Mechanically reinforcement of optically functional
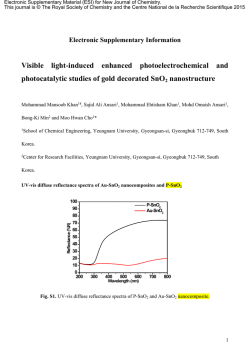
OPTICALLY TRANSPARENT NANOCOMPOSITE REINFORCED WITH MODIFIED CELLULOSE NANOFIBERS Yaser Dahman* and Amir Sani Department of Chemical Engineering, Ryerson University, Toronto, ON, Canada Abstract: In this work we report a novel example of optically transparent nano-structured composite which consists of surface-modified bacterial cellulose (B.C.) nanofibers reinforced in poly(hydroxyethyl methacrylate) (PHEMA) hydrogel matrix. To promote the interfacial strength between the nanofibers and the PHEMA phases, surface of the cellulose nanofibers was modified by fibrous acetylation to preserve the B.C. nanofibrillar morphology. The modified nanofibers were then graft copolymerized with acrylic based PHEMA hydrogel via free-radical mechanisms using a benzoyl-peroxide initiator. Several samples of grafted nanofibers were prepared with different degrees of acetylation and graft yields, which were characterized using 13C solid-state NMR, FTIR, and gravimetric method. The maximum degree of acetylation was quantified to be 2.3, while the gravimetrical graft yield was 82.35 %. The modified nanofibers were then reinforced into a polymeric matrix of PHEMA to form the final designed biocomposite. The nanofiber-network-reinforced PHEMA polymer composite maintained its transparency up to more than 1% nanofibrillar content. The biocomposite material transmitted more than 80 % of the light when the nanofiber network content was 1 wt%. The light transmittance was higher with samples with lower nanofibrillar content. The loss of transparency in B.C.-based nanocomposite was small, despite the differences in B.C. and PHEMA refractive indices. This clearly indicates the size effect of the nanoscaled fibres. These results clearly indicate the advanced properties of the nanocomposite which allow it to be combined with various optically functional materials having significantly different refractive indices and maintain the transparency of the final material. This novel nanocomposite is light in weight, highly flexible, and easy to be mould. Considering the unique properties of the cellulose nanofibers, in addition to the optically transparency of the final material, the nanocomposite is expected to have significant improvements in the thermal and mechanical properties, which will allow a wide range of applications. Keywords: nanobiomaterials, nanofibers, cellulose, fibrous acetylation, surface grafting, transmittance, refractive index. * Corresponding author, email: [email protected], Tel: (416) 979-5000 ext. 4080, Fax: (416) 979-5083 1 1.0 INTRODUCTION Bacterial cellulose (B.C.) is a nanomaterial produced by Acetobactor xylinum, with a diameter of less than 50 nm and a high degree of crystallinity. It is a linear polymer of glucose linked by β–(1-4)-glycosidic linkages that are similar to plant cellulose in chemical structure and has a degree of polymerization of 2000-6000. B.C. is produced in the form of pure nanofibres with unique physical properties. High surface:volume ratio combined with its unique poly-functionality, hydrophilicity and biocompatibility make B.C. a potential material for a range of biomedical applications. With its high elastic modulus of 78±17 GPa and breaking strengths of up to 1GN/m2 (10,000 MPa), B.C. can be used to form nanocomposites (Fontana et al., 1990; Deinema et al., 1971; Ross et al., 1991; Römling, 2002). Although, B.C. nanofibers have useful properties, it lacks properties of synthetic polymers, hence, drawing the attention to be used in “biocomposites”. Cellulose has been investigated for possible biocomposites with acrylic based polymers including poly(hydroxyethyl methacrylate) (PHEMA). PHEMA is a porous, cross-linked inert polymer hydrogel with excellent swelling behaviour in water and solvents. It has a little structural value, being ripped and deformed extremely easily. Optically clear PHEMA hydrogels are used as soft contact lenses. HEMA has also been tested for pharmaceutical applications (Karlson and Gatenholm, 1997; Seidel and Malmonge, 2000; Koo et al., 2002; Tsou et al., 2005). There has been special interest in grafting acrylic based monomers onto plant cellulose, while a little information is available on research using HEMA. Karlson et al. (1997) have controlled the degree, the place and uniformity of grafting PHEMA onto plant cellulose under swollen conditions. Nho et al. (2003) found an application for HEMA-grafted plant cellulose in hemi-dialysis using irradiation. They attached heparin to HEMA strands on the cellulose and measured anti-thermobic properties. Nishioka et al. (1992) found the thermal stability to be about that of the constituents in graft polymers with a low weight in grafts, and the thermal stability to be heavily impaired in the case of extensive grafting. They also found that a blend of graft polymer and homopolymer actually had a higher water capacity than any of the constituents of this blend. The only research involved in B.C. based biocomposite with PHEMA has produced a transparent composite with a low thermal-expansion coefficient similar to that of silicon crystal with a mechanical strength of five times that of engineered plastics, being an excellent material for a variety of applications (Iwamoto et al., 2005; Nogi et al., 2005; Yano et al., 2005). There has been no material that combined B.C. and PHEMA hydrogels or little work has done towards grafting of HEMA onto the cellulose. On this background, we postulated the importance of developing a novel method to combine B.C. and PHEMA in a pre-designed way to produce a stable biocomposite material with advanced properties. An approach was in surface/fibrous modification of the nanofibers in addition to grafting copolymerization, in which PHEMA can be polymerized as side chains of the B.C. hydroxyl groups. The technique allow for surface modifications of cellulose, which would increase water holding capacity, tear and temperature resistance. With this wealth of possible modifications, the applications of B.C. would grow from the commercially produced plastic celluloid to contact lenses, drug delivery systems, biosensors, pervaporation membranes and even the removal of heavy metals from water systems. 2 2.0 MATERIALS AND METHODS 2.1 Bacterial Cellulose Biosynthesis B.C. was produced in shake flasks using a fructose based medium using A. xylinum BPR 2001 (ATCC # 700178) at an optimum temperature of 28oC, in the form of beads (Joseph et al., 2003). B.C. was then treated with 1% (w/w) of NaOH in a bath of boiling water for 30 min and washed repeatedly with deionized water. The extracted B.C. has been kept in deionized water at 4oC. 2.2 Surface Acetylation of Bacterial Cellulose B.C. was partially acetylated to preserve the nanofibrillar morphology and improves the transparency of the targeted final biocomposites. It was transferred into acetic acid by stepwise solvent exchange and then was swollen for 72 h. B.C. was acetylated in a solution consisting acetic acid (20 parts), acetic anhydride (20 parts), and 97% sufuric acid (0.04 parts). The mixture was shaken vigorously for 1 h, and thereafter was allowed to stand for 1 h at room temperature (~25oC). B.C. was washed several times with methanol and water while being transferred back in aqueous solution by stepwise solvent exchange. 2.3 Graft Copolymerization The graft copolymerization of HEMA monomers onto cellulose acetate (CA) nanofibers was carried out with a benzoyl-peroxide (BPO) free-radical initiator. The aqueous solution of CA of known concentration was transferred to 100 mL of acetone by stepwise solvent exchange. Polymerization was carried out in a 500 mL glass reactor equipped with a reflux condenser and an overhead stirrer which was operated at 300 rpm under controlled temperature and N2. CA was continuously stirred in the 500-mL reaction vessel under N2 while adding 5% (wt dry cellulose) of BPO initiator. The initiator was allowed to interact with the cellulose acetylated fibres for 15 min at 60oC to form free radicals. This was followed by the addition of monomer(s). Free-radical graft polymerization was continued for 6 h at 60oC under N2 and the reactor temperature was brought to 25oC. The un-reacted monomer was removed by washing the fibres with a methanol/water (30:70 v/v), by stepwise solvent exchange. The un-reacted acrylic homopolymers were removed with benzene through repeated extraction. Finally, the CA grafted with PHEMA (CA-gPHEMA) was stored in water at 25oC. 2.4 Reinforcement Composites of the Grafted B.C. PHEMA crosslinked matrix was synthesized by free-radical mechanism at presence of CA-g-PHEMA nanofibers at different initial concentrations (0-1.0 % by weight). The homopolymerization reactions were carried out in a 500 mL glass reactor with BPO as initiator and ethylene glycol dimethacrylate as crosslinking agent. The final biocomposite material was soaked in water for several weeks before examining its transparency. 2.5 Characterization Experiments Nanostructured biocomposite samples were quantified for the amount of grafting using gravimetric methods. Compositions were characterized using FTIR and 13C Solid-State NMR. FTIR spectra recorded in Diffuse Reflectance mode performed on the nanofibers before and after modification. Spectra were analyzed using a Spectra-Tech Baseline Drifts accessory. The 13C Solid-State NMR spectra were recorded using a 600 MHz Varian Inova-600 spectrometer. 2.6 Transmittance Testing The final nanobiocomposite samples were cast on flat surfaces to form thin sheets of ~ 1 - 2 mm thickness. The transmittance of the final nanobiocomposite sheets was measured at wavelengths from 200 to 800 nm using a UV/ Visible Spectrophotometer (DU 520, Beckman Coulter). Regular transmittance was measured by placing the polymer specimens at ~20 cm from the entrance port of the UV lamp. 3 3.0 RESULTS AND DISCUSSIONS 3.1 Surface Acetylation of Bacterial Cellulose. Figure 1: (a) and (b) show the FTIR spectra of B.C. nanofibers before and after conducting the fibrous acetylation, respectively. Examination of this Figure reveals a monotonous decrease in the OH band at 3350 cm-1 and an increase in the three major bands of cellulose triacetate, i.e. the C=O stretching band at 1750 cm-1, the C-O band at 1240 cm-1 and the C-CH3 bands at 1375 and 1450 cm-1 respectively. The degree of substitution (DS) was quantified using back titration method. Results obtained showed that the DS had initially a steep rise as the additions of acetic anhydride were increased. Thereafter, DS gradually leveled off as the amount of reagent was increased, reaching the maximum DS obtained. The nonlinearity mainly indicates the fibrous heterogeneous surface acetylation. Moreover, this represents an initial rapid surface acetylation and a slow inside acetylation with the collapsing crystal structure of cellulose I. This proves that the reaction proceeds from the surface to the core of the semicrystalline nanofibers. Degrees of acetylation of the nanofibers for the different samples were quantified using the back titration method and found to be in the range of 1 – 2.3. C-CH3 C-O C=O (b) O-H (a) 3500 3000 2500 2000 Wavenumbers (cm-1) 1500 1000 Figure 1. FTIR spectra of B.C. nanofibers before (a) and after (b) acetylation (DS by titration was 2.3). 3.2 Graft Copolymerization of CA Nanofibers Graft copolymerization reactions were initiated by free-radical mechanism as follows: C6H5OCO–OCO6H5 2C6H5OCO* 60 o C 2C6H5OCO* 2C6H5*+2CO2 CA–OH + C6H5* CA–O* + C6H6 CA–O* + M CA–O–M* CA–O–M* + nM CA–O–M*(n+1) CA–O–M*(n+1) + CA–O–M*(m+1) Grafted Polymer where, CA-OH is cellulose acetate, CA-O* are cellulose acetate radicals, CA-OM* is the graft copolymer radical, and M is HEMA monomer. 4 The mechanism shows that when BPO initiator molecules were heated to 60oC, they were decomposed yielding phenyl radicals. The OH groups on CA nanofibers are the targeted grafting sites. The C5H5* radicals interact with the OH on CA surface that were not acetylated producing CA macroradicals, which initiate grafting on the fibres surface. A weight increase was observed in the benzene extracted grafted fibres compared to the weight of the CA before grafting. This indicates possible grafting of the B.C. nanofibers. Three samples of the grafted CA were prepared with different HEMA to CA weight ratios of 0.5, 1.0, and 2.0 in the feed; see Table 1. All the prominent peaks pertaining to polyHEMA arose clearly in the FTIR spectra (not shown) compared to what is in Figure 1. These peaks are: OH stretching at 3300 cm-1, ester methyl stretching vibrations at 2900 cm-1, asymmetric and symmetric CH2 stretching vibrations at 2800 cm-1, and carbonyl vibrations at 1740 cm-1. In addition to these peaks, some of the characteristic peaks of CA were also observed confirming the grafting. Quantitative analysis of the polyHEMA grafted on the nanofibres was done based on the characteristic carbonyl groups arose at 1740 cm-1. Figure 2 shows the 13C solid-state NMR of the nanofibers after surface graft-copolymerization with HEMA compared with the unmodified BC. Peak assignment of the 13C solid-state NMR spectra of PHEMA and CA-g-polyHEMA fibres that had initial weight ratios of 2:1 of HEMA:CA are summarized in Table 1. Figure 2. 13C solid-state NMR of surface grafted CA nanofibers with HEMA with initial weight ratios of 2:1 of HEMA:CA compared to unmodified B.C. nanofibers. The degree of acetylation and the amount of grafted PHEMA were obtained by comparing the peaks arising from the fast rotating methyl carbons (a8) at 28.8 ppm and carbonyl carbon (b1) at 178.8 ppm, with the peak arising from the carbons adjacent of ether linkage in cellulose at 71.07 ppm (Rana et al., 2006; Lim et al., 1999; Udhardt et al., 2005; Princi et al., 2005). The maximum degree of acetylation of cellulose nanofibers was found to be 2.5, which was quantified as 2.3 using the surface back titration method as indicated above. The PHEMA graft yield obtained gravimetrically was 82.35 %. Table 1 lists the different graft parameter calculated from the weight results. The diameter of the fibres was calculated from SEM pictures (not shown) results of which are summarized in Table 1. Examining results reveals that cellulose nanofibers’ diameter increased as the amount of HEMA used initially for the graft copolymerization increased. 5 Table 1. Characterization results for the samples of modified cellulose nanofibers based nanocomposite. Monomer/ Monomer Graft Graft HomoFibre Cellulose ratio Conversation yield Efficiency polymer diameter (w/w) (%) a (%)b (%)c (%)c (nm) d CA-g-PHMEA-0.5 0.5 96 11.76 11.76 88.24 75 - 90 CA-g-PHMEA-1.0 1.0 88 25.49 13.0 87.00 90 – 200 CA-g-PHMEA-2.0 2.0 86 82.35 21.0 79.00 150 - 200 a Calculated from the change in the weight after removal of un-reacted monomer by washing with methanol/ water mixture, b Calculated from the change in the weight after removal of homopolymers by washing with benzene through repeated extraction, c Calculated from FTIR spectra based on the carbonyl peaks, d Diameter of pure cellulose fibres ~ 27 - 40 nm. Sample ID 3.3 Optical Transmittance of Biocomposites Results for the light transmittance versus wavelength measurements (not shown) of the nanocomposite sheets of PHEMA reinforced with CA-g-polyHEMA in the wavelength range of 500-800 nm showed that the biocomposite material transmitted more than 80 % of the light, including surface reflection (Fresnel’s reflection). When comparing the light transmittance of the nanocomposite material against that of pure PHEMA, the degradation in light transmission due to the nanofiber network content of 1% (by weight) is ~ 10%, while it was less than 5% for the sample that had 0.05%. It is well-known that composite materials suffer from increased light scattering, resulting in a loss of transparency, caused by differences in the refractive indices of the materials in the composites. For the B.C. based nanocomposite, this loss of transparency is small, despite the differences in BC and PHEMA refractive indices (refractive index of cellulose fibre is 1.618 along the fibre and 1.544 in the transverse direction, and that of PHEMA is 1.49) (Stickler and Rhein, 1992). These results clearly indicate the size effect of the nano-scaled fibres, which allows it to combine with various optically functional materials having significantly different refractive indices. CONCLUSIONS Novel optically transparent nano-structured composites were synthesized based on PHEMA hydrogel matrix reinforced with surface modified B.C. nanofibers. This novel class of biocomposite has many improved thermal and mechanical characteristics such as lightness, high flexibility and high transparency. These characteristics will allow for a wide range of applications. BC nanofibers were first acetylated heterogeneously (i.e., without dissolution) to improve the transparency. Then, the acetylated fibers were then graft copolymerized with HEMA, and finally the modified nanofibers were reinforced into continuous matrix of PHEMA hydrogel with different nanofibrous contents. The nanofiber-network-reinforced PHEMA polymer composite maintained its transparency up to 1wt. % of the modified cellulose nanofiber content, as it transmitted more than 80 % or the light. For the BC based nanocomposite, this loss of transparency is small, which clearly indicates the size effect of the nano-scaled fibres. This characteristic allows the cellulose nanofibers to combine with various optically functional materials having significantly different refractive indices keeps the optical transparency of the final composites. ACKNOWLEDGMENT This work was financially supported by awards from the Natural Sciences and Engineering Research Council of Canada (NSERC). 6 REFERENCES 1. Fontana, J., de Souza, A., Fontana, C., Torriani, I., Moreschi, J., Gallotti B., de Souza, S., Narcisco, G., Bichara, J. and Farah L. The Future Prospects of Microbial Cellulose in Biomedical Applications; J Appl Biochem Biotechnol, 25/25 (1990), 253-264. 2. Deinema, M, and Zevehvizen, L. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation, Arch Microbiol, 78 (1971), 42-51. 3. Ross, P., Mayer, R., and Benzimann, M. Cellulose biosynthesis and function in bacteria. Microbiol, 55 (1991), 35-58. 4. Römling, U. Molecular biology of cellulose production in bacteria. Microbiol Res, 153 (2002), 205-212. 5. Karlson, J. and Gatenholm, P. Preparation and characterization of cellulose-supported HEMA hydrogels, Polymer, 38/18 (1997), 4727-4731. 6. Seidel, J. and Malmonge S. Synthesis of polyHEMA hydrogels for using on biomaterial. Bulk and solution radical-initiated polymerization techniques, Mater Res, 3/3 (2000), 79-83. 7. Koo, J., Smith, P. and Williams, R. Synthesis and characterization of methacrylate-based copolymers for integrated optical applications; Chem Mater, 14 (2002), 5030-5036. 8. Tsou, T., Tang, S., Huang, Y., Wu, J., Young, J., and Wang, H. Poly(2-hydroxyethyl methacrylate) wound dressing containing ciprofloxacin and its drug release; J Mater Sci: Mater Med, 16/2 (2005), 95-100. 9. Nho Y. and Kwon, O. Blood compatibility of AAc, HEMA, and PEGMA-grafted cellulose film; Radiat Phys Chem, 66 (2003), 299-307. 10. Nishioka, N., Yoshida, N. Thermal Decomposition of Cellulose/Synthetic Polymer Blends Containing Graft Copolymers; Polymer J, 24/10 (1992), 1009-1015. 11. Iwamoto, S., Nakagaito, A. N., Yano, H., Nogi, M. Optically transparent composites reinforced with plant fiber-based nanofibers; Appl Phys, 81 (2005), 1109 – 1112. 12. Nogi, M., Handa, K., Nakagito, A. N., Yano, H. Optically transparent bionanofiber composites with low sensitivity to refractive index of the polymer matrix; Appl Phys Lett, 87, 243110 (2005); DOI:10.1063/1.2146056 13. Yano, H.; Sugiyama, J., Nakagaito, A. N.; Nogi, M.; Matsuura, T.; Hikita, M.; Handa, K.; Optically transparent composites reinforced with networks of bacterial nanofibres. Adv Mater, 17 (2005), 153 – 155. 14. Rana, D., Matsuurra, T., Khulbe, K. C.; Feng, C. Study on the spin probe added polymeric dense membranes by 13C solid-state nuclear magnetic resonance spectroscopy; J Appl Polym Sci, 99 (2006), 3062-3069. 15. Lim, A., Schueneman, G. T., Novak, B. M. The T1ρ13C spin-lattice relaxation time of interpenetrating networks by solid state NMR; Solid State Commun, 109 (1999), 465-470. 16. Udhardt, U., Hesse, S., Klemm, D. Analytical Investigations of Bacterial Cellulose; Macromol Symp, 223 (2005), 201–202. 17. Princi, E., Vicini, S., Proietti, N., Capitani, D. Grafting polymerization on cellulose based textiles; A 13C solide state NMR caractérisation; Eur Polym J, 41 (2005), 1196 – 1203. 18. Guruprasad, K. H., Shashidhara, G. M. Grafting, blending, and biodegradability of cellulose acetate; J Appl Polym Sci, 91 (2004), 1716–1723. 19. Stickler, M.; Rhein, T., “Polymethacrylates” in Ullmann’s Encyclopedia of Industrial Chemistry, 5thed., Elvers, B., Hawkins, S.; Schultz, G. Eds., VHS: New York, 1992, A21, 473" 20. Joseph G., Rowe G., Margaritis A., Wan, W. Effects of polyacrylamide on bacterial cellulose production; J Chem Technol Biotechnol, 78 (2003), 964 - 970. 21. Kim, D., Nishiyama, Y., Kuga, S. Surface acetylation of bacterial cellulose; Cellulose, 9 (2002), 361–367. 7
© Copyright 2026