Protective action of the peroxisome proliferator
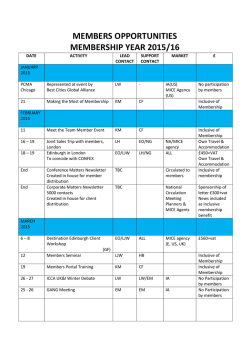
Journal of Neurochemistry, 2002, 82, 615–624 Protective action of the peroxisome proliferator-activated receptor-c agonist pioglitazone in a mouse model of Parkinson’s disease T. Breidert,* J. Callebert, M. T. Heneka,à G. Landreth,§ J. M. Launay and E. C. Hirsch* *INSERM U289, Experimental Neurology and Therapeutics, Hoˆpital de la Pitie´-Salpeˆtrie`re, Paris, France Service de Biochimie et Biologie Mole´culaire, Hoˆpital de Lariboisie`re, Paris, France àDepartment of Neurology, University of Bonn, Bonn, Germany §Department of Neurosciences and Neurology, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA Abstract We examined the effect of pioglitazone, a peroxisome proliferator-activated receptor-c (PPARc) agonist of the thiazolidinedione class, on dopaminergic nerve cell death and glial activation in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. The acute intoxication of C57BL/6 mice with MPTP led to nigrostriatal injury, as determined by tyrosine hydroxylase (TH) immunocytochemistry, and HPLC detection of striatal dopamine and metabolites. Damage to the nigrostriatal dopamine system was accompanied by a transient activation of microglia, as determined by macrophage antigen-1 (Mac-1) and inducible nitric oxide synthase (iNOS) immunoreactivity, and a prolonged astrocytic response. Orally administered pioglitazone ( 20 mg/kg/day) attenuated the MPTP-induced glial activation and prevented the dopaminergic cell loss in the substantia nigra pars compacta (SNpc). In contrast, there was little reduction of MPTP-induced dopamine depletion, with no detectable effect on loss of TH immunoreactivity and glial response in the striatum of pioglitazone-treated animals. Low levels of PPARc expression were detected in the ventral mesencephalon and striatum, and were unaffected by MPTP or pioglitazone treatment. Since pioglitazone affects primarily the SNpc in our model, different PPARc-independent mechanisms may regulate glial activation in the dopaminergic terminals compared with the dopaminergic cell bodies after acute MPTP intoxication. Keywords: glia, inflammation, MPTP, Parkinson’s disease, peroxisome-proliferator-activated receptor-c, pioglitazone. J. Neurochem. (2002) 82, 615–624. Parkinson’s disease (PD) is characterized by a progressive loss of dopaminergic neurons in the substantia nigra (SN). The cause of this cell death is largely unknown, but recent evidence suggests a role of glial cells and inflammatory processes in the pathogenesis of PD. A microglial activation has been demonstrated in the SN of PD patients (McGeer et al. 1988; Hirsch 2000), in human patients exposed to 1-methyl-4-phenyl1,2,3,6-tetrahydropyridine (MPTP; Langston et al. 1999), in an MPTP-induced mouse model of PD (KurkowskaJastrzebska et al. 1999), and in 6-hydroxydopamine-induced parkinsonian rats (Akiyama and McGeer 1989). Activation of microglia becomes apparent by proliferation, recruitment to the site of injury, and by morphological, immunohistochemical and functional changes (Kreutzberg 1996). Activated microglia is believed to contribute to neurodegeneration through the release of cytotoxic compounds including reactive oxygen intermediates, nitric oxide, proteases and pro-inflammatory cytokines (Chao et al. 1992; Hunot et al. 1996; Bal-Price and Brown 2001; Le et al. 2001). Peroxisome proliferator-activated receptor-c (PPARc) is a member of the nuclear receptor superfamily that regulates Received December 14, 2001; revised manuscript received April 16, 2002; accepted April 19, 2002. Address correspondence and reprint requests to T. Breidert, INSERM U289, Neurologie et The´rapeutique Expe´rimentale, Hoˆpital de la Salpeˆtrie`re, 47 boulevard de l’Hoˆpital, 75651 Paris CEDEX 13, France. E-mail: [email protected] Abbreviations used: DA, dopamine; DOPAC, dihydroxy-phenylacetic acid; GFAP, glial fibrillary acidic protein; HVA, homovenillic acid; iNOS, inducible nitric oxide synthase; Mac-1, macrophage antigen-1; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MW, molecular weight; NE, norepinephrine; PB, phosphate buffer, PBS, phosphatebuffered saline; PD, Parkinson’s disease; PPARc, peroxisome proliferator-activated receptor-c; SN, substantia nigra; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase. 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 615 616 T. Breidert et al. carbohydrate and lipid metabolism (Delerive et al. 2001). Recently, a new role has been described for PPARc receptors in the regulation of inflammation (Delerive et al. 2001). Thus, PPARc agonists have been shown to inhibit inflammatory processes in a variety of cell types in vitro, including monocytes/macrophages (Jiang et al. 1998; Ricote et al. 1998) and microglial cells (Combs et al. 2000). In vivo, PPARc agonists have been shown to modulate inflammatory responses in brain (Heneka et al. 2000). Here, we sought to determine whether the inhibition of glial activation would reduce neuronal death in the MPTP model of PD. To this end, we used the PPARc agonist pioglitazone, a synthetic ligand of the thiazolidinedione class currently used as an antidiabetic agent because of its insulinsensitizing effect. Pioglitazone has been shown to have antiinflammatory effects in animal models of autoimmune diseases (Takamura et al. 1999; Feinstein et al. 2001) and, when administered orally, it is rapidly absorbed and crosses the blood–brain barrier (Maeshiba et al. 1997). We found that orally administered pioglitazone led to an attenuation of glial activation and dopaminergic cell loss in the SN of MPTP-treated mice, whereas it had little effect on MPTP-induced changes in the striatum. Low levels of PPARc expression could be detected in brain target areas of MPTP. These findings suggest that PPAR-sensitive inflammatory processes contribute to neuronal death in the MPTP mouse model of Parkinson’s disease, but PPARc-independent mechanisms may regulate glial activation in the dopaminergic terminals compared with the dopaminergic cell bodies. Materials and methods Animals and treatment Ten to twelve-week-old male C57BL/6 mice (Janvier, Le Genest St Isle, France), weighing 24–27 g, were housed (two to five animals per cage) with free access to food pellets and water, and were maintained at a constant temperature on a 12-h light–dark cycle. The animal treatments and care protocols conformed to National Institutes of Health guidelines. Mice received four intraperitoneal injections of MPTP-HCl (15 mg/kg; Sigma Aldrich, St Quentin, France) in saline at 2- h intervals in 1 day. Control mice received saline only. Animals were killed 2–8 days after the last injection. Pioglitazone (Takeda Chemical Industries, Osaka, Japan) was administered in rodent chow (#5002, Ralston Purina, St Louis, MO, USA) at 120 p.p.m., which was estimated to yield 20 mg/kg/day, beginning 3 days prior to MPTP intoxication, and continuing throughout the entire experiment. This treatment protocol has previously been shown to be useful for investigating PPARc mediated effects of pioglitazone (Tang et al. 1999). Neurochemical analysis Determination of dopamine (DA), norepinephrine (NE) and the DA metabolites (DOPAC, HVA) levels in the striatum was performed using high-performance liquid chromatography (HPLC). All structures were homogenized in ice-cold 0.1 M acetic acid containing sodium metabisulphite (10 lM), EDTA (10 lM) and ascorbic acid (10 lM). After centrifugation, the supernatant was passed through a 10 000-MW filter (Nanosep 10 k, Pall). A 50-lL aliquot of sample was analysed for monoamines and metabolites by isocratic elution and electrochemical detection on a serial electrode array of coulometric flow-through graphite electrodes (Coularray, ESA). Monoamines and metabolites were then identified based on their retention time as well as their electrochemical behaviour across the arrays. The analysis, data reduction and peak identification were fully automated. Western blot analysis For protein extracts, mice were killed by cervical dislocation, and striatum and ventral mesencephalon were rapidly dissected. The samples were homogenized in 10 volumes (w/v) of ice-cold lysis buffer (50 mM TRIS, pH 8; 150 mM NaCl; 1 mM EDTA; 1% NP40) by trituration using a syringe (18- and 26-gauge needles). A cocktail of protease inhibitors, COMPLETE (Roche Molecular Biochemicals, Meylan, France), Pefabloc (1 mM) (Uptima, Montluc¸on, France) and pepstatin A (1 lg/mL) was included in all extractions. Samples of each group were pooled (n ¼ 5) and put on ice for 30 min. After centrifugation (13 000 g, 20 min, 4C), supernatants were collected and stored at ) 80C until analysis. Protein concentrations were determined using the Bradford reagent (Biorad, Hercules, CA, USA). For western blot analysis, samples (200 lg protein) were loaded on a 10% sodium dodecyl sulfate–polyacryamide electrophoresis gel. As a positive control, protein extract of rodent adipose tissue (40 lg per lane) was used. After separation, proteins were blotted on a nitrocellulose membrane and subsequently incubated overnight at 4C in PBS containing 5% skimmed milk. After washing in phosphate-buffered saline (PBS), membranes were incubated with primary antibody (rabbit polyclonal anti-PPARc; Calbiochem, San Diego, CA, USA; 1 : 1000) in PBS containing 0.05% Tween 20 for 1 h at room temperature. Primary antibody was detected by using an HRP-coupled secondary antibody (anti-rabbit; Jackson Laboratories, San Harbor, MA, USA; 1 : 50 000). Bound secondary antibodies were visualized by using enhanced chemiluminescence (Super Signal west pico; Pierce, Rockford, IL, USA). Immunohistochemistry Animals were anaesthetized with pentobarbital (130 mg/kg; Sigma, St. Quentin, France) and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed, postfixed, and cryoprotected. Immunohistochemistry was performed as described previously (Hirsch et al. 1988) on free-floating cryomicrotome-cut sections (20 lm in thickness) encompassing the entire striatum and midbrain. After incubation in 3% H2O2/20% methanol, followed by 0.2% Triton X-100 and by 2% bovine serum albumin in 0.1 PBS, the sections were stained overnight at 4C using a polyclonal antibody against tyrosine hydroxylase (1 : 1000; Pel Freez, Rogers, AR, USA) for dopaminergic neurons, a mouse monoclonal antibody against inducible nitric oxide synthase (iNOS; 1 : 250; BD Transduction, Point de Claix, France), a rat antibody against macrophage antigen-1 (Mac-1; 1 : 250; Serotec, Raleigh, NC, USA) for microglia, and a rabbit antibody against glial fibrillary acidic protein (GFAP; 1 : 250; Dako, Trappes, France) for astrocytes. The specificity of these antibodies has already been demonstrated and tested by western blot analysis (Viale et al. 1991; Vodovotz et al. 1993; Liberatore et al. 1999). Sections were then 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 Pioglitazone attenuates MPTP toxicity 617 treated with secondary antibodies (Vectastain; Vector Laboratory, Burlingame, CA, USA), and subsequently incubated with avidinbiotinylated horseradish peroxidase complex. The peroxidase was revealed by incubation with 0.05% 3,3¢-diaminobenzidine tetrahydrochloride containing 0.015% hydrogen peroxide, and for iNOS staining 0.16 g/L NiSO4. For Nissl cell counts TH sections were counterstained with cresyl violet. All sections for a given marker were stained simultaneously for all animals using the same solutions. Cell counting and statistical analysis SNpc TH- and Nissl-positive neuron counts were performed in 20 lm coronal mesencephalic sections 300 lm apart (bregma )2.06 mm to )4.04 mm; Franklin and Paxinos 1996). The total number of process-bearing, TH- and Nissl-stained cells with clearly visible nuclear borders was estimated using a previously described method and an image analysis system (Hunot et al. 1997; Visioscan, BIOCOM, Les Ulis, France). All sections were coded and examined blind. As an index of microglial activation, Mac-1-stained cells with a diameter > 17 lm (cell body) were counted in a similar fashion in the mesencephalon (bregma )2.06 mm to )4.04 mm). For analysis of astrocytes, GFAP-positive cells were counted in a 500 lm · 500 lm frame overlying the SN at bregma )5.20 mm, and cell number per mm2 was calculated. In the striatum, measurement of optical density of TH immunostaining as an index of the density of dopaminergic axons and nerve terminals was performed using the same image analysis system. Differences between the four treatment groups (control versus MPTP versus Pioglitazone versus Pioglitazone/MPTP) for TH-, Nissl-, Mac-1-, and GFAP-positive cell counts were analysed using ANOVA, followed by Newman– Keuls’s post-hoc analysis or, in the event of failure in normality test, by Kruskal–Wallis’ one-way analysis of variance on ranks. In all analyses, the null hypothesis was rejected at the 0.05 level. Results In the SNpc, where the somata of dopaminergic neurons are located, the loss of TH-positive cells in MPTP-treated animals compared with vehicle-treated controls was 23%, 30% and 19% at days 2, 5 and 8 after intoxication, (a) Fig. 1 Effect of pioglitazone on MPTPinduced dopaminergic cell death: number of TH-positive neurons (a) in the SNpc of C57BL/6 mice treated with MPTP (4 · 15 mg/kg) in the absence (black) or presence (dark grey) of pioglitazone at day 2 (D2; n ¼ 10 per group), day 5 (D5; n ¼ 5 per group) and day 8 (D8; n ¼ 4–5 per group) post MPTP intoxication and in saline-injected (white) and pioglitazonetreated (light grey) control animals, respectively. Data are presented as means ± SEM (*p < 0.05 and **p < 0.01 compared with MPTP intoxicated animals; Newman–Keuls’ post-hoc analysis). (b–e) Photomicrographs of TH immunoreactivity in the SNpc of saline-injected control mice in the absence (b) or presence (c) of pioglitazone and of MPTP-treated mice at day 5 post MPTP in the absence (d) or presence (e) of pioglitazone. There is a loss of TH-positive neurons and fibres in MPTP-treated mice (arrowheads). Scale bar represents 300 lm (b–e). (b) (c) (d) (e) 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 618 T. Breidert et al. respectively (Fig. 1). The loss of TH-positive neurons was confirmed by Nissl staining (data not shown). Pioglitazone prevented the MPTP-induced loss of TH-positive neurons in the SN. In the striatum, the area of projection of dopaminergic neurons from the SN, the MPTP-induced loss of striatal TH immunoreactivity compared with vehicle-treated controls reached 76% at days 2 and 5, and decreased to 53% at day 8 (Fig. 2). In contrast to the protective effect in the SN, piogliatzone had no detectable effect on the loss of TH-positive terminals in the striatum of MPTP-treated animals. To test striatal nerve terminal injury under pioglitazone treatment in more detail, HPLC measurements for dopamine and its metabolites DOPAC and HVA were performed. Compared with untreated controls, dopamine, DOPAC and HVA baseline contents were reduced by 37%, 48%, and 44% in pioglitazone-treated animals (Figs 3a–c), while there was no significant difference in striatal noradrenaline levels between those two groups (Fig. 3d). In mice that received MPTP, a significant reduction in striatal dopamine, DOPAC, and HVA levels was observed (Figs 3a–c). Striatal dopamine levels were depleted by 85% at day 2, by 84% by day 5, and by 89% by day 8 after MPTP intoxication, compared with 72%, 77%, and 79% in pioglitazone-treated animals, respectively (Fig. 3a). Two days after MPTP intoxication (a) (b) (d) (c) (e) Fig. 2 Absence of pioglitazone effect on MPTP-induced loss of TH-positive fibres in the striatum: striatal TH immunoreactivity determined by optical densitometry (a) in MPTP-treated mice in the absence (black) or presence (dark grey) of pioglitazone at day 2 (D2; n ¼ 5 per group), day 5 (D5; n ¼ 5 per group), and day 8 (D8; n ¼ 4–5 per group) post MPTP injections, and in pioglitazone-treated mice (light grey) expressed as percentage of saline-injected controls. Data are presented as means ± SEM (p-values between MPTP-treated animals and controls were < 0.001 at all three time points; Newman–Keuls’ post-hoc analysis). (b–e) Photomicrographs of TH immunoreactivity in the striatum of salineinjected control mice in the absence (b) or presence (c) of pioglitazone, and of MPTPtreated mice at day 5 post MPTP in the absence (d) or presence (e) of pioglitazone. Scale bar represents 400 lm (b–e). 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 Pioglitazone attenuates MPTP toxicity 619 (b) (a) (c) Fig. 3 Striatal dopamine (a), dihydroxyphenylacetic acid (DOPAC; b), homovanillic acid (HVA; c), and noradrenaline (d) levels of MPTP intoxicated [two (D2), five (D5) and eight days (D8) post MPTP] and salineinjected mice (Co) under pioglitazone treatment (black bars) compared with mice that received no pioglitazone (grey bars). Data are presented as means ± SEM (n ¼ 5). The significance of the differences between control and MPTP-treated groups is indicated by symbols (*p < 0.05, **p < 0.01, and ***p < 0.001). (e) Immunoblot detection of PPARc protein expression in ventral mesencephalon (vM) and striatum (ST) of pioglitazone and MPTP-treated mice. Murine adipose tissue served as positive control. The filter was stripped and reprobed with anti-actin antibody to confirm equal protein loading. (d) (e) DOPAC levels were reduced by 81%, and HVA levels by 77%, compared with 60% and 43% in the pioglitazone group, respectively (Figs 3b and c). Since pioglitazone is a potent agonist of PPARc, and this receptor is thought to represent the main pharmacological target of this drug, PPARc protein expression was determined in the SN and striatum of animals after MPTP intoxication treated with or without pioglitazone by western blot analysis. PPARc was faintly detectable in both SN and striatum compared with the strong expression in the adipose tissue used as control sample, and PPARc expression was unaffected by MPTP or pioglitazone treatment at day 2, 5, and 8 after MPTP administration (Fig. 3e). Since PPARc agonists are implicated in regulation of microglial cells, we next tested the effect of pioglitazone on the glial response. The complement receptor Mac-1 was used as a specific marker of microglia. In saline-injected mice, ramified resting microglia were faintly stained with the Mac-1 antibody in the SN, and to an even lesser extent in the surrounding ventral midbrain (Fig. 4g). Pioglitazone treatment had no effect on resting microglia (Fig. 4h). In MPTPinjected mice, Mac-1 staining in the SN increased markedly after intoxication and this was accompanied by typical morphological changes, such as cell body enlargement, shortening of processes, and loss of ramification (Figs 4a, c and e and Table 1). The MPTP-induced microglial response was maximal at day 2 after MPTP injection and spanned the entire SN, predominating in the SNpc (Fig. 4a). At day 5 post MPTP, the total number of cells with activated morphology was reduced. The remaining cells showed an increased staining intensity and an irregular shape, characteristic of phagocytic cells, and their anatomical distribution was more confined to the region of the SNpc (Fig. 4c). At day 8, Mac-1 expression was no longer different from control sections (Fig. 4e and Table 1). In pioglitazone-treated mice, there was a strong reduction of Mac-1 expression at day 2 post MPTP, 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 620 T. Breidert et al. (a) Table 1 MPTP-induced microglial activation in the ventral mesencephalon (b) MPTP MPTP + pioglitazone (c) (d) (e) (f) (g) (h) (i) (j) Day 2 Day 5 Day 8 1596 ± 289 185 ± 78*** 711 ± 355 0±0 0±0 0±0 Total number of Mac-1-stained cell bodies with a diameter >17 lm per ventral mesencephalon was estimated as an index of microglial activation 2, 5 and 8 days after MPTP intoxication. Activated cells were confined to the region of the SN, as determined by TH immunoreactivity of adjacent mesencephalic sections. The cell counts between the two groups (MPTP vs. MPTP + pioglitazone) were compared using a nonparametric statistical test because distribution differed significantly from normality. Data are represented as means ± SEM (n ¼ 5 per group). No Mac-1-stained cells with a diameter >17 lm were detected in control animals, with or without pioglitazone treatment. ***Significant difference (p < 0.001) between pioglitazone treated group and control group. Fig. 4 Attenuation of MPTP-induced iNOS-positive, activated microglia in the SN in the presence of pioglitazone: Mac-1 immunoreactivity in MPTP-treated mice at day 2 (a), day 5 (c), and day 8 (e) post MPTP; in MPTP and pioglitazone-treated mice at day 2 (b), day 5 (d) and day 8 (f) post MPTP; and in saline-injected control mice in the absence (g) or presence (h) of pioglitazone. Magnification of Mac-1 immunostaining in MPTP-treated mice reveals typical morphology of activated microglial cells 2 days after MPTP injections (inset of panel a). iNOSpositive microglia in MPTP-treated animals (i) is absent in MPTP + pioglitazone-treated animals (j) at day 2 post MPTP. Scale bars represent 200 lm (a–j). Insets of panel (a) and (I): 7 · magnification of the respective panel. and no difference in Mac-1 staining in comparison to control sections thereafter (Figs 4b, d and f and Table 1). MPTP also induced the expression of iNOS in the SN, revealing cells with cytoplasmic staining and morphology of activated microglia at day 2 post MPTP injections (Fig. 3i), which were absent in pioglitazone-treated animals (Fig. 4j). Fewer iNOS-positive cells than Mac-1-positive cells were found in the SN of MPTP-treated animals. Since the anatomical distribution and morphology of iNOS immunoreactive cells was virtually identical to that of Mac-1-positive microglia, no double staining was performed. MPTP increased the number and size of cells expressing GFAP in the entire SN, but predominately in the SNpc (Fig. 5; Table 2). The density and staining intensity of GFAP expressing cells in the SN was maximal at day 5 post MPTP treatment compared with vehicle-treated controls and decreased thereafter. GFAP-immunoreactive cells in MPTPtreated animals shared the characteristics of reactive astrocytes, i.e. labelled astrocytes were hypertrophic with shortening and thickening of cytoplasmic processes (Fig. 5c). Pioglitazone treatment attenuated the MPTPinduced increase in GFAP-positive astrocytes in the mesencephalon by 59% at day 2, 36% at day 5, and 53% at day 8 (Table 2), and GFAP-positive cells were rather evenly distributed in the SN of pioglitazone-treated animals compared with the MPTP-treated group (Fig. 5). In the striatum, MPTP intoxication led to a transient increase of Mac-1 expression with a similar time course as that of microglial activation observed in the ventral mesencephalon (Fig. 6). In contrast to the midbrain, pioglitazone had no effect on striatal Mac-1 expression (Fig. 6). As in the mesencephalon, MPTP intoxication led to an astrocytic response in the striatum, estimated by the increased number of GFAP expressing cells with a reactive morphology, which was maximal 5 days after the treatment (Fig. 7). In the 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 Pioglitazone attenuates MPTP toxicity 621 (a) (b) Table 2 Density of glial fibrillary acidic protein (GFAP)-positive cells in the substantia nigra Saline Saline + pioglitazone MPTP MPTP + pioglitazone (c) (d) (e) (f) (g) (h) Fig. 5 Reduction of MPTP-induced reactive astrocytosis in the mesencephalon in the presence of pioglitazone: glial fibrillary acidic protein (GFAP) immunostained astrocytes in the ventral mesencephalon of MPTP-treated mice at day 2 (a), day 5 (c), and day 8 (e) post MPTP; in MPTP and pioglitazone-treated mice at day 2 (b), day 5 (d), and day 8 (f) post MPTP; and in saline-injected control mice in the absence (g) or presence (h) of pioglitazone. Magnification of GFAP immunostaining in MPTP-treated mice shows typical morphology of reactive astrocytes at day 5 post MPTP (inset of panel c). Scale bar represents 200 lm (a–h). Inset: 14 · magnification of panel c. striatum, pioglitazone showed no effect on MPTP-induced changes in GFAP expression (Fig. 7). Discussion We used the thiazolidinedione pioglitazone, an agonist of the nuclear receptor PPARc, to test our hypothesis that the blockade of the inflammatory activation would attenuate neuronal damage in the MPTP rodent model of Parkinson’s disease. In the present study, we demonstrated a protective effect of pioglitazone on MPTP-induced neuronal cell death and a concomitant attenuation of glial activation in the SNpc of C57BL/6 mice. Day 2 Day 5 Day 8 41 ± 3 41 ± 8 115 ± 31** 47 ± 7 173 ± 16*** 110 ± 9à 94 ± 15* 44 ± 8 The number of GFAP stained perikarya per mm2 in the region of the SN (at bregma –5.20 mm) was estimated as an index of astrogliosis 2, 5 and 8 days after MPTP intoxication. Data are represented as means ± SEM (n ¼ 5–7 per group). *,**,***Significant difference (p < 0.05, 0.01, 0.001, respectively) between MPTP-treated and saline-injected control group. ,àSignificant difference (p < 0.05/0.01, respectively) between the time matched MPTP group and MPTP + pioglitazone-treated group. Acute intoxication with MPTP led to a rapid decrease of TH immunoreactivity in the SNpc (Fig. 1a), consistent with the mitochondrial accumulation of MPP+, the active metabolite of MPTP, in the dopaminergic nigrostriatal cells via the dopamine uptake system, leading to energy failure and consecutively to inhibition of protein expression (Przedborski and Jackson-Lewis 1998). The reduction of TH-positive neurons, determined 1 week after MPTP intoxication, serves as a good marker for dopaminergic cell loss (Jackson-Lewis et al. 1995). The greater loss of TH-immunoreactive cell bodies in MPTP-treated mice at day 2 compared with mice at day 8 post-intoxication (Fig. 1a) does not imply a neogenesis of dopaminergic nerve cells, since the loss of TH immunoreactivity can exceed the degree of neuronal cell death during the active phase of MPTP-induced neurodegeneration, so that TH-positive neuronal counts during the first days postinjection do not accurately reflect the number of living neurons (Jackson-Lewis et al. 1995). Thus, the loss of TH-positive neurons at day 8 is likely to represent the real loss of dopaminergic neurons rather than a loss of TH expression. To estimate microglial activation, the complement receptor Mac-1 was used because it is expressed on all types of microglia and its expression is significantly increased on activated microglia (He et al. 1997). In agreement with previous results, intraperitoneal injection of MPTP led to a rapid activation of microglia followed later by an astrocytic response (Kurkowska-Jastrzebska et al. 1999; Liberatore et al. 1999). The activation of glia may lead to a release of cytotoxic substances, such as pro-inflammatory cytokines, oxygen-derived reactive species, and nitric oxide, that are able to induce neuronal cell death in vitro (Chao et al. 1992; Le et al. 2001) and in vivo (Liberatore et al. 1999; Dehmer et al. 2000). Indeed, lipopolysaccharide-induced microglial activation has been associated with the destruction of dopaminergic neurons in the SNpc (Liu et al. 2000; Lu et al. 2000), and microglia suppressing factors may retard 6-hydroxydopamine-induced dopaminergic cell death in the SN (He et al. 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 622 T. Breidert et al. (a) (b) (a) (b) (c) (d) (c) (d) (e) (f) (e) (f) (g) (h) (g) (h) Fig. 6 MPTP-induced transient microglial activation remains unchanged in the striatum in the presence of pioglitazone: photomicrographs of Mac-1 immunostained microglia in MPTP-treated mice at day 2 (a), day 5 (c), and day 8 (e) post MPTP; in MPTP and pioglitazone-treated mice at day 2 (b), day 5 (d), and day 8 (f) post MPTP; and in vehicle-treated control mice in the absence (g) or presence (h) of pioglitazone in the dorsal striatum. Scale bar represents 50 lm (a–h). 2001). In the present animal model of PD, the administration of the thiazolidinedione pioglitazone led to a significant reduction of iNOS-positive activated microglia in the SNpc after MPTP injection, accompanied by the protection of dopaminergic neurons in the SN, supporting the idea of a deleterious role of microglial activation in neuronal cell death. Thiazolidinediones such as pioglitazone are agonists of PPARc (Smith 2001), and PPARc is expressed in cells of monocyte/macrophage lineage including brain resident microglia (Ricote et al. 1998; Bernardo et al. 2000). PPARc ligands have been shown to prevent microglial activation in vitro by inhibiting the expression of a number of PPARc regulated genes that become up-regulated during cellular activation (Ricote et al. 1998; Combs et al. 2000). Fig. 7 MPTP-induced astrocytic response remains unchanged in the striatum in the presence of pioglitazone. Photomicrographs of glial fibrillary acidic protein (GFAP)-positive astrocytes in MPTP-treated mice at day 2 (a), day 5 (c), and day 8 (e) post MPTP; in MPTP and pioglitazone-treated mice at day 2 (b), day 5 (d) and day 8 (f) post MPTP; and in saline-injected control mice in the absence (g) or presence (h) of pioglitazone in the dorsal striatum. Scale bar represents 50 lm (a–h). Mediators that were affected by PPARc ligands are proinflammatory cytokines such as tumour necrosis factor (TNFa) and iNOS that have been implicated in dopaminergic cell death in vitro (Hunot et al. 1996) and in vivo (Boka et al. 1994). Thus, in the present study, the protection of dopaminergic cells by pioglitazone could be caused by PPARcmediated inhibition of microglial activation. The inhibition of iNOS by pioglitazone is consistent with the ability of PPARc to interact with coactivators to repress iNOS promoter activity in response to ligand binding (Li et al. 2000). Since isolated suppression of iNOS is sufficient to protect neurons in the MPTP model without altering activated microglial morphology, as assessed by Mac-1 immunoreactivity (Liberatore et al. 1999), the inhibition of 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 Pioglitazone attenuates MPTP toxicity 623 iNOS expression may be responsible for the neuroprotective effect of the PPARc ligand pioglitazone. Another possible explanation for the preservation of SNpc dopaminergic neurons in pioglitazone-treated MPTP mice could be a direct protective effect of pioglitazone on dopaminergic nerve cells, leading secondarily to reduced microglial activation, which cannot be ruled out from the present data. Pioglitazone is currently in use as an antidiabetic agent which improves insulin resistance. Like other thiazolidinediones, pioglitazone has been shown to enhance basal glucose uptake by regulation of glucose transporters in peripheral tissue (el-Kebbi et al. 1994; Smith 2001). In neuronal tissue, the effect of thiazolidinediones on glucose homeostasis has not yet been investigated. Administration of MPTP increases acutely glucose utilization in the substantia nigra in primates and rodents (Palacios and Wiederhold 1984; Palombo et al. 1988), consistent with an increased dependence on anaerobic glycolysis to meet metabolic demand after blocking oxidative respiration by MPP+. A pioglitazone-induced increase in glucose uptake could therefore lead to an increased resistance of SNpc neurons to MPTP toxicity. Although the main action of pioglitazone is mediated via binding to PPARc in vitro, it remains possible that other activities are influencing its effects in vivo. The use of other, structurally unrelated PPARc agonists might help to clarify the attribution of PPARc unrelated effects. In the striatum, the MPTP-induced loss of TH-immunoreactivity and depletion of dopamine and metabolites, was accompanied by a glial reaction with similar time course as seen in the SN (Fig. 3). Unexpectedly, in pioglitazone-treated animals the attenuation of microglial activation and protection of dopaminergic neurons in the SN was not accompanied by a similar effect on the glial response and dopaminergic fibre degeneration in the striatum. A similar differential response of glial activation and damage to dopaminergic structures of the striatum compared with the ventral midbrain has been shown in other in vivo studies using the MPTP model (Liberatore et al. 1999; Dehmer et al. 2000; Ghulam et al. 2001). Regional heterogeneity in the number and morphology of resting microglia in the central nervous system has been described (Lawson et al. 1990), and this heterogeneity might contribute to unequal sensitivity to microglia-mediated neurotoxicity in different regions of the brain (Kim et al. 2000). In agreement with the low PPARc mRNA levels in murine brain (Cullingford et al. 1998), the western blot analysis in the present study revealed only low expression levels in ventral mesencephalon and striatum, with no evidence of differential expression of this receptor in these two brain regions (Fig. 3e). Thus, other microglial regulating factors may be responsible for the postulated differences in microglial regulation in SN and striatum. Since, in contrast to the SN, striatal glucose uptake remains unchanged or is even reduced in MPTPtreated primates and rodents (Palacios and Wiederhold 1984; Palombo et al. 1988), it is also possible that differences in glucose metabolism or the higher energy demand of the striatal nerve terminals compared with the SNpc cell bodies (Sokoloff 1993) could make the striatum more vulnerable to MPTP toxicity and mask the protective effect of pioglitazone. The observed glial activation might be secondary to neuronal damage and hence persist in the unprotected striatum. At present, the cause of the selective nerve cell death in idiopathic PD remains unknown, and the therapeutic approaches currently in use have no effect on the progressive neurodegeneration. In the present study we present evidence that nerve cell loss and glial activation in the SN in an animal model of PD is attenuated by an agonist of the nuclear receptor PPARc. Since inflammation, such as activated microglia, increased cytokines, and nitric oxide synthase expression have been demonstrated in PD (McGeer et al. 1988; Boka et al. 1994; Mogi et al. 1994; Hunot et al. 1996), the present findings might help to develop new therapeutic strategies for the treatment of PD. Acknowledgements This study was supported by Institut de la Sante´ et de la Recherche Me´dicale (INSERM, France) and the National Parkinson Foundation (Miami, FL, USA). TB is a postdoctoral fellow of the Deutsche Forschungsgemeinschaft. We are grateful to Karine Parain and Estelle Rousselet for their help with animal care, and Elena Galea for carefully reading the manuscript. References Akiyama H. and McGeer P. L. (1989) Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 489, 247–253. Bal-Price A. and Brown G. C. (2001) Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 21, 6480–6491. Bernardo A., Levi G. and Minghetti L. (2000) Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-delta12,14-prostaglandin J2 in the regulation of microglial functions. Eur. J. Neurosci. 12, 2215–2223. Boka G., Anglade P., Wallach D., Javoy-Agid F., Agid Y. and Hirsch E. C. (1994) Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 172, 151–154. Chao C. C., Hu S., Molitor T. W., Shaskan E. G. and Peterson P. K. (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 149, 2736–2741. Combs C. K., Johnson D. E., Karlo J. C., Cannady S. B. and Landreth G. E. (2000) Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J. Neurosci. 20, 558–567. Cullingford T. E., Bhakoo K., Peuchen S., Dolphin C. T., Patel R. and Clark J. B. (1998) Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J. Neurochem. 70, 1366–1375. Dehmer T., Lindenau J., Haid S., Dichgans J. and Schulz J. B. (2000) Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J. Neurochem. 74, 2213–2216. 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624 624 T. Breidert et al. Delerive P., Fruchart J. C. and Staels B. (2001) Peroxisome proliferatoractivated receptors in inflammation control. J. Endocrinol. 169, 453–459. Feinstein D. L., Brosnan C. F., Sharp A., Landreth G. E., Gavrilyuk V., Wullner U. and Heneka M. T. (2001) Suppression of experimental autoimmune encephalomyelitis by pioglitazone, a PPAR-gamma agonist. Soc. Neurosci. Abstracts 27, 102.15. Franklin K. B. J. and Paxinos G. (1996) The Mouse Brain in Stereotaxic Coordinates. Academic Press, Sydney. Ghulam N., Sager T., Laursen H. and Vaudano E. (2001) Minocycline reduces microglia activation in the chronic MPTP mouse model of Parkinson’s disease. Soc. Neurosci. Abstracts 27, 887.8. He B. P., Tay S. S. and Leong S. K. (1997) Microglia responses in the CNS following sciatic nerve transection in C57BL/Wld(s) and BALB/c mice. Exp. Neurol. 146, 587–595. He Y., Appel S. and Le W. (2001) Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 909, 187–193. Heneka M. T., Klockgether T. and Feinstein D. L. (2000) Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J. Neurosci. 20, 6862–6867. Hirsch E. C. (2000) Glial cells and Parkinson’s disease. J. Neurol. 247, II58–II62. Hirsch E., Graybiel A. M. and Agid Y. A. (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334, 345–348. Hunot S., Boissie`re F., Faucheux B., Brugg B., Mouatt-Prigent A., Agid Y. and Hirsch E. C. (1996) Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience 72, 355– 363. Hunot S., Brugg B., Ricard D., Michel P. P., Muriel M. P., Ruberg M., Faucheux B. A., Agid Y. and Hirsch E. C. (1997) Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc. Natl Acad. Sci. USA 94, 7531–7536. Jackson-Lewis V., Jakowec M., Burke R. E. and Przedborski S. (1995) Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4, 257–269. Jiang C., Ting A. T. and Seed B. (1998) PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391, 82–86. el-Kebbi I. M., Roser S. and Pollet R. J. (1994) Regulation of glucose transport by pioglitazone in cultured muscle cells. Metabolism 43, 953–958. Kim W. G., Mohney R. P., Wilson B., Jeohn G. H., Liu B. and Hong J. S. (2000) Regional difference in susceptibility to lipopolysaccharideinduced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 20, 6309–6316. Kreutzberg G. W. (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318. Kurkowska-Jastrzebska I., Wronska A., Kohutnicka M., Czlonkowski A. and Czlonkowska A. (1999) The inflammatory reaction following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication in mouse. Exp. Neurol. 156, 50–61. Langston J. W., Forno L. S., Tetrud J., Reeves A. G., Kaplan J. A. and Karluk D. (1999) Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6tetrahydropyridine exposure. Ann. Neurol. 46, 598–605. Lawson L. J., Perry V. H., Dri P. and Gordon S. (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170. Le W., Rowe D., Xie W., Ortiz I., He Y. and Appel S. H. (2001) Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson’s disease. J. Neurosci. 21, 8447–8455. Li M., Pascual G. and Glass C. K. (2000) Peroxisome proliferatoractivated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol. Cell Biol. 20, 4699–4707. Liberatore G. T., Jackson-Lewis V., Vukosavic S., Mandir A. S., Vila M., McAuliffe W. G., Dawson V. L., Dawson T. M. and Przedborski S. (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 5, 1403–1409. Liu B., Jiang J. W., Wilson B. C., Du L., Yang S. N., Wang J. Y., Wu G. C., Cao X. D. and Hong J. S. (2000) Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J. Pharmacol. Exp. Ther. 295, 125–132. Lu X., Bing G. and Hagg T. (2000) Naloxone prevents microgliainduced degeneration of dopaminergic substantia nigra neurons in adult rats. Neuroscience 97, 285–291. Maeshiba Y., Kiyota Y., Yamashita K., Motohashi M. and Tanayama S. (1997) Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneim-Forschung/Drug Res. 47, 29–35. McGeer P. L., Itagaki S., Boyes B. E. and McGeer E. G. (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291. Mogi M., Harada M., Kondo T., Riederer P., Inagaki H., Minami M. and Nagatsu T. (1994) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 180, 147–150. Palacios J. M. and Wiederhold K. H. (1984) Acute administration of 1-N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a compound producing parkinsonism in humans, stimulates [2–14C] deoxyglucose uptake in the regions of the catecholaminergic cell bodies in the rat and guinea pig brains. Brain Res. 301, 187–191. Palombo E., Porrino L. J., Bankiewicz K. S., Crane A. M., Kopin I. J. and Sokoloff L. (1988) Administration of MPTP acutely increases glucose utilization in the substantia nigra of primates. Brain Res. 453, 227–234. Przedborski S. and Jackson-Lewis V. (1998) Mechanisms of MPTP toxicity. Mov Disord 13, 35–38. Ricote M., Li A. C., Willson T. M., Kelly C. J. and Glass C. K. (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391, 79–82. Smith U. (2001) Pioglitazone: mechanism of action. Int. J. Clin. Pract. supplement 13–18. Sokoloff L. (1993) Sites and mechanisms of function-related changes in energy metabolism in the nervous system. Dev. Neurosci. 15, 194–206. Takamura T., Ando H., Nagai Y., Yamashita H., Nohara E. and Kobayashi K. (1999) Pioglitazone prevents mice from multiple low-dose streptozotocin-induced insulitis and diabetes. Diabetes Res. Clin. Pract 44, 107–114. Tang Y., Osawa H., Onuma H., Nishimiya T., Ochi M. and Makino H. (1999) Improvement in insulin resistance and the restoration of reduced phosphodiesterase 3B gene expression by pioglitazone in adipose tissue of obese diabetic KKAy mice. Diabetes 48, 1830– 1835. Viale G., Gambacorta M., Coggi G., Dell’Orto P., Milani M. and Doglioni C. (1991) Glial fibrillary acidic protein immunoreactivity in normal and diseased human breast. Virchows Arch. A Pathol. Anat. Histopathol. 418, 339–348. Vodovotz Y., Bogdan C., Paik J., Xie Q. W. and Nathan C. (1993) Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J. Exp. Med. 178, 605–613. 2002 International Society for Neurochemistry, Journal of Neurochemistry, 82, 615–624
© Copyright 2026