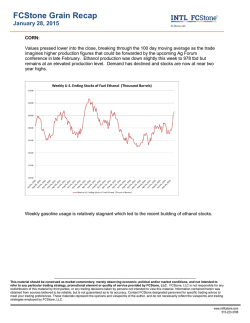
o_, R = CHC=CCHC=CH b_, R = n_
Tetrahedron Letters No. 5, pp 411 - 414. @Pergamon Press Ltd. 1979. Printed in Great Britain. A FORMAL SYNTHESIS OF (*)-PERIIYDR~HIsTRI~NIC~T~IN -via a-ACYLIMMONIUM ION-OLEFIN CYCLIZATIONS D. A. Evans and E. W. Thomas’ Contribution No. 5903 from the Laboratories California Institute of Technology, Pasadena, Histrionicotoxin of Chemistry California 91125 (HTX) (la), ’ an alkaloid recently isolated from the skin secretions the Colombian frog Dendrobates histrionicus, has recently attracted considerable interest in relation to its total synthesis. 3 The interest in H’IX and perhydrohistrionicotoxin (lb) stems from their unique properties and 2 as neurotoxins. It has been shown that both 2 of block post synaptic membrane depolarization while not interfering with acetylcholine binding. It has been postulat4ed that these toxins prevent membrane depolarization by reversible binding to the ion channel. L 2 o_, R = CHC=CCHC=CH 2, R=H b_, R = n_-C4Hg ta, R=$H 0 Recent studies directed towards the total synthesis of perhydrohistrionicotoxin (lb) have established that spirolactam our own interest in devising simplified investigated the olefin cyclization desired spirolactam the stereochemical E is a versatile to $&3b In conjunction with approaches to the synthesis of PHTX analogs we have of a-acylimmonium g or its epimer $ (Scheme I). outcome of these competitive to 2, would insure a considerable precursor (PHTX) improvement ion 5 which could lead to either the In spite of the uncertainty associated with cyclization modes, in the previously a successful ring closure published route to lactam 2,.3 During the course of tf present study Speckamp reported the related cyclization of 5 to give c in 45% yield. This communication has prompted us to report our own observations on the cyclizations summarized in Scheme I. The requisite precursor 411 g was prepared in good overall yield via the route outlined in Scheme II. The Grignard 6 7a, was added to the iodomagnesium After work-up (sat. aq. NH4C1, O’C) a mixture of reagent 7b, prepared from alkyl chlorip salt of glutarimide (Et20, 1 h, reflux). hydroxy lactam c and keto amide g, mp 90-91°C, were obtained in 66% yield.’ runs this mixture was directly dehydrated in a 75% yield to a 9:1-mixture In preparative of enamides g and fi respectively acid in refluxing toluene-DMF if”ethe presence of a catalytic amount of ptoluenesulfonic (50:1, 48 h). Nu: (I) 0 0 Nu: k NH (2) H H Nu 4, HCOlH (3) OCHO 6, Chromatographic separationof enamide isomers z and g: this mixture on Silica Gel (l:l, For $Z EtOAc:hexane) IR (neat) 3220 (NH), 1687, 1665 cm-’ afforded the pure (C=O, C=C); ‘H NMR IR (neat) 3220 (NH), 1682, 1665 cm-l (C=O, (CDC13) 4.75 (multiplet, -CH) - _ and for g 'H NMR 6 (CDC13) 4.32 (t, J = 6 Hz, C=Cg). Upon acid treatment (CF3C02H, CDC13), C=C); 6 each isomer equilibrated -via the presumed acylimmonium ion z to a 8:1-ratio of g and E. After exploring a range of cyclization conditions it was found that a 0.1 molar solution of g and 2 in anhydrous formic in a 40% yield after chromate acid (25’C, 32 h) afforded the desired formate 2k, mp 148-149’C, raphy:7 lR (CHC13) 1716 (C=O), 1650 cm-:$C=O lactam); +I NMR 6 (CDC13) 4.95 (m, lH, W1 B2 = 15 Hz, C8-H), 8.08 (s, lH, formate); C NMR 6 (CDC13), 171.9 (C,), 160.1 (formate), 73.9 (C8), 58.5 (C6), 50.7 (C,), 36.1 (C,). No. 5 OH Scheme__e C4Hs f-- X ,7 g,x=cr t& X=MgCI H2Nrc4Hs 0 TsOH A * Alcoholysis (MeOH, NaOMe, 25’C, give the beautifully crystalline alcohol z 13C NMR 6 (CDC13), 171.4 (C,), + 0.5 h) of formate 2&was carried mp 133-134’C; 69.7 (C,,, 57.4 (C,), 49.3 (C,), 33.1 (C,), to be identical in all respects to an authentic sample provided by Professor chromatographic of enamide l0, isomeric separation’ and identification of tf a 10% yield of the enamide dimer, 6, 5-spirans out ixi’95% yield to (C=O); IR (CHC13), 1623 cm which proved Y. Kishi. Careful reaction products revealed a 10% recovery and a 30% yield of two separable diastereo- (2:1-ratio) The minor 6, 5-spirocyclic shown (vide -_I_ infra) to possess the general structure E. formate ester E was obtained as an oil while the major isom_elr Ewas subseqyently crystallized: mp 118-12O’C; IR (CHC13), 1708 (formate), 1650 cm 5.13 (d, d, J1 = 5.4 Hz, J2 = 10.8 Hz, W ‘j2 = 12 Hz, 1H, H NMR 6 (CDCl (lactam C=O); 325 C NMR 6 (CDC13), 72.0 (C,), 160.3 (formate), 7.28 (Cl,), Cll-H), 8.05 (s, lH, formate); 64.2 (C,), 52.2 (C,), 40.0 (C,). No. 5 414 Degradation of each spirolactsm both g and E olefin cyclization cyclization afforded the keto la&am l4tlthus (eq. 1) proceeds stereocenters confirming that These results indicate that there are two competing modes which proceed with nearly equal facility. Nonetheless, in those product manifold (Scheme I, eq. 1, 2) the with high stereoselectivity for perhydrohistrionicotoxin Am parison. and s modes leading to the 6,6-azaspirane desired ring closure requisite g were diastereosiomers. to establish three of the four (l&) in a single step. We wish to thank Professor Y. Kishi for a sample of 22 for com- Support from the National Institutes of Health is gratefully acknowledged. REFERENCES 1. National Institutes of Health Postdoctoral 2. (Ta);o~yGoampa) FAer;nya, 2z, 1121 (1971); (b) J.W. Daly, I. Kafle, W. Myers, a ers, and B, Witkop, Proc. Nat. Acad. Sci., 62, 1870 (1971); (cj T. Tokuy&na’ K. Uenoyama, G. Brown, J W Dsly, and B W ifkop, Helv. Chim. Acta, z, 2597 (i974); (d) I. Karle, J. Am. &em. Sot., E, 4036 (1973). 3. a) E. J. Corey, J.F. Arnett, and G.N. Widiger, J. Am. Chem. Sot., 97, 430 (1975); b) M. Aratani, L. V. Dunkerton, T. Fukuyama, Y. Kishi, H. Kakoi, S.%igiura, and S. moue, J. Org. Chem. 40, 2009 (1975); T. Fukuyama, L.V. Dunkerton, M. Aratani, and Y. Kishi,ibid (1975); (c) E.J. Corey, M. Petrzilka, and Y. Ueda, Helv. Chim. Acta, K&-$294$977), and references cited therein. 4. J. Elliott and M. A. Raftery, Biochem. and references cited therein. 5. H. E. Schoemaker Fellow. and Biophys. Res. Commun., Tetrahedron Lett., and W. N. Speckamp, z, 1347 (1977), 1515 (1978). 6. Compound 7a was synthesized from 1-hepten-3-01 in an overall yield of 66% via 3 orthoester Claiss rearrangement; 4 lithium aluminum hydride reduction; 3 maanesulfonation and sodium chloride displacement. 7. Satisfactory 8. M. Sekiya and Y. Terao, 9. Preparative separations were performed on a Waters Prep 500 chromatogra h (Silica Gel, EtOAc). Analytical HPLC was carried out with a p-Poricil (30 cm) column PEtOAc, 6 ml/ (11.2 ml), l2& (13.1 ml), 2& (14.3 ml). min) . Observed retention volumes: s spectral and analytical data were obtained for all new compounds reported. Chem. Pharm. Bull., g, 10. For related dimerizations see: J.B. P. A. Wijnberg, Tetrahedron Lett., 4077 (1974). 11. IR (CHC13), 1716 (C=O) 1650 cm -l (C=O lactam); 167.093. Found: 167.695. (Received in USA 27 November 1978) 391 (1971). W.N. Speckamp, Exact mass calcd. and J. J. J. de Boer, for CgHl3NO2:
© Copyright 2026