BrdU-HOECHST-ETHIDIUM BROMIDE (EB) QUENCHING
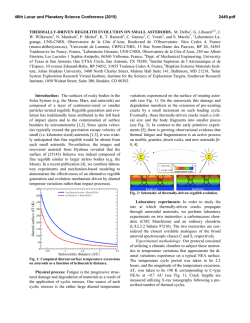
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 289 CORRESPONDENCE BrdU-HOECHST-ETHIDIUM BROMIDE (EB) QUENCHING TECHNIQUE FOR STUDYING KINETICS OF HEMATOPOIESIS To the Editor: In vitro stimulatory or inhibitory effects on hematopoietic bone marrow (BM) progenitors are mostly assessed through evaluation of the progeny at later stages (ie, colony scoring after 2 to 3 weeks). The immediate kinetic effects are mostly ignored, although the evaluation of the progenitor cell cycle status and kinetics of response can be performed by a variety of methods such as 3H-thymidine incorporation and/or suicide 3H-thymidine technique, viable (Hoechst) or nonviable (EB, PI, etc) DNA staining. These techniques have the disadvantage of detecting only the S phase compartment at a given moment: a limitation shared by all these techniques is indeed that continued growth of cells beyond a single cell cycle and the kinetics of cell proliferation cannot be further discriminated. Therefore, we want to draw attention to the flow cytometric BrdU/Hoechst propidium iodide (or EB) technique as a powerful alternative for cell kinetic aaalysis, which is capable of not only discriminating between cycling and noncycling cells but also between cells that have replicated once, twice, or more times within a given time interval: this novel analysis is capable of showing the proliferative history of cells. The technique was reviewed recently' and has already found considerable interest in the immunological (mitogen response) and pharmaceutical (drug effects) science. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 290 CORRESPONDENCE In our opinion, the wealth of cell kinetic information and the ease by which this information can be obtained is left underestimated in the field of experimental hematology. We have adapted some of the experimental conditions (which were originally developed for mitogen-stimulated lymphocyte proliferation) to make the technique more suitable to analyze in greater detail growth factor-induced proliferative response of hematopoieticprogenitors (ie, by loweringthe BrdU concentration of 5 kmol/L and using serum-deprived conditions). We here present two examples that illustrate the power of this technique for analysisof hematopoietic cell kinetics. 77te first eromple (Fig 1, top) represents the 72-hour culture status of sorted CD34+ BM progenitors,grown in Ixove's modified Dulbecco's medium (IMDM) supplemented with either granulocyte colony-stimulating factor (G-CSF) (Fig lA), granulocytemacrophage CSF (GM-CSF) (Fig le). or a combination of both cytokines (Fig. IC). BrdU was added to the culture medium at culture set-up and was present until harvest. The Row cytometric BrdUlHoechst technique significantly discriminates between responders (R2 + R3 + R4). nonresponders (Go, RI), and dying cells (R5). Within the responder compartment differentiation between fast responders (R4. after 72 hours they have already entered the third cell cycle), intermediate rate responders (R3. second cell cycle), and slow responders (R2,still in the first cell cycle) is possible. Because the sum of responders to G-CSF stimulation (6296, Fig 1A) and to GM-CSF stimulation (50%. Fig 1B) is significantly higher (A of 47%) than the responders to simultaneous G-and GM-CSF stimulation (65%. Fig lC), one can conclude in a first approximation that the CD34+ compartment in this particular experiment consists of target cells for both G-and GM-CSF (47%), target cells for G-CSF alone (62-47 = lS%), Eh: CD34+ bD: LIN- + G-CSF 1 %: target cells for GM-CSF alone (50-47 = 3%). and cells unresponsive to either G- or GM-CSF (region R1 = 35%. Fig 1C). Comparing the number of cells in the individual cell cycles, it is evident that the combination of both growth factors speeds up the cell cycle traverse rate for a significant fraction of the target cells (cells in R4 of Fig 1, A through C). In contrast to the first example where most BM progenitors start from an almost synchronous CD34+ sorted fraction, the second a m p l e (Fig 1. D through F) illustrates a total human BM culture that is asynchronouslyproliferating after 1 week of culture using a growth factor cocktail (GM-CSF. erythropoietin (Epo], interleukin-3 [IL-31). The data shown are restricted to all but the mature cells (gatingon negative cells after CD3, CDl9, CDl lb, and CD14 MAB labeling), and show the cell kinetic status after 18 hours of BrdU-supplemented culture. Although the analysisof the asynchronous BM culture is more complicated than in the previous example, one can discriminate (Fig 1D) between GI nonresponders, and proliferating cells in the first (S,Gz/M) and second cell cycle (Gi and an S' region). Further explanation on interpretation of asynchronousgrowing cultures can be found in references 2 and 3. Figure 1E displays the result after treatment of human BM cells by cis-platinum. From this data it is evident that inhibition of proliferation by this chemotherapeutic agent is accomplished by the slowing down of GI and early and mid S-phase cells. Late S and G2M cells divide; however, most of them die after division (see nuclear decay line). However. addition of the putative chemoprotective agent diethyldithiocarbamate (DDTC) alone results in a similar toxic response of the proliferating BM cells, except a slightly higher cell viability of dividing late S and GzM cells, which is shown by the presence of G i cells (Fig 1F). CD34+ + GM-CSF hE: LIN- + CIS-PLRTINUM 1 E]C: CD34+ + 0-CSF + GM-CSF qF: LIN- + DDTC czm .i :i F l - 1 ~ n. 1 -> Fk 1. flow cytometrlc bivariate Hoechat versua EB fluorescema ytognnn from BM progsnlton (sod CD34+cella In A, B, and Cllinerg. mathfafraction of total BM mononuclear population In D, E, and F) c u b d in the presence of G-CSF (A), GM-CSF (E), 0- GM-CSF (C), or OM-CSF IL-3 + Epo (D through F). Cells in region Rl(38% in A, 50% in B, 35% in C), R2 (7% in A, 9% in B, 5% in C), R3 (28% in A, 15% in B, 28% in C), R4 (14% in A 3% in B. 19%in C) and RS (14% in A 23% in B. 13% in C) represent cells in quiescence,in first, second, third cell cycle, or in decay, + rerpecttvely. + From www.bloodjournal.org by guest on February 6, 2015. For personal use only. CORRESPONDENCE Both examples illustrate the potential power of this novel flow cytometric analysis in unraveling the complexity of the cell kinetic response of hematopoietic BM cells under influence of a variety of different agents (growth factors, growth inhibitors, drugs, toxic compounds, etc): the wealth of early event information and its refined biologic interpretation (detection and quantification of subsets of target cells, cell cycle progression rate, cell-cycle blockage position, cellular stability, etc) may be a valuable supplement to final outcome information (colony scoring). The mild staining procedure results in little cell loss and allows for simultaneous analysis of intracellular and extracellular epitopes. We also have proven that this technique can easily be performed, even at higher resolution, using the relatively inexpensive He-Cd laser instead of the expensive Argon la~er.4,~ We suggest that this 291 technique becomes more popular and available in in vitro research of cell cycle kinetics of hematopoiesis. DIRK R. VAN BOCKSTAELE FILIP LARDON HANS-W. SNOECK MARC E. PEETERMANS Experimental Hematology University ofAntwerp (UZA)Edegem, Belgium MANFRED KUBBIES Cell Biology Boehringer Mannheim Penzberg, Germany REFERENCES 1. Kubbies M, Hoehn H, Schindler D, Chen YC, Rabinovitch cell populations by flow cytometry using bromodeoxyuridine label PS: Cell cycle analysis via BrdU-Hoechst flow cytometryand Hoechst-Propidiumiodide stain. Cytometry 1992 (in press) 4. Van Bockstaele DR, Lardon F, Snoeck H-W, Peetermans Principles and applications, in Yen A (ed): Flow Cytometry: ME: BrdU-Hoechst flow cytometry using a 20 mW He-Cd laser as Advanced Research and Clinical Applications,vol 11. Boca Raton, UV light source: II/Evaluation of growth factor induced bone marrow FL, CRC, 1989, p 5 progenitor (CD34+)proliferation. Cytometry Suppl5:54,1991(abstr) 2. Kubbies M, Ormerod MG: Cell cycle analysis of asynchronous cultures using the BrdU/Hoechst-PI flow cytometric method. 5. Kubbies M, Goller B, Van Bockstaele DR: Improved BRDUCytometry Suppl5:77,1991 (abstr) Hoechst bivariate cell kinetic analysis by Helium-Cadmium single laser excitation. Cytometry 1992 (in press) 3. Ormerod M, Kubbies M: Cell cycle analysis of asynchronous From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1992 80: 289-291 BrdU-Hoechst-ethidium bromide (EB) quenching technique for studying kinetics of hematopoiesis [letter] DR Van Bockstaele, F Lardon, HW Snoeck, ME Peetermans and M Kubbies Updated information and services can be found at: http://www.bloodjournal.org/content/80/1/289.citation.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026