Evaluation of invitro free radical scavenging potential of various
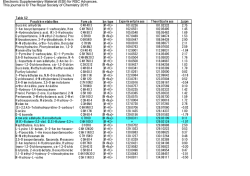
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(1):860-864 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Evaluation of invitro free radical scavenging potential of various extracts of whole plant of Calycopteris floribunda (Lam.) Bhuvaneswari Santharam*, Ganesh P.1, Soranam R.2, Divya V. V.3 and Packia Lekshmi N. C. J.4 * Department of Biochemistry, KR College of Arts and Science, Kovilpatti, Tamilnadu 1 Department of Microbiology, Annamalai University, Annamalai Nagar, 2 Department of Environmental Biotechnology, M. S. University, Alwarkurichi 3 Department of Biotechnology, Manonmaniam Sundaranar University, Tirunelveli, Tamilnadu 4 Department of Microbiology, Udaya College of Arts and Science, Vellamodi, Kanyakumari district, Tamilnadu _____________________________________________________________________________________________ ABSTRACT The present study was aimed to investigate the free radical scavenging potential of various extracts from whole plant of Calycopteris floribunda with the help of two in-vitro antioxidant models were carried out for total antioxidant activity (phosphomolybdic acid method), ferric reducing antioxidant potential (FRAP) assay and estimation of total flavonoids using spectrophotometric methods. Ascorbate was used as standard and positive control for above models. Ethyl acetate extract of Calycopteris floribunda was found to be extremely effective in total antioxidant activity. The IC50 values of ethyl acetate extract of Calycopteris floribunda and ascorbate were found to be 360µg/ml and 410µg/ml respectively. The ethyl acteate extract of Calycopteris floribunda was found more efficient in FRAP assay than that of petroleum ether and methanolic extract. Results indicate that ethyl acteate extract have marked amount of flavonoids, which could be responsible for antioxidant activity. Our findings revealed that ethyl acetate extract of Calycopteris floribunda possess interesting antioxidant activity, which may provide protection against free radicals induced damage to biomolecules. It is also worth noting that these results validate with invitro tests, the therapeutic use of the plant in traditional medicine. Key words: Calycopteris floribunda, antioxidant activity, FRAP assay, flavonoids, free radicals. _____________________________________________________________________________________________ INTRODUCTION Free radicals or ROS are formed in our body as result of biological oxidation; over production of the same contributes to the oxidative stress, [1-2] which leads to the damage of proteins, DNA and lipid that is associated with the chronic degenerative diseases including cancer, coronary artery diseases, hypertension and diabetes etc [3]. Iron is known to be involved in the generation of reactive oxygen species (ROS) and in the formation of highly toxic hydroxyl radical from other active oxygen species such as hydrogen peroxide [4,5]. Antioxidant compounds may function as free radical scavengers, complexing agents for pro-oxidant metals, reducing agents and quenchers of singlet oxygen formation [6]. Naturally occurring antioxidants in leaf vegetables and seeds, such as ascorbic acid, Vitamin E and phenolic compounds possess the ability to reduce the oxidative damage associated with many diseases [7-9]. So many researchers have focused on natural antioxidants and in plant kingdom numerous crude extracts and pure natural compounds were previously reported to have antioxidant properties. 860 Bhuvaneswari Santharam et al J. Chem. Pharm. Res., 2015, 7(1):860-864 ______________________________________________________________________________ Calycopteris floribunda Lam. (Combertaceae) commonly known as kokkarai in hindi, minnarakoti in tamil, a scadent woody and climbing shrub which is 5 - 10 cm long with slender brown streaked branches with vine storing water abundantly. So it is referred as a life - saver by the forest dwellers during summer when streams dry up, people quench their thirst by using this plant [10-12]. The leaves have reported to posses anti - diabetic activity [13]. The hepato productive activity of various stem and leave extracts have been reported [14 -15] and even fruits claimed to treat jaundice. Calycopterone, Isocalycopterone and 4 - dimethyl-calycopterone showed a wide range activity against solid cell lines [16]. Antioxidants are important in the prevention of human diseases. The leaves are reported to have medicinal uses as a laxative and anti - helmintic while the juice derived from the young twigs is used for the treatment of diarrhea, dysentery and malaria [17]. Volatile oil extracted from the leaves of C. floribunda and reported it to exhibit high antimicrobial activity [18]. Previous phytochemical studies have reported on the isolation of the flavonoids, calycopterin, quercetin and five bi flavonoids [19 - 20]. An ethnomedicinal survey conducted in Uttara Kannada district; evidence the wound healing activity [21]. The calycopterin is used to synthesize many flavones displaying high antiproliferative activity [22]. Toxicity studies of C. floribunda reported in calf, rabbit and rats [23]. As far as our literature survey could ascertain, no reports concerning the in vitro free radical scavenging activities of the whole plant of C. floribunda given here. Therefore we undertook the present study to investigate the free radical scavenging activities of various extracts of the whole plant of C. floribunda through various in vitro models. EXPERIMENTAL SECTION Collection and identification of plant materials The whole plant of C. floribunda (Lam.) was collected form Pulliyankudi, Nellai district of Tamil nadu, India. Taxonomic identification was made from Botanical Survey of Medical Plants Unit Siddha, Government of India, Palayamkottai. The whole plant material of C. floribunda (Lam.) was dried under shade, segregated, pulverized by a mechanical grinder and passed through a 40 mesh sieve. Preparation of extracts The above powdered materials were successively extracted by hot continuous percolation method in soxhlet apparatus [24] for 24 hrs with petroleum ether (40-60oC) followed by ethyl acetate (76-78oC) and methanol. The extracts were concentrated by using a rotary evaporator and subjected to freeze drying in a lyophilizer till dry powder was obtained. Evaluation of antioxidant activity by in vitro techniques Total antioxidant activity (Phosphomolybdic acid method) The antioxidant activity of the sample was evaluated by the transformation of Mo (VI) to Mo (V) to from phosphomolybdenum complex [25]. An aliquot of 0.4 ml of sample solution was combined in a vial with 4 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4mM ammonium molybdate). The vials were capped and incubated in a water bath at 95oC for 90 min. After the samples had cooled to room temperature, the absorbance of the mixture was measured at 695 nm against blank. The antioxidant activity was expresses relative to that of ascorbic acid. FRAP assay A modified method of Benzie and Strain [26] was adopted for the FRAP assay. The stock solutions included 300mM acetate buffer, pH 3.6, 10 mM TPTZ (2, 4, 6-tripyridyl -s -triazine) solution in 40 mM HCL and 20mM FeCl3. 6H2O. Fresh working solution was prepared by mixing 25 ml acetate buffer, 2.5 ml TPTZ and 2.5 ml FeCl3, 6 H2O. The temperature of the solution was raised to 37oC before using plant extracts (0.15ml) were allowed to react with 2.85 ml of FRAP solution for 30 min in the dark condition. Readings of the coloured product (ferrous tripyridyltriazine complex) were taken at 593 nm. The standard curve was linear between 200 and 1000 mM FeSO4. Results are expressed in mM (Fe (II) /g dry mass and compared with that of ascorbic acid. Total flavonoids 0.2 g of the plant material was ground with ethanol - water in 2 different ratios namely 9: 1 and 1: 1 respectively. 861 Bhuvaneswari Santharam et al J. Chem. Pharm. Res., 2015, 7(1):860-864 ______________________________________________________________________________ The homogenate was filtered and these 2 ratios were combined. This was evaporated to dryness until most of the ethanol has removed. The resultant aqueous extract was extracted in a separating funnel with hexane or chloroform. The solvent extracted aqueous layer was concentrated, 0.5 ml of aliquot was pipetted out in a test tube. 4 ml of the vanillin reagent (1% vanillin in 70% conc.H2SO4) was added and kept in a boiling water bath for 15 mins. The absorbance was read at 360 nm. A standard was run by using catechol (110mM/ml) [27]. RESULTS AND DISCUSSION Phenolic compounds and flavonoids are widely distributed in plants which have been reported to exert multiple biological effects, including antioxidants, free radical scavenging abilities, anti inflammatory, anti carcinogenic activity etc., [28]. Antioxidant compounds may function as free radical scavengers, initiator of the complexes of pro-oxidant metals, reducing agents and quenchers of singlet oxygen formation [29]. Therefore the importance of search for natural antioxidants has increased in the recent years so many researches focused the same [30]. Total antioxidant activity (Phosphomolybdic acid method) The percentage of total antioxidant activity of petroleum ether, ethyl acetate and methanolic extracts of Calycopteris floribunda was depicted in Table- 1. The petroleum ether extract of C. floribunda exhibited a maximum total antioxidant activity of 50.79 % at 1000 µg/ml, whereas for ascorbate (standard) was found to be 55.23% at 1000 µg/ml. The IC50 values of petroleum ether extracts of C. floribunda and ascorbate were found to be 988µg/ml and 410µg/ml. The ethyl acetate extract of C. floribunda exhibited a maximum total antioxidant activity of 76.53 % at The methanolic 1000 µg/ml. The IC50 values of ethyl acetate extract of C.floribunda was found to be 360µg/ml. extract of C. floribunda exhibited a maximum total antioxidant activity of 69.67% at 1000 µg/ml. The IC50 value of methanolic extracts of C.floribunda was found to be 495µg/ml. Table 1: Total antioxidant activity of solvent extracts of Calycopteris floribunda S.No Concentration (µg/ml) 1 2 3 4 125 250 500 1000 % of activity(±SEM)* Petroleum ether extract Ethyl acetate extract Methanolic extract 11.88 ± 0.056 36.24 ± 0.046 23.34 ± 0.012 29.65 ± 0.018 46.15 ± 0.045 35.30 ± 0.078 45.76 ± 0.035 69.55 ± 0.052 50.45 ± 0.072 50.79 ± 0.021 76.53 ± 0.040 69.67 ± 0.034 IC50 = 988 IC50 = 360 µg/ml IC50=495µg/ml *All values are expressed as mean ± SEM for three determinations. Standard (Ascorbate) 26.87 ± 0.076 30.30 ± 0.054 60.64 ± 0.022 55.23 ± 0.014 IC50 = 410µg/ml Based on the result clearly indicated the ethyl acetate extract of C. floribunda was found to be more effective than petroleum ether and methanolic extract. But when compared to all the extracts with standard the ethyl acetate of C. floribunda was found to possess strong antioxidant activity. The IC50 value of the ethyl acetate of C. floribunda was found to be 360µg/ml. Table 2: FRAP assay of solvent extracts of Calycopteris floribunda % of activity(±SEM)* S.No 1 2 3 4 Concentration (µg/ml) 125 250 500 1000 Petroleum ether extract Ethyl acetate extract Methanolic extract 18.39 ± 0.077 33.33 ± 0.044 20.12 ± 0.035 23.42 ± 0.027 53.43 ± 0.029 31.34 ± 0.012 33.97 ± 0.022 64.59 ± 0.036 48.95 ± 0.068 37.52 ± 0.041 78.82 ± 0.013 67.56 ± 0.086 IC50 = 190 µg/ml IC50=560 µg/ml IC50= 1300 µg/ml *All values are expressed as mean ± SEM for three determinations Standard (Ascorbate) 72.04 ± 0.014 82.05 ± 0.034 86.04 ± 0.026 98.07 ± 0.041 IC50 = 50 µg/ml FRAP assay The antioxidant potential of C. floribunda was ascertained from FRAP assay based on their ability to reduce TPTZ Fe (III) complex to TPTZ - Fe (II). The reducing ability of the petroleum ether, ethyl acetate and methanolic extracts of C. floribunda and ascorbate at various concentration (125, 250, 500 and 1000 µg/ml) were examined and the values were depicted in table 2. The maximum reducing ability at 1000 µg/ml for petroleum ether extract and ascorbate was found to be 37.52 % and 98.07 % respectively. The IC50 value of petroleum either extract and ascorbate were recorded as 1300µg/ml and 50µg/ml respectively. The maximum reducing ability at 1000 µg/ml for 862 Bhuvaneswari Santharam et al J. Chem. Pharm. Res., 2015, 7(1):860-864 ______________________________________________________________________________ ethyl acetate extract was found to be 78.82 %. The IC50 value of ethyl acetate extract was recorded as 190µg/ml. The maximum reducing ability at 1000 µg/ml for methanolic extract was found to be 67.56 %. The IC50 value of methanolic extract was recorded as 560µg/ml. Based on the above results indicated, the ethyl acetate extract of C.floribunda was found to more effective than that of petroleum ether and methanolic extract. But when compared to all the three extracts with ascorbate (standard), ethyl acetate extract of C.floribunda showed the moderate activity. Total flavanoids Most beneficial effects of flavonoids are attributed to their antioxidant and chelating abilities [31]. Flavonoids present in food of plant origin are also potential antioxidants [32,33]. The total amount of flavonoids content of various extract of whole plant of C.floribunda was depicted in table 3. Table 3: The total flavonoids content of various extracts of whole plant of Calycopteris floribunda S.No 1 2 3 Extracts of Calycopteris floribunda Total flavonoids content (mg/g) (±SEM)* Petroleum ether extract 0.038 ± 0.076 Ethyl acetate extract 3.545 ± 0.023 Methanolic extract 1.489 ± 0.043 *All values are expressed as mean ± SEM for three determinations Based on the result, the ethyl acetate extract showed high content of flavonoids than that of petroleum ether and methanolic extract of C.floribunda. CONCLUSION From the results obtained in the present study, its concluded that ethyl acetate extract of C. floribunda showed strong antioxidant activity by total antioxidant activity and FRAP assay when compared with standard ascorbate. In addition, the ethyl acetate extract of C. floribunda was found to contain noticeable amount of flavonoids that could have great importance as a therapeutic agent in preventing or slowing oxidative stress related degenerative diseases. Therefore the plant can be further harnessed for novel antioxidant / bioactive compound which is very well evidence by the present study. Acknowledgement The authors are grateful to the authorities of KR College of Arts and Science, Kovilpatti and SB College of Pharmacy Sivakasi, Tamilnadu, India for providing required facilities. REFERENCES [1] AT Diplock. Free radical damage and control. In: Rice Evans CA and Burden RH (eds).Antioxidant and free radical scavengers. New york, USA: Elsevier, 1994. [2] MJ Thomson, Critical Reviews of Food Sci.Nutri., 1995, 35, 21 - 29. [3] KG Lee; AE Mitchell; T Shibamoto. J. Agri. food chem.., 2000, 48, 4817 - 4820. [4] OI Aruoma; B Halliwell; E Gajewski; M Dizdaroglu, J Biol Chem., 1989, 264, 20509-12. [5] B Halliwell; JMC Gutteridge, Methods Enzymol., 1990, 186, 1 - 85. [6] W Andlauer; P Furst, Cereal foods world, 1998, 43, 356-359. [7] P Pietta; P Simonetti; P Mauri, J. Agric. Food Chem., 1988, 46, 4487 - 4490. [8] KG Lee; AE Mitchell; T Shibamoto, J. Agric. Food Chem., 2000, 48, 4817-4820. [9] E Middleton; C Kandaswamy; TC Theoharides, Pharmacol. Rev., 2000, 52, 673 - 751. [10] RN Chopra; SL Nayar; I Chopra, Glossary of Indian Medicinal plants. New Delhi : 1956. [11] WHO. Assessment of risk of hepatotoxicity with kava products, 2001. [12] Indian Medicinal Plants: A Compendium of 500 Species. Chennai: Orient Longman, 1995. [13] T Sreenu; T Jyothibasu; N Delhiraj; K Suresh Kumar, Int. J. Pharmaceut. Sci. Drug Res., 2012, 4 (4), 250 -2. [14] EM Chinna; T Satyanarayana, Int. J. Pharmacog. Phytochem. Res., 2010, 2, 53-7. [15] S Thalla; B Pentela, Int. J. Chem. Pharm. Sci., 2011, 2, 15-21. [16] MC Wall; MC Wani; F Fullas; JB Oswald; M Brown; T Santisuk; V Reutrakul; AT McPhail; NR Farnsworth; JM Pezzuto; AD Kinghorn; JM Besterman, J. Med. Chem., 1994, 37, 1465 - 70. 863 Bhuvaneswari Santharam et al J. Chem. Pharm. Res., 2015, 7(1):860-864 ______________________________________________________________________________ [17] RN Chopra, Indigenous Drugs of India. 3rd ed. Kolkata: Academic publishers, 2006. [18] JJ Liu; DL Yang; Y Zhang; Y Yuan; FX Cao; JM Zhao; XB Peng, J. Cent. Sou. Univ. of Tech., 2009, 16, 9315. [19] ZE Rodrigue; G Vander Velde; TJ Mabry; SS Subramanian; AGR Nair, J. Phytochem., 1972, 11, 2311-12. [20] R Mayer, Phytochem., 2004, 65, 593-601. [21] P Bhat; G Hegde; GR Hegde, J. Ethnopharmacol., 2012, 143, 501-14. [22] G Lewin; NB Shridhar; G Aubert; S Thoret; J Dubosi; T Cresteil, Bio. Med. Chem., 2011, 19(1), 186 - 96. [23] P Sreekanth; K Narayana; NB Shridhar, J. Ethnopharmacol., 2006, 107 (2), 229 - 33. [24] JB Harborne. Phytochemical methods 2nd ed. New York: In Chapman & Hall, 1984. [25] P Prieto; M Pineda; M Aguilar, Anal. Biochem., 1999, 269, 337-341. [26] IEF Benzie; JJ Strain, Anal. Biochem., 1996, 239, 70-76. [27] GR Cameron; RF Milton; JW Allen, Lancet., 1943, 179. [28] AL Miller, Alt.Med.Rev., 1996,1,103. [29] W Andlauer; P Furst, Cereal Foods World, 1998, 43, 356-359. [30] GK Jayabrakasha; T Selvi; KK Sakariah, Food Res.Int., 2003, 36, 117-122. [31] A Hassig; WX Liang; K Shwabl; K Stampfl, Med. Hypotheses., 1999, 52, 471-481. [32] N Salah; NJ Miller; G Paganga; L Tijpurg; GP Volwell; C Rice Evans, Arch. Biochem. Biophys., 1995, 322(2), 339-346. [33] VA Sabe; V Vijgh; F Bast, Free Rad. Bio. Med., 1996, 20(3), 331-342. 864
© Copyright 2026