Home Treatment With Intravenous Enzyme Replacement
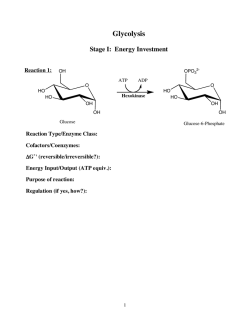
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. Home Treatment With Intravenous Enzyme Replacement Therapy for Gaucher Disease: An International Collaborative Study of 33 Patients By A. Zimran, C.E.M. Hollak, A. Abrahamov, M.H.J. van Oers, M. Kelly, and E. Beutler Intravenous enzyme replacement therapy (Alglucerase; Ceredase; Genzyme Corp, Boston, MA) is an effective and safe treatment for patients with type 1 Gaucher disease. In an attempt to reduce its high cost, a ”low-dose high-frequency” protocol (30 U/kg/mo, 3 times a week) was introduced and found to be as effective as the original high-dose protocol (60 U/kg every 2 weeks). Because receiving frequent infusions creates a burden for many patients, we have implemented a program of home treatment for our patients. W e now report the safety and feasibility of lowdose/high-frequency home intravenous enzyme-replacement therapy in 33 patients with Gaucher disease. The chronic nature of the treatment, its safety, lack of adverse effects, the stable condition of most patients, and the need to reduce the high cost make enzyme replacement for Gaucher disease a good candidate for intravenous home therapy. 0 1993 by The American Society of Hematology. I Instructions and performance of home treatment. Instructions for the safe and sterile administration of the drug were given to all NTRAVENOUS ENZYME replacement therapy (Alglucerase; Ceredase; Genzyme Corp, Boston, MA) has been introduced recently as an effective treatment for patients with type l Gaucher disease.‘ It has been shown to produce reversal of symptoms and signs of the disorder.’-’ The treatment has been found to be generally safe, and there are no contraindications to its administration. However, this treatment is enormously expensive and, therefore, cannot be available for many patients with Gaucher disease. In an attempt to reduce its cost while maintaining full therapeutic effectiveness,one of us (EB) has proposed the administration of a lower dose of the enzyme at a higher frequency.*,’ The experience of the investigators from three medical centers, using this low-dose/high-frequency protocol has been very g~-atifying.~,’,~ However, this altered treatment protocol creates a burden for some patients who need to come to the hospital 3 times a week with additional costs associated with each visit. In an attempt to reduce the disruption of the quality of life of our patients and to further reduce the cost ofthe administration of enzyme therapy, we have conducted a program of home treatment for our patients. We now report the feasibility, safety, and advantages of low-dose/high-frequency home intravenous enzyme-replacement therapy in 33 patients with Gaucher disease. PATIENTS AND METHODS Patients studied: Diagnosis and indications for treatment. All study patients had been diagnosed by at least two of the three diagnostic methods: histologic demonstration of typical Gaucher cells in a bone marrow specimen,low leukocyte acid @-glucosidase activity, and molecular diagnosis of a defined genotype associated with Gaucher disease. All patients suffered from symptomatic disease and had at least one of the following indications for enzyme therapy: moderate to massive organomegaly,symptomatic cytopenia, moderate to severe bone involvement and pulmonary involvement. A severity score index was assigned to each patient based on age at diagnosis and extent of organ involvement, as described previously.’ Treatment protocols. All patients received the low-dose/highfrequency protocol that usually consisted of 30 U/kg body weight per month, divided into equal doses given 3 times a week. Three patients received the 30 U/kg divided into daily equal doses for 6 months (2 patients) and 3 months; 4 patients received 50 U/kg/ month (3 times a week); and 4 other patients received a “very lowdose protocol” of 15 U/kg/month (3 times a week). All patients received the first infusions in the hospital before starting home treatment. Venous access devices (mainly Port-a-cath; Pharmacia, St Paul, MN) were surgically implanted into 24 of our patients; 9 patients received the infusion through a peripheral vein. BOCI~, VOI 82,NO4 (August 15).1993:pp 1107-1 109 patients during the initial in-hospital therapy. After this period, they were usually able to perform an infusion either into a peripheral vein or into the Port-a-cath system by themselves or with the help of another person. Although most of the Dutch patients were trained to perform the insertion ofthe needles by themselves, many of the Israeli and American patients received help from either a family member or a visiting nurse (Table 1). Most of the patients received Ceredase vials containing 50 U of the enzyme (10 U/mL). In other cases, when 50 U vials were not available, strict guidelines for the preservation of undiluted enzyme (in the standard 400 U/ mL vials) were given. The appropriate quantity of the enzyme was diluted aseptically in 100 mL of saline (0.9%),followed by administration by gravity or infusion-pumps either immediately or within 1 week. The enzyme, diluted in 100 mL of physiologic saline for administration, was stable for several weeks (Fig 1). RESULTS Our patient population included 17 women and 16 men, ranging in age from 4 to 53 years old, with a mean age of 29.5. Their mean seventy score index was 15.7 (range, 5 to 30), indicating moderate-to-severe phenotypic expression of the disease. They received a total of approximately 4,500 infusions during a period that ranged between 13 and 82 weeks per patient. Side effects. One patient complained of some abdominal discomfort after Ceredase infusion. Another patient reported 2 episodes of a visual disturbance occuring during From the Gaucher Clinic, Shaare-Zedek Medical Center, Jerusalem Israel; the Department of Internal Medicine and Hematology, Academic Medical Center, Amsterdam, The Netherlands; and the Department of Molecular and Experimental Medicine, Scripps Clinic and Research Foundation. La Jolla, CA. Submitted February 1, 1993; accepted April 13. 1993. Supported by the Helen Manuel Foundation (A.Z.), by National Institutes of Health Grants No. DK36639 and RR00833, and the Sam Stein and Rose Stein Charitable Trust Fund (E.B.). This is manuscript 7474-MEMfom the Scripps Research Institute. Address reprint requests to E. Beutler, MD, Department ofMolecular and Experimental Medicine, The Scripps Research Institute, 10666 N Torrey Pines Rd, La Jolla, CA 92037. The publication costs of this article were defayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact. 0 1993 by The American Society of Hematology. 0006-4971/93/8204-0032$3.00/0 1107 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. ZIMRAN ET AL 1108 Table 1. Person Performing the Infusion Person Performing Infusion No. of Infusions Patient Parent Brother/Sister Son/Daughter Spouse Nurse 19 8 1 1 2 4 In 3 patients the infusions were performed by more than one person. early infusions in the course of his treatment. These were diagnosed by an opthalmologist as “ocular migraine.” Subsequent infusions were not associated with any symptoms. There were no reports of side effects among any of the other patients during home therapy. Complications. There have been no serious complications associated with either the insertion of the needles or with the infusions ofthe enzyme itself. Phlebitis of a peripheral vein without systemic symptoms occurred in a single case. In another case, infection of a central line required a brief hospitalization and was resolved by intravenous antibiotics without the need to remove the line. Replacement of the Port-a-cath device was performed in a single case, because initially it had been placed on a painful spot on the chest. In another case, repositioning of the Port-a-cath line into the subclavian vein was required shortly after the implantation. Patient satisfaction. Patient satisfaction was not specifically addressed in this study. However, because all the patients received the treatment at home by choice, the patients, in general, reported satisfaction with the home treatment and preferred it to frequent visits to the hospital. There were no reports of fears or anxiety associated with the fact that the treatment was administered at home. Therapeutic responses. Every patient experienced a satisfactory response to treatment as judged by changes in blood counts and of organomegaly, measured either by ultrasound scanning or by quantitative magnetic resonance imaging. Indeed, as had been reported previously, the results of low-doselhigh-frequency therapy were indistinguishable from the much more costly low-frequencylhigh-dose therapy usually recommended by Genzyme Corp and by some investigators. Details of the responses of the patients treated are presented elsewhere. lo, Costs. When the treatment is performed in a hospital, the approximate costs of hospital visits three times a week in Israel, the United States, and the Netherlands are, in United States dollars, $1,700, $4,810, and $2,750, respectively, per month. The costs of home treatment plus a monthly visit to the hospital for follow-up are estimated to be $240, $500, and $320, respectively, representing approximately a 90% reduction in the costs (excluding the cost of the enzyme). antibiotics, y-globulin, chemotherapy, blood, parenteral nutrition, heparin, and factor VIII.’*-I9Although these treatments are associated with some risk of complications, they were still found suitable for home therapy. Among the major advantages of home therapy are the ability to maintain a near-normal lifestyle, including work or school attendance, in a comfortable and private environment; the patient’s choice of the most convenient time for therapy; and cost-effectiveness. With a proper selection of patients, basic education, and periodic monitoring and with the advent of various access devices that provide an easy way to insert a needle, intravenous home treatment has become feasible for very sick patients who otherwise would be hospitalized for long periods of time. Although originally used mainly by adults, recent reports have shown the applicability of this approach to children.” Low-dose enzyme-replacement therapy for Gaucher disease seems to be an ideal candidate for intravenous home therapy. The treatment, administered over a long period of time with high frequency, is very safe and practically free of significant complications. Most of the patients are clinically stable and, therefore, do not require frequent examinations by a physician. In some of the patients, especially children, contact with other patients with various hematologic and malignant disorders may create additional fears. The lowering of the cost of the treatment is particularly important, because alglucerase is presently one of the most expensive drugs in the world. Because, safety is a major consideration, after a single report of a putative anaphylactic reaction in a patient treated by the high-dose protocol in hospital (G. Pastore, Mount Sinai Medical Center, New York, NY, personal com- a 60 -- 9’ DISCUSSION Increasing numbers of patients suffering from different major diseases are receiving intravenous therapy at home. These home treatments include hemodialysis, intravenous 20 40 60 80 Days of Storage Fig 1. The stability of Alglucerase diluted to a concentration of approximately 1 U/mL in preservative-free isotonic sodium chloride solution for infusion into a patient with Gaucher disease. The residual enzyme from the IV tubing was stored at 4°C and assayed for acid @-glucosidaseactivity using 4-methyl umbelliferyl @-glucoside as substrate accordingto the method of Raghaven et aL9 Even after 60 days of storage, over 70%of the enzyme activity was still present. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. CEREDASE HOME THERAPY munication), we reviewed the course of our population of 6 1 Gaucher patients treated with Ceredase (both in the hospital and at home) and found 1 patient with a single minor episode of hives and pruritus that did not reoccur during later infusions. Combining the minimal adverse effects with the large number of infusions (-9,000 to date), we suggest that the safety profile of low-dose Ceredase compares very favorably with that of other medications that are administered at home. The absence of severe infectious complications is also remarkable in this population of Gaucher patients, which includes some who are relatively immunocompromised because of splenectomy or defective neutrophil function.’’ Thus, we find that home intravenous enzyme-replacement therapy for Gaucher disease is safe, feasible, and well accepted by the patients and their families. REFERENCES 1. Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE, Grewal RP, Yu K-T: Replacement therapy for inherited enzyme deficiency: Macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med 324:1464, 1991 2. Beutler E, Kay A, Saven A, Garver P, Thurston D, Dawson A, Rosenbloom B: Enzyme replacement therapy for Gaucher disease. Blood 78:1183, 1991 3. Parker RI, Barton NW, Read EJ, Brady RO: Hematologic improvement in a patient with Gaucher disease on long-term enzyme replacement therapy: Evidence for decreased splenic sequestration and improved red blood cell survival. Am J Hematol 38:130, 1991 4. Fallet S , Sibille A, Mendelson R, Shapiro D, Hermann G, GrabowskiGA: Enzyme augmentation in moderate to life-threatening Gaucher disease. Pediatr Res 31:496, 1992 5 . Figueroa ML, Rosenbloom BE, Kay AC, Garver P, Thurston DW, Koziol JA, Gelbart T, Beutler E: A less costly regimen of alglucerase to treat Gaucher’s disease. N Engl J Med 327:1632, I992 1109 6. Zimran A, Hadas-Halpern I, Abrahamov A: Enzyme replacement therapy for Gaucher’s disease. N Engl J Med 325:1810, 1991 7. Mistry PK, Davies S, Cofield A, a x o n AK, Cox TM: Successful treatment of bone marrow failure in Gaucher’s disease with lowdose modified glucocerebrosidase. Q J Med 83541, 1992 8. Zimran A, Sorge J, Gross E, Kubitz M, West C, Beutler E: Prediction of severity of Gaucher’s disease by identification of mutations at DNA level. Lancet 2:349, 1989 9. Raghavan SS, Topol J, Kolodny EH: Leukocyte beta-glucosidase in homozygotes and heterozygotes for Gaucher disease. Am J Hum Genet 32:158, 1980 IO. Zimran A, Hadas-Halpern I, Zevin S, Levy-Lahd E, Abrahamov A: Low dose high frequency enzyme replacement therapy for very young children with Gaucher disease. Br J Haematol (in press) 11. Hollak CEM, Aerts JMFG, van Oers MHJ: Treatment of Gaucher’s disease. N Engl J Med 328: 1565, 1993 12. Comprehensive Management of Hemophilia. Philadelphia, PA, F.A. Davis, 1976 13. Chapel H, Brennan V: Self-infusion with immunoglobulins at home. J Clin Pathol44:358, 199I 14. Homza SA: Continuous home heparin infusion therapy in the ambulatory patient. J Intraven Nurs 14:37, 1991 15. Davies MG, Feeley TM, Moore DJ, Shanik GD: Home parenteral nutrition using a totally implanted subcutaneous venous access device. Br J Clin Pract 44:750, 1990 16. Fox E, Peace K, Neale TJ, Momson RB, Hatfield PJ, Mellsop G: “Quality of life” for patients with end-stage renal failure. Ren Fail 13:31, 1991 17. New PB, Swanson GF, Bulich RG, Taplin GC: Ambulatory antibiotic infusion devices: Extending the spectrum of outpatient therapies. Am J Med 91:455, 1991 18. Portez DM: High tech comes home; comment. Am J Med 91:453, 1991 19. WernikowskiJT, Rotheney M, Dawson S , Andrew M: Evaluation of home intravenous antibiotic program in pediatric oncology. Am J Pediatr Hematol Oncol 13:144, 199I 20. Aker M, Zimran A, Abrahamov A, Horowitz M, Matzner Y: Abnormal neutrophil chemotaxis in Gaucher disease. Br J Haemato1 83:187, 1993 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1993 82: 1107-1109 Home treatment with intravenous enzyme replacement therapy for Gaucher disease: an international collaborative study of 33 patients A Zimran, CE Hollak, A Abrahamov, MH van Oers, M Kelly and E Beutler Updated information and services can be found at: http://www.bloodjournal.org/content/82/4/1107.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026