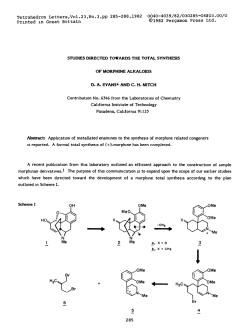
1,3-Asymmetric Induction in Hydride Addition Reactions to P
Terrahedron Leuers, Vol. 35. No. 46, pp. 8541-8544, 1994 Pergamon Elswier Science Ltd Printed in Great Britain oo40-4039/94 0040-4039(94)0 1,3-Asymmetric $7.m+o.otl 189I-X Induction in Hydride Addition Reactions to P-Substituted Ketones. A Model for Chin&y Transfer David A. Evans,* Depmmenr Michael J. Dart, and Joseph L. Duffy of Chemisrry, Harvard University. Cambridge. Massachusetts 02138, USA We report the 1.3~asymmetric induction observed in the additions of various hydride reagents to /jAbstract: substituted ketones. Both the nature of the &substituents and the size of the achiral alkyl group attached to the carbonyl moiety have a significant effect on the direction aad degree of carbonyl diastereofaciai selectivity. A revision of cram’s polar mode1 for 1.3~asymmetric induction is proposed to account for these results. The purpose of this Letter is to highlight the turnover in stereoselectivity that is dependent upon the nahlre of the B-substituent (alkyd vs. alkoxy) in the reductions of acyclic p-substituted ketones. Two representative reductions that illustrate this observation are provided below (Scheme I).t.* We propose that electrostatic effects3 due to the presence of the phetemamm substituent are responsible for the observed reversal in z-facial selectivity, rather than internal chelation.4 The stericallydemanding bornhydride reagent, lithium tri-set-butylbomhyd, has been compared with other common nucleophilic and electrophilic reducing agents, and is generally the most stereoselective of the metal hydrides evaluated. Revision in the Cram polar and steric transition state models for 1,3-asymmeaic induction’. is presented to rationalize the trends observed for these and related processes. Scheme I k -+ U(S-BU)@H RL THF, -783‘c * -pRL RL=CMe2CH=Cb 0 ‘&&- (1) Anbi:Spl=S2:oB Ad stereochamiatry Ml: -- Canl stelic Model TBS L&s-Bu)sBH Me -rpMe yrJh Rb Rp - CMe3 TBB THF. -78°C - I-&Me& - yvq yrJIy Anursyn-a3:97 ~c$J++&_ (2) I Crawl Polar Model P_Alkoxyketones. Representative metal hydrides. including the sterically demanding reagents Li(s-BuTsBH (L-Selectride) and Li(t-BuO)jAlH and the electrophilic reducing agents diisobutylalumiuum hydride @IBALE%) and 9-borabicyclo[3.3. llnonane (9-BBN), were evaluated against a series of &alkoxyketones.5 Permutations in the steric requirements of the Balky1 substituent (R@, the alkyl group appcnded to the carbonyl moiety (R), and the hydmxyl protecting group (P) wen made. Ketones 1 and 4 (eq 3) were selected to gauge the influence of the hydroxyl protecting group on the course of the reaction. Reduction of these substrates with the illustrated hydride reagents (Table I) revealed that formation of the 1.3~syn products 2 and 5 was preferred brespcctive of the nature of the hydroxyl protecting group. While internal chelation4 of the substrate followed by external hydride delivery accounts for formation of the 1,3-syn diastereomer,6 we believe that reaction through such a chelated intermediate, particularly in the strong donor solvent THF. is not responsibk for the observed stemoinduction in these cases. This assertion is supported by the fact that the highest syn selectivity shown in Table I (946) was achieved in the reduction of the silyl protected hydroxy ketone 4.7 It is also significant that the highest levels 1,3-syn st ereoselection in these reactions were achieved with L,i(s-Bu)3BH, a reagent which is generally not disposed toward chelation-controlled reduction.* 854 1 8542 Tdle ow OP i-r&A, Svn 2 5 1 P= PMB IpMoQC&i&Hp) 4 P =TBB (I-BuMezSi-) I. Rdotions d hVab Jx 3 6 (P : :A@ U(s-Bu)@H Li(t-RuO)@H DIBAL-H 9-BEN (3) Anff Ketortos (eq 3). p-Substituted 51 : 19 77 : 23 75: 22 59:43 5:s (P-J-W 94: cs 57: 13 73: 27 50: 50 ‘DclermkwdbyOLCulrlyr*dthupwlllrd~mtatt~ Tabk Il. lnnuuloeof Alkylaroup (R) on Rf@duabn (5q 4r .M;w,., L ax.x;tiph syn 8 $1 14 7 R=Me 10 R-i-i% 13 R=t-Bu 8:9 RIM5 hydride REx~:~*~(4) Anti 9 12 15 11:12 R-I-R 14: 15 R-t-& Li(MlJ~BH 51 : 49 79: 21 73: 27 U(t-BuO)sAlH 54: 44 89: 31 75: 25 DIBM-H 54; 45 50: 41 76: 24 51 : 49 xi: 46 66: 14 9.BBN %aIumlnodty OLCmAydnd thaupurl(lrdmadbnmlxtums In order to probe the influence of the alkyl substituent (R) appended to the carbonyl moiety, substrates 7,lO. and 13 were subjected to the standard set of rcduction conditions (eq 4, Table II). These substrates were also designed to evaluate the impact of the polar alkoxy substituent in a setting where the steric requirements of both & substituents (C!HzCI-IzAr and OCHzAr) are comparable. The data in Table II document that enhanced selectivity is observed as the size of the carbonyl substituent (R) is increased with the rerr-Bu ketone 13 generally exhibiting face selectivity the highest levels of syn dlastereoselection. In contrast, methyl ketone 7 exhibits poor cartiny irrespective of the hydride source. The influence of the size of the p-alkyl group (Re) on reaction diastereoselectivity was examined in the reduction of p-OTBS ketones 16,19,4, and 225b (eq 5, Table III). For both of the nucleophilic hydride reagents, Li(s-l3u)3BH and Li(r-BuO)gAlH, a strong correlation between syn diastereoselection and the steric demands of Ra is evident from the data. 0 Me % GMe H- Me*@ My&qp (5) 16 RB=Me 17 18 19 Rg = cH&yPh 30 21 5 6 4 Fte=i-Pr 23 24 22 Rp = t-Bu T5biaIII. Influenceof Substltutent (Rg) In the Illustrated HydrideReductions(eq Sy hydride 17: 19 %-Me 290:21 Rg=ui@#h Li(e-Bu)$H Ll(t-BuO)+JH 74: 26 78: 22 92 : 08 93: 17 5:8 R@~i” 94: 06 23:24 = t-&l 97 : 03 87: 13 91 : 09 DBAL-H 69: 31 69: 31 73: 27 76 : 24 9-Bed 72: 26 87: 33 50: 60 79: a Mastmlvily wm delamllned by QLC dyxb 21 of ihe Mpwlned maabll mlxlur. Based on the above observations, the following generalizations may be made: A) a turnover in carbonyi xfacial selectivity is observed upon changing the P-substituent (alkyl+OP); B) enhanced 1,3-syn stereosclectivlty in the reduction of kalkoxy ketones is observed with an increase in either the size of the B-alkyl moiety (Rp), the acyl substituent (R), or the hydroxyl protecting group (P). A transition state model that accounts for this reversal in diastereofacial selectivity as well as the other trends outlined above is currently lacking. A revision of the Cram polar model for 1,3+tereoinduction Ia has been suggested in the preceding Letter.9 The specific objection to this model hinges on the utilization of eclipsed rather than staggered transition structures (see Scheme I). As for predictive capacity, the Cram polar model does not correlate the influence of the carbonyl substituent (R) on reaction diastereoselectivity (Table II). On the other hand, electrostatic effects are clearly 8543 playing an important role in governing the sense of asymmetric induction as highlighted in the cases cited in eq 1 and 2. The following revision of Cram’s original polar model for 1,3-asymmetric induction is pmposed below. Revised Polar Model. It is proposed that those transition structures wherein the fiarbon <C# is oriented perpendicular to the <Tframework of the carbonyl moiety be considered in recognition of the Felkin postulate, supported by subsequent computational studies, that the staggered conformation between Co and the trigonal carbon undergoing reaction is preferred in such addition processes. to Staggered transition structures A and B the dipoles associated with C+-OR and the account for the data reported in this study. In both of these SEUCN~, In distinguishing between these two alternatives, structure A transforming carbonyl moiety sre stabilizing. accounts for the dependence of 1,3-induction on the size of the carbonyl substituent (R) that is not handled by Cram’s original proposal. The principal destabilizing element in B is the nonbonding m(R)C=O interaction. However, transition structure B might well bc favored in addition reactions to those substrates having sterically demanding Rp substituents (e.g. eq 2). For purposes of comparison, from the preceding Letter we have concluded that the preferred transition structure in the Mukaiyama aldol addition to B-alkoxyaldehydes is that corresponding to B (R = H). where the indicated nonbonding interaction m(H)C=O has been substantially diminished. Examination of potential transition states leading to the anti product diastereomerlt lead us to conclude that D is disfavored on the basis of steric considerations (Rgc)(R)C=O), while destabilizing electrostatic interactions are present in C. We also suggest that it is the electrostatic destabilization of C which differentiates the present polar model from the steric model E provided in Scheme El (OR = RM). Scheme II on OP Finally, why is reaction diastereoselectivity generally elevated with an increase in steric requirements of the hydride reagent? We speculate that the illustrated atui, rather than g~h,Iu relationship between nucleophile and C8 is enforced with sterically demanding nucleophiles. The Cram steric model (1968) for 1.3-induction in Steric Models for l&Asymmetric Induction. carbonyl addition has been widely recognized (Scheme I). la A less highly cited, but nonetheless significant study by Jacques and co-workerstb in the same year rationalized the stcreochemical course of the hydride reductions of P_alkyl substituted ketones (eq 1) through transition structures which may be closer to the consensus view of the preferred geometries for these processes. lo In view of the relevance of the Jacques proposal to the present investigation, it is reinterpreted here: A) Staggered rather than eclipsed transition structures are preferred having anti orientation between C8 and the forming bond (Fe&in). B) The dominant destabilizing interactions are between the acyl carbon substituent (R) and the ~substituents. These interactions are minimized in E where the illustrated relationship between (R) and the smallest @ubstituent @I in the present case) is established. 8544 A restatement of this model has recently been tqorted by Ohnold to account for the SteEOchemical course of nucleophilic additions to @wbstituted acylsilanes (eq 6). Some years ago we also anploycd an analysis related to that described by Jacques to rationalize the stcreochcrnical course of the illustrated hydroboration rea&on (eq 7).** SehemeN ohfw (1994) Evans (1982) The models for 1,3-asymmetric induction presented above and in the previous Letter rationalize the r-facial selectivity that is observed in the absence of inte.rnal chelation in the nucleophilic additions to &substituted aldehydes and ketones. Ongoing theoretical and experimental studies to further investigate the nature of 1,3induction will be reported in due course. References and Footnotes 1) For examples of nuclcqhilic additions to Balky1 substituted ketones w: (a) Leitereg. T. J_; Cram, D. J. /. Am. C&m. Sot. 2) 3) 4) s) 6) 7) 8) 9) 10) 11) 12) 1968.90,4011-4018. and 4019-4026. (b) Brienne, M-J.; Ouannea, C.; Jacques, J. Bull. Sot. 0&n. Fr. 1968.1036-1047. (c) Fleming I.; Lawrence, N. J. Chcm. Sot. Perkin Trans. I 1992.3309-33 18. (d) NaLada, M.: Urano. Y .: Kobaya&. S.: Ohno. M. Tetrakdron Lett. 1994.3.5, 741-744. For examples of the nucleophilic additions to protected p-aJkoxy ketones see: (a) Ukaji, Y; Kanda. K.: Yamameto. K, Fujisawa, T. Chum. Lea 1998.597-600. (b) Arco. M. J; Trammel, M-H.; White, J. D. 1. Org. Ckm. 1976.41.20752083. (c) Danishefsky. S. 1.: Annistead. D. M.; Wincott. F. E.: Selnick, H. G.: Hungate. R. J. Am. Chum. Sot. 1989. 111.2%7-2980. For reductions of p-amino ketcmca see: (a) Pilli, R. A.; Russowsky. D.; Dias. L. C. 1. Ckm. Sot. Perkin Trans. 1 1998.1213-1214. (e) Tnnnontini, M. Synrhti 1982.605644. Fur aa example of the lnflucnce of remute electrauatic effects on earbony x-facial r&ctivIty see; Wu. Y.-D.; Tucker,I. A.; Ho&K. N. 1. Am. Chem. Sot. 1991.113.5018-5027. (a) Cram, D. J.: Kopccky. K. R. J. Am. Ckm. Sot. 1959.81.2748-2755. (b) Reetz. M. T. Act. Cheat. Res. 1993.26. 462468andrefuenccs &cd tbetein. (c) Ager, D. J.: East, M. B. Tetrakdroti 1992,48 2803-2894. (a) The raductions were carried out at the following tempemtuma in THFz Li(s-BuhBH and DIBAL-H at -78 Oc, Li(tBuG)3AlH and 9-BBN dimer at 0 “c to room tcmpemttue. Stemo&zn ical assignments htvolved calversiou of the l$diols into the curwaponding accmnides or benzylidene &I, and analysis by NMR spectroscopy (see nf. 9). (b) The DIBAL.-H reduction of subatmtc 22 was cawied out in a vari#y of solvents to 8ive the folbwing ratios (l&yn : l&z&): THF (76 : 24). Et20 (63 : 27). CH2CI2 (62 : 38).andtolueme (64 : 36). (a) Fa a review of hydride additions to carbonyl annpounds see: Graves, N. in Co~e&nsivc Or+c Syntksis,; Trost. B. M.; Fleming. I. Eds.; Pergamon Press: New York. 1991: Vol 1. Chapter 1. (b) For examples of &as&rwAective hydride addi* to ketoneaseez Nogradi. M. in Stereoselecrivc SynthesS. VCH. New York, 1986;Ch+u 3.2 (a) Bloch. R.: Gilbert, L.: Gii C. Ternah&on Len. 1988.29. _loZl-1024. (b) Keck.G. E.: Andms, M. B.: CasteU&, S. J. Am. Chem. Sot. 1989.111,8136-8141.Howeves.for mcentemdenca in suppon of chelationby a vicinal OTBS ~poupsee: (c) Chen, X.; Holtelano,E. R.; Eliel, B. L.; Frye, S. V. 1. Am. Chem. Sot. 1992,114, 1778-1784. For examples illustrating the lack of chelation in the rcduelions of potected hydmxy-ketmx?s with Li(s-BuhBH &e: (a) Takaha&i. T.; Miyazawa, M.; Tsuji, J. Tetrakdron L&r. 1985.26.5139-5142. (b) Larcbeveque. M.; L&n&. J. J. Ghan. Sot., Chem. Common. 1985.83-84. Evans. D. A.; Duffy, J. L; Dart, M. J. Tetrakcdron Lcrr. 1994.35, thii issue. (a) Cbereat, M.; Aelkin. H.; Prudent, N. Tetrakdron Lctt. 1968. 2199-2204. Fa compuuriollpl evidence supparine the impormncc ofnonc&ps& gtomenies in carbonyl add&ion seez (b) Anh. N. T.; Elsensteht. 0. None. 1. C/&n. 1977.1.61-70. (c) Padden-Row. M. N.: Rondan. N. G.: Hot&, K. N. 1. AJPI.Chem. Sot. 1982.104.7162-7166. (d) Houk. K. N.: PaddenRow, M. N.; Rondan, N. G.; Wu. Y.-D.; Brown, F. K.; Spellmeyer, D. C.; Mea. J. T.; Li. Y.; Loncbarich. R. J. Science 1986,231, 1108-1117. Itisnotsuggts~edCCDDareeonly~ilionsllUeswhichmightgiverisetotheminorproductdiawer#wm Itis reasonablethateansiti~structurespoasessingagovckenlacionshipbetwwncgandnucleophileshwld~be~. (a) Evans, D. A.; Bartroli, J.: Godel. T. Tewakdron L&r. 1982.23. 45774580. (b) For a relatedexnmplesee ref. Ic. (c)COmputatiaRalJupportfa~modelpopostdbyushesbeen~byHouk,rcf.lOd (Re&ved in UsA 24 August 1994; revised 15 September 1994; accepted 21 September 1994)
© Copyright 2026