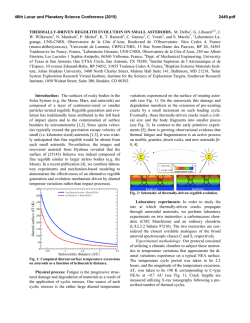
Platelet Glycoprotein IIb-III, (ffIIbp3 Integrin) Confers Fibrinogen
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. Platelet Glycoprotein IIb-III, ( f f I I b p 3 Integrin) Confers Fibrinogen- and Ac tiva tion-Dependen t Aggregation on Heterologous Cells By Mony M. Frojmovic, Timothy E. O‘Toole, Edward F. Plow, Joseph C. Loftus, and Mark H. Ginsberg To analyze molecular mechanisms of platelet aggregation, w e have studied the aggregation of Chinese hamster ovary (CHO) cells expressing between 1 and 4 x lo5 recombinant human glycoprotein (GP) 11,-111, molecules per cell (A5 cells). These cells aggregated as measured by the disappearance of single cells during rotary agitation. Aggregation was dependent on the presence of extracellular fibrinogen ( ~ 5 0 0 nmol/L) and divalent cations, and required prior activation of the GPII,-Ill,. A synthetic peptide (GRGDSP) and monoclonal anti-GPII,-Ill, antibody (2G12) that block platelet aggregation also blocked aggregation of these cells. Parent CHO cells or those expressing recombinant GPII,-Ill, containing a point mutation that causes variant thrombasthenia both failed to aggregate when stimulated in the presence of fibrinogen. These data show that GPII,-Ill, is the only unique platelet surface component required for aggregation. o 1991 by The American Society of Hematology. P KL).These two Abs, respectively, report on the activation dependent allbP3receptor for Fg” and on the resting heterodimer c~mplex.’~ After incubation at room temperature for 30 to 60 minutes, samples were diluted to 0.5 mL with Tyrodes immediately preceding FCM ana lyse^.'^ HYSIOLOGIC platelet aggregation is essential for normal hemostasis. Such aggregation requires the presence of glycoprotein (GP) IIh-IIIa’ in the “activated ~tate,”’.~ ie, in a state competent to bind fibrinogen (Fg) or other adhesive macromolecules with high affinity. Although the aggregation response requires adhesive protein binding centered on Fg,’,2,4,7 I’ it is clear that additional “postoccupancy” event^'.^^^^" Is are also required for normal aggregation. In particular, contributions from other unique platelet components from the surface or internal to the aggregation response have been difficult to evaluate. We therefore analyzed the capacity of recombinant GPI1,111, to support aggregation of heterologous cells. In the present report, we have used two novel tools to “isolate” the role of GPI1,-111, in supporting cellular aggregation: (1) recombinant GPI1,-111, inserted into a non-platelet cellular environment (Chinese hamster ovary [CHO] cells); and ( 2 ) activating antibodies directed against GPIII, previously shown to directly transform GPI1,-111, into a high-affinity Fg receptor.I6 The aggregation of CHO cells required functional GPII,,-IIIa,activation, and divalent cation-dependent Fg binding. Thus, GPI1,-111, is the only unique platelet component required for Fg-mediated platelet aggregation. MATERIALS AND METHODS Generation and Analysis of Stable Cell Lines CHO cells were cotransfected with equal amounts of CD3a and CD2b,” and a CDM8 vector containing the neomycin resistance gene (CDNeo) in a 3 0 1 ratio as described.” Cloned cell lines were established by single cell sorting in a FACStar (Becton Dickinson, San Jose, CA) and maintained in media without G418 (Geniticin; GIBCO, Grand Island, NY). Cell lines were established using normal human platelet allbP3 (A5),and for allbP3 containing a point mutation in p3abrogating Fg binding (BCAM). These two cell lines were readily distinguishable by the presence (A5) and absence (BCAM) of upregulation of binding of the anti-LIBS1 monoclonal antibody (MoAb) following incubation of the cells with RGDcontaining peptides.’, Fluorescence Measurements by Flow Cytometry (FCM) Cells prepared in suspension (see below) at 2.5 x lo5 in 25 FL Tyrodes buffer (NaC1 140 mmol/L, KC12.7 mmol/L, NaHCO, 0.4 mmollL, containing albumin [ l mgimL], Ca” [2 mmol/L], Mg” [2 mmol/L], glucose [l mgimL], all adjusted to pH 7.4; hereafter referred to as Tyrodes), were incubated with fluoresceinated (F1TC)-PAC1 (10 pg/mL)I8 or FITC-4F10I9 in the presence or absence of additional agonists andlor inhibitors (final volume, 50 Blood, Vol78, No 2 (July 15). 1991: pp 369-376 Activating MoAbs The principal MoAb used was one previously raised against purified al,,Ps,Ab6220;we used both the IgG and Fab fragments of this Ab (IgG62 and Fab62). In a few initial experiments we also used the Fab fragment of the IgG, P41, which was raised against intact platelets for its capacity to stimulate PAC1 (and Fg) binding.l6 For one study (Table l),we also used the Fab fragments of Mab33 IgG (Fab33), which bind to GPIII,, as previously reported.*’ The IgGs were purified from ascitic fluid on protein A-sepharose (BioRad, Richmond, CA); the Fab fragments were prepared by digestion of the IgGs with papain (2001 wtiwt of IgG to papain) for 6 hours at 37T, and finally purified with protein A sepharose (sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] showed the presence of less than 8% undigested heavy chain). Immunoprecipitation: Analysis of GPII, and 111, Stable allbP3transfectants and wild-type CHO cells were surface labeled by the lactoperoxidase-glucose oxidase method and octylglucoside extracts prepared. Immunoprecipitations with specific antibody were preformed as previously described” and analyzed by reducing and nonreducing SDS-PAGE, followed by autoradiography. The recombinant GPI1,-111, is antigenically and functionally similar to platelet GPII,-III,.16 To compare the molecular size of these two proteins, recombinant GPI1,-111, was immunoprecipitated from surface labeled A5 cells and its mobility on SDS-PAGE From the Department of Physiology, McGill University, Montreal, Quebec, Canada. Submitted October 22, 1990; accepted March 12, 1991. Supported in part by the Medical Research Council of Canada (M.M.F.),and by National Institutes of Health Grant Nos. HL-28235 and HL-31950. Presented in part at the 63rd Scientific Sessions of the American Heart Association, Dallas, TX, November 1990. Address reprint requests to Mony M. Frojmovic, PhD, Department of Physiology, McGill University, 3655 Drummond, #1102, Montreal, Quebec, Canada H3G lY6. The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact. 0 1991 by The American Society of Hematology. 0006-4971I9117802-0012$3.00/0 369 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. FROJMOVIC ET AL 370 Table 1. Comparison of Fg-Dependent Aggregation of A5 Cells Activated With Antibodies Against GPIII, %PAmax* Antibody Mean 2 SD Range Fab62 IGg62 Fab (P41) 65 f 13 (8)t 61 2 15 (8) 50 (1) 40-80 38-84 - 'PAmax values for a given preparation were obtained by comparing particle counts for A5 cells activated with and without Fg (500 nmol/L); each of these samples was preactivated with Ab (6 pmol/L in all cases) for 30 minutes and then rotated for 15 to 20 minutes with subsequent fixation: PAmax values are the average for PA, and PA, (see Materials and Methods). tThe number of different A5 cell suspensions evaluated is shown in parentheses; four common preparations were used for Fab62 and lgG62, yielding 57 f 13and 55 f 7 %PAmax, respectively. was compared with purified platelet GPI1,-111, electrophoresed in the adjacent lane. Recombinant GPIII, exactly comigrated with platelet GPIII,, but the recombinant GPII, had a slightly greater mobility (apparent Mr = 125,000) than the platelet protein (apparent Mr = 133,000), consistent with altered glycosylation in the CHO cells. Preparation of Cell Suspensions Monolayers of cells in culture were harvested at room temperature by one wash with phosphate-buffered saline (PBS), pH 7.4; incubated with 3.5 mmol/L EDTA (5 minutes); and washed via pelleting (2X) with Tyrodes to give suspensions containing = 5 x lo7particledml. Such suspensions usually contained unacceptably high levels of aggregated cells, with greater than 75% of cells in aggregates of greater than 4 cells per aggregate; moreover, the preparations varied from day to day, as reported by others?' Attempts to grow and maintain single cells directly in suspension culture, as described by Harper and Juliana,= proved unsuccessful. Therefore, we determined the minimal final concentration of TPCK-Trypsin needed to yield largely single cells with negligible higher-order aggregates. We used 0.01% (final concentration) TPCK-Trypsin, added directly for a further 5 minutes of incubation J to the 3.5 mmol/L EDTA above. A solution of 10% fetal calf serum and soybean trypsin inhibitor (1 mg/mL) in Tyrodes was then added (1 vol), followed by pelleting and two washes with final resuspension in Tyrodes at = 10' cells/mL, to optimize neutralization of trypsin. These trypsin-resuspended cells typically contained 74% f 10% singlets, 18% f 7% doublets, 4% f 3% triplets, negligible higher-order aggregates for A5 (n = 5 ) , CHO, or BCAM cells; 96% f 2% of all cells were viable (Trypan blue exclusion test). The cell suspensions were incubated and gently mixed at room temperature for 1/2 hour before activatiodaggregation assays, and were normally used within 3 hours. Aggregation of A5 Cells in Wells Rotated At 75 rpm A modification of the procedure recently described for studies of aggregation of L cell transfectants was sed.'^ Typically, 124 FL of unactivated A5 cells (2 x lO'/mL) was added to one side of each well of a 24-well tissue culture plate plus 77 FL of buffer; 24 pL of Ab62 was added to a second side of each well and all mixed by gentle swirling at 0 times; the mixture was incubated for greater than 30 minutes at room temperature; and then 75 p L of buffer or Fg solution was pipetted in, mixed with rapid hand-swirling ( < 3 seconds), and then rotated at 75 rpm on a American Rotator V (American Dade) gyrotory shaker at R T for greater than 20 minutes. To arrest aggregation, 400 FL of 1% glutaraldehyde (GA) (or 2% formaldehyde [FA] on two occasions yielding the same results) was added directly into the rotating suspension and allowed to rest at room temperature for greater than 15 minutes before analysis. In a few experiments, Fg was added at the same time as activating Ab. Parameters of Aggregation Three parameters were typically used to describe cell aggregation in this study: the percent of particles aggregated (%PA), derived from the changes in singlet counts ( % P A ) or from the changes in total particle counts (%PA,), and aggregate size. The general equation for %PA due to changes in particle counts is: %PA = (1 - N,/N,) x 100 (equation 1); where No and N,are the number of particles per unit volume at time 0 (control sample) and t (time for aggregation). Thus, %PA can be determined by microscopy from changes in singlet counts (%PAJ, as well as in 100 h 8 Y PAS PAT Aggregate size 40 20 0 A 5* CHO* BCAM* Fig 1. Extent of aggregation of A5 venus"control" CHO cells incubated with Ab62 and Fg. The A5*, CHO*, and BCAW are t h e same cells preactivated with Fab62 as described in t h e Legend t o Fig 7. Sub-aliquots of these suspensions were taken from t h e activated cells with and without Fg addition, and the particle distribution and size of aggregates determined by microscopic counting as in Materials and Methods. The actual percent of aggregation due to decreases in singlets (%PA,) or total particles (%P&) is shown, while the mean sizes of aggregates determined from the average number of cells per aggregate have been normalized to that seen for the A5* cells = 3 f 1 cells per aggregate seen for CHO* or BCAM" cells versus 250 cells per aggregate seen for A5* cells. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. a& AND CELL AGGREGATION 37 1 120 BUnu ae Y -m C 0 60- 0) aLI 0 0 a 40- 0 PAS PAT Aggregate size Fig 2. Aggregation of Ab62 (IgG)-actbated A5 cells is Fg-dependent. The aggregation and size of aggmgates formed is presented as % P A , %PA,, and relative aggregate size (see Materials and Methods). Cell counts and aggregate size were determined for A5 cells; A5 cells plus Fg (500 nmol/L); A5 cells plus lgG62 (6.1 pmol/L); or A5 cells plus lgG62 (30 minutes) followed by addition of Fg (SO0 nmollL). Each of these suspensions (300 pL) was incubated for 30 minutes without Fg in the microtiter wells, followed by addition of buffer or in one case, Fg (500 nmol/L); gyrotated at 75 rpm for 15 minutes; fixed with glutaraldehyde and analyzed (see Materials and Methods). Cell counts before gyrotation were 7 x l b / m L for all suspensions. The %PA, values are mean values determined both by microscopy and FCM. Aggregate size was determined as the number of cells per aggregate and normalized t o 50 cells/aggregate as 100%. The bars represent one standard deviation for three t o four values determined for any one parameter (see Materials and Methods). Similar results were obtained with a second A5 preparation. total particle counts (%PAT), and by FCM for %PA, (see below). All three methods generally gave very similar results, but FCM was a much faster method. Cross-checkingof methods was conducted in a number of experiments 10 avoid artifacts, including possible disaggregation due to flow in the FCM. Microwopic and FCM estimates of %PA gave, respectively. less than 10% and F / r variations in duplicates for the same suspension. Unless othenvise specified, %PA, in the Results is an average for duplicate samples determined and FCM and refers an average for all three methods. The data is presented graphically as average values when only duplicate measurements have heen made for a given parameter (Fig I ) , or as mean 2 standard deviation (SD)when three or more values have been pooled (Figs 2 and 3). Aggregation Determined by Particle Counting purfide Co"n'inR M ; , - ~ , ~ c oCell ~ . suspensions were viewed on a hemocytometer with a phase-contrast &iss microwope ( 2 0 magnification); ~ singlets and multiplets (2 or more cells per aggregate) were counted as individual particles ( 21 0 particles per sample) for determinations of the total singlet count (N,) and total particle count (NT) present per Of cell suspension* as PreviouslY reported in platelet studies." For the evaluation of size distribution of particles, the frequency distribution of singlets and of doublets to higher-order aggregates was recorded ( L I 0 particles per "1 100 Fig 3. Fg-dependent aggregation of A5 cells requires calcium and actbated GPllb-llla. The %PA, %PA,, and relative aggregate size are shown for (1) A5 cells maximally aggregated with lgG62 (6.1 pmollL) and Fg (500 nmol/L) as in Fig 2, and (2) for inhibitory added to the At cells immediately before Ab62 addition: EDTA (5 mmol/L), RGDSP (1 mmollL), 2G12, and a non-blocking Ab15 (60 pglmL). The experimental and assay conditions are otherwise identical t o those described in Fig 2. PAS PAT J T 1 Aggregate 9120 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. FROJMOVIC ET AL 372 sample): phase-contrast optics ( 2 0 ~was ) found more useful than bright field for counting cells within aggregates, with an uncertainty of 2 10% to 20% for aggregates containing greater than =50 cells per aggregate. Aggregate size was obtained by averaging the number of particles containing greater than 2 cells per aggregate ( 2 2 0 particles counted), and then generally expressed as a percentage of maximal aggregate size, normally 250 cells per aggregate. FCM. For large experiments, we used the more efficient FCM to determine the particle count per unit volume (N,) of the A5 cell suspensions, as recently reported for studies of neutrophils.26 Briefly, the Becton Dickinson FACScan was used by determining the flow time (FT) required to count a fixed number of A5 particles (typically 3,000). The glutaraldehyde-fixed A5 suspensions in the above aggregation mixture were generally diluted a further threefold with particle-free Hematall (N, = 5 x 10'/mL); 500 FL was added to Becton Dickinson Falcon tubes (#2052), and taken up into the FACScan at the high flow rate (FR) setting. Cells were discriminated from noise by gating on forward scatter. Using FACScan Research software; the FT to measure 3,000 particles was recorded for the different A5 cell preparations. Because the FACScan draws a sample at a constant FR, the number of particles per unit volume (N) = 3,0004FR x FT). It follows that N,/N, = FTJFT,. Substituting into equation 1 yields: %PA, = (1 - FTJ FT,) x 100 (equation 2); yielding %PA, directly from a comparison of FTs for control (FTJ and aggregating (FT,) samples. Other Reagents Monoclonals IgG 4F10 and 2G12, specific for the a,,,,P,complex, were generous gifts from Virgil Woods (Univ. of California, San Diego). Other monoclonals were produced or obtained as described."' The peptide GRGDSP was prepared as previously described." These proteins and antibodies were stored at -70°C. thawed at 37°C just before use, and diluted with Tyrodes-albumin; they were spun in a microfuge (Eppendorf; Brinkman, Westbury, NY) at room temperature for 5 minutes to remove any large aggregates. TPCK-Trypsin (230 f 5 U/mg protein; Worthington, NJ) was made up in Tyrodes (free of albumin) just before use. All other reagents or chemicals obtained were of the highest available quality. RESULTS Characterization of Recombinant GPII,-Ill, and its Activation in CHO Cells We found that the treatment of A5 cells with low doses of trypsin was required to dissociate small aggregates in EDTA suspensions. This treatment had little effect on the quantity (Fig 4A) or structure of GPI1,-111, in A5 cells (Fig 4B). A5 cells treated in this manner could also be stimulated to express binding sites for the PAC1lRantibody (Fig 5) or Fg (Fig 6). Fab fragments of IgG 62 produced maximal PAC1 binding at = 6 pmol/L, Fab and 4 pmol/L IgG. Ab62-stimulated specific Fg binding reached steady state by 60 minutes, whether activated by IgG62 or by Fab62 fragments lacking the Fc portion (not shown), with 2 75% of maximal binding within 30 minutes (Fig 6). The time course for Fg binding was similar when the antibody and Fg were added simultaneously or the cells were preactivated with Fab62 for 30 minutes before Fg addition. In succeeding experiments we routinely preactivated with Ab62 for 30 to 60 minutes before both Fg binding and A5 aggregation studies to ensure optimal Fg-receptor expression. Fig 4. (A) Fluorescence histograms showing GPllb-llla on A5 cells resuspended with and without trypsin. A5 cells were resuspended from the same preparation of cells in culture using EDTA (-) and EDTA plus trypsin (---); incubated with buffer (A) or with FITC-AMF10 (recognizing the q,,p3 complex) (9) for 60 minutes, and analyzed with the FACStar FCM (see Materials and Methods). (9) Analysis of GPllb-llla subunits from A5 cells resuspended with and without trypsin. Detergent lysate was prepared from surface-iodinated A5 cells resuspendedwith EDTA without (0) and with trypsin (TRYP).The lysate was immunoprecipitatedwith an anti-GPII,-Ill, antibody (454) (lanes 1 through 4), or treated with control serum (NRS) (lanes 5 through 6). The resulting products were analyzed by SDS-PAGE for nonreducing (-pME) and reducing (+pME) 7% gels, with subsequent autoradiography. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. u , ,AND ~ ~CELL ~ AGGREGATION 373 60 at 75 rpm, large visible aggregates formed within = 5 to 12 minutes of gyrotation. The actual percent of single cells or particles maximally recruited into aggregates, normally evaluated after 15 to 30 minutes gyrotation, was identical for Ab62, whether evaluated for the Fab or IgG form (Table 1). This was the case even with the large variations in the absolute %PA max values, which varied by up to twofold (see range in Table 1).Similar aggregation was also observed with activation induced by Fab fragments of an additional anti-GPIII, antibody (P41 in Table 1). Although trypsinization of cells grown in culture is a widely used technique to obtain a monodispersed cell suspension, causing minor (Fig 4) or negligible (Fig 5) changes in the ciIIbp3receptor on the A5 cells, we examined the possible effects on aggregation due to this isolation procedure. We found similar results as shown in Table 1 when we evaluated A5 cells resuspended only with EDTA and no trypsin: %PA (average of %PA, and %PA,) was 50% 2 5% for preactivation with 6 kmol/L Fab62 (conditions as in Table 1). It must be emphasized that such studies without trypsin were not usually feasible due to variations in the degree of aggregates present before activation, but in the above example greater than 70% of particles contained 5 3 cells per aggregate, with the balance containing from 4 to greater than 10 cells per aggregate. As observed for the Fg binding time course (Fig 6), similar maximal aggregation was obtained for A5 cells whether the Fab62 was added together with 500 nmol/L Fg (60% 2 5%, 230 minutes rotation) or cells from the same A5 preparation were first preactivated for 30 minutes before Fg addition (71% at 30 minutes rotation). Almost identical behavior was also found with a distinct Fab (P41) previously reported16to yield similar activation and expression of Fg-binding sites on A5 cells as for Fab62: 50% -C 2% and 58% aggregation, respectively, for joint or preaddition of the Fab (P41) to A5 cells and Fg. 40 Functional GPII,-Ill, Is Required for Cell Aggregation 300 I I 1o1 1oo 1o2 103 104 Fluorescence Intensity Fig 5. Expression of PAC-1 binding sites on A5 cells activated with lgG62. Trypsinized A5 cell suspensions were incubated with (A) buffer, (B) FITC-PAC1, and (C through E) FITC-PAC1 plus 1.2,4.1, and 6.1 pmol/L of lgG62 for 35 minutes and analyzed with a FACStar FCM (see Materials and Methods). Aggregation of CHO Cells Bearing Recombinant GPII,-I Ila When A5 cells were preactivated with Ab62, followed by Fg addition (500 nmol/L) and gyrotation in the plastic wells 100 1 .... ........... -TI /. 1. I 1 E m LL 80 20 0 P , , , 0 20 40 60 80 100 120 140 Time (min) Fig 6. Time course for Fg binding t o A5 cells activated with lgG62. The specific binding of '"I Fg t o A5 cells is plotted as a percentage of maximal binding attained at 60 t o 120 minutes versus the time of incubation of the A5 cells with 500 nmol/L Fg at room temperature, conducted as previously described." This was determined for 7.1 t o as 7.2 pmol/L Fab 62 (--; open symbols) and 6.1 pmol/L lgG62 (-; closed symbols) as activators. For one cell preparation, Fab 62 was and added together with the '9 Fg at 0 time (0);for a second (0,O) third (A, A)preparation, the Ab62 was added t o the A5 cells for 30 t o 45 minutes before addition of the '"I Fg. The 100% values for '"I-Fg-specific binding corresponded t o mean values of 185,000 and 133,000 Fg molecules per A5 cell, respectively, for activation with lgG62 and Fab62. Nonspecific binding of Fg, determined by incubating the A5 cells with GRGDSP (1 mmol/L) and Fg, was linear over time and typically 25% t o 30% of total (uncorrected) Fg binding found at 15 minutes of incubation. To determine if aggregation required functional GPI1,III,, the aggregation of wild-type CHO cells, CHO cells expressing transfected normal aIIbp3 (A5),and CHO cells expressing transfected aImps(BCAM) incapable of binding Fg, were c~mpared.'~ The A5 cells used in these studies contained between 1 to 4 x 10' recombinant GPI1,-111, molecules per cell as assayed from the binding of antibody 2Gl2.I' In addition, we confirmed by FCM analysis, using FITC-labeled antibodies directed at the GPI1,-111, complex, that the A5 and BCAM cells compared in our aggregation studies had similar surface-expressed quantities of GPIIb-IIIa,as previously r e p ~ r t e d . ' ~ Addition of 500 nmol/L Fg to antibody-activated cells caused large visible aggregates of A5 cells to form within = 10 minutes of gyrotation (Fig 7C and D). The large visible aggregates formed with the A5 cells + Fg persisted with the fixation used for the photomicroscopy, with many aggregates containing greater than 50 cells per aggregate. The micrograph in Fig 7C may, in fact, underrepresent the very large aggregates seen in Fig 7D, because the mixing and From www.bloodjournal.org by guest on February 6, 2015. For personal use only. FROJMOVIC ET AL 374 Fig 7. CHO cells expressing normal recombinant GPII,-III, can aggregate in the presence of Fg and Ab62 (Fab). Photomicrographs of cells fixed after 60 minutes of preactivation at room temperature with 7.2 pmol/L Fab62 and placed in gyrotation for 20 minutes at room temperature with 500 nmol/L Fg were obtained for (A) CHO cells, (B) BCAM cells, and (C and 0 ) A5 cells observed in t w o different fields of the same preparation. Total particle counts before gyrotation and Fg addition were 1 t o 3 & 0.1 x lO'lmL for all cell suspensions. Micrographs of activated cells without Fg, or of unactivated cells -c Fg were indistinguishable from (A) and (B) for all three cell types. Pictures were taken using a bright field lox objective and 1.6~optovar (Zeiss) for 50 pL of fixed cell suspensions placed on similar sized wells on glass slides prepared with vacuum grease, with cover glass sealing this well. The data for particle size and aggregation is given in Table 1. fixation appeared to diminish the size of unusually large aggregates. In contrast, neither the CHO nor BCAM cells showed aggregate formation (Fig 7A and B). Thus, aggregation requires functional GPII,-III,. This concept is further supported by the quantitative data for platelet aggregation measured from changes in singlets (%p&) Or in total Particle count (%PAT), and from relative m ~ aggregate n size, obtained for CHO, A5,or BCAM cells (Fig 1). Aggregation of A5 Cells Requires Both Activation and Fg We next demonstrated that negligible aggregation occurs for A5 cells in the presence of only Fg (500 nmol/L) or only IgG62 (6.1 kmol/L). However, the combination of preactivation with IgG62, and subsequent addition of Fg to the A5 cells undergoing collisions with gyrotation of the suspension at 75 rpm, gave similar extent and size of aggregate formation (Fig 2), as reported for Fab62-induced aggregation (Fig 1). This was again the case whether evaluating %PA, %PA,, or relative aggregate size (Fig 2). Aggregation of Ab62-Activated A5 Cells Requires Divalent Cations and Fg Binding Sites To determine if Fg binding to activated recombinant GPII,-III, was required for aggregation, we examined the effects of inhibition of Fg binding on this reaction. We found that EDTA (5 mmol/L), GRGDSP (1 mmol/L), and monoclonal 2G12 (60 kg/mL), which all inhibit Fg binding to GPIIh-IIIa,'4~R all effectively blocked the aggregation of A5 cells induced by IgG62 and Fg (Fig 3). A control Ab15 (60 p,g/mL), known to bind to GPIII, without affecting Fg binding,'" did not affect the A5 cell aggregation (Fig 3). The elevated aggregation seen with GRGDSP inhibition is not significantly different from that seen for unactivated A5 cells treated with GRGDSP, which gave %PA, %PAT,and relative size of 31%, 25% ? 6%, and 26% ? 2%, respectively. DISCUSSION Human recombinant GPI1,-111, can mediate cell aggregation in the membrane environment of CHO cells if the following conditions are satisfied: (1) the GPI1,-111, is From www.bloodjournal.org by guest on February 6, 2015. For personal use only. q e P B AND CELL AGGREGATION 375 creted from a-granules), thought to form a TSP-mediated capable of binding Fg; (2) this integrin is activated to form a complex with Fg that stabilizes macroaggregates; (3) GP1,high-affinity receptor for Fg, previously shown to have kd = 11,, a receptor for collagen, which may modulate GPIIb110 & 18 pmol/L16; (3) divalent cations and Fg are both IIIa3’; and (4) the PADGEM protein or GMP-140, whose present; and (4) the Fg binding site on the activated appearance on the platelet surface parallels TSP secretion GPI1,-111, is accessible. Based on these requirements, from ~u-granules.”~~’ In addition, the platelet contains intertransfected GPI1,-111, confers an aggregation response on CHO cells that mimics aspects of human platelet aggreganal membrane pool^,"^^^ and secretable adhesive proteins, including TSP, vWf, and additional Fg, all of which may tion. Physiologic platelet aggregation triggered by agonists confer unique and complex contributions to the functional such as thrombin require internal signalling.z8The antibodexpression of GPI1,-111,-Fg in supporting platelet aggregaies used here to activate GPI1,-111, do so without signal tion. It has also been shown that the membrane lipid transduction.I6 Thus, cellular aggregation appears to reenvironment may modulate the functional activity of intequire GPI1,-111, activation and may not depend on the grim3‘ multitude of other cellular responses to agonist. Further, Our results demonstrating aggregation of A5 cells in the this approach now makes possible the systematic evaluation absence of many of the above platelet components suggest of the structural elements of GPI1,-111, required for arlbP3- that these platelet factors serve in the expression and mediated aggregation. In addition, the relative contriburegulation of the Fg receptor, but are not directly required tions to aggregation of intracellular signalling, and of for the minimal aggregation reported here. Nevertheless, in surface-changes in platelets can now be dissected from the stirred suspensions, as normally used to study platelet activation of GPI1,-111, by comparing platelets with the aggregation,” no significant aggregation of A5 cells was GPI1,-111, transfected cells. The binding of Fg or another observed. Thus, the more complex platelet may have GPI1,-111, ligand is required for platelet aggregati~n.’,’,~,’.’~ properties not present in A5 cells needed to support high Events following Fg binding are needed to allow its funcshear aggregation. Future studies comparing platelet and tional expression in eliciting platelet-platelet aggregation, A5 cell efficiencies of aggregation under well-controlled likely involving (1) further conformational changes in the conditions33 may help to further define these ancillary occupied ( 2 ) interactions with cytoskelet~n,’~~~~ elements. (3) clustering of the receptors in activated cells before cell-cell on tact,''^^^^^^ and (4) localization of Fg occupied ACKNOWLEDGMENT receptors on surface projection^.^^'^^^^ The platelet also contains many other membrane glycoproteins,’,’ including: We gratefully acknowledge the technical help of Jane Forsythe, (1) about 25,000 GPIb molecules in the plasma membrane Alison Glass, and Tim Halloran, and the use of the FACScan that can bind von Willebrand factor (vWf); ( 2 ) GPIV generously provided by Dr L. Sklar (Research Institute of Scripps Clinic, La Jolla, CA). believed to be a receptor for thrombospondin (TSP; seREFERENCES 1. Plow EF, Ginsberg MH: Cellular adhesion: GPI1,-111, as a prototypic adhesion receptor. Prog Hemost Thromb 9:117, 1989 2. Nurden AT: Platelet membrane glycoproteins and their clinical aspects, in Verstraete M, Vermylen J, Lijnen R, Arnout J (eds): Thrombosis and Haemostasis. Leuven, Belgium, Leuven University, 1987, p 93 3. Phillips DR, Charo JF, Parise LV, Fitzgerald L A The platelet membrane glycoprotein 11,-111, complex. Blood 71331, 1988 4. Peerschke EIB: The platelet fibrinogen receptor. Semin Hematol22:241, 1985 5. Coller BS: A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein 11,-111, complex. J Clin Invest 76:101,1985 6. Shattil SJ, Hoxie JA, Cunningham M, Brass L F Changes in the platelet membrane glycoprotein 11,-111, complex during platelet activation. J Biol Chem 258:10240, 1985 7. Asch AS, Leung LLK, Polley MJ, Nachman RL: Platelet membrane topography: Colocalization of thrombospondin and fibrinogen with the glycoprotein 11,-111, complex. Blood 66:926, 1985 8. Gartner TK, Power JW, Beachey EH, Bennett JS, Shattil SJ: The tetrapeptide analogue of the alpha chain and decapeptide analogue of the gamma chain of fibrinogen bind to different sites on the platelet fibrinogen receptor. Blood 66:3050,1985 9. Shattil SJ, Budzynski A, Scrutton MC: Epinephrine induces platelet fibrinogen receptor expression, fibrinogen binding, and aggregation in whole blood in the absence of other excitatory agonists. Blood 73:150,1989 10. Loftus JC, Albrecht RM: Redistribution of the fibrinogen receptor of human platelets after activation. J Cell Biol 995322, 1984 11. Isenberg WM, McEver RP, Phillips DR, Shuman MA, Bainton DF: The platelet fibrinogen receptor: An immunogold surface replica study of agonist-induced ligand binding and receptor clustering. J Cell Biol104:1655, 1987 12. Peerschke EIB: Decreased accessibility of platelet-bound fibrinogen to antibody and enzyme probes. Blood 74582,1989 13. Frelinger AL 111, Lam SC-T, Plow EF, Smith MA, Loftus JC, Ginsberg MH: Occupancy of an adhesive glycoprotein receptor modulates expression of an antigenic site involved in cell adhesion. J Biol Chem 263:12397,1988 14. Kakaiya RM, Kiraly TL, Cable RG: Concanavalin A induces patchingkapping of the platelet membrane glycoprotein IIJIII, complex. Thromb Haemost 59:281,1988 15. Santoso S, Zimmerman U, Neppert J, Mueller-Eckhardt C: Receptor patching and capping of platelet membranes induced by monoclonal antibodies. Blood 67:343,1986 16. O’Toole TE, Loftus JC, Du X, Glass AA, Ruggeri ZM, Shattil SJ, Plow EW, Ginsberg MH: Affinity modulation of the From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 376 allbP3 integrin (platelet GPIIb-IIIa)is an intrinsic property of the receptor. Cell Regulation 1:883, 1990 17. O’Toole TE, Loftus JC, Plow EF, Glass AA, Harper JR, Ginsberg MH: Efficient surface expression of platelet GPI1,-111, requires both subunits. Blood 74:14,1989 18. Taub R, Gould RJ, Garsky VM, Ciccarone TM, Hoxie J, Friedman PA, Shattil SJ: A monoclonal antibody against the platelet fibrinogen receptor contains a sequence that mimics a receptor recognition domain in fibrinogen. J Biol Chem 264:259, 1989 19. Woods VL, Wolff LE, Keller DM: Resting platelets contain a substantially centrally located pool of glycoprotein 11,-111, complex which may be accessible to some but not other extracellular proteins. J Biol Chem 261:15242,1986 20. Frelinger AL 111, Cohen I, Plow EF, Smith MA, Loftus JC, Ginsberg MH: Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J Biol Chem 265:6346,1990 21. Plow EF, Loftus J, Levin E, Fair D, Dixon D, Forsyth J, Ginsberg MH: Immunologic relationship between platelet membrane glycoprotein GPII,JII, and cell surface molecules expressed by a variety of cells. Proc Natl Acad Sci USA 83:6002,1986 22. Kaesberg PR, Ershler WB, Esko JD, Mosher DF: Chinese hamster ovary cell adhesion to human platelet thrombospondin is dependent on cell surface heparan sulfate proteoglycan. J Clin Invest 83:994,1989 23, Harper PA, Juliano RL: Isolation and characterization of Chinese hamster ovary cell variants defective in adhesion to fibronectin-coated collagen. J Cell Biol87:755,1980 24. St John T, Myer J, Idzerada R, Gallatin WM: Expression of CD44 confers a new adhesive phenotype on transfected cells. Cell 60:45,1990 25. Frojmovic MM, Milton JG, Gear AL: Platelet aggregation FROJMOVIC ET AL measured in vitro by microscopic and electronic particle counting. Methods Enzymol169:134,1989 26. Rochon YP, Frojmovic MM: Dynamics of human neutrophil aggregation dynamics evaluated by flow cytometry. J Leuk Biol (in press, 1991) 27. Loftus JC, O’Toole TE, Plow EF, Glass A, Frelinger AL 111, Ginsberg MH: A P3integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science 249:915, 1990 28. Haslam RJ: Signal transduction in platelet activation, in Verstraete M, Vermylen J, Lijnen R, Arnout J (eds): Thrombosis and Haemostasis. Leuven, Belgium, Leuven University Press, 1987, p 147 29. Hensler ME, Frojmovic MM, Taylor RG, Hantgan RR, Lewis JC: Platelet morphological changes and fibrinogen receptor localization: Initial responses in ADP-activated human platelets. Am J Pathol (submitted) 30. Coller BS, Beer JH, Scudder LE, Steinberg MH: Collagenplatelet interactions: Evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIbiIIIa mediated by adhesive proteins. Blood 74:182, 1989 31. George JN, Pickett EB, Saucerman S, McEver RP, Kunicki TJ, Keiffer N, Newman PJ: Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest 78:340, 1986 32. Conforti G, Zanetti A, Pasquali-Ronchetti I, Quaglino D Jr, Neyroz P, Dejana E: Modulation of vitronectin receptor binding by membrane lipid composition. J Biol Chem 265:4011,1990 33. Bongrand P: Physical Basis of Cell-Cell Adhesion. Boca Raton, FL, CRC, 1988 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1991 78: 369-376 Platelet glycoprotein IIb-IIIa (alpha IIb beta 3 integrin) confers fibrinogen- and activation-dependent aggregation on heterologous cells MM Frojmovic, TE O'Toole, EF Plow, JC Loftus and MH Ginsberg Updated information and services can be found at: http://www.bloodjournal.org/content/78/2/369.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026