The Identification and Characterization of a Novel Human
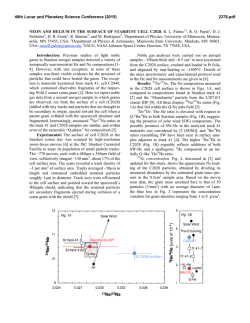
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. The Identification and Characterization of a Novel Human Differentiation-Inhibiting Protein That Selectively Blocks Erythroid Differentiation By J.P. Durkin, J.M. Biquard, J.F. Whitfield, N. Morardet, J. Royer, P. Macdonald, R. Tremblay, J.D. Legal, R. Doyonnas, J.P. Blanchet, and V. Krsmanovic We have isolated a novel inhibitor of erythropoieticdifferentiation from the plasma of a patient suffering from idiopathic pure red cell aplasia. This differentiation-inhibitingprotein (DIP) specifically blocked the differentiation of human burstforming unit-erythroid (BFU-E), but not colony-forming uniterythroid (CFU-E) cells. DIP also blocked the maturation of murine BFU-E cells, but not CFU-E or CFU-granulocytemacrophage cells, and it inhibited the dimethyl sulfoxide (DMSO)-induceddifferentiation of Friend murine erythroleukemia cells (FLC) at levels between lO-’’and IO-’’ mol/L. DIP activity was not detectable in the plasma of normal, healthy subjects. Unlike other known inhibitors of hematopoiesis, DIP appears to directly inhibit erythropoietic differentiation, because it did not affect the proliferation of untreated FLC and it effectively blocked FLC hemoglobinization without affecting the ability of the blocked cells to proliferate. DIP blocked FLC differentiation only when added to the culture medium within 1 hour of inducing the cells with DMSO, suggesting that the protein inhibited an early, but critical, DMSO-induced cellular procesd. DIP appears to be at least partially responsible for the patient’s anemia, and its unique activity suggests a role in the early development of some erythroleukemias. o 1992 by The American Society of Hematology. T precursor cells suggested the possible existence of a human homolog that could potentially play a role in normal erythropoiesis as well as aplastic anemias and erythroleukemia. The strategy used to identify such a factor was based on the proposition that its overproduction would specifically block erythrocytk development in otherwise normal bone marrow, a condition found in the blood disorder known as pure red cell aplasia (PRCA).16Therefore, blood samples from patients suffering from PRCA of unknown cause were screened for a novel erythroid differentiationinhibiting activity. We report here the isolation of the first human erythroid differentiation-inhibiting protein (DIP) from the blood of a 60-year-old woman suffering from PRCA. The isolation, purification, and inhibitory activity of this novel protein are described. HE PROLIFERATION and differentiation of cells of the different hemopoietic lineages is driven by a variety of factors such as erythropoietin (EPO), granulocytemacrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and macrophage colony-stimulating factor (M-CSF).’ On the other hand, the proliferation and differentiation of a wide variety of hematopoietic cells can be inhibited by agents that primarily block cell cycle progression, such as transforming growth factor-p (TGF-p): tumor necrosis factor-a (TNF-CX),~.~ and several other less welldefined A different type of highly selective hematopoietic inhibitor was found by Fasciotto et all4 in the conditioned medium of tsAEV-LSCCHD3 chicken erythroleukemia cells, which had been transformed by the erb-B gene of the avian erythroblastosis virus (AEV). This 45-Kd protein, subsequently named autocrine differentiation-inhibiting factor (ADIF), inhibited the EPO-induced differentiation of the producer chicken cells and the dimethyl sulfoxide (DMSO)-induced differentiation of Friend murine erythroleukemia cells (FLC).14,15 The unique characteristic of this protein was its ability to block differentiation without directly affecting cell cycle progression.” Moreover, chicken ADIF selectively blocked the maturation of burst-forming unit-erythroid (BFU-E) erythroid progenitor cells without affecting the more advanced colony-forming unit-erythroid (CFU-E) cells, or cells of the granulocyte-macrophage lineage in human and murine bone marrow culture^.'^^^^ Thus, with respect to their sensitivity to ADIF, both the tsAEV-LSCCHD3 chicken cells and the clone of FLC cells used in the present study have at least a partial BFU-E phenotype. Another ADIF-related protein has recently been identified that inhibits both tlie macrophage differentiation of the murine myeloid leukemia cells that secrete it and blocks the maturation of other murine (but not human) myeloid cells without affecting cell proliferation.’ In principle, agents that block the differentiation of hemopoietic cells without affecting the ability of the blocked cells to proliferate can be seen as contributing to the first of a series of steps leading towards leukemia. The ability of the chicken erythroid ADIF to selectively inhibit the differentiation of human BFU-E erythroid Blood, Vol79, No 5 (March I), 1992: pp 1161-1171 MATERIALS AND METHODS Leukemia Cell Lines FLC, a clone of Friend C19X10 cells,14 were cultivated in a complete medium consisting of 90% RPMI (Flow Laboratories, McLean, VA), 10% decomplemented fetal bovine serum (FBS; GIBCO BRL, Grand Island, NY), and penicillin (100 U/mL)/ streptomycin (0.1 mg/mL). The human promyelocytic cell line, HL-60, was grown in the same medium except that 20% decomplemented FBS was used. From the Cell Signals Group, Institute for Biological Sciences, National Research Council of Canada, Ottawa, Ontario, Canada, the Unite de Recherches en Imagerie Medicale Morphologique et Fonctionnelle, Institut Gustave Roussy (INSERM U66), villejuij France; and the Centre de Genetique Moleculaire et Cellulaire, Universite de Lyon 1, Villeurbanne C e d e France. Submitted July 22,1991;accepted October 24,1991. Address reprint requests to J.P. Durkin, PhD, Cell Signals Group, Institute for Biological Sciences, National Research Council of Canada, Ottawa, Ontario, Canada KIA OR6. The publication costs of this article were defiayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C.section 1734 solely to indicate this fact. 0 1992 by The American Sociev of Hematology. 0006-497ild2/7905-OOO5$3.OO/O 1161 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1162 FLC dijierenfiafionassay. FLC cells, taken from stock cultures at 1.5 to 2.0 x lo6cells/mL, were plated at 2.0 x lo5cells/mL in 1.5 mL of complete medium in 24-well dishes (Falcon 3047; Becton Dickinson, New Jersey). In some cultures, the cells were induced to differentiate into hemoglobin-synthesizing erythroid cells by adding 2% DMSO to the culture medium. Samples (15 pL) to be tested for differentiation-inhibiting activity were added to the cultures 5 minutes before the DMSO. The proportion of hemoglobin-synthesizing cells was determined by the benzidine staining technique” on day 4, or when the percentage of hemoglobinized cells reached 70% to 85% in control (DMSO only) cultures. Typically, the percentage of benzidine-positive (b+) cells in uninduced FLC cultures was less than 2%. Human eiytkroid and myeloid cells. Human erythroid precursor cells from bone marrow were enriched by Ficoll density centrifugation and washed twice with Iscove’s modified Dulbecco medium (IMDM). The cells were allowed to adhere to the bottom of T-25 tissue culture flasks for 1.5 hours at 37°C in an atmosphere consisting of 95% air and 5% CO,. Cells adhering to the flasks were discarded and only the nonadherent cells were used. Where required, DIP was added at the indicated concentrations to lo6 nonadherent bone marrow or peripheral blood cells that were preincubated for 1.5 hours. For BFU-E and CFU-E progenitor cell assays, lo6 DIP-treated or untreated cells were seeded in 35-mm Petri dishes containing 1 mL of a semi-solid medium consisting of 85% IMDM, 15% FBS, 1% deionized bovine serum albumin, 2 U/mL human recombinant EPO, 0.075 mmol/L a-thioglycerol, and incubated at 37°C in a humidiand 0.8% methylcellulose,’8~~a fied atmosphere of 95% air and 5% CO,. The FBS provided enough IL-3 for BFU-E and CFU-E growth and differentiation. The numbers and appearance of CFU-E colonies were determined on day 7, and BFU-E colonies on days 14 and 18. The cells were examined unstained with an inverted microscope. All orange-red aggregates having more than 50 cells were counted as a burst. Colonies were removed and stained with the May-GriinwaldGiemsa stain for further examination. Murine eiytkroid and myeloid cells. Freshly isolated mouse bone marrow from 6- to 8-week-old C57 Black/6 mice was used as the source of BFU-E, CFU-E, and CFU-granulocyte-macrophage (CFU-GM) progenitors. Semi-solid cultures of CFU-E were established in IMDM supplemented with 30% FBS, 1% deionized bovine serum albumin, 75 pmol/L P-mercaptoethanol, 0.2 U/mL porcine EPO (Centre National de Transfusion Sanguine, Paris, France), and 0.8% methylcellulose?0 Cells were plated at a final concentration of 2 X 104/mL in a volume of 100 pL in 16-mm diameter wells and incubated for 2 days at 37°C in a watersaturated atmosphere consisting of 97.5% air and 2.5% CO,. Colonies of more than 8 cells derived from CFU-E were enumerated after benzidine staining using an inverted microscope. BFU-E and CFU-GM cells were grown in IMDM supplemented with 20% FBS, 1% deionized bovine serum albumin, 75 pmol/L P-mercaptoethanol, 45 pmol/L hemin, 0.5 U/mL porcine EPO (specific activity, 1,030 U/mg), 50 U/mL purified mlL-3 (lo6U/mg; Genzyme Corp, Boston, MA), and 0.8% methylcellulose. Cells were plated at a final concentration of 2 x 10‘/mL in a volume of 500 pL in microtitration plates and incubated at 37°C. Colonies derived from BFU-E precursor cells, and granulocyte-macrophage colonies produced by CFU-GM cells were counted at 7 days without staining?’ Fractionation of plasma. One hundred milliliters of PRCA or normal plasma was dialyzed overnight against 1 mol/L acetic acid and the resulting precipitate was removed by centrifugation at 10,OoOg for 5 minutes. The supernatant was dialyzed extensively against 0.15 mol/L NaCI, 10 mmol/L HEPES, pH 9.0, and the dialyzed protein extract (1.5 g) was applied to a Chelating DURKIN ET AL Sepharose 6B column (1.2 x 15 cm; Pharmacia LKB, Uppsala, Sweden), charged with 100 mmol/L ZnSO,, and equilibrated with 150 mmol/L NaC1-10 mmol/L HEPES (pH 9.0) buffer solution, at a flow rate of 50 mL/min. The unadsorbed pH 9.0 flow-through fraction (100 mL) was collected, and the material adsorbed to the column was eluted using a stepwise pH gradient from pH 8.0 to 4.0. Each of the eluted fractions (100 mL) was dialyzed against phosphate-buffered saline (PBS), and 20 pL samples of each fraction were then tested for their abilities to inhibit the differentiation of human BFU-E colony development or FLC hemoglobinization. The pH 9.0 fraction, containing most of the inhibitory activity, was dialyzed against 1 mol/L acetic acid and lyophilized. Samples of this material (8 to 10 mg) were redissolved in 1 mL of 1 mol/L acetic acid and applied to a P-60 gel filtration column (20 X 100 cm; Bio-Rad Laboratories, Richmount, CA) equilibrated in 1 mol/L acetic acid. Samples (50 pL) of each eluted fraction (1.5 mL) were pooled into groups, lyophilized, resuspended in an equal volume of PBS, and tested for differentiation-inhibiting activity. The most active fractions (fractions 39 to 41) were lyophilized, resuspended in 1mL of PBS, and rechromatographed on a P-60 gel filtration column (20 X 100 cm) equilibrated in PBS. The inhibitory activity of each fraction (1.5 mL) was measured by the FLC differentiation assay. The fraction of peak activity (fraction 33) was dialyzed against 1 mol/L acetic acid, lyophilized, resuspended in 100 pL solubilization buffer (20% glycerol, 6% sodium dodecyl sulfate [SDS], 10% P-mercaptoethanol, 100 mmol/L Tris-HC1, pH 6.8) and heated to 80°C for 5 minutes. The sample was applied to several wells of a reducing SDS-polyacrylamide (10%) gel and electrophoresed under standard Laemmli conditions.2z Rainbow protein molecular weight markers (Amersham Corporation, Arlington Heights, IL) served as visual landmarks on unstained gels. After electrophoresis, the gel was halved and the protein bands on one-half of the gel were visualized by silver stain (Bio-Rad). The regions of interest were cut from the unstained portion of the gel, divided into 1-mm slices, and each gel slice was eluted overnight at 10 mA (Bio-Rad Model 422 Electo-eluter) into 50 mmol/L ammonium bicarbonate-0.1% SDS at 4°C. The electroeluted proteins were dialyzed for 4 hours against water, lyophilized, resuspended in RPMI medium, and then tested for differentiationinhibiting activity. RESULTS Purification of the Differentiation-ZnhibitingActivity The anemia of the 60-year-old woman suffering from idiopathic PRCA was not caused by an EPO deficiency; her serum EPO levels were actually 8 to 9 times above normal, as measured by standard bioassay. Despite the high concentration of EPO, the patient’s serum only feebly stimulated fetal liver erythroblast proliferation, which suggested the presence of a circulating inhibitor of erythropoiesis. As shall be shown, there was no evidence that this inhibitor was a circulating autoantibody (the most common cause of PRCA23,24), nor was the inhibition linked to a detectable thymoma or other malignancy that often accompanies PRCA.16 Finally, physical examination of the patient and electron microscopic examination of the particulate and soluble fractions of her plasma did not show an underlying viral infection. Collectively, the clinical evidence pointed to the presence of a novel circulating inhibitor of erythropoiesis that we proceeded to isolate. Because of the chicken ADIF’s proven stability in acid conditions, the patient’s plasma was initially treated with 1 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1163 INHIBITORS OF ERYTHROID DIFFERENTIATION mol/L acetic acid, a step that precipitated the majority (-80%) of the plasma protein. The supernatant was clarified by centrifugation, and small samples (20 FL) were lyophilized to remove acetic acid and then tested for biologic activity. The samples inhibited the DMSO-induced hemoglobinization of FLC (Fig 1A) but, in this crude state, they could not block the development of human BFU-E erythroid cells in soft-agar assay (Fig 1B). By contrast, 501A7 200 . ' - 40 - 150 30 - F 100- - El 0 Z 20 + 0 U n 10 50 - 0 - 0 B 100 1 80 C r AP 9.0 8.0 6.5 5.0 4.0 pH OF ELUANT Fig 1. Metal chelate affinity chromatography of PRCA plasma. was Acidified PRCA plasma (a)(1.5 g protein) or normal plasma (0) applied t o a Zn*+-chargedchelating Sepharose 68 column equilibrated with 150 mmol/L NaCI-10 mmol/L HEPES, pH 9.0. The eluate (100 mL) was collected and the proteins bound t o the column were eluted with a step-wise pH gradient from pH 8.0 t o 4.0, as described in Materials and Methods. Each fraction, along with the unfractionated acidified plasma (AP), was dialyzed against PBS and samples (20 pL) were tested for their ability (A) t o inhibit the hemoglobinization of DMSOinduced FLC. The percentage of b+cells was determined 5 days after adding 2% DMSO t o the culture medium as described in Materials and Methods. The percentage of b' cells in DMSO-treated and untreated control cultures was 75.3 f 1.6 and 2.4 f 1.0, respectively. Each point is the mean f SEM of four cultures. The fractions and AP were also tested for their ability (B) t o inhibit the development of human BFU-E-derived colonies in soft-agar assay. Human bone marrow cells were cultured as described in Materials and Methods and the number of BFU-E-derived bursts containing greater than 50 cells was determined on days 14 and 18. The number of colonies in untreated, control cultures averaged 69 per well. The data are representative of three separate determinations performed in duplicate. identically processed plasma from normal subjects with the same type-A blood as the PRCA patient did not affect FLC differentiation. The PRCA and normal acidified plasmas were dialyzed against a solution containing 150 mmol/L NaCl and 10 mmol/L HEPES (pH 9.0) and lyophilized. The resuspended material was then subjected to chelating SepharosedB chromatography, on a column (1.2 x 15 cm) charged with 100 m ZnSO, and equilibrated with the 150 mmol/L NaCl-10 mmol/L HEPES (pH 9.0) solution. The flow-through fraction (100 mL) was collected, and the protein that bound to the column was eluted by a pH step-gradient (ranging from pH 9.0 to 4.0). The pH 9.0 flow-through fraction was the only one that inhibited significantly the DMSO-induced differentiation of FLC (Fig 1A). Unlike the unfractionated material, this pH 9.0 fraction inhibited by about 75% the development of bursts derived from human BFU-E progenitors (Fig lB), and the bursts that did develop were smaller and, on average, were about 75% less hemoglobinized than bursts from control cultures (data not shown). Acidified normal plasma treated in the same way only marginally inhibited BFU-E and FLC differentiation (Fig 1). As shown in Fig lB, other fractions eluting from the chelating SepharosedB exhibited relatively low levels of BFU-E-inhibiting activity, which did not inhibit FLC differentiation. For these reasons, only the pH 9.0 fractions were subjected to further purification. Although human BFU-E erythroid cells were more responsive than FLC to the differentiation-inhibiting activity of the PRCA factor, a 70% to 80% reduction of human BFU-E differentiation translated consistently into a 35% to 40% reduction in FLC differentiation. Cells transformed by the Friend virus (a complex of spleen focus forming virus and helper murine leukemia virus) are thought to exhibit a phenotype that is a mixture of late BFU-E and early CFU-E.Z5,26 The specific FLC clone used in these studies most certainly manifests the BFU-E property of being inhibitable by chicken ADIF, a protein that blocks the differentiation of chicken and murine BFU-E, but not CFU-E.14,15 Because of its greater speed and convenience, and its reliable reflection of a larger effectiveness on human BFU-E cells, the FLC differentiation assay was mainly used to measure the differentiation-inhibiting activity during the subsequent purification steps. The differentiation-inhibiting activity in the pH 9.0 fraction was dialyzed against 1 mol/L acetic acid, lyophilized, resuspended in a minimum volume of 1 mol/L acetic acid and applied to a Bio-Gel P-60 column (20 x 100 cm) that had been equilibrated in 1 mol/L acetic acid. Samples of each eluted fraction (1.5 mL) were pooled into a series of groups, and each group tested for its ability to inhibit DMSO-induced FLC differentiation. When tested at a 1:lo4dilution, most of the differentiation-inhibiting activity eluted in two peaks with mean apparent molecular weights of approximately 45 Kd and 66 Kd (Fig 2A). By comparison, fractionated normal plasma contained virtually no differentiation-inhibiting activity when tested at the same dilution (Fig 2A), or even at levels 100 times that shown. Although not shown in Fig 2, low molecular weight material ( < 15 Kd) eluting in fractions 55 to 70 had no more inhibitory From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1164 DURKIN ET AL ' i E 40 tA 0.6 45kDa 66kDa I W LL 4 0 LL 0 0.4 30 W V Z Z 2 DL 0 t- 20 m I -z g W 0 LL 2 0 VI 0.2 t m I z W 0 10 z P 10 u DL a -I 20 30 40 50 0.0 60 ap FRACTION Fig 2. P-60 (acid) gel filtration chromatography of the differentiation-inhibitingactivity. (A) The pH 9.0 fraction of the PRCA (EA) and control (0) plasmas was dialyzed, lyophilized, and a portion (8 to 10 mg protein) applied to a P-60 gel filtration column equilibratedin 1 mol/L acetic acid as described in Materials and Methods. The eluted fractions (1.5 mL) were pooled into groups and tested at a 1:1O,OOO dilution for their ability to inhibitthe hemoglobinizationof DMSO-induced FLC as described in Materials and Methods and the legend to Fig 1. The percentage of b+cells in DMSO-treated control cultures was 76.3 f 2.5. Each point is the mean f SEM of four cultures. (6)The 45-Kd regions (pooled fractions 37 to 42) of both PRCA and control plasma were tested at a 1:lO.OOO dilution for their ability to inhibit the development of human BFU-E-derivedcolonies in soft-agar assay as described in Materials and Methods and the legend to Fig 1. The number of colonies in untreated,control cultures averaged 58 per well. The data are representativeof five separate determinations performed in duplicate. activity than the corresponding fractions of control serum. Because the active 66-Kd fractions coeluted with a major contaminating protein peak (Fig 2A), we focused on the 45-Kd peak, which clearly had the higher specific activity. This 45-Kd activity strongly inhibited the differentiation of human BFU-E progenitor cells in soft-agar assay by greater than 80% (Fig 2B), and the few bursts that did develop were both smaller and much less hemoglobinized. The same fractions from the control plasma had no differentiation-inhibiting activity (Fig 2B). The separate fractions that made up the 45-Kd peak were tested for differentiationinhibiting activity and also subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The peak activity was restricted to those fractions (fractions 39 to 41) in which a protein with an apparent molecular weight of 65 Kd appeared on the corresponding SDS-PAGE gels (Fig 3). This protein, which was detectable in all three fractions, was most intensely stained in that fraction (fraction 40) which contained the maximum differentiation-inhibiting activity. The appearance of the 65-Kd protein was unexpected, because proteins of this size would normally have eluted much earlier from the P-60 column in fractions 26 to 30. It may well be that the 65-Kd band reflects the aberrant mobility of the protein under the conditions of either SDS-PAGE or gel filtration. Fractions 39 to 41 were pooled, lyophilized, and subjected to further purification. Attempts to purify the protein further by ion-exchange chromatography resulted in unacceptable losses of differentiation-inhibiting activity. Therefore, the pooled active fractions were rechromatographed on a Bio-Gel P-60 column (20 x 100 cm) that had been equilibrated with PBS instead of 1 mol/L acetic acid. The rationale for this - - approach was that the migration pattern of the protein could vary if eluted from the column under neutral, instead of acidic, conditions. Indeed, most of the FLC differentiation-inhibiting activity eluted from the P-60 (PBS) column with an apparent molecular weight of 58 Kd (fractions 31 to 37), which coincided with the major protein peak (Fig 4A). The material purified in this way inhibited the formation of bursts from human BFU-E erythroid progenitor cells by greater than 85% (data not shown). The fraction with peak differentiation-inhibiting activity (fraction 33), when subjected to SDS-PAGE, was found to be greatly enriched in the 65-Kd protein with a minor protein appearing at about 80 Kd (Fig 4B). We had previously shown that the differentiationinhibiting activity of the PRCA plasma was stable to SDS-PAGE conditions and that it retained its biologic activity after electroelution from unstained gels. Thus, the region on unstained gels containing both the 65-Kd and 80-Kd bands was cut into 1-mm slices, and the protein in each slice was electroeluted from the gel into 50 mmol/L NH,HCO,-0.1% SDS solution. The eluted samples were dialyzed against water and tested for differentiationinhibiting activity. As shown in Fig 5, both the 65-Kd and 80-Kd bands had moderate differentiation-inhibiting activity when tested at a 1:104 dilution against DMSO-induced FLC. At a 1:105 dilution the activity associated with the 80-Kd, but not the 65-Kd, band was lost (data not shown), suggestingthat the majority of the differentiation-inhibiting activity was associated with the 65-Kd protein. Several other regions of the gel that were similarly excised and electroeluted had no differentiation-inhibiting activity. The protein content of the gel slice containing the greatest From www.bloodjournal.org by guest on February 6, 2015. For personal use only. INHIBITORS OF ERYTHROID DIFFERENTIATION 1165 Fig 3. A comparison of the differentiation-inhibking activity, and the SDS-PAGE profile, of the individual fractions of the 45-Kd peak area. The individual fractions surrounding the region of maximum inhibitory activity (fractions 34 t o 44; Fig 2) were tested at a 1:lO.OOO dilution for their ability t o inhibit the hemoglobinization of DMSO-induced FLC as described in Materials and Methods and the legend t o Fig 1. The percentage of benzidine-positive cells in DMSO-treated control cultures was 85.5 2 4.2. Samples (50 pL) of each fraction were also analyzed by SDS-10% PAGE and the proteins visualized on the reducing gel by silver stain. The arrow indicates the 62-Kd protein whose appearance coincides with the fraction of peak inhibitory activity. A weak band corresponding t o a 80-Kd protein was also present. amount of differentiation-inhibiting activity in the 65-Kd region (ic, slice 10, Fig 5) migrated as a single band of the same molecular weight when reanalyzed by SDS-PAGE (Fig SB), indicating that the inhibitory activity was attributable to a single protein identified as a 65-Kd band on SDS-PAGE gels. DIP Is a Unique Differentiation-InhibitingProtein DIP activity was lost when the protein was treated with low levels of trypsin, but its activity was unaffected by heating to 100°C for 5 minutes even in the presence of the levels of SDS and p-mecaptoethanol found in electrophoretic sample buffer. The protein’s migration rate on reducing gels was clearly differentfrom other differentiationinhibiting activities such as the homodimeric TGF-P (molecular weight 25 Kd) or TNF-a (molecular weight 17 Kd). Unlike DIP, TGF-P cannot be extracted from rcducing gels in an active state.” Moreover, DIP was unable to inhibit the proliferation of human K562, human erythroleukemia (HEL) and HL-60 cells, as does TNF-CX.~ DIP‘S unique electrophoretic pattern and stability in both acid and reducing SDS-PAGE conditions indicated that the protein was not a differentiation-inhibiting autoimmune antibody (the most common cause of PRCA). In addition, while the main peak of differentiation-inhibiting activity eluted from the P-60 column at 45 Kd, there was no inhibitory activity in the k-rich void volume (Fig 2A), and the k G fractions of the PRCA patient’s blood had no detectable differentiationinhibiting activity (data not shown). AS will be shown, DIP is structurally and functionally distinct from inhibitory activities that have been identified in lcukemia Cells and in the conditioned medium of leukemia cell lines. DIP Is a Potent Inhibitor of Etythroid Differentiation P-60 (PBS)-purified DIP maximally suppressed the hemoglobinization of DMSO-induced FLC at concentrations of From www.bloodjournal.org by guest on February 6, 2015. For personal use only. DURKIN ET AL 1166 z 0 I- .010 9 I- z W LT 66KdA 4 I W LL i 45KdA D IL W .008 (v 2 W 0 0 .004 m I kDo C Z k - E 0 03 .006 0 f - -97 -66 Z U m K z 0 in t- .002 m U Z w 0 LT W a 0 -45 0 20 40 60 80 FRACTION Fig 4. P-60 (PES) gel filtration chromatography of the fractions of peak differentiation-inhibitingactivity (fractions 39 to 41). (A) Fractions39 to 41 (Fig 3) were pooled (4.5 mL), dialyzed against PES, lypholized, and applied to a P-60 gel filtration column equilibrated in PES as described in Materials and Methods. The eluted fractions (1.5 mL) were pooled into groups and tested at a 1:10,000 dilution for their ability to inhibit the hemoglobinization of DMSO-induced FLC as described in Materials and Methods and the legend to Fig 1. The percentage of b' cells in DMSO-treated control cultures was 76.3 2 2.5. Each point is the mean f SEM of four cultures. (E) ReducingSDS-10% PAGE of a sample (50 ILL)of the fraction of peak differentiation-inhibiting activity (fraction33) from the P-60 (PES) column showing a 62-Kd and 80-Kd protein. 500 pg/mL ( < lo-'' mol/L) (Fig 6). In addition to its effects on thc maturation of human BFU-E cells, DIP effectively blocked the diffcrentiation of BFU-E cells in murine bone marrow cultures (Fig 7A). DIP inhibited the development of murine BFU-E-dcrived bursts by 57% when added as a single dose to mouse bone marrow cultures at 100 ng/mL (Fig 7A). By comparison, the inhibitor maximally blocked by more than 80% the development of human BFU-Ederived bursts at concentrations as low as 25 ng/mL (Fig 7B). An interesting finding was that higher concentrations of P-60 (PBS)-purified DIP were consistently less inhibitory for FLC (Fig 6), mouse BFU-E (Fig 7), and human BFU-E differentiation. It is possible that a high-affinity DIP receptor mediatcs the protcin's inhibitory activity, while the activation of a low-affinityreceptor overrides this inhibitory effect. Such a mechanism would, in principal, allow the cell to restrict the inhibitory cffects of DIP to a very narrow concentration range. human BFU-E burst formation, it did not affect human CFU-E progenitor cell development in colony assays (Fig 7B). Further support for DIP being an erythroid-specific factor was providcd by its inability to block the differentiation of HL-60 human promyelocytie leukemia cells induced by either DMSO or 12-0-tetradecanoylphorbol-13-acetate (TPA) (data not shown). IL-3, GM-CSF, and EPO stimulate the proliferation of early and/or late BFU-E progenitor cells.s33 The presence of IL-3, GM-CSF, and EPO (all from Genzyme Corp) added singularly or in combination at levels 100 to 1,000 times above saturation, did not affect the ability of DIP to block the differentiation of human BFU-E cells or DMSOinduced FLC (data not shown). Thus, DIP does not function by interfering with the binding of these hematopoietic growth factors to their respective receptors. Specificity of DIP Inhibition Most of the known hematopoietic inhibitors affect precursor cell maturation by primarily blocking cell-cycle processes needed for the initiation of DNA synthesis and proliferation. Included in this list of growth-inhibiting factors are TGF-P,' TNF-a,'.J superoxide dismutase;' and inhibin." These inhibitory proteins all block the cell-cycle progression of a variety of myeloid progenitor cells and lcukcmia cell lines. A second, and very select, group of hematopoietic inhibitors appears to block cell differentiation directly, not as a secondary response to the inhibition of Human DIP is not species-specific because it effectively blocks the differentiation of mouse as well as human BFU-E crythroid precursor cells. However, the inhibitor appears to act selectively on BFU-E precursor cells. While DIP blocked mouse BFU-E burst development, it did not significantly affcct the development of colonies from the morc advanced CFU-E erythroid precursors nor the development of granulocyte-macrophage (CFU-GM) colonies (Fig 7A). Similarly, whilc DIP was a potent inhibitor of DIP Directly Blocks Cell Differentiation and not Prolifeation From www.bloodjournal.org by guest on February 6, 2015. For personal use only. INHIBITORS OF ERYTHROID DIFFERENTIATION 1167 I 40tA t 30 - 80kDa kDa - 65kDa I -97 -66 20 -45 10 0 2 4 6 8 10 12 FRACTION NUMBER , The differentiation-inhibiting activity of the 62-Kd and 80-Kd protein bands electroeluted from a reducing SDS-10% PAGE gel. (A) The fraction of peak activity (fraction 33) was dialyzed against 1 mol/L acetic acid, lyophilized, resuspended in 100 pL solubilization buffer (20% glycerol, 6% SDS, 10% p-mercaptoethanol, 100 mmol/ L Tris-HCI, pH 6.8) and heated t o 80°C for 5 minutes. The sample was run on several lanes of a reducing SDS-lO% PAGE gel, and the region containing the 80-Kd and 62-Kd bands was c u t from the unstained gel as described in Materials and Methods. This section of gel was cut into 12 1-mm slices, and the protein present in each gel slice was electroeluted as described in Materials and Methods. The eluted proteins were dialyzed against water, lyophilized, resuspended in RPMl medium, and tested at a 1:lO.OOO dilution for their ability t o inhibit the hemoglobinizationof DMSO-induced FLC as described in Materials and Methods and the legend t o Fig 1. The percentage of b’ cells in DMSO-treated control cultures was 90.1 2 4.5. Each point is the mean 2 SEM of four cultures. (B) The eluted material from the gel slice containing the 62-Kd protein (slice no. 4) was rerun on a reducing SDS-10% PAGE gel, and the protein visualized by silver stain. proliferation.'."^" As shown in Fig 8, adding DIP to the medium of FLC cultures at the time of DMSO induction effectively blocked the hemoglobinization of the cells from day 4 onwards. However, DIP did not inhibit the proliferation of uninduccd FLC, nor did it affect the proliferation of the more slowly growing DMSO-treated cells (which ceased proliferating on day 8 [data not shown]) during the period that differentiation was affected (Fig 8). Similarly, DIP did not affect the prolifcration of HL-60 cells, or tsAEV-LSCC HD3 chicken erythroleukemia cells. To our knowledge, DIP is the first example of a human hematopoietic inhibitor protein that directly blocks cell differentiation rather than the cell cycle. DIP R I Q c Ear(v ~ Events in DMSO-Induced Differentiation It took approximately 3 days for DMSO to induce hemoglobin production in 50% of the cells of the FLC line used in this study (Fig 9, insert). The continuous presence of DMSO was ncedcd in the culture during the first 48 to 72 hours to fully commit the cells to hcmoglobinization (Fig 9, insert). The question arose as to whcrc in this commitment phase DIP acted in order to block differentiation. DIP inhibition was maximally effective when added to the culture shortly before (data not shown) or at the same time as DMSO (Fig 9A). As indicated in Fig 9A, the “window” of DIP action was extremely narrow; the inhibitor was effective only when added to cultures within 1 hour after adding DMSO. DIP did not block the differentiation of FLC when added later than 1 hour after DMSO (Fig 9A). These results were confirmed by washout experiments that showed that DIP had to be in the culture medium only during the first hour in order to inhibit the hemoglobinization of DMSO-induced FLC 5 days later (Fig 9B). Thus, DIP appears to function by inhibiting an early and crucial From www.bloodjournal.org by guest on February 6, 2015. For personal use only. DURKIN ET AL 1168 step in the process by which DMSO stimulates FLC differcntiation. 50 z 0 + 4I- z DISCUSSION 40 W IY L W k 0 LL 30 0 z 0 k m I z 5 W V 20 10 a W a 0 I 0.001 . .......I . 0.01 . .......I .......I . 1 0.1 .......I 10 . .......I 100 LOG PROTEIN (ng/ml) Fig 6. The effect of Increasing concentrations of DIP on DMSOinduced FLC differentiation. Various concentrations of P-60 (PES). purified DIP (V),or identicallytreated normal plasma (L)were tested for their ability to inhibit the hemoglobinization of DMSO-induced FLC as described in Materials and Methods and the legend to Fig 1. The percentage of b‘ cells in DMSO-treated control cultures was 82.9 4.1. Each point is the mean 2 SEM of four cultures. + BI 60 A 65-Kd protein isolated from the plasma of a PRCA patient was found to bc a potent and specific inhibitor of erythroid differentiation. It was able to block the maturation of human and mousc BFU-E crythroid cclls, but not human or mouse CFU-E cclls or mouse CFU-GM cells. Consistent with these findings, DIP inhibited the DMSOinduced differentiation of Friend erythroleukemia cclls (arrested at the late BFU-E/early CFU-E stagc of dcvclopmerit"." and exhibiting at least a partial BFU-E phenotype“.”), but not the promyelocytic cell line, HL-60. Collcctively, these data indicate that DIP is a novcl inhibitor of differentiation, which is likely to be responsiblc for the patient’s PRCA. This cortclusion is strongly supportcd by preliminary experiments that indicatc that injccting DIP into normal mice selcctivcly induccs a profound crythroblastopenic condition in which the maturation of othcr hematopoietic lineages is not significantly affcctcd (manuscript in preparation). To our knowlcdgc, this is thc first diffcrentiation-inhibiting activity to bc purificd from human plasma with the potcntial to cause hcmatopoictic discasc. The involvement of this protein in othcr ancmias remains to bc determined. c B 6 I 100 - A MOUSE a s a E 50 5 40 0 30 n s 0 LL 0 Z HUMAN T 20 80 - 60 - 40 - 20 - 0 t m I z 10 M 0 L 10 L 100 1000 LOG PROTEIN (ng/ml) Fig 7. The effects of DIP on the development of IA) m(8)BFU-E, (0) CN-E, and (0)CFU-OM, and (B) human BFU-E and CFU-E progenitor cells. Various concentrations of P.80 (PBS)-purified DIP were tested for their ability to inhibit the development of mouse hematopoietic progenitorsin colony assays as described in Materials and Methods. The number of EFU-E bunts and CFU-E and CFU-GMcolonies in untreated, control mouse cultures averaged 24, 200, and 37 per well, respecthrely. DIP was tested on human EFU-E and CFU-E cells at 0.25 nglmL, the optimal concentration for human EFU-E inhibition (data not shown). The data are representative of three separate determinations each of which was performed in triplicate. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. INHIBITORS OF ERYTHROID DIFFERENTIATION 40 1169 A T CI VI ? 30 X v IL: W m 20 25 3 z -I -I W 0 10 0 1 2 3 4 5 0 6 1 2 3 4 5 6 DAYS DAYS Fig 8. A comparison of the effects of DIP on the (A) proliferation and the (B) differentiation of FLC cells. FLC cells were plated at 70,000 cells/mL (in 24-well dishes) in RPMl medium containing 10% FBS. DIP (0.5 ng/mL) was added to the medium of some (V, V),but'not other (0.0) FLC cukyres at t = 0. DMSO was then added to a final concentration of 2% to some (V,0).but not other (7,0 )dishes and the cultures were days, small gamples were removed from each well and (A) stained with trypan blde and the number of viable incubated at 37"E On the in@icated cells determined, and (B) the proportion of hemoglobin (b+)-producingcells was determined as described in Materials and Methods. The proliferation of DMSO-treated, but not untreated, cells ceased on days. The plues are the mean f SEM from triplicate cultures. such as TNF-a and TGF-f3 are potent inhibitors of human and mouse CFU-E cell differentiation but they rapidly inhibit the proliferation of a wide variety of cell lines.24 $imilarly, the erythroid inhibitory activity (EIA) isolated DIP is a novel differgntiation-inhibiting factor because of its apparent specificity for BFU-E progenitorsand its ability to inhibit the differenfiation of DMSO-induced FLC without being a cell cycle blocker. Hematopoietic inhibitors I- B 60 30 z P $ z e $ 5O z w I): f z w E E 40 LL 8 z P L 30 0 z o_ c- y 20 E 0 t m I 20 E z - be w 10 0. l o0 0 1 2 TIME OF DIP ADDITION (HRS) 3 Il p 1 2 TIME OF DIP REMOVAL (HRS) Fig 9. DIP blocks an early and crucial step in the process by which DMSO stimulates FLC differsntiation. (A) FLC cells were induced to differentiate into hemoglobin-producing cells by the addition of 2% DMSO as described in Materials and Methods. DIP (0.5 ng/mL) was added tq the culture medium at the indicatedtimes after OMSO aadition, and its effect on hemoglobinization was 9Pasured 4 days later. The percentage of b+ cells in DMSO-treated control cultures was 72.3 k 3.0. Each point is the mean f SEM of four cultures. (Inrert) The reqirdmgnt for DMSd throughout the c9urse of hemoglobin production in differentiating FLC. Cells were plated in complete medium containing $% DMSO and th,e tells were (0)maintained in this medium, or the medium was replaced with fresh, DMSO-free medium (0) 24, (0)48, or 72 hburs (0) later. The percentage of b+cells was determined at the indicattd times. (B) FLC were plated in complete medium containing DIP (0.5 ng/mL) and 2% DMSO. At the indicated times, excess DIP was removed by washing the cells with complete medium, and fresh, complete medium containing 2% DMSO was added. The effect of these treatments an FLC hemoglobinization was determined 4 days later as described in Materials and Methods. The percentage of b' cells in control cultures not exposed to DIP was 78.3 f 3.7. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. DURKIN ET AL 1170 from murine macrophage cell lines is a potent inhibitor of FLC pr~liferation.~~ DIP is functionally different from inhibitory proteins such as leukemia inhibitory activity (LIA) and leukemia-associated inhibitor (LAI), both of which mainly affect granulopoie~is.'~~'~ LAI, originally isolated from the conditioned medium of promyelocytic HL-60 cell cultures,'* migrates as a 125-Kd protein band on SDS-PAGE gels, cannot be eluted from gels in an active state, and inhibits CFU-GM but not BFU-E differentiation.I3 LIA is an acidic isoferritin, a metal binding protein complex of 24 subunits (-500 Kd) composed of a higher proportion of heavy ( 21 Kd) to light ( 19 Kd) subunits, which suppresses colony formation by human and mouse CFU-GM and BFU-E progenitor cells in vitro.'",'' LIA migrates as a pair of 20-Kd bands on reducing gels that are not biologically active after elution from the gel.'" Finally, DIP is different from the inhibitor isolated from C57 BL/BG mouse bone marrow cultures that reversibly inhibits the initiation of DNA replication in BFU-E precursor The inhibitory activity of cells and a variety of cell this 16-Kd protein, recently identified as superoxide dismutase? is readily suppressed by excess IL-3. Clearly, the physical and functional characteristics of these, and other, hematopoietic inhibitors are clearly distinct from those of DIP. DIP blocks the hemoglobinization of FLC, which occurs 4 to 5 days after the cells are exposed to DMSO. The protein appears to do this within the first hour after DMSO addition by blocking a crucial, transient early step in the long process leading to hemoglobinization. The inhibitory effect of DIP persists even when the inhibitor is removed from the medium after the first hour, suggesting that the DIP-sensitive step(s) must occur in a coordinated and timed manner for hemoglobinization to normally occur several days later. DIP is a third (and the first human-derived) member of a group of proteins able to directly inhibit hematopoietic cell differentiation. These proteins are distinguished by their ability to inhibit the differentiation of leukemia cell lines in culture without directly affecting cell proliferation. The first member of this group to be identified was chicken ADIF, which blocks the differentiation of both chicken and murine erythroleukemia cells into hemoglobin-producing cells without inhibiting pr~liferation.'~.'~.~~ The other member of this group was isolated from the conditioned medium of R-1 mouse myeloid leukemia cells and inhibits the differentiation and increases the oncogenicity of murine myeloid cell - - cultures without directly blocking cell pr~liferation.~ Unlike DIP, this protein is species-specific and does not affect human cells.' While DIP is functionally similar to ADIF, the two proteins are not the same because human DIP does not affect the differentiation of the ADIF-producing chicken tsAEV-LSCCHD3 cells. If DIP acts primarily by inhibiting cell differentiation, why does it not affect the proliferation of FLC but does block the growth of bursts derived from BFU-E progenitor cells in vitro? The answer probably lies in the bidirectional coupling of differentiation and proliferation in normal hematopoietic cells such as BFU-E. It is well understood that, in most cases, the differentiated phenotype develops only in those cells that are proliferating. What is less clearly understood is the reverse scenario-the dependence of proliferation/viability on normal cell differentiation. This dependence, lost in erythroleukemia cell lines that proliferate persistently without differentiating, may operate to safeguard normal hematopoiesis. By this mechanism, the proliferation of BFU-E cells would be halted (perhaps by apoptosis) if the cells were prevented from differentiating into CFU-E within a prescribed number of cell divisions. This would safeguard the organism from the disastrous consequences of allowing uncontrolled proliferation of undifferentiated cells to occur simply because of a block in BFU-E maturation. If this is true, then the in vivo effects of DIP would be expected to cause erythroblastopenia, the condition found in the PRCA patient. Thus, a block in erythroid differentiation is in itself unable to cause erythroleukemia. In principle, these cells could become erythroleukemic only if they acquire the ability to proliferate despite the block in differentiation. This uncoupling of proliferation from differentiation is characteristic of erythroleukemia cell lines, such as FLC, that proliferate continuously as immature precursor cells. It follows, therefore, that DIP can inhibit FLC differentiation without affecting proliferation, but that proliferation of normal BFU-E is halted as a secondary response to the inhibitor. Finally, it is of interest that a significant proportion of patients suffering from prolonged idiopathic PRCA go on to develop erythroleukemia.I6 If DIP-like proteins are responsible for a proportion of idiopathic PRCA, then their inhibition of erythropoiesis may be an early step in the process leading to leukemia. Studies are in progress to determine the frequency at which DIP occurs in patients suffering from PRCA and other hematopoietic disorders, such as leukemias. REFERE:NCES 1. Dexter TM, Garland JM, Testa NG (eds): Colony-Stimulating Factors. Molecular and Cellular Biology. New York, NY, Dekker, 1989 2. Lawrence D A Transforming growth factor+: A multifunctional growth factor, in Krsmanovic V, Whitfield JF (eds): Malignant Cell Secretion. Boca Raton, FL, CRC, 1990, p 234 3. Munker R, Koeffler HP: In vitro action of tumor necrosis factor on myeloid leukemia cells. Blood 69:1102,1987 4. Roodman GD, Bird A, Hutzler D, Montgomery W Tumor necrosis factor-a and hematopoietic progenitors: Effects of tumor necrosis factor on the growth of erythroid progenitors CFU-E and BFU-E and the hematopoietic cell lines. Exp Hematol15:928,1987 5. Del Rizzo DF, Eskinazi D, Axelrad AA: Negative regulation of DNA synthesis in early erythropoieticprogenitor cells (BFU-E) by a protein purified from the medium of C57BL/6 mouse marrow cells. Proc Natl Acad Sci USA 85:4320, 1988 6. Metcalf D, Hilton DJ, Nicola N A Clonal analysis of the actions of the murine leukemia inhibitory factor on leukemic and normal murine hemopoietic cells. Leukemia 2216,1988 7. Okabe-Kado J, Kasukabe T, Honma Y, Hayashi M, Hozumi M: Purification of a factor inhibiting differentiation from condi- From www.bloodjournal.org by guest on February 6, 2015. For personal use only. INHIBITORS OF ERYTHROID DIFFERENTIATION tioned medium of nondaerentiating mouse myeloid leukemia cells. J Biol Chem 263:10994,10999,1988 8. Olsson I, Arnljots K, Gullberq U, Lantz M, Peetre C, Richter J: Myeloid cell differentiation:The differentiation inducing factors of myeloid leukemia cells. Leukemia 2165,1988 9. Smith AG, Heth JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D: Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336688, 1988 10. Broxmeyer HE, Bognacki J, Dorner MH, Sousa M: Identification of leukemia associated inhibitory activity as acidic isoferritins. J Exp Med 153:1426, 1981 11. Broxmeyer HE, Lu L, Bicknell DC, Williams DE, Cooper S, Levi S, Salfeld J, Arosio P: The influence of purified recombinant human heavy-subunit ferritins on colony formation in vitro by granulocyte-macrophage and erythroid progenitor cells. Blood 68:1257,1986 12. Olofsson T, Olsson I: Suppression of normal granulopoiesis in vitro by a leukemia associated inhibitor (LAI) derived from a human promyelocytic cell line (HL-60). Leuk Res 4:437,1980 13. Olofsson T Leukemia associated inhibitor: Biological characterization and purification of the active subunit, Najman A, Guigon M, Gorin NC, Mary JY (eds): The Inhibitorsof Hematopoiesis. Paris, France, John Libbey Eurotext Ltd, 1987,p 177 14. Fasciotto B, Kanazir D, Durkin JP, Whitfield JF, Krsmanovic V: AEV-transformed chicken erythroid cells secrete autocrine factors which promote soft agar growth and block erythroleukemia cell differentiation. Biochem Biophys Res Commun 143:775, 1987 15. Krsmanovic V, Morardet N, Biquard JM, Mouchiroud G, Fasciotto B, Ristic A, Parmentier C, Blanchet JP, Kanazir D, Durkin JP, Whitfield JF: Autocrine differentiation-inhibitingfactor (ADIF) from chicken erythroleukemia cells acts on human and mouse early BFU-E erythroid precursors. Biochem Biophys Res Commun 157:762,1988 16. Dessypris E N Pure Red Cell Aplasia. Baltimore, MD, Johns Hopkins University, 1988 17. Orkin SH, Harosi FI, Leder P: Differentiation in erythrleukemia cells and their somatic hybrids. Proc Natl Acad Sci USA 72:98,1975 18. Dainiak N, Kreczko S, Cohen A, Pannel R, Lawler L Primary human marrow cultures for erythroid bursts in a serumsubstituted system. Exp Hematol13:1073,1985 19. Kobari L, Dainiak N, Najman A, Kreczko S, Gorin NC, Duhamel G, Frindel E Stimulatory effects of plasma from patients with acute nonlymphoblasticleukemia on early erythroid progenitors and pluripotent stem cells. Exp Hematol15:838,1987 20. Iscove NN, Sieber F: Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol3:32, 1975 21. Blanchet JP, Arnaud S, Samarut J, Bouabdelli M: A factor present in normal mouse serum stimulates later erythroid precursor proliferation. Exp Hematol 12595,1984 1171 22. Laemmli U K Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680,1970 23. Koenig HM, Lightsey AL, Nelson DP, Diamond LK Immune suppression of erythropoiesisin transient erythroblastopenia of children. Blood 53:742,1979 24. Dessypris EN, Krantz SB, Roloff JS, Lukens JN: Mode of action of the IgG inhibitor of erythropoiesis in transient erythroblastopenia of children. Blood 59114,1982 25. Kabat D: Molecular biology of friend viral erythroleukemia. Curr Top Microbiol Immunol148:1,1989 26. Ostertag W, Stocking C, Johnson GR, Kluge N, Kollek R, Franz T, Hess N Transforming genes and target cells of murine spleen focus-formingviruses. Adv Cancer Res 48:193,1987 27. Roberts AB, Anzano MA, Meyers CA, Wideman J, Blacher R, Yu-Ching E, Stein S, Lehrman SR, Smith JM, Lamb LC, Sporn MB: Purification and properties of a type transforming growth factor from bovine kidney. Biochemistry225692,1983 28. Iscove NN, Sieber F, Winterhalter KH: Erythroid colony formation in cultures of mouse and human bone marrow: Analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose con A. J Cell Physiol30983,1974 29. Tojo A, Fukamachi H, Saito T, Kasuga M, Urabe A, Takaku F Induction of the receptor for erythropoietin in murine erythroleukemia cells after dimethyl sulfoxide treatment. Cancer Res 48:1818,1988 30. Sawyer ST, Krantz SB, Luna J: Identificationof the receptor of erythropoietin by cross-linking to Friend virus-infected erythroid cells. Proc Natl Acad Sci USA 84:3690,1987 31. Donahue RE, Emerson SG, Wang EA, Wong GG, Clark SE, Nathan DG: Demonstration of burst promoting activity of recombinant human GM-CSF on circulating erythroid progenitors using an assay involving the delayed addition of erythropoietin. Blood 66:1479,1985 32. Ihle JN, Keller J, Greenberger JS, Henderson L, Yetter RA, Morse H C Phenotypic characteristics of cell lines requering interleukin 3 for growth. J Immunol129:1377,1982 33. Westbrook CA, Gasson JC, Gerber SE, Selsted ME, Golde DW: Purification and characterization of human T-lymphocytederived erythroid-potentiatingactivity. J Biol Chem 259:9992,1982 34. Pluthero FG, Shreeve M, Eskinazi D, van der Gaag H, Huang KS, Hulmes JD, Blum M, Axelrad A Purification of an inhibitor of erythroid progenitor cell cycling and antagonist to interleukin 3 from mouse marrow cell supernatants and its identification as cytosolic superoxide dismutase. J Cell Biol111:1217,1990 35. De Jong FH: Inhibin. Physiol Rev 68:555, 1988 36. Sassa S, Wolpe S, Cerami A Inhibition of erythroid differentiation of mouse erythroleukemia cells by a macrophage product(s). Blood Cells 13:161,1987 37. Krsmanovic V, Biquard J M Erythroleukemia cell secretion and erythroid cell differentiation-inhibitingfactors, in Krsmanovic V, Whitfield JF (eds): Malignant Cell Secretion. 1990, p 151 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1992 79: 1161-1171 The identification and characterization of a novel human differentiation-inhibiting protein that selectively blocks erythroid differentiation JP Durkin, JM Biquard, JF Whitfield, N Morardet, J Royer, P Macdonald, R Tremblay, JD Legal, R Doyonnas and JP Blanchet Updated information and services can be found at: http://www.bloodjournal.org/content/79/5/1161.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026