low incidence of activity of p-glycoprotein (p-170) in de novo
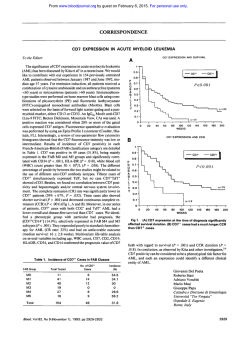
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. CORRESPONDENCE 3505 LOW INCIDENCE OF ACTIVITY OF P-GLYCOPROTEIN (P-170) IN DE NOVO ACUTE LYMPHOBLASTIC LEUKEMIA DETERMINED BY A FLOW CYTOMETRIC ASSAY To the Editor: Recently, two articles of the journal discussed the clinical significance of P-glycoprotein (P-gp)-mediated multidrug resistance (MDR) in human malignancies.’,’ As stated in the Editorial, thus far there has been no agreement on standardized screening method for the detection of MDR expressingtumor cells. Techniques determining the level of MDRl mRNA or immunohistochemicalstaining of P-gp have been widely used to examine MDR expression in human malignancies.Because posttranslational phosphorylationof P-gp may influence its function,’ we think that there is still a demand for investigational methods that give information concerning the “pumping” activity of P-gp. We investigated the P-gp expression in acute leukemia by means of a functional assay! Based on the fluorescent propertiesof the dye rhodamine 123 (Rh123), which is transported by P-gp, we measured the efflux/retention of this drug by flow cytometry in 29 patients with acute myeloid (AML) and 19 patients with acute lymphoblastic leukemia (ALL). Investigations were performed at time of diagnosis. For dual-fluorescence analysis with Rh 123 the leukemic cells were stained with the following phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs): CD34/CD 13/CD33(AML) and CDlO/CD19/CD7 (ALL). Fluorescence gates were placed around the PE-labeled blast cells and Rh 123 efflux was selectively assessed in these cells in the presence or absence of 10 pmol/L verapamil. Seventeen of 29 (59%) AML cases showed a significant Rh123 efflux that was completely blocked in the presence of verapamil (Fig 1). On the contrary, we could detect Rh123 efflux, and thus, P-gp “pumping” activity only in 1 ( 5 % ) of 19 ALL patients analyzed. This surprising observation clearly contradicts the results currently published by Goasguen et al: who found 38% P-gp expression in ALL. In this study P-gp expressionwas determined by immunocytochemistry using two MoAbs (C2 19 and JSBl ). In our ALL seriesthe immunologic subtype of the single Rh 123-positive case was of BALL. Until now we evaluated four ALL samples (including the Rh 123-positiveand three Rh 123-negativesamples) for the expression of MDRl mRNA by quantitative polymerase chain reaction (PCR). All four cases-Rh 123-positive as well as Rh 123-negative -expressed MDRl mRNA at quite the same level. The MDRl mRNA levels were quantified to the @,-microglobulinamplification product by means of high-performance liquid chromatography. Although we are aware that our results are preliminary, we want to point out that the detection of P-gp expression by immunocytochemistry or at the mRNA level does not necessarily mean that P-gp is functionally active. Posttranslational modification of P-gp, as described in tissue cultures,’ may also alter its function in clinical From www.bloodjournal.org by guest on February 6, 2015. For personal use only. CORRESPONDENCE 3506 Pt.#26 Rh I23 neg. Pt.#25 Rh 7 23 pos. 0 min M M d- 0 0 M n n M d- Cr) M 0 n 0 n dm M M n 0 -1. 1’. ’1. 1 9 t.4 Rhodamin 123 Mean Fluoreszenz samples and could explain the discrepancy between the findings of Goasguen et aI2and our results with the Rh 123 efflux assay in ALL. Consequently, we suppose that investigations which intend to define the clinical role of MDR expression in human malignancies should include MDR screening techniques that provide information about the “pumping” activity of P-gp. Chnstof Ludescher Wolfgang Hilbe Wolfgang Eisterer Josef Thaler Department of Internal Medicine Markus Gotwald Johann Hofmann Institute of Medical Chemistry and Biochemistry University of Innsbruck Innsbruck, Austria 120 min + Verap. 120 min - Verap. Fig I. TWO parameter counter plots of leukemic cells in an MDR - (patient no. 26) and an MDR+ (patient no. 25) AML case, stained with Rhl23 and the PE-labeled markers CD33 and CD34. In patient no. 25 Rh123 efflux (cells left to the cursor) is evident after 120 minutes in Rhl23-free medium (-V). Efflux is completely blocked in the presence of verapamil ( +V). Patient no. 26: no Rhl23 efflux is seen after 120 minutes. REFERENCES 1. Arceci RJ: Clinical significance of P-glycoprotein in multidrug resistance malignancies. Blood 8 1:22 15, 1993 2. Goasguen JE, Dossot JM, Fardel 0, Le Mee F, Le Gall E, Leblay R, Le Prise P, Chaperon J, Fauchet R: Expression of the multidrug resistance-associated P-glycoprotein (P- 170) in 59 cases of de novo acute lymphoblastic leukemia: Prognostic implications. Blood 81:2394, 1993 3. Hait WN, Aftab DT: Rational design and pre-clinical pharmacology of drugs for reversing multidrug resistance. Biochem Pharmacol43:103, 1992 4. Ludexher C, Thaler J, Drach D, Drach J, Spitaler M, Gattringer C, Huber H, Hofmann J: Detection of activity of P-glycoprotein in human tumour samples using rhodamine 123. Br J Haematol 82: 16I, 1992 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 3507 CORRESPONDENCE RESPONSE It is clear that detection of P- 170 on the cell membrane, or the mRNA level does not necessarily mean that P-glycoprotein (P-gp) is functionally active as stated by Ludescher et a1 in the previous letter. Two important comments can be made with respect to their remarks. Firstly, Ludescher et a1 showed the P-170 (so-called multidrug resistance [MDR]) phenotype in only four acute lymphoblastic leukemia (ALL) cases (all positive)by using a molecular biology technique that cannot be compared strictly with the immunocytochemical staining that we used. It is clear that a standardized technique is necessary. Many previously published reportd4 have addressed this subject. Moreover, we chose this test in view of its easiness and rapidity in a standard laboratory, and because its reliability given that fixation, incubation, antibody specificity: and secondary staining (APAAP technique) are well defined. Secondly, in our series: we showed that 6 of the 2 1 P- 170-positive cases had less than 11% positive cells; therefore, we can easily suppose that cases with such low number of P-170-positive cells cannot demonstrate Rh 123efflux by flow cytometry. For the same reason, cases with few P- 170-positive cells cannot be detected by flow cytometry, even when using appropriate antibodies. These cases (with low number of P- 170-positive cells) have been reported to be resistant cases or cases with early relapse by Musto et al.’ In our series, two of the six P- 170-positive cases (with <1 1% positive cells) did not achieve complete remission, and three of the four other had a relapse. This shows the high prognostic value of this methodology. Chaudhary and Roninson* have demonstrated a strong inverse correlation between the level of P-gp expression and the retention of fluorescent dyes in lymphoid cells, but in their study P-gp was detected in approximately 55% to 65% of the lymphoid cells and in a “large” fraction of the CD34+ cells. These results are not inconsistent with our interpretation. In oncology, prognostic criteria of value are those that predict complete remission and relapse. However, we agree with Ludescher et al in that posttranslational modification of P-170 can occur in leukemic cells, modifying the role ofthe transmembrane protein. This point is now under investigation in our laboratory. Jean E. Goasguen Beatrice Ly Sunaram Jean-Marc Dossot Erwan Mordelet Renee Fauchet Laboratoire d’Himatologie Hopital SUD Rennes, France REFERENCES 1. Grogan T, Dalton W, Rybski J, Pier C, Meltzer P, Richter L, Gleason M, Pindur J, Cline A, Scheper R, Tsuruo T, Salmon S Optimization of immunocytochemical P-glycoprotein assessment in multidrug-resistantplasma cell myeloma using three antibodies. Lab Invest 63315, 1990 2. Kartner N, Pore11 E, Bradley G, Ling V Detection of P-glycoprotein in multidrug resistant cell lines by monoclonal antibodies. Nature 316:820, 1985 3. Pileri SA, Sabattini E, Falini B, Tazzari PL, Gherlinzoni F, Michieli MG, Damiani D, Zucchini L, Gobbi M, Tsuruo T, Baccarani M: Immunohistochemical detection of the multidrug transport protein P-170 in human normal tissues and malignant lymphomas. Histopathology 19: 131, I99 1 4. Michieli M, Raspadori D, Damiani D, Geromin A, Gallizia C, Michelutti A, Fanin R, Fasola G, Russo D, Tazzari P, Pileri S, Mallardi F, Baccarani M: The expression of the multidrug resistance-associated glycoprotein in B-cell chronic lymphocytic leukemia. Br J Haematol 77:460, 1991 5. van der Valk P, van Kalken C, Ketelaars H, Broxterman HJ, Scheffer G, Kuipper CM, Tsuruo T, Lankelma J, Meijer CJLM, Pinedo HM, Scheper RJ: Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Ann Oncology 1:56, 1990 6. Goasguen JE, Dossot J-M, Fardel 0, Le Mee F, Le Gall E, Leblay R, Le Prise PY, Chaperon J, Fauchet R: Expression of the multidrug resistance-associatedP-glycoprotein (P- 170) in 59 cases of de novo acute lymphoblasticleukemia: Prognostic implications. Blood 81:2394, 1993 7. Musto P, Melillo L, Lombardi G, Matera R, di Giorgio G, Carotenuto M: High risk of early resistant relapse for leukaemic patients with presence of multidrug resistance associated P-glycoprotein positive cells in complete remission. Br J Haematol 7750, 1991 8. Chaudhary PM, Roninson I B Expression and activity of Pglycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell 66235, 1991 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1993 82: 3505-3507 Low incidence of activity of P-glycoprotein (P-170) in de novo acute lymphoblastic leukemia determined by a flow cytometric assay [letter; comment] C Ludescher, W Hilbe, W Eisterer, J Thaler, M Gotwald and J Hofmann Updated information and services can be found at: http://www.bloodjournal.org/content/82/11/3505.citation.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026