Chemokine Regulation of Human Megakaryocytopoiesis
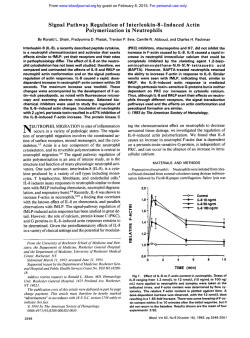
Chemokine Regulation of Human Megakaryocytopoiesis By Alan M. Gewirtz, Jin Zhang, Janina Ratajczak, Mariusz Ratajczak, Kwang Sook Park, Changqing Li, Zhanqing Yan, and Mortirner Poncz We have previously shown that platelet factor 4 (PF4). a platelet-specific CXC chemokine, can directly andspecifically inhibit human megakaryocyte colony formation. We therefore hypothesized that PF4 might functionas anegative autocrine regulator of megakaryocytopoiesis. Herein we present additional studies characterizing the inhibitory effect of CXC chemokines on humanmegakaryocyte development. We first corroborated our initial studies by showing that recombinant human (rH1 PF4, like the native protein, inhibited megakaryocytopoiesis. We then examined the inhibitory properties of other CXC family members. Neutrophil activating peptide-2 (NAP-2). a naturally occurring N-terminally cleaved PTG peptide, was foundt o inhibit megakaryocytopoiesis with two t o three orders of magnitude greater potency thanPF4. Structure functionstudies showed thatan N-terminal mutation, which eliminated NAP-2's neutrophil activating properties (NAP-2mA), also abrogated its ability t o inhibit megakaryocyte development. Further investigations of this type demonstrated that a chimeric PF4 protein (AELR/PF4) in which PF4's N-terminus wasreplaced with the first four amino acids of NAP-P was also a potent inhibitorof megakaryocytopoiesis. lnterleukin (IL)-8, another CXC chemokine, and three CC chemokines (macrophage inhibitory protein-l& [MIP-la], MIP-1/3, and C101 also specifically inhibited megakaryocyte colonyformation atNAP-2 equivalent doses. CXC and CC chemokine inhibition was additive suggesting that the effects might be mediated through a common pathway. The inhibitory effects of NAP-2 and MIPl a could not overcome be by adding physiologically relevant amounts ofrecombinant human megakaryocyte growth and development factor (MGDR) (50 ng/mL) t o t h ecultures. UsingNorthernblotand reverse transcriptase-polymerase chainreaction (RT-PCR) based analyses, we documented mRNA expression of IL-8 receptor isoforms a and /3 in total platelet RNA and in normal humanmegakaryocytes, respectively. Based on these results, we hypothesize that chemokines play a physiologic role in regulating megakaryocytopoiesis. Because chemokines are elaborated by ancillary marrow cells, both autocrine and paracrine growth control exerted, in part, is suggested, the effects of which might be through a and /3 IL-8 receptors. 0 7995 by The American Society of Hematology. H for example factor V mRNA expression, could be inhibited by nanogram quantities of PF4, colony inhibition occurs in the range of -25 pg/mL.I6 Though such concentrations are routinely observed in serum, whether marrowconcentrations of this magnitude can really be achieved in vivo is debatable. Accordingly, we entertained the possibility that other members of the chemokine family to which PF4 and PTG belonged might possess similar, or perhaps even more potent, inhibitory activity against the megakaryocyte lineage.I9-*' Chemokines are 70 to 100 amino acids long and contain four cysteine r e s i d ~ e s . l The ~ - ~ family ~ has diverged into two main subgroups. PF4 and PTG are members of the CXC or a chemokine family subgroup. In this group the first two of the four conserved cysteine residues are separated from each other by one additional amino acid residue. This subfamily also includes interleukin-8 (IL-8), a potent neutrophil activating agent. PF4 and PTG are poor neutrophil activators, but a cleavage product of PTG, neutrophil activating peptide- UMAN MEGAKARYOCYTOPOIESIS and platelet production are complex processes whose regulation remains incompletely understood. The identification and cloning of the c-mpl ligand, now known as thrombopoietin (TPO) or MGDR, represents an important advance in this area because this protein appears to be the major regulator of megakaryocyte development. MGDR has been shown, for example, to increase the number, size, andploidy of megakaryocytes, and in vivo administration markedly increases platelet count in recipient anirnal~."~ Nevertheless, MGDR is clearly not the sole regulator of the megakaryocytelplatelet axis, as innumerable cytokine experiments5"' and knockout experiments of the MGDR receptor, c-Mpl," have shown. Besides positive effectors, it is possible that megakaryocytopoiesis is regulated by inhibitory influences as well. In this regard, it has been observed that megakaryocyte colony growth is inferior in serum than in platelet-poor plasma suggesting that a platelet released product inhibits megakaryocyte g r o ~ t h . ' ~Megakaryocytes "~ also appear to release products that inhibit their own development. We,and others, have shown that one of the platelet-specific a-granule chemokines, platelet factor 4 (PF4), can inhibit megakaryocyte colony formation in a lineage-specific fashion.I6"' Inhibition is manifested by a decrease in the number and size of megakaryocyte colonies. In our hands,16 the related platelet-specific a-granule chemokine, PTG, does not exert similar effects on cultured cells, though conflicting findings have been reported by others." In aggregate, these studies suggest that megakaryocytopoiesis and thrombopoiesis maybe controlled to an as yet undetermined extent by a negative autocrine growth loop. One caveat in attributing a physiologic role to PF4 in the regulation of human megakaryocyte development is the amount of material required to observe the negative growth effect. Though some markers of megakaryocyte maturation, Blood, Vol 86, No 7 (October l), 1995: pp 2559-2567 Fromthe Departments of Pathology, Medicine and Pediatrics, University of Pennsylvania School of Medicine, Philadelphia; The Children's Hospital of Philadelphia, Philadelphia, PA; and the Department of Microbiology, Korea University School of Medicine, Seoul, Korea. Submitted January 9, 1995; accepted June 2, 1995. Supported by Grants No. CA36896 to A.M.G. and HL.37419 to M.P. from the National Institutes of Health and the Tobacco Research Council Grant No. 3152 to M.P. Address reprint requests to Mortimer Poncz, MD, The Children 'S Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104. The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" in accordance with I 8 U.S.C. section 1734 solely to indicate this fact. 0 1995 by The American Society of Hematology. 0006-4971~.5/8607-0019$3.00/0 2559 2560 GEWIRTZ ET AL 2 (NAP-2), is a potent neutrophil a c t i v a t ~ r . ~Of ~ ”note, ~ two different IL-8 receptors (IL-8R) are expressedbyneutrophils. One is designated the a or type I IL-8R and binds IL-8 efficiently, but NAP-2 poorly. A second /? or type 2 IL-8R has also been described, which binds IL-8 well, and NAP-2 with moderate affinity.20.26,27The C C o r p chemokines, in which the first two cysteine residues are immediately adjacent to each other, represent the other branch of this family. CC chemokines such as macrophage inhibitory protein (MIP)-la**appear to be important monocyte stimulating agents andmay also selectively suppress human megakaryocyte development. A CC chemokine receptor has been defined.*’ The PF4 and PTG studies described above suggested that neutrophil activating potency and megakaryocyte colony inhibitory activity paralleled each other. To test this hypothesis, we examined the effect of the neutrophil activatorNAP2 on human megakaryocyte development in vitro. We also sought to determine the megakaryocytecolonyinhibitory activity of other members of this chemokine family. Herein, we present data showing that a number of CXC and CC chemokines are potent inhibitors of human megakaryocytopoiesis and that megakaryocytes express a number of chemokine receptors. These data suggest that chemokines, perhaps elaborated by ancillary cells in the marrow microenvironment, may play a physiologically significant role in regulating human megakaryocyte development by both autoctine and paracrine mechanisms. MATERIALS AND METHODS Cells Light-density bone marrow mononuclear cells (MNC) were obtained from normal remunerated consenting donors and depleted of adherent cells and T lymphocytes (A-T-MNC) as described.3o CD34’ were enriched from the A-T-MNC population by incubation with anti-human progenitor cell antigen (HPCA)-1 murine monoclonal antibodies (Becton Dickinson, San Jose, CA) and subsequent immunoselection of antibody-labeled cells with magnetic beads according tothe manufacturer’s protocol (Dynal, Oslo, Norway) as described.” Purity of CD34’ cells selected in this manner exceeded 85%. Hematopoietic Colony Assays All assays were performed with either peripheral blood or bone marrow obtained from normal consenting volunteers. Hematopoietic In colonies were grown and identified as previously brief, colony assays were performed with either unseparated lightdensity marrownuclear cells (MNC) or with MNC depleted of monocyte-macrophages and T-lymphocytes as previously described.” Final plated cell concentrations were 2 X 10’lmL. Cultures were supplemented with 30% vollvol of aplastic anemia serum, except when supplemented with recombinant human MGDF (generously provided by Dr Pamela Hunt, Amgen Corp, Thousand Oaks, CA).4 Megakaryocyte and granulocyte colonies were supplemented with rH IL-3 (-29 U/mL) and rH granulocyte-macrophage colonystimulating factor (GM-CSF) (-5 ng/mL) (Genetics Institute, Cambridge, MA), except for the megakaryocyte studies that tested MGDF effect. Erythroid colonies were supplemented with recombinant erythropoietin (5 U/mL) (Amgen Corp). Recombinant human chemokines were added just before plating. Megakaryocyte colonies were enumerated after 12 days in culture by indirect immunofluorescence using either a highly-specific rabbit antihuman platelet membrane glycoprotein antiserum’h~3”.31 or :I monoclonal anti-GPIIbflIIa complex antibody A2AY.” Binding of the probe antibody was detected with a species appropriate Ruorescein-conjugated secondary antibody (Meloy, Springfield, VA). A cluster of three or more intensely fluorescent cells were defined as one colony. Plates were read by two separate individuals, including one blinded toplate designation. Results were in agreement between both readers within 5% to 10%. Results are reported as the mean L standard error (SE) of colonies enumerated. Colony forming units-erythroid (CFU-E) were cultured for 7 days and thenenumerated after staining with I % benzidine and hematoxylin as previously described.’” Colony-forming unit granulocyte-macrophage (CFU-GM) were cultured and identified as previously described.3” In Vitro Synthesis of Recombinant Human Chemokines The construction of the T,-promoter expression vectors for PF4, NAP-2, AELRPF4, in which the N-terminal amino acid sequence of PF4 preceding the first cysteine residue is replaced with the amino acids A-E-L-R, and NAP-2“2’A in whichthe second amino acid residue ofNAP-2is mutated from glutamic acidto alanine has been previously described.” These vectors wereusedto express BL21(DE3)pLys S (Novarecombinant proteins in Escherichia coli gen. Madison, WI), which were allowed to grow to an optical density (OD),,of -0.9, which was followed byan additional 3 hours of growth in 1 mmol/L isopropyl-thiogalactopyranoside that induced recombinant protein expression. deThe recombinant proteins were processed aspreviously ~ c r i b e d . ’Bacteria ~ . ~ ~ were centrifuged at 3,OOO rpm in a Sorvall GSA rotor for 10 minutes andthen resuspended in %,,th the volume of TED (50 mmollL Tris HCI, pH 8.0; I mmol/L EDTA; and I mmotl L dithiothreitol [D=]). The cells were recentrifuged andresuspended in the same volume of TED with 0.1 mg/mL of lysozyme added for 30 minutes. The lysate was sonicated at 4°C three times with a Branson Soniiier (Branson Ultrasonics, Danbury, CT) using a microtip at a power of 6 for 1 minute each cycle. The samples were centrifuged for 10 minutes in a Sorvall GSA rotor for l 0 minutes at 10,000 rpm, andthe supernatants were collected and stored. Recombinant proteins were purified from the supernatants of bacterial lysates described above by a two-step procedure. Initially, they were applied to a heparin agarose column (Sigma Chemical CO, St Louis, MO), washed with TE buffer (50 mmoln Tris HCl, pH 8.0. and 1 m m o a EDTA) containing 0.15 m o l n NaCl and eluted with TE buffer containing 0.5 mollL NaCl for all the NAP-2 proteins and 1.5 moULNaCl for all the PF4 proteins. The eluted proteins were concentrated usingYM3 Amico ultrafiltration filters (W.R. Grace C O ,Beverly, MA), and the buffer was switched to 0.1% trifluoroacetic acid. The concentrated samples were further fractionated on reverse phase high performance liquid chromatography (HPLC). All purified proteins wererun on 20% precast sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) underreduced condition followed by Coomassie blue staining. Further identification was done by enzyme-linked immunosorbent assay (ELBA1 as previously described.” Recombinant protein concentrations were determined using a Coomassie Protein Assay Reagent Kit with bovine serum albumin as a standard (Pierce, Rockford, IL). Recombinant 72 amino acid IL-8 protein was purchased (R & D Inc, Minneapolis, MN), and MIP- la and 0 were generous gifts from Drs Barbara Sherry and Anthony Cerami (Rockefeller University, New York, NY).” Medium containing the CC chemokine C-l0 and control medium were generously provided by Dr Mark Berger (University of Pennsylvania, Philadelphia). 2561 CHEMOKINEREGULATION OF HUMAN MEGAKARYOCYTOPOIESIS similar to those we previously reported with highly purified, serum-derived material. Mature rH PF4 protein was syntheThe IL-8Ra and IL-8RP cDNAs were kindly provided by Dr sized in Escherichia coli as described in Materials and MethIngrid U. SchraufsWer (Scripps Institute, La Jolla, CA) in the exods. Its amino acid sequence was identical to the native pression vector ~ s F F V . n e oThe . ~ ~ 1.2-kb coding regions for both protein, except that the recombinant material retained an the IL-8Ra and IL-8RP cDNAs, the 3.3-kb platelet glycoprotein IIb initiating methionine residue.34Biologic integrity of the engi(GPIlb), and the 0.4-kb P G cDNA inserts”-38 were released from their respective vectors by EcoRI digestion. All of the above cDNA neeredprotein was demonstrated in chemotactic studies inserts were purified from an agarose gel using GENECLEAN where the rH PF4 was as effective as serum-derived PF4.34 (BiolOl, Vista, CA). These inserts were then labeled using ( Y - ~ ~ P - In a plasma clot assay, both recombinant and native material dCTP, random primers, and Klenow to -lo* CPM/pg DNA. inhibited megakaryocyte colony formation in an identical Total platelet RNA was prepared from platelet rich plasma (PRP) manner throughout the dose range tested (Fig 1A). In obtained from 1 0 0 mL of sodium citrate anticoagulated blood. Only agreement with our previously reported results, inhibition the top two-thirds of the PRP was used. After spinning down the was dose-dependent and most significant at concentrations platelets, platelet RNA was prepared using the guanidinium thiocya2 10 pg/mL.I6 Also in agreement with our previous results, The amount natdacid phenol single-step RNA isolation te~hnique.’~ the numbers of cells/colony (Fig 1B) and the size of cells of RNA applied per lane was equivalent to -25% of the total yield. The RNA was fractionated on gels containing formaldehyde folcomprising the colonies (Fig 1C) were also diminished in the lowed by transfer to Genescreen Plus membrane (DuPont CO, Wilpresence of exogenous PF4. Finally, inhibition was megamington, DE) and hybridization to the labeled probe as previously karyocyte lineage specific (data not shown). IL-8Ra and IL-8RP Platelet Northern Blot - described.40 Reverse Transcriptase-Polymerase Chain Reaction of Purijied Megakaryocyte mRNA Human megakaryocytes were isolated from the marrow of remunerated, normal addt volunteers. The isolation of greater than 99% pure megakaryocytes was accomplished as previously described using counterflow centrifugal elutriation (Beckman J2-21M; Standard elutriation motor; Beckman Instruments; Mountainview, CA) to obtain an initial megakaryocyte enrichment:’ followed by isolation of morphologically recognizable mature megakaryocytes with a micromanipulator. RNA was isolated from -50 megakaryocytes in less than 3 hours’ time. mRNA was isolated from these cells using the Quick-Pre mRNA Purification Kit (Pharmacia, Piscataway, NJ). The final mRNApellet was washed with 75% ethanol and resuspended in 10 pL of triple distilled autoclaved water. Reverse transcription of the megakaryocyte mRNA was performed using 4 pL of the original sample heated to 65°C for 10 minutes and then cooled on ice for 3 minutes. A total of 100 U of Moloney murine leukemia reverse transcriptase (RT) (GIBCO BRL, Gaithersburg, MD), 50 ng of random primers (Boehringer Mannheim, Indianapolis, IN), 40 U of RNAzin (Promega, Madison, WI), and dNTPs (50 pmoVL each) were added to the tube and incubated for 1 hour at 37°C. Specific oligo primer pairs used for the polymerase chain reaction (PCR) amplification with the megakaryocyte random cDNA were as follows: II-8Ra: 5“ATGTCAAATATTACAGATCC-3’ (sense oligonucleotide; 1 to 20 base, beginning at the transcriptional start site) and 5”AGATTCATAGACAGTCCCCA-3’ (antisense, 500 to 481 base);and IL-8Rp: 5’GAGGACCCAGGTGATCCAGG-3’ (sense, 816 to 835 base) and 5‘-GAGAGTAGTGGAAGTGTGCC-3‘ (antisense, 1065 to 1046 The anticipated PCR products are, therefore, 500 bp and 249 bp for IL-8Ra and P, respectively. Ten microliters of the reverse transcription reaction or 100 ng of the appropriate IL-8R cDNA or water was used in a 100-pL PCR reaction using 30 ng of both sense and antisense primers for each receptor and 2.5 U Taq polymerase (Promega) using manufacturer’s supplied buffer and 2.5 pmoVL MgClz. PCR conditions were melting at 95°C for 1 minute, annealing at 60°C, and extension at 72°C for 30 rounds. A total of 10 pL of each final PCR product was size-fractionated on a 1.2% agarose gel. RESULTS Hematopoietic Effects of Recombinant PF4 We firstdetermined whether rH PF4 made in a prokaryotic system would manifest effects on megakaryocytopoiesis NAP-2, AELWPF4 and Mutation Proteins We have previously shown that PTG does not inhibit in vitro megakaryocytopoiesis.’6However, it is now known that a neutrophil cathepsin G N-terminal cleavage product of PTG, a 70-amino acid protein termed NAP-2, is a biologically active form of this protein. NAP-2 is a potent activator of neutrophils, binding to the IL-8RP on neutrophils.26To extend our structure/function studies and to begin to understand the mechanismby which chemokines inhibit megakaryocyte development, we tested whether NAP-2, as opposed to PTG, would inhibit megakaryocytopoiesis. As shown in Fig 2A, megakaryocyte colony formation wasmuchmore sensitive to the inhibitory effects of NAP-2 than PF4. Where PF4 inhibited in the pg/rnL range, half maximal inhibitory concentrations of NAP-2 were in the 10 to 100 ng/mL range. These concentrations were comparable to those needed for NAP-2 to activate neutrophil^.^^ As was the case with PF4, NAP-2’s inhibitory effect was lineage-specific. It has previously been reported that at PF4 concentrations 2 10 pg/mL inhibition of CFU-E and C m - G M might be ~bserved.”.’~ However, we did not observe such effects in our cultures and no definitive changes in CFU-E or CFU-GM colony formation were observed with increasing doses of NAP-2. We also sought to determine if NAP-2 inhibited megakaryocyte development directly, or indirectly via a secondary effect on accessory marrow cells. To address this question, marrow mononuclear cells were depleted of monocyte-macrophages and T-lymphocytes and then enriched for CD34+ cells using immunomagnetic beads before exposure to NAP2. Purity of the CD34+ cells in this population consistently exceeded 85%. At 50 pg/mL, PF4 decreased the number of megakaryocyte colonies to 8% of the control, and at 750 ngl mL, NAP-2 decreased expression to 10% of the control. These results support the hypothesis that the chemokines tested exerted a direct inhibitory effect on megakaryocyte progenitor cells. Because NAP-2 is thought to activate neutrophils through IL-8R0, we tested other NAP-2 and PF4 mutant constructs whose interactions with the neutrophil IL-8 receptors have been previously defined.33For example, alanine substitutions GEWIRTZ ET AL 2562 I I 3 5 3 00 2 5 2 5 0 15 10 I PF4 I I I I I 40 @g/ml) no PF4 supplement 0 3 5 53 0 2 51 02 0 1 5 PF4 40 (pg/ml) PF4 supplement Fig 1. Inhibition of megakaryocytopoiesis by rPF4. (A) Percent decrease in the number of colonies seen per lo5 marrow cells plated at increasing concentrations of PF4. (01,rH PF4; (0).native PF4. (B)The total numbers of cells seen per colony with increasing concentrations of rPF4 are shown. (C) Immunofluorescence of typical megakaryocyte colonies with (bottom) and without rH PF4 (top), demonstrating the decrease in the size of the colony andof the megakaryocytes within thecolony. Data in (A) and (B) represent analysis of two or moreseparate experiments with four separate plates for each data point. The number of megakaryocyte colonies seen without added chemokine ranged from 56 t o 107 per plate. at the N-terminus of NAP-2 markedly inhibit its ability to activate neutrophils. Interestingly, NAP-2""*, in which the second amino acid is mutated from glutamic acid to alanine (Table l), also loses its ability to inhibit megakaryocytopoiesis (Fig 2B). In contrast, AELRPF4, in which PF4's first eight amino acids proximal to the first cysteine residue are replaced with the four NAP-2 N-amino acid residues preceding its first cysteine residue, is equivalent to NAP-2 in terms of its ability to activate neutrophils and to inhibit megakaryocyte colony formation (Fig 2B). Inhibitory Effects of Other Chemokines We tested additional chemokines to see whether they too could inhibit megakaryocytopoiesis. IL-8 was almost as effective as NAP-2 in inhibiting megakaryocytopoiesis in the 5 to 500 ng/mL concentration range (Fig 3A). Surprisingly, the CC chemokine MIP-la was equally effective (Fig 3A). Inhibition with both of these chemokines was again noted to be lineage specific (data not shown). Two additional CC chemokines were tested and also inhibited megakaryocytopoiesis. MIP- 10 decreased megakaryocyte colony formation to 52% of the untreated control value at 500 ng/mL, and a culture supernatant containing C-IO, another member of the CC chemokine family, decreased megakaryocyte colonies to 35% of control values at a concentration of 2% (vol/vol). We thensought to determine whether the inhibitory effects of the CXC and CC chemokines might be additive or synergistic. Progenitor cells were therefore cultured with the individual chemokines at varying doses, or with the two added together. As shown in Fig 3B, the combination of NAP-2 and MIP-la had greater inhibitory effect than either chemokine individually. There does not appear to be a synergistic effect. REGULATIONCHEMOKINE OF HUMAN MEGAKARYOCYTOPOIESIS chemokine dose, megakaryocyte colony formation was not completely extinguished. When compared with an untreated control group, -30% of maximal colony formation was still observed even atthe highest combined dose level. The nature of the difference@)between this resistant subpopulation and those progenitor cells that were sensitive to the chemokine inhibitory effects remains to be determined. Finally, we also determined whether thechemokine inhibitory effect could be abrogated by MGDF stimulation. We first established that, in our culture system, megakaryocyte colony growth was maximal at MGDF doses -20 ng/mL. At such doses, the number ofcolonies observed in the culture dishes was comparable to the number observed when optimal concentrations of aplastic anemia serum plus L - 3 and E-6 were used as stimulators. We then cultured progenitor cells in the presence of 50 ng/mL of MGDF and varying doses of either NAP-2 or MIP-la. As shown inFig 4,in comparison to the numbers of colonies obtained in the presence of MGDF alone, NAP-2 at 2 5 ng/mL and MIF"1a at >50 ng/ mL significantly inhibited colony formation. Therefore, even in the presence of a significant physiologic stimulator, used at maximally effective concentrations, these chemokines appeared capable of blunting megakaryocyte colony growth. " - 1 B 2563 '"7 Demonstration of the Expression of IL-8Ra and IL-8Rp by Megakaryocytes 0 ' d ;OO 1'01 1'02 NAP-2 1'0s 1'04 1'0s 1'06 1'07 (ng/ml) Fig 2. inhibition of m.gakaryocytopoi.rh by NAP-2 and related constructs. (A) Percant decr.rw in the number of megakaryocyte colonies aeon par I C marrow caih plated at incroasing concentrations of NAP-2. (01,CFU-Meg colonies; (m), CFU-GM colonies; and (AI, CFU-E colonin. PM studlrat 25 pglmL were also done. (0). CFU-Mag colonies; (U), CFU-GM colonies; and (A), CFU-E colonies. Error bar nfm to OM standard devlatlon for r m p l n studied four or mora times. IBI Analyois of CFU-Meg colony n u m h forPF4 (01. NAP-2 (01,NAP-2(X), and AEWPF4 (t). Data In (AI and (B) analysis of two or mora .rpernte exporimento wlth four soparota plates for r c h doto point. The numbor of megakaryocyte colonin m n without added chemokine rangedfrom 51 to 128 per pinta. Rather, the combination of NAP-2 and MIP-la better approximates an additive effect. If the inhibition observed is additive, CXC and CC chemokines may inhibit megakaryocyte development by signaling through a common pathway. It should also be noted that even at the highest combined The above studies suggested that chemokines exert a direct effect on megakaryocyte development. If our interpretation ofthe experiments was correct, it follows that megakaryocytes should express the receptors that bind these ligands. To provide these important data, we pursued two independent, but complementary approaches. First, we isolated total platelet RNA and looked for E-8Ra and IL-8R@-A. The presence of these messages in the total RNA pool would provide evidence that these receptors might, in fact, be expressed by megakaryocytes. We also performed RT-PCRfor these same messages on an essentially pure population of normal human megakaryocytes. Platelet RNA was extracted from the top two-thirds of platelet-rich plasma of low-speed centrifuged blood. White cell contamination was approximately 1 cell per 5,000 platelets. Duplicate lanes of total RNA were hybridizedto cDNAs for the L-8Ra and IL-8RP and platelet GPIIb and P G as platelet-specific positive controls. As seen in Fig 5A, there were single detectable bands of the expected size of -3 kb for both of the L - 8 receptors and 3.3 kb for GPIIb.38.42 An intense signal was detected with pTG at 0.8 kb with additional bands seen at 1.2 and 1.8 kb.Total peripheral blood neutrophils detect two major L-8R bands of 2.4 and 3.0 kb, - Table 1. Recombinant PF4 and NAP-2 Chemoklnes Name Amino Acid Sequence NAP-2 (wild type) NAP-2EW PF4 (wild type) AELWPF4 AELRCMC THCN DGRKICLDPDAPRIKKIVQKLAGDESAD +RCMC. THCN. . DGRKICLDPDAPRIKKIVQKLAGDESAD EAEEDGDLQCLC PHCP . NGRKICLDLQAPLYKKIIKKLLES e C L C . . PHCP .NGRKICLDLQAPLYKKIIKLLES Amino acid differences from wild type are underlined. ... .. ... ... . . . . .. GEWIRTZ ET AL 2564 2 3 o 7 0- -\ a 0 2 ; 0 0 O 3 m . a m a \ 2 ag\ - 5 " 0 O Ya d MIP-1 a IL-8 a g m = z Y g 0 z 2.5 NAP-2 while HL60 cell lines only express the 3.0 kb band, similar to the single band we detected in platelet RNA.4' Even though the blots depicted in Fig 5 were hybridized under high stringency conditions, it is possible that we were only detecting a single IL-8R species because of the -70% homology in the coding regions of the IL-8R cDNAs. Therefore, to provide additional proof that we were, in fact, detecting both IL-8Ra and IL-gRP, weperformedRT-PCR reactions on mRNA isolated from a small number of 100% pure normal human megakaryocytes!3 Primers chosen were unique for the two IL-8 receptors, as shown by the fact that the primers do not yield cross-amplify using IL-8 receptor cDNAs (data not shown). These RT-PCR studies demonstrate that both IL-8 receptors are expressed in megakaryocytes (Fig 5B). DISCUSSION o k T L X O Chemokine added (ng/ml) Fig 3. Inhibitory effects of CXC and CC chemokines on megakaryocytopoiesis. (AI The inhibitory effect of 11-8 and MIP-la onmegakaryocyte formation relative t o an untreated control. NAP2 data done simultaneously are also included. Data represent the average of two experiments with four plates readeach for point in each experiment. Errorbars refer t o one SD. At every concentration shown, the inhibitory effect was significant atP c .003. (B)Effect of combined treatment withNAP-2 and MIP-la.Megakaryocyte colony formation was tested withNAP-2 ( W , MIP-la (01,or both (XI. Error bar refers t o 1 SD for samples studied four or more times.Data in (A) and (El represent analysis of two or more separate experiments with four separate plates for each data point. 100461 0.04 0 T T 0.009 S NAP-2 We have previously shown that PF4, a platelet-specific CXC chemokine, can directly inhibit in vitro megakaryocytopoiesis. We now show that other CXC chemokines, NAP2, IL-8, and even the more distantly related CC chemokines, MIP-la and P, and C10, have a similar direct inhibitory effect on in vitro megakaryocyte development as manifested by the appearance of fewer colonies composed of smaller numbers of less mature cells. Inhibition is observed when chemokines are added to progenitor cell cultures at concentrations equivalent to those at which theyactivate neutrophils and monocytes,-" suggesting that the inhibitory signals they generate maybeof physiologic significance. Studies performed with mutated NAP-2 and PF4 proteins also support this hypothesis as the ability to activate neutrophils and to inhibit megakaryocytopoiesis closely parallel each other. Another highly suggestive finding is that megakaryocytes express both a and isoforms of the IL-8 receptor. Nevertheless, although the presence of these receptors provides a mechanism for the observed inhibitory effects of some of the CXC chemokines, further studies will be needed to define the full repertoire of megakaryocyte chemokine receptors and, in particular, whether CC chemokine receptors also exist on these cells. .L8 10 0.y02 5'0 5 0 0 (ng/ml) 5 5'0 560 M I P - l a (ng/rnl) Fig 4. Interaction of MGDF and chemokines. Inhibitory studies with NAP-2 and MIP-la were performed in the presence of 50 ng/mL of rH MGDF. Data represent the average of two experiments with four plates read for each point in each experiment. Error barsrefer t o one SD. The Pvalue foreach study is shownabove the error bar. CHEMOKINE REGULATION OF HUMAN MEGAKARYOCYTOPOIESIS 2565 Although many stimulatory cytokines have been described, relativelyfewinhibitoryproteinshavebeenelucidated.The best-studied examples of these include the interferons (INFs), W tumor necrosis factor-a (TNF-a),transforming growth factorlp (TGF-p), and MP-la.""' Thephysiologicroleofthese c?! cytokines in the regulation ofhuman hematopoiesis remains speculative.44 This is, in part, because the biologic effects of kb thesecytokinesarecontextdependent, a situationthathas tendedtogenerateapparentlycontradictoryreportsontheir activity. For example, M P - l a appears to specifically inhibit 4.4 the growth of cycling hematopoietic cells,46 but when admixed with IL-3 or GM-CSF appears to promote colony growth in a synergistic manner:'In addition, TNF-a and the INFs appear to be general suppressers of hematopoietic cell growth, while 2.4 the activity of TGF-p is similar to MIP-la in that it appears to block proliferation of growth factor stimulated progenitor cells, but alone may stimulate CFU-GM and CFU-E.These observationshavepromptedspeculationthatsomeofthese inhibitors, in particular TGF-p and MP-la, may play a role in maintaining stem cells in a Go state when hematopoiesis is otherwise stimulated." In support of this hypothesis,it has been reportedthatinhibitionof TGF-p expression with antisense DNA increases the apparent numberof assayable multilineage progenitor cells in cord blood?R These apparently discordant results may also be explained by the observation that the inhibitory effects of M P - l a and TGF-p depend on the maturation of theprogenitorthathasbeenexposedtothischemokine.4".49.%1 Many investigatorshavereportedthat MP-la either has no effect on, or actually stimulates the growth of morematureprogenitors,whileitsuppressesthegrowthof more primitive C ~ I I The S ~ fact ~ ~ that ~ ~ the chemokines we have investigated have no apparent effect on CFU-GM derived colony formation is in accord with these results. Why the apparently mature CFU-Meg are inhibited by these chemokines when CFU-GM are not, remains unknown. Whether the findings we report are of physiologic significance remains unclear. The fact that members of this family can inhibit megakaryocyte development at concentrations three orders of magnitude lower than PF4 is suggestive of physiologic relevance because concentrations in this range are likely achievable in vivo. Further, the ability to have significant inhibition despite maximal levels of TPO also supports a potential in vivo role. Nevertheless, establishing whether there is a correlation between megakaryocyte/platelet mass and chemokine concentrations, either in plasma or in the marrow, will have to be investigated. This willbe particularly important in patients with inflammatory states who maybe expected to have relatively high circulating " chemokine levels, and perhaps paradoxically, reactive thrombocytosis. Measurement of MGDF levels will be of Ra equal importance then, as we have already speculated that Fig 5. Expression of IL-8Ra and f3 RNA in megakaryocytes. (A) stimulatory growth factors may overdrive the effects of apNorthern blot analysis of totalplateletRNAprobed with the parently weaker negative regulators.'" Indeed, we have also cDNAs for pTG, GPllb, IL-8Rrr, and IL-8Rf3. (B) Agarose gel of the hypothesized that negative regulatory loops, whether autoRT-PCR products of IL-8Ra and p. Lane 1, mRNA from isolated megakaryocytes, Lane 2, the cDNA for appropriate receptor, and crine or paracrine in nature, are more likely to play a role Lane 3, water control. Size markers are indicated at the side of in regulating basal, or nonstimulated, megakaryocyte proboth (A) and (B). duction." If shown to be of physiologic significance, these studies may represent a significant advance in the development of new pharmacologic strategies to regulate disordered or inappropriate thrombopoiesis. A 7.5 m I t B M 1 2 3 1 2 3 I L-8 IL-8RR 2566 GEWIRTZ ET AL N, Izaquirre C: The growth of large 14.MessnerHA,Jamal megakaryocyte colonies from human bone marrow. J Cell Physiol We thank Dr Pamela Hunt at AmgenCorp (Thousand Oaks,CA) 1:45, 1994 for the MGDF, Ingrid U. Schraufstlitter at the Scripps Institute (La JS, Harker 15.Kimura HS, BursteinSA,ThorningD,Powell Jolla, CA) for the E-8R cDNAs, and Dr Mark Berger at the UniverLA, Fialkow PJ, AdamsonJ W : Human megakaryocytic progenitors sity of Pennsylvania (Philadelphia, PA) for the C10-containing me(CFU-M) assayed in methylcellulose: Physical characteristics and dium. requirements for growth. J Cell Physiol 118:87, 1984 16. Gewirtz AM, Calabretta B, Rucinski B, Niewiarowski S, Xu REFERENCES WY: Inhibition of human megakaryocytopoiesis in vitroby platelet 1. De SauvageFJ,HassPE,SpencerSD,MalloyBE,Gurney factor 4 (PF4) and a synthetic COOH-terminal PF4 peptide. J Clin Invest 83:1477, 1989 AL, SpencerSA,DarbonneWC, Hemel WJ, Wong SC, Kuang W-J, Oles KJ, Hultgren B, Solberg LA, Jr., Goeddel DV, Eaton DL: 17.HanZC,SenskbeL,AbgrallJF, Bribe J: Platelet factor 4 Stimulation of megakaryocytopoiesis and thrombopoiesis by the cinhibits human megakaryocytopoiesis in vitro. Blood751234, 1990 Mpl ligand. Nature 369:533, 1994 18. Han ZC, Bellucci S,Tenza D, Caen JP: Negative regulation 2. Luk S, Kaushansky K, HollyRD, Kuijper JL, Lofton-Day CE, of human rnegakaryocytopoiesisby human platelet factor 4 and beta Oort PJ, Grant FJ, Heipel MD, BurkheadSK, Kramer JM, Bell LA, thromboglobulin:Comparativeanalysisinbone m o w cultures Sprecher CA, Blumberg H, Johnson R, Prunkard D, Ching AFT, from normal individuals and patients with essential thrombocythaeMathewes SL, Bailey MC, Forstrom JW, Buddle MM, Osbom SG, mia and immune thrombocytopenic purpura. Br J Haematol 74:395, Evans SJ, Sheppard PO, F’resnell SR Cloning and expression of 1990 murine thrombopoietin cDNA and stimulation of platelet production 19. Baggiolini M, Dewald B, Moser B: Interleukin-8 and related in vivo. Nature 369565, 1994 chemotactic cytokines-CXC and CC chemokines. Adv Immunol 3. Kaushansky K, Lok S. Holly R D , Broudy VC, Lin N, Bailey 55:97, 1994 MC, ForstromJ W , Buddle MM, Oort PJ, HagenFS,Roth GI, Papa20.Horuk R: The interleukin-8-receptorfamily:Fromchemoyannopoulou T, Foster DC: Promotion of megakaryocyte progenitor kines to malaria. Immunol Today 15:169, 1994 expansion and differentiation by the c-Mpl ligand thrombopoietin. 21. Mantovani A, Sozzani S : Chemokines. Lancet 343:923, 1994 Nature 369:568, 1994 22. Walz A, Baggiolini M: A novel cleavage product of 0-throm4. Bartley T D , Bogenberger J, Hunt P, Li Y-S, Lu HS, Martin boglobulin formed in cultures of stimulated mononuclear cellsactiF, Chang M-S, Samal B, Nichol L,Swift S , JohnsonMJ,Hsu vates human neutrophils. Biochem Biophys Res Commun 159:969, R-Y, Parker VP, Suggs S , Skrine JD, Merewether LA, Clogston C, 1989 Hsu E, Hokom MM, Hornkohl A, Choi E, Pangelinan M, Sun Y, 23. Walz A, Dewald B, Tscharner V, Baggiolini M: Effect of Mar V: Identification and cloning of a megakaryocyte growth and neutrophil-activating peptide NAP-2,platelet basic protein, connecdevelopment factor that is a ligand for the cytokine receptor Mpl. tivetissue-activatingpeptide m,andplatelet factor 4onhuman Cell 77:1117, 1994 neutrophils. J Exp Med 170:1745, 1989 5. Leary AG, Yang YC, ClarkSC, Gasson JC, GoldeDW, Ogawa 24. Walz A, Baggiolini M: Generation of the neutrophil-activatM: Recombinant gibbon Interleukin 3 supports formation of human ing peptide NAP-2 from platelet basic protein or connective tissue multilineage colonies and blast cell colonies in culture: Comparison peptide 111 through monocyte protease. J Exp Med 171:449, 1990 with recombinant human granulocyte-macrophage colony stimulat25. Holt JC, Yan Z, Lu W, Stewart GJ, Niewiarowski S: Isolation, ing factor. Blood 701343, 1987 characterization, and immunological detection of neutrophil activat6. WarrenMK,ConroyLB.Rose JS: Therole of interleukin ing peptide 2: A proteolytic degradation product of platelet basic 6 and interleukin 1 in megakaryocyte development. Exp Hematol protein. Proc Soc Exp Biology Med 199:171, 1991 17:1095, 1989 26. Leonard El, Yoshimura T, Rot A, Noer K, Walz A, Baggiolini 7. Williams N, De Giorgio T, Banu N, Withy R, Hirano T, KishiM: Chemotactic activity and receptor binding of neutrophil attractmot0T:Recornbinantinterleukin 6 stimulatesimmaturemurine ant/activation protein-l (NAP-l) and structurally related host demegakaryocytes. Exp Hematol 18:69, 1990 fense cytokines: Interaction of NAP-2 with the NAP-l receptor. J 8. Quesenberry PJ, McGrath HE, Williams M E , Robinson BE, Leukoc Biol49:258, 1991 Deacon DH,Clark S, Urdal D, McNiece I K Multifactor stimulation 27. Murphy PM, Tiffany HL: Cloning of complementary DNA of megakaryocytopoiesis: Effects of interleukin 6. Exp Hematol encoding functional a human interleukin-8 receptor. Science 19:35,1991 253:1280, 1991 9. Takahashi T,Tsuyuoka R, Ueda Y, Suzuki A, IchibaS, Okuno 28. Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines Y, Nakamura K,Imura H: Megakaryocyte potentiating activity of GK, BurdickMD, Pope RM, Strieter RM: Macrophage inflammatory IL-l, IL-6 and GM-CSF as evaluated by their action on in vitro protein-la. A novel chemotactic cytokinefor macrophages in rheuhuman megakaryocytic colonies. Br J Haematol78:480, 1991 matoid arthritis. J Clin Invest 93:921, 1994 10. Kaushansky K, O’Hara P, Bemer K, Segal GM, Hagen FS, 29. Neote K, Digregorio D, Mak JY, Honk R, Schall TJ: MolecuAdamson W. Genomic cloning, characterization and multilineage lar-cloning, functional expression, and signaling characteristics of a growth-promoting activity of human granulocyte macrophage colC-C chemokine receptor. J Immunol72415, 1994 ony-stimulating factor. Proc Natl Acad Sci USA 83:3101, 1986 30. Ratajczak M Z , Kuczynski W,Onodera K, Moore J, Ratajc11. Briddell RA, Bruno E, Cooper RJ, Brandt J E , Hoffman R zak J, Kregenow DA, DeRiel K, Gewirtz AM. A reappraisal of the Effect ofc-kit ligand on in vitro human megakaryocytopoiesis. Blood role of the insulin like growth factor-I (IGF-I) in the regulation of 78:2854, 1991 the human hematopoiesis. J Clin Invest 94:320, 1994 MW: 12.Gurney AL, Carvermoore K, DesavageFJ,Moore 31. Gewirtz A M , Xu WY, Mangan KF: Role of natural killer Thrombocytopenia in c-Mpl-deficient mice. Science 265:1445, 1994 cells, in comparisonwith T lymphocytes and monocytes in the regu13. Vainchenker W, ChapmanJ, Deschamps JF, Vinci G, Bouget lation of normal human megakaryocytopoiesis.J Immunol 139:2915, J, Titeux M, Breton-Gorius J Normal human serum contains a fac1987 tor(s) capable of inhibiting megakaryocyte colony formation. Exp 32.BennettJS, Hoxie JA,Leitman SF, Vilaire G, CinesDB: Hematol 10650, 1982 ACKNOWLEDGMENT CHEMOKINEREGULATION OF HUMAN MEGAKARYOCYTOPOIESIS Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci USA 80:2417, 1983 33. Yan Z, Zhang J, Holt JC, Stewart GJ, Niewiarowski S, Poncz M: Structural requirements of platelet chemokines for neutrophil activation. Blood 84:2334, 1994 34. Park KS, Rifat S, Eck H, Adachi K, Surrey S, Poncz M: Biologic and biochemic properties of recombinant platelet factor 4 demonstrate identity with the native protein. Blood 75:1290, 1990 35. Wang JM, Sherry B, Fivash MJ, Kelvin DJ, Oppenheim JJ: Human recombinant macrophage inflammatory protein-l alpha and -beta and monocyte chemotactic and activating factor utilize common and unique receptors on human monocytes. J Immunol 150:3022, 1993 36. Schraufsttitter IU, Barritt DS, Ma M, Oades ZG, Cochrane CG: Multiple sites on IL-8responsible for binding to Q and p IL8 receptors. J Immunol 151:6418, 1993 37. Majumdar S, Gonder D, Koutsis B, Poncz M: Characterization of the human P-thromboglobulin gene. Comparison with the gene for platelet factor 4. J Biol Chem 2665785, 1991 38. Poncz M, Eisman R, Heidenreich R, Silver SM, Vilaire G, Surrey S, Schwartz E, Bennett JS: Structure of the platelet membrane glycoprotein IIb: Homology to the alpha subunits of the vitronectin and fibronectin receptors. J Biol Chem 262:8476, 1987 39. Newman PJ,Gorski J, White GCII, Gidwitz S, Cretney CJ, Aster RH: Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest 82:739, 1988 40. Alwine JC: Hybridization analysis of specific RNAs by electrophoretic separation in agarose gels and transfer to diazobenzyloxymethyl paper. Gene Amplif Anal 2565, 1981 41. Gewirtz AM, Keefer M, Doshi A, Annamali HC, Chiu C, Colman RW: Biology of human megakaryocyte factor V. Blood 67:1639, 1986 42. Moser B, Barella L, Mattei S, Schumacher C, Boulay F, Colombo MP, Baggiolini M: Expression of transcripts for two in- 2567 terleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J 294285, 1993 43. Gewirtz A, Boghosian-Sell L, Catani L, Ratajczak M, Shen YM, Schreiber AD. Expression of FcyRII and CD4 receptors by normal human megakaryocytes. Exp Hematol20:512, 1992 44. Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood 81:2844, 1993 45. Keller JR, Bartelmez SH, Sitnicka E, Ruscetti F W , Ortiz M, Gooya JM, Jacobsen SEW Distinct and overlapping direct effects of macrophage inflammatory protein-la and transforming growth factor p on hematopoietic progenitor/stem cell growth. Blood 84:2175, 1994 46. Jacobsen SEW, Ruscetti F W , Oniz M, Gooya JM, Keller JR: The growth response of Lin-Thy-l+ hematopoietic progenitors to cytokines is determined by the balance between synergy of multiple stimulators and negative cooperation of multipIe inhibitors. Exp Hematot 22:985, 1994 47. Mantel C, Kim YJ, Cooper S, Dwon B, Broxmeyer HE. Polymerization of murine macrophage inflammatory protein l a inactivates its myelosuppressive effects in vitro: The active form is a monomer. Proc Natl Acad Sci USA 90:2232, 1993 48. Cardoso AA, Li ML, Batard P, Hatzfeld A, Brown EL, Levesque JP, Sookdeo H, Panterne B, Sansilvestri P, Clark SC, Hatzfeld J. Release from quiescence of CD34TD38- human umbilical cord blood cells reveals their potentiality to engraft adults. Proc Natl Acad Sci USA 908707, 1993 49. Broxmeyer HE, Sherry B, Lu L, Cooper S, Oh K-0, TekampOlson P, Kwon BS, Cerami A. Enhancing and suppressing effects of recombinant murine macrophage inflammatory protein la, an inhibitor of primitive hematopoietic cells. Roc Natl Acad Sci USA 90:12015, 1990 50. Mayani H, Little MT, Dragowska W, Thornbury G, Lansdorp PM. Differential effects of the hematopoietic inhibitors MP-la, TGF-p, and TNF-a on cytokine-induced proliferation of subpopulations of CD34+ cells purified from cord blood and fetal liver. Exp Hematol 23:422, 1995
© Copyright 2026