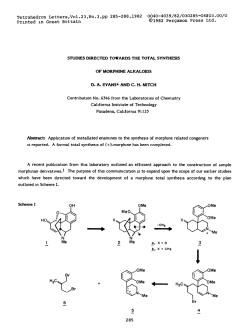
1,3-Asymmetric Induction in the Aldol Addition Reactions of Methyl
Tetrahedron
Letters,
Vol.
35, No. 46. PP. 8537-8540.
1994
Elsevia Science Ltd
F’rinted in Great Britain
txm-do39l94 $7.oo+o.00
1,3-Asymmetric Induction in the Aldol Addition Reactions of Methyl Ketone
Enolates and Enolsilanes to P-Substituted Aldehydes. A Model for Chirality Transfer
David A. Evans,*
Joseph L. Duffy, and Michael J. Dart
Department of Chemistry, Harvard University, Cambridge, Massachusetts 02338, USA
Abstract:
The direction and degree of 1,fasymmetric
induction have be&t evaluated in the addition of metal
enolates and enolsilanes to aldehydes substituted at the p position with both polar (OR, OAc. Cl) and nonpolar
(Me) substituents. A model for 1.3~asymmetric induction for polar addition processes such as the Mukaiyama
aldol reaction is proposed to account for the documented trends in reaction diistereoselection
for polar psubstituents.
Nucleophilic carbonyl addition lurctions continue to be ranked among the premier chemical transformations in
organic synthesis. With regard to the stereochemical aspects of this process, significant effort has been expended
in the development of transition state models that account for the impact of proximal substituents on carbonyl Rfacial selectivity. Hetemamm substituents positioned either a or p to the reacting carbonyl moiety raise the potential for transition state chelate organization, and cram’s chelation models have been both well recognized and
heavily exploited in the prediction of reaction diastereoselection. 1 In those substrates lacking the potential for
chelate organization, the interplay of steric and electronic effects are accounted for by the Felkin-Anh paradigm
which generally provides a useful stereoinduction model for substrates bearing stereogenic centers a to theCBTbony1 moiety.2 In contrast, comparable models acknowledging the influence of psubstituents
on reaction diasteteoselectivity have been less well developed3
The purpose of this Letter is to address the issue of 13-asymmetric induction in the addition of metal enolate
and enolsilane nucleophiles to p-substituted aldehydes (eq 1) with the objective of identifying the relative
importance of polar, steric. and chelate substituent effects in dictating reaction diastereoselection.
In this study,
enolate structure, with the exception of the metal ion (M), has been held constant.
M
0’
Me
Ju9
+
-
h*%
-+&Ll
(‘1
Me
M - U, TKTn,
BRz,SJMes
X - OPMB.
OTBB. Ok.
Cl
The addition of 3-methyl-2-butanonederived
enolates to p-oxygen substituted aldehydes 1 and 4 was carried
out under a variety of conditions (cq 2, Table J.). It is assumed that the Li, Ti, and B enolates m
through closed
transition states, but that only the Li and Ti enolatc nucleophiles might exhibit the potential for chelate oqanixation.4 In contrast, the BF3aEt2-promoted
enolsilane al&l reaction (M = SiMe# is presumd to proceed through
an open transition state where chelate organization is again precluded due to the limitations of four-coordinacy at
bomn.6 The data in Table I indicate that the formatiion of the 1.3-M products 2 and 5 is generally preferred irxespective of the nature of the oxygen protecting group. 7 The formation of the 1.3-anti product diastereomer is
consistent with the intervention of the illustrated internal chelabe. and this may be one possible explanation for the
results of the lithium and titanium mediated aldol reactions of aldehyde 1 (entries A, B).
However, for aldehyde 4 internal chelation is strongly disfavored by the tert-butyldimethylsilyl
(TBS) protecting group.* In addition, it is highly improbable that the anti stereochemical
outcome of the BFsaEtz-promoted
aldol reaction9 (entry D) is also chclate controlled. Reetz has
8537
9.
R
Mm
Ii
*
Ma
8538
postulated that anti stereoinduction for this process probably arises from transition state
polar effectssf and has invoked the illustrated Cram polar modeWe to account for the &
results. From these and related Lewis acid pmmoted addition reactions,*0 it is evident that
remote electrostatic effects can play a significant role in influencing the stereochemical
outcome of these processes and that this stereocontrol element will assume greater
importance for those reactions proceeding through more polar transition states.
-6
-qyc&hb
H+
1, x - OPMB
(Ocl+C&pOMe)
4,XEOTBS
(osI~&BU)
conditions
_
TC& IIRzNEt
UICln
C
S-BBNOTf I iPr#lEt
M - mz
BF,.OEb
1%
“Tax
‘__N~
H
Cram-Red2
(2)
2
2
5
6
Em&teas with fi-Substttuted Atdahydeet (aq 2).
metel (M)
LDA
D
*
H
-+&y
A
0
‘The abw
c
. -
Tabk 1. Afdol Fteaott~~ of 3-Methyl-P-butsnow
entry
H
M=Si&
2 :3
(X - PMB)
(%)
S:6
(X =TBB)
(%)
71 :29
(ltxl)
76:24
(91)
80:4Q
(Qe)
se:42
(68)
42 :58
Q2:a
(82)
g;z
1;;
W)
toTable II,footnotea
footnote-ponds
In order to further evaluate the interplay of steric and electrostatic effects on the addition process, aldehyde 7
was synthesized with P-substituents (OCH2Ar vs. CHsH2Ar) of similar size but different electronic properties
(eq 3). The aldol reactions employing the Li, Ti. and B enolates generally exhibited low diastereofacial selectivity
with this substrate (Table II). These results are consistent with the generalization that electrostatic effects alone do
not provide a strong diastereofacial bias for these aldol processes. In contrast, synthetically useful levels of anti
diastereoselection were obtained in the more polar Lewis acid promoted enolsilane aldol variant (entry D) with
aldehydes 7, 10,and 16 containing p-OPMB, -OTBS, and -Cl substituents, respectively.
As a conttol
experiment, the SnCb-promoted enolsilane aldol reaction was also carried out under conditions whete chelate
organization, if intervening, should be expected to afford good levels of anti diastereoselection. From the data
presented, we conclude that with SnCLq, only the -0PMB and -0Ac substituents might be involved in chelation in
the reactions with aldehydes 7 and 13. On the other hand. the poor diastereoselection observed with aldehyde 10
again provides qualitative support for the conclusion that the -0TBS substituent does not participate in chelate
organization even under favorable circumstances.*
To assess steric contributions to 1,3-asymmetric induction in the absence of any electrostatic effects, aldehyde
19 was subjected to the representative set of aldol reactions {eq 4, Table III). From the data in Table III, it is
Me dM
+
Me
.L
Ph
-
-+Ph
-+I%
1.3-Anti
lS,X-cl
TabIf.
Conditions
A
fDA
TfCW tf=tzNEt
O-BBNoTf I R&Et
D
BF9-OE19
E
sncG=
::
WSyn
metal (M)
In Uw Illuatmbd AIdol Mdftbn
(X =“dLB,
M-U
53:47
(Q4)
M-ma,
58:42
(S4)
M-B&
u:56
(77)
12
,5
18
17
InRuawa of the B AJdehyda Substkwnt
entry
:
(3)
7,x -0PMB
lO.X=OTBS
13,X=0&
11:12
(X = OTBS)
Reaction (eq 3).
14:1s
(X - OAO)
17:w
(x -
a+
61:s
(96)
58 :41
(82)
62 :38
(93)
En:20
31:6Q
(7Q)
6Q:s7
(75)
(se)
(W)
52:48
(82)
(W)
27:73
43:67
(77)
(78)
23:77
83:17
(6.9)
Bs:11
(85)
-
M-Btfl&
81 :lQ
(87)
73:27
M-StMe3
gs:5
(79)
48:52
~AJnrkn.~arrl~outL-70~InetlhrMF(~A)aCnrCC[nV*rEq.
PmdUatmtimwuod.mm~wGU:~w~
of the sityiaw mactienmixtumr. Yii
am reportad far tha mixnma of product diuDreaSn.
bsonochmkal
UdPrmentl
aecumd h,,,gh independent synlhmds. GM8 add 131; txwamfhed
WHJIthe 8ldhydB plor to encW=~ -.
(66)
W
8539
&dent that the psteric component of 1,34nduction provides low diasttl~selectivity
of variable direction.tl The
observations outlined above imply that cooperative steric and electrostatic effects combine to influence the
direction and degree of 1.3~induction in these processes.
Me&
Me
“&
-
+&+
10
-*
l,Ak4l,
-r&la
WI. Add
ReacuoM
CCWdtlOn~
entry
A
B
C
with -v
D
E
Tfleaov.faunaes
mad
w
M-U
T&&l
0-m
w
DrnwenUated
/ iR&JEt
BFvOEh
B&l,=
1.3~spa. 21
B-substnuenta
20:
21
64:20
26:64
::z;
(41
bm 4).
(SC)
(W
(100)
24:BB
M-BMeJ
M-SIMeJ
SB:42
!iB:42
g;
(79)
~lofhmeloundhT&.laII.foUM~a.b.
The Model. We propose a revised model for 1,3-asymmetric
induction for nucleophilic additions to
aldehydes bearing polar substituents in the p-position (Scheme I). In the illustrated transition structures, the
descriptor 4 denotes the p-carbon alkyl substituent, while X denotes the “polar” heteroatom substituent (OR,
Cl, etc.). For transition structures A, B, and C, the @carbon (Cp) is oriented perpendicular to the Q framework
of the carbonyl moiety. This is in accord with the Fe&in assertion that the staggered conformation between C,
and the trigonal carbon undergoing reaction is preferred in such addition processes.”
Complementary
minimization of interacting dipoles and nonbonded interactions favors reaction through transition state A.
Structure B suffers from a destabilizing gauche interaction (R~++C=O), which should be amplified with larger
Rp substituents. Transition state C is disfavored due to electrostatic interactions between the C=O and polar psubstituent.13
It follows that 1,3-anti diastereoselection
should be enhanced with an increase in the steric
requirement of Rp, and this trend is evident in the data presented in Tables I-II. particularly for the Mukaiyama
aldol reaction.
This model modifies the Cram polar model by replacement of the destabilizing
gauche
(R~wC=O) and (C~HNu) and eclipsing (Cg*(H)C=O)
interactions with the illustrated staggered geometry.
Scheme I
NW
)
favored
1,3-syn
Pmducl
Of the reaction variants examined, the Lewis acid-promoted Mukaiyama aldol process generally exhibits the
most synthetically useful levels (3-12~1) of 1,3-asymmetric induction (Tables I-III), even with those substrates
wherein the size difference between the X and Rg is minimal CLgble II). It is presumed that electrostatic effects
more strongly influence this family of polar transition states than the less polar enolate based processes. A more
complex dipole at the p-position, as in j%OAc aldehyde 13, may complicate the electrostatic influence of the polar
substituent X and thereby provide less predictable results.14
Complex Aldehydes.
For aldehydes such as 22 and 23 bearing stereocenters at both the a and B positions,
Analysis of the respective transition
the Felkin and 1,3-asymmetric
induction models may be integrated.
structures for the two Mukaiyama aldol reactions cone&y predicts that 22 should exhibit a more pronounced
facial bias (eq 5). since both stereoccn ters mutually reinforce addition from the same carbonyl diitereoface
(replace H1 for Me in A). In contrast, the two stereogenic centers in aldehyde 23, which are not reinforcing
(replace H2 for Me in A), should lead to diminished fcaction diastereoselection
below provide a gratifying illustration of this projection
ak
k
k
Felkln:Anll F&in = 98:02
Me
L
(al 6). The results summarized
the
2s
Felkln:AnUFelkin I 5634
reduction of P-alkoxyketones
and a related model
Complementary
studies on the diastereoselective
rationalizing
thestenochemical
control clin these reactions may be found in the accompanying
_ _ - Letter.‘5
References and Footnotes
D. J.; KopecLy.K. R. J. Am. Ghan. SOC. 1959,81.2748-2755.
(b) Rcetz. M. T. Act. Chem. Res. 1993.26, 46%
468andmfmwlces
1992.48.2803-2894.
cited therein. (c) Agex, D. J.; East, M. 3. ?&&&a
(a) w,
M.; Feikh H.: hdent.
N. Tetrahedron Lrn. 1968.2199-2204.
(b) Anh. N. T.; Eiaenstein. 0. Nouv. /. Clrim .
1977.1.61-70.
J.; Go&l, T.
(a) Bfienne. M-J.: G~anncs. C.; Jacques, J. Bull. Sot. Chim. Fr. 1968. 1036-1047. (b) Evans, D. A.; B-Ii,
Tetr&&on
Len. 1982.23, 45774580.
(c) Nalrrde, M.; Urano, Y.; Kobayashi. S.; Ghno, M. Te~rahe&on krt.
1!194,35,
741-744. (d) Leitereg. T. J.: Cram. D. J. J. Am. Clan. SDC. 1968.90.4011-4018.
(c) Ldtereg. T. J.: Cram, D. J. J. em.
Chm. Sot. I96&%
4019-4026. (t-)Reetz. M. T.; Kesaekr. K.; Jung, A. Tetrahedron L.ctr. 1984.25.729-732.
(a) Martin. S. F.: Lee. W.-C. T&&&on
Leit. 1993.34.2711-2714.
(b) Sviridov. N. D.; Bomdkin, V. S.; Errnolcnko, M.
(c) Masamune. S.; Ellingboe, J. W.; C!hoy. W. 1.
S.: Yashmsky, D. V.; Kochctkov. N. K. Te@&&on
1991,47,2317-2336.
Am. Chem. Sot. 1982.104.5526-5528.
(a) Heathcock. C. H.; Davidson. S. K.; Hug, K. T.; Flippin, L. A. J. Org. Chem. 1986,51.3027-3037.
(h) Gennari, C. in
Cornprehcnsfve Organic Synrkesis, Trost, B. M.: Fleming. I; Heathack. C. H. Eds.: F%rgamonPress NY, 1991: Vol2. ch 4.
Hypwknt
baron species have been characm.
but only under strongly msonamx stabilized conditions. Lee, D. Y.: Martin,
J. C. 3. Am. Chem. Sot. 1984.106.5745-5746.
The relative stereochemistry of 1,3diol moieties was established unambiguously via either conversion to the corresponding 13. benzylidii ketal and analysis of %I NMR
acetonidc aod analysis of the 13C NMR spectrum. or conversion to the ce
nCk data Rychnovsky. S. D.: Rogers, B.: Yan. 0. J. Org. Chem. 1993.58.3511-3515.
(a) Bloch, R.; Gilbert. L.; Girard, C. Tetrahedron Len. 1988.29. 1021-1024. (b) Kcck, G. E.; At&us, M. B.; castcllitto, 8.
J. Am. Chum. Sot. lw19. I I J,8 136-8141. However, for a recent evidence in support of chelation by a vicinal GTBS group see:
tc) Chen, X.; Herb&no, E. R.; Eliel, E. L.; Frye, S. V. J. Am. Chem. Sot. 1992,114, 1778-1784.
For related eXamp1e.s see: (a) ref. 3f. (b) Paterson. I.: Smith. J. 1. Org. Chem. 1992.57. 3261-3264. (c) Haneasian. S.:
Tehim, A.; Chen, P. J. Org. Chem. 1993,58,7768-7781.
Simihu trends have been reported in MuLaiysma &I a&atg
u) pddo-substituted aldehydes: (d) Annunziata R.; Cinquini, AI.; Cozzi. F.; Cozzi. F. G.; ConsoIandi. E. J. Org. Chem. 1992.57.
456-461.
Allylsilane addition. nf. 3r; allylstannanc addition. Nakatsuka. M.; Ragan, J. A.; Sammakia, T.; Smith, D. B.: Uehliig. D. E.:
Schreibef, S. L. J. Am. Chem. Sot. 1990.112, 5583-5601; [2+2] cycloaddition, Pans, J.-M.: Pommier. A.; Lerpiniere, I.;
Kocienski, P. J. Chem. Sot.. Perk& Trans. I 1993, 1549-1551.
The relative stexeuchemistry of the 13-a& product was established by conversion to the corresponds‘ngvandanalysisof
rhe ‘II NMR nOe and coupling constant data.
(a) Ref. 2a. For compuWonal evidence supportingthe importanceof none&peed geanehies in carbonyl addition see: (b) ref.
2b. (c) padden-Row. M. N.; Rondan. N. G.: Houk, K. N. 1. Am. Chem. Sot. 1982,104.7162-71666.
(d) Houk. K. N.: PaddonRow. M. N.; Rondan. N. G.: Wu. Y.-D.; Brown, F. K.: Spellmeyer, D. C.; Mea, J. T.; Li, Y.; Loncharich. R. J. Science
1986.231.1108-1117.
It is not suggcatcd that B and C are the only transition states which might give rise to the minor product diasrmeom er. It is
teason&lethatean&iam
stnrctures~ag~~relMionshipbchveurC8andnuclsophileshouMalsobeconsidaed
Low or vadabk. mlectivity
in BF39Bt2 mediated Mukaiyama aIdol nactions has bctn obsxved elaewti
fcr WberearrJ
with complex dipole protecting groups at the jGoxygen. For B-O-methoxymethyl (MOM). see: (a) Roy, R.: Rey, A. W.
Synlert 1990.448450.
No 1,3-induction waa observed in the BF3-OEt2 promoted Mukaiyama aIdol addition to a B-O-pmcthoxybenzyloxymethyl @OM) substituted aldehyde: (b) Evans, D. A.: Carter. P. H. unpublished results.
Evans, D. A.; Dart, M. J.; Duffy, J. L. TerraJtedron Len. 1994,35, this issue.
1) (a) k.
2)
3)
4)
5)
6)
7)
8)
9)
10)
11)
12)
13)
14)
1%
(Received
in USA 24 August
1994; revised
15 September
1994; accepted 2 1 September
1994)
© Copyright 2026