THF:H2O:HOAc, 3:1:1)7 17 via base catalyzed l3C NMR and Infrared sources, generously by Lemleux-Johnson to ketone synthesis of morphme. 8 Verlflcatlon to the Cl4-eplmer 16. 9 The lo, from natural of (+ )-morphme condltlons lfib which of the structure equlhbratlon was of and subsequent spectra of 17 were found to be ldentlcal provided by A. Brossl and I+. SchmldhammerJO 288 Acbowledgement. Lilly This work has been supported Company. The use of the Southern by the Natlonal Callfornla lnstltutes Reglonal NMR of Health (GM-261 I I) and the El1 Faclhty (NSF-7916324AI) IS also acknowledged. REFERENCES AND NOTES D. A. Evans, C. H. Mitch, (I) R. C. Thomas, D. M. Zimmerman, and R. L. Robey, J. Am. Chem. Sot., 102, 5955 (1980). R. K. Smgh and S. Damshefsky, J. Org. Chem., (3) R. 0. Hutchms, and C. Maryanoff, (4) D. E. Applequlst (2) D. Masllamaru, and J. D. Roberts, 40, 2969 (1975). J. Am. Chem. ZnBr2 and anhydrous condltlons J. Org. Chem., (5) Subhmed (6) H. Cllman, (7) H. Vorbrueggen (8) M. Gates and G. Tschudi, (9) M. Gates and R. Helg, J. Am. Chem. Sot., 75, 379 (1953). J. SWISS, and L. C. Cheney, and C. Dlerassl, (a) A. Brossl et., (IO) 41, 1071 (1976). Sot., 78, 874 (1956). were found to be essential for the desired transformation. J. Am. Chem. Sot., 62, 1963 (1940). J. Am. Chem. J. Am. Chem. Helv. Chum. Acta, Sot., 84, 2990 (1962). Sot., 78, 1380 (1956). 64, 1298 (1981). (b) C. Oheman, L. Maat, and H. C. Beyerman, Rec. Trav. Chum., 99, 169 (1980). Selected 17: 13C NMR Data: (CDC13) 6 210.5, (22.5 MHz) 151.5, 148.9, 130.4, 130.2, 122.9, 111.4, 60.3, 57.0, 55.8, 51.3, 46.5, 45.6, 42.6, 41.5, 41.1, 40.0, 27.0, 24.0. 1%: (C6D6) 6 209.6 (s), 151.9 (s), 148.7 (s), 136.8 (s), 131.1 (s), 123.2 (d), 111.8 (d), 60.0 (q), 57.8 (d), 55.6 (q), 51.9 (t), 47.9 (t), 42.8 (q), 41.7 (d), 40.8 (t), 40.4 (s), 31.3 (t), 28.5 (t), 26.9 (t). 14: 6 152.7, 147.4, 137.0, 136.3, 123.8, 111.8, 110.1, 69.7, 63.3, 60.1, 55.4, 47.0, 46.7, 45.5,42.4, (C6D6) 40.3, 35.4, 30.2, 25.4. 11 _trans: (CD3CN) 6 182.4, 154.6, 148.8, 145.1, 131.2, 123.9, 122.3, 113.7, 111.8, 61.3, 56.5, 52.4, 48.8, 45.7, 43.0, 35.2, 34.7, 27.0. 11 -as: (CD3CN) 6 181.4, 154.6, 148.9, 144.6, 136.2, 124.8, 120.2, 113.7, 111.9, 61.2, 56.5, 52.2, 49.3, 43.1, 42.6, 39.3, 33.2, 27.6, 27.1. 12: (CD3CN) 6 154.5, 148.8, 145.7, 138.3, 124.1, 121.3, 113.3, 111.4, 60.8, 56.5, 53.5, 50.8, 50.7, 46.2, 43.3, 39.4, 34.8, 30.8, 30.4, 27.6. 10: (CDC13) 6 153.2, 147.7, 147.3, 138.9, 133.7, 42.7, 37.8, 36.1, 31.8. ‘(Received in USA 9 October 1981) 125.1, 122.0, 112.8, 110.9, 108.4, 60.2, 55.7, 46.7, 46.2, 44.5,
© Copyright 2026