FeS Grains with Abundant Fe
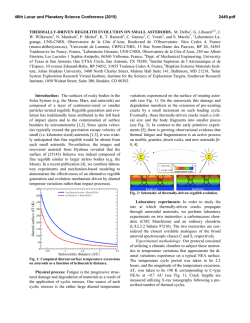
46th Lunar and Planetary Science Conference (2015) 1516.pdf FeS GRAINS WITH ABUNDANT Fe1-xO-Fe3O4 CORE-SHELL CRYSTALS IN THE ANGRITE D´ORBIGNY. Hwang S-L1, Shen P2, Chu H-T3, Yui T-F4, Iizuka Y4 and Varela M.E 5. 1Department of Materials Science and Engineering, National Dong Hwa University, Hualien, Taiwan, ROC ([email protected] ); 2 Department of Materials and Optoelectronic Science, National Sun Yat-sen University, Kaohsiung, Taiwan, ROC; 3 Central Geological Survey, PO Box 968, Taipei, Taiwan, ROC; 4Institute of Earth Sciences, Academia Sinica, Taipei, Taiwan, ROC, 5Instituto de Ciencias Astronómicas de la Tierra y del Espacio (ICATE) Avenida España 1512 sur, J5402DSP, San Juan, Argentina Introduction: The angrites are rocks as old as the solar system [1] with unusual petrological and geochemical characteristics. Notwithstanding their controversial genesis, they are considered to be igneous rocks of basaltic composition [e.g., 2 and references therein]. D´Orbigny is by far the largest (16.55 kg) angrite known and is peculiar in several respects. Its bulk chemical composition, mineralogical composition, mineral chemistry, rare gas contents and O isotope abundances are in line with other members of this meteorite class. However, the shape of D’Orbigny, its structure with highly porous lithologies alternating with compact ones and its mineralogical heterogeneity strongly suggest that the rock was not formed by simple crystallization of a basaltic melt [3, 4]. Although the source of angritic melts remains unknown, melts chemically resembling those of bulk angrites were produced by partial melting of Allende under very high oxygen fugacity conditions (IW+2) [5]. Albeit none of the experimentally produced melts match angritic melts in all parameters, these rocks are believed to have been formed under oxidizing conditions (e.g., [2 and references therein]). A previous chemical and petrological study of D'Orbigny suggests formation of this rock under redox changing conditions, ranging from reducing to highly oxidizing [3, 4]. Our study of high Ni-bearing metal and sulfides phases hosted by anorthite or anorthite and olivine in the groundmass of this rock strongly indicates that redox conditions in angrites need to be revised [6]. As another contribution towards this revision, we report in this abstract the presence of FeS grains with abundant Fe1-xO-Fe3O4 core shell crystals in the angrite D´Orbginy. Sample and Results: The polished thin section D´Orbigny NDHU-1 (National Dong Hwa University, Taiwan) was studied by optical microscopy and analytical scanning electron microscopy (FE-SEM). TEM samples were prepared by the FIB technique for selected area electron diffraction (SAED) using a JEOL 3010 AEM. The FeS grains hosted by anorthite are small (2040 µm), irregular in shape and host scarce metal (15%Ni) grains and abundant round oxides (Fig. 1 a), or host abundant metal (pure Fe) and few oxides (Fig. 1b). An analytical scanning electron microscopy study reveals that the round oxides are mainly composed by two phases: an euhedral (cubic-octahedral) FeO core surrounded by a Fe3O4 shell (Fig. 2). Figure 1a: BSE image of a FeS grain holding a polycrystalline metal pocket and abundant oxides. Figure 1b: BSE image of a FeS grain with abundant inclusions of metal (Fe) and some oxides. Figure 2, SEM-BSE micrographs showing a detail view of the round oxides. 46th Lunar and Planetary Science Conference (2015) A TEM study reveals that whereas the larger oxide particles always possess the FeO-Fe3O4 core-shell structure (Fig. 3), the smaller oxide particles are exclusively Fe3O4 (Fig. 4). 1516.pdf wards pentlandite, the Fe (10-15% Ni) phase is always polycrystalline with “weir” domain boundaries. A TEM image of the FeS phase also reveals the presence of well-developed domains (Fig. 5). Such domain boundaries are possibly due to nucleation from non-equivalent lattice sites of a more primitive phase to be identified in the future. Figure 3, TEM bight field image showing the euhedral FeO and the Fe3O4 shell. Figure 5, TEM bight field image showing the structure of troilite. The metal-sulfide and the FeS-oxide associations show the existence of redox changing conditions, which apparently changed in only one direction: the events became increasingly more oxidizing from one to the other. The presence of FeS with Fe1-xO-Fe3O4 coreshell crystals show that the latest event was highly oxidizing and possibly lasted only a short time. Figure 4, TEM bright field image showing the small Fe3O4 particles. These observations imply that it was an oxidation process from Fe (10% Ni) to nearly stoichiometric FeO as indicated by the absence of side band diffractions characteristic of paracrystalline distribution of defect clusters in non-stoichiometric Fe1-xO, and then to Fe3O4. These metal-sulfides-oxide associations are very different from those described by [6]. Whereas the euhedral Fe (50% Ni) phase hosted by pentlandite (see Fig. 2, [6]) is single crystal (fcc) with sharp edge to- References: [1] Wasserburg G. J. et al. (1977) Earth Planet. Sci. Lett. 161, 684; [2] Keil (2012) Chemie der Erde 72, 191-218; [3] Kurat et al., (2004) GCA 68, 1901-1921; [4] Varela et al., (2003) GCA 67, 5027–5046; [5] Jurewicz et al., (1993) GCA 57, 2123– 2139 ; [6] Varela et la., (2015), this conference.
© Copyright 2026