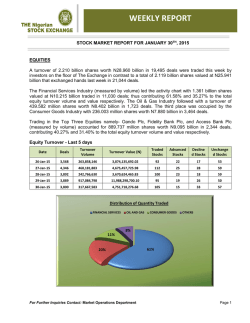
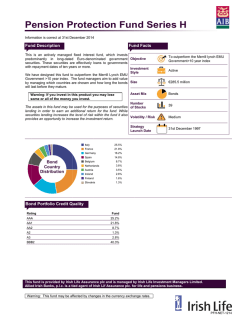
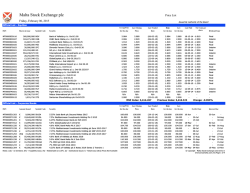
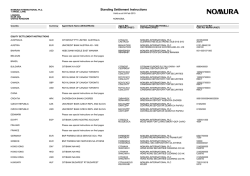
Sphere Medical Corporate Presentation January 2015
Corporate Presentation
© Sphere Medical Holding plc
Disclaimer
The information contained in this presentation is strictly confidential, and is supplied on the understanding that it will be held in confidence, and not copied,
reproduced, distributed, published or disclosed to third parties without the prior written consent of Sphere Medical Holding plc ("Sphere Medical" or the "Company",
which term includes Sphere Medical Limited, a wholly owned subsidiary). The recipient agrees that on request from Sphere Medical it will return or destroy all copies
of this presentation.
This presentation does not constitute or form part of any invitation to purchase, sell or subscribe for, or any solicitation of any such offer to purchase, sell or
subscribe for, any securities in Sphere Medical nor shall this presentation or any part of it, or the fact of its distribution, form the basis of, or be relied on in
connection with, any contract therefor.
No reliance may be placed, for any purposes whatsoever, on the information contained in this presentation or on its completeness and this presentation should not
be considered a recommendation by Sphere Medical or any of its affiliates in relation to any purchase of or subscription for securities of Sphere Medical. No
representation or warranty, express or implied, is given by or on behalf of Sphere Medical or any of its directors, officers, employees, advisers or any other persons
as to the accuracy, fairness or sufficiency of the information or opinions contained in this presentation and none of the information contained in this presentation has
been independently verified. Save in the case of fraud, no liability is accepted for any errors, omissions or inaccuracies in such information or opinions.
The securities of the Company will not be registered under the US Securities Act of 1933, as amended (the "Securities Act") and may not be offered, sold, resold,
pledged, delivered, distributed or otherwise transferred, directly or indirectly, in or into the United States or to or for the account or benefit of US persons (as such
terms are defined in Regulation S under the Securities Act ("Regulation S")) unless registered under the Securities Act or pursuant to an exemption from, and in
compliance with any applicable securities laws of any state or jurisdiction of the United States. No offer of securities will be made to the public in the United States or
to or for the account or benefit of US persons. Distribution of this presentation may be restricted or prohibited by US law and the laws of certain other jurisdictions.
Recipients are required to inform themselves of, and comply with, all such restrictions or prohibitions and the Company or any other person accepts liability to any
person in relation thereto.
The securities of the Company have not been approved or disapproved by the US Securities and Exchange Commission, any state securities commission in the
United States or any US regulatory authority, nor have any of the foregoing authorities passed upon or endorsed the merits of any proposed offering of the securities
of the Company, or the accuracy or adequacy of this presentation. Any representation to the contrary is a criminal offence in the United States. There is currently no
public market in the United States for the securities of the Company and none is expected to develop in the foreseeable future. As a result, potential investors
should be aware that they may be required to bear the financial risks of any investment in the securities of the Company for an indefinite period of time.
The distribution of this presentation in certain jurisdictions may be restricted and accordingly it is the responsibility of any person into whose possession the
presentation comes to inform themselves about and observe such restrictions. This document is intended for distribution (A) in the United Kingdom only to persons
who (i) have professional experience in matters relating to investments who fall within the definition of "investment professionals" in Article 19(5) of the Financial
Services and Markets Act 2000 (Financial Promotion) Order 2005 (as amended) or, high net worth companies, unincorporated associations or partnerships or
trustees of high value trusts as described in Article 49(2) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (as amended) and
investment personnel of any of the foregoing (each within the meaning of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005) and (ii)
are "qualified investors", as defined in section 86 of the Financial Services and Markets Act 2000 (the "FSMA"); (B) in member states of the European Economic
Area ("EEA") other than the UK only to "qualified investors"; (C) outside the US to non-US persons (as defined in Regulation S) in reliance upon Regulation S; and
(D) in the United States to persons reasonably believed to be “qualified institutional buyers” as defined in Rule 144A under the Securities Act (“QIBs”) who are also
“qualified purchasers” as defined in Section 2(a)(51) of the Investment Company Act (“QPs”); and (E) otherwise, only to persons to whom it may be lawful to
communicate it (each such person in (A) to (E) above being a "Relevant Person").
© Sphere Medical plc
2
Disclaimer (con’t)
This presentation and any offer if made subsequently is directed only at persons in member states of the EEA who are qualified investors within the meaning of
Article 2(1)(e) of the Prospectus Directive (Directive 2003/71/EC as amended (including amendments by Directive 2010/73/EU), to the extent implemented in the
relevant member state) (the "Prospectus Directive") (Qualified Investors). Any person in the EEA who acquires the shares in any offer or to whom any offer of the
shares is made will be deemed to have represented and agreed that it is a Qualified Investor. Any investor will also be deemed to have represented and agreed that
any shares acquired by it in the offer have not been acquired on behalf of persons in the EEA other than Qualified Investors or persons in the UK and other member
states (where equivalent legislation exists) for whom the investor has authority to make decisions on a wholly discretionary basis, nor have the shares been
acquired with a view to their resale in the EEA to persons where this would result in a requirement for publication by the Company or any other manager of a
prospectus pursuant to Article 3 of the Prospectus Directive.
By accepting this presentation and not immediately returning it, by your action, you warrant, represent, acknowledge and agree to and with the Company that: (i) you
are (a) outside the United States, not a US person, a Relevant Person and a Qualified Investor or (b) a QIB and a QP; (ii) you have read, agree to and will comply
with the contents of this disclaimer, you will keep the information in this presentation and any accompanying document confidential and information about the
Company confidential, and will not reproduce or distribute, in whole or in part (directly or indirectly) any such information, until such information has been made
publicly available and will take all reasonable steps to preserve such confidentiality; and (iii) you are permitted in accordance with applicable laws, to receive such
information.
This presentation contains certain forward looking statements relating to the Company’s future prospects, developments and business strategies. Forward looking
statements are identified by their use of terms and phrases such as “targets” “estimates”, “envisages”, “believes”, “expects”, “aims”, “intends”, “plans”, “will”, “may”,
“anticipates”, “would”, “could” or similar expressions or the negative of those, variations or comparable expressions, including references to assumptions. The
forward looking statements in this presentation are based on current expectations and are subject to risks and uncertainties which could cause actual results to differ
materially from those expressed or implied by those statements. If one or more of these risks or uncertainties materialises, or if the underlying assumptions prove
incorrect, the Company’s actual results may vary materially from those expected, estimated or projected. Given these risks and uncertainties, potential investors
should not place any reliance on forward looking statements. These forward looking statements relate only to the position as at the date of this presentation. Neither
the directors nor the Company undertake any obligation to update forward looking statements or risks, other than as required by the AIM Rules for Companies or by
the rules of any other applicable securities regulatory authority, whether as a result of the information, future events or otherwise.
All logos, graphics and trademarks contained in this presentation are recognised and remain the property of the respective organisation. Sphere’s word mark is a
trademark of Sphere Medical, where the term trademark means the word or mark however represented, including stylised representations, all associated logos and
symbols and combinations of the foregoing with another word or mark.
Sphere Medical Holding plc is incorporated in England with registered number 4179503 and registered address Harston Mill, Harston, Cambridge CB22 7GG.
© Sphere Medical plc
3
Investment Highlights
Proxima is a CE marked, patient-attached, arterial blood gas analyser for
the critical care market
Disruptive technology represents a potential game changer
Global market potential validated by partner, Ortho Clinical Diagnostics
Strong KOL support
Sales launch of Proxima 3 in September 2014
Clear growth catalysts over next 18 months
£48 million invested to date via blue-chip and specialist investors
Commercially focused management team with relevant med tech
experience
© Sphere Medical plc
4
Unmet Need – Two KOL opinions
“… one of the things we have learnt is that the
careful control of patient physiology has a
huge potential to improve outcome”
Member of Sphere Medical Advisory Board and
Chief Investigator Proxima Method Comparison Study
Dr. Tom Clutton-Brock
Senior Lecturer and Head of the
Department of Anaesthesia and
Intensive Care,
University of Birmingham, UK
“… Proxima has the potential to bring about a
paradigm shift in the intensive and critical
care setting …”
Chairman of Sphere Medical Advisory Board
Professor Vincent is Professor of Intensive
Care at the Université Libre de Bruxelles
and the Head of the Department of
Intensive Care, Erasme University Hospital
(University of Brussels)
© Sphere Medical plc
5
Current Practice
Current clinical practice for blood gas
measurements (1)
On average blood gases are measured every 3
hours
For critically ill patients, up to 19 measurements
are made per day
Unstable patients would benefit from more
frequent measurements
Detect onset of critical conditions earlier to
prevent complications
Detect improvements earlier to wean off
ventilation
Current barriers to more frequent measurements
Increased workload for nursing staff
Rise in blood anaemia
Rise of infection risk
(1) Nursing in Critical Care 2008
© Sphere Medical plc
6
Current Players
Laboratory blood gas analysers
Large instruments sited in a central laboratory
High volume measurements with high accuracy
Key players include: Radiometer, Siemens, Instrumentation
Labs, Roche
Benchtop blood gas analysers
Smaller instruments sited ‘near patient’ in the ward
Medium volume measurements with high accuracy
Key players include: Radiometer, Siemens, Instrumentation
Labs, Roche
Point of Care analysers
Transportable or hand held device used at patient bedside
Single use cartridge with high accuracy
Key players include: Abbott POC
© Sphere Medical plc
7
Proxima – The Potential Game Changer
Disposable patient-attached
device
Closed system mounted in
arterial line
Blood returned to patient
Integrates into existing
practices and workflow
Designed to measure up to
72 hours, as frequently as
possible
Sensor panel = pH, pCO2,
pO2, Haematocrit, K+
© Sphere Medical plc
8
Proxima Benefits
Keeps nurse at the patient’s bedside
Conserves patient blood
Reduces infection risk
Easy to make more frequent measurements
Enables closer control of therapeutic response
Expected to improve patient outcome
© Sphere Medical plc
9
Attractive Market
$3.2 billion market worldwide for blood gas and
electrolyte testing in 2013(1)
Instruments, service, consumables
CAGR 3.1%
$0.9 billion Point of Care market worldwide in 2013
CAGR 4.9%
$0.3 billion Point of Care market Europe in 2013
CAGR 4.8%
(1) Blood Gas and Electrolyte Analysers, Global Industry Analysts Inc. 2012
© Sphere Medical plc
10
Market Launch
Proxima 3 launched in UK at the Association of
Anaesthetists of Great Britain and Ireland (AAGBI)
conference 17-19 September 2014
Presented Proxima 3 at the British Association of
Critical Care Nurses (BACCN) conference in early
September 2014 and received several hospital
evaluation requests
Appointed new VP Sales direct from Abbott Point of
Care to lead Proxima’s sales activities
© Sphere Medical plc
11
Target Markets
Target market segments where patient management
requires frequent measurements
Severe sepsis and septic shock
Acute respiratory distress syndrome (ARDS)
Major trauma
Neuro trauma
Target early adopters which treat patients within target
segments from 2014 onwards
83 hospitals in the UK(1)
Expand sales to Europe via own focused sales team
Germany, Benelux in H1 2015
(1) Source NHS critical care bed report 2013
© Sphere Medical plc
12
Our Global Partner OCD
Strong global presence
World leading sales and marketing capabilities
Shared vision
Estimated sales opportunity > $200 million at full market
penetration
Collaborative product enhancements based on market
feedback
Partnership structure
Development deal signed in 2013
Opportunity to negotiate a potential distribution deal H2
2015, upon completion of development milestones
Hold 13.7% of Sphere Medical’s shares
New ownership
Carlyle Group has acquired OCD from J&J (June 2014)
© Sphere Medical plc
13
Catalysts for Growth
Geographical sales expansion
Germany, Benelux H1 2015
Potentially sign worldwide distribution partnership H2 2015
Market penetration
Proxima 4 (launch expected in Europe H1 2016)
Add glucose and sodium to sensor panel
Connectivity to HIS/LIS systems
Obtain FDA approval
Future product evolution planned
Expand analytes on sensor panel
Penetrate new target market segments
Method comparison study
Birmingham H2
Proxima 3 UK launch
September
2014
Proxima 3 EUR launch
H1
Potential worldwide distribution deal
H2
2015
© Sphere Medical plc
Proxima 4 EUR launch
H1
2016
14
Clear Investment Opportunity
Proxima is a CE marked, patient-attached, arterial blood gas analyser for the
critical care market
Disruptive technology represents a potential game changer
Global market potential validated by partner, Ortho Clinical Diagnostics
Strong KOL support
Sales launch of Proxima 3 in September 2014
Clear growth catalysts over next 18 months
£48 million invested to date via blue-chip and specialist investors
Commercially focused management team with relevant med tech experience
© Sphere Medical plc
15
Frost and Sullivan Point-of-Care Technologies
award for Technology Innovation of the Year
“The One to Watch” investment award, March
2011, at IBIZ 2011, organised by UKTI
Appendix
17
Presentation team
Dr. Wolfgang Rencken
CEO
Matthew Hall
CFO
Appointed CEO in February 2014
More than 15 years’ experience in
healthcare and medical devices industries
Joined Sphere in 2011 as CFO
the
Proven track record in developing and
commercialising medical devices and driving
significant product revenue growth
Previous roles:
CEO of MAQUET Cardiopulmonary AG, an
international medical devices group with over
1,000 employees. Over three years as CEO, he
oversaw revenue growth of over 50% and more
than doubled EBITDA
Chartered Accountant
economics
a
degree
in
Previous roles:
CFO of IS Pharma, specialist pharmaceutical
group listed on AIM, which merged with Sinclair
Pharma in May 2011
20 years corporate finance experience – held
positions at Noble Group, Close Brothers
Group, Hill Samuel Group, Merrill Lynch,
Deloitte Touche Tohmatsu
COO and a director of Definiens AG
Siemens AG over a 15 year period
© Sphere Medical plc
with
18
Non-Executive Directors
Non-executive Chairman
Non-executive Director
Non-executive Director
Dr Anthony Martin
Stephen H. Mahle
John Gregory
Joined Sphere in 2005
Joined Sphere in December 2011
Joined Sphere in December 2011
Senior Independent Director
Current roles
Non-executive Chairman - Phico Therapeutics
Ltd
Non-executive Director- Abcam plc, Orthofix
International N.V.
Previous roles
Current roles
Previous roles
Former Executive Vice President of Medtronic
Inc. and President of Medtronic’s largest
division, Pacing and subsequently President of
Cardiac Rhythm Disease Management - retired
after 37 years
Non-executive Chairman –
Immunodiagnostics Systems Holdings plc,
NeuTec Pharma plc, Tepnel Life Sciences,
Molecular Probes Inc.
Non-executive Director
Non-executive Director
Dr David Martyr
Meinhard Schmidt
Current roles
CEO - Tecan AG
Non-Executive Director - ALDA (Analytical Life
Science Diagnostics Association), Washington
DC
Previous roles
Group President Leica Microsystems and VP
& Group Executive, Danaher Corporation
Previous roles
Non-executive Chairman – IS Pharma,
Sinclair IS Pharma
Non-executive Director - Noble Fund
Managers
CEO – Molecular Probes Inc., Invitrogen
Corporation, Celsis International
Joined Sphere in 2015
Non-executive Chairman – Foresight VCT
Joined Sphere in 2015
Current roles
Founder and Managing Partner - mt:onyx AG,
Director of Quanta Fluid Solutions Ltd,
ValuationLab AG, Promimic AB (Sweden)
Previous roles
Director of Cellnovo Ltd, CEO, SVP & Head
Business Unit Prosthetics/Digitalization Institut Straumann AG and Open Digital
Dentistry AG, SVP Head Roche Decentralized
Solutions – Roche Diagnostics
© Sphere Medical plc
19
Medical Advisory Board
Medical Advisory Board
Professor Jean-Louis Vincent
Professor of Intensive Care at the Université
Libre de Bruxelles
Head of the Department of Intensive Care,
Erasme University Hospital (University of
Brussels)
President of the World Federation of Societies
of Intensive and Critical Care Medicine
Medical Advisory Board
Medical Advisory Board
Dr Tom Clutton-Brock
Professor Michael Quintel
Senior Lecturer and Head of the Department of
Anesthesia & Intensive Care, University of
Birmingham Medical School
Director of the Department of Anaesthesiology,
Emergency and Intensive Care Medicine at the
University of Göttingen, Germany
Programme Director PGDip & MSc Physicians’
Assistant (Anaesthesia)
Member of the European Society of Intensive
Care Medicine (ESICM) group on respiratory
failure
Consultant in Anaesthesia and Critical Care,
University Hospitals NHS Foundation Trust
Associate Medical Director for Audit & Clinical
Effectiveness, University Hospitals NHS
Foundation Trust
Elected member of Council, Royal College of
Anaesthetists
Past President of the German Interdisciplinary
Society of Intensive Care Medicine (DIVI) and a
past council member of the German Society of
Anaesthesia and Intensive Care (DGAI).
Professor Quintel is also a member of the
German Sepsis Society and an Investigator in
the SepNet Sepsis Clinical Trial Network in
Germany and has participated in numerous
sepsis and critical care trials.
Member of Interventional Procedures
Committee, NICE
© Sphere Medical plc
Member of the Scientific Committee of
International ECMONet
20
Customers
The champion for adoption in the ICU will be the
physician
Clinical effectiveness of the product
Other key influencers in the purchase will be
Critical care nurses – usability and workflow
impact
Finance/procurement – cost-benefit analysis
Laboratory Point of Care Committee –
analytical performance of the product
© Sphere Medical plc
21
Competition
Laboratory benchtop analysers
Radiometer, Siemens, Instrumentation Labs,
Roche
Near patient benchtop analysers
Radiometer, Siemens, Instrumentation Labs,
Roche
Point of Care analysers
Abbott POC
© Sphere Medical plc
22
Sales Process
Each geographical region will have dedicated sales
teams
Each sales team will consist of a commercial and a
technical team member
Every prospective customer will receive an
evaluation of the product
Training of the staff (1 week)
Continuing in use support (1 week)
Support to prepare business case for investment
proposal
Revenue models
Purchase monitor hardware and disposables
separately
Up charging model (premium on the disposables)
© Sphere Medical plc
23
Marketing Activities
Rebalance Medical Advisory Board with leading
European KOLs
Professor Jean-Louis Vincent appointed in August
2014
Gather clinical evidence
Conduct 2 clinical studies in 2014
Proxima 3 method comparison study QE
Hospital Birmingham 20-40 patients (ongoing)
Time and motion study (planned)
2015 onwards
Engage various KOLs and clinicians to
conduct case studies and publish results in
targeted market segments
Raise market awareness
2014 Attend AAGBI, BACCN, ICS trade shows in
UK
2015 Attend ISICEM, Brussels, 3 national shows
(UK, DE, NL)
© Sphere Medical plc
24
Interim Results - 6 months to June 30, 2014
Operating expenses were £3.1 million (H1 2013: £3.0 million)
Product Development and Realisation costs £1.5 million (H1
2013: £2.0)
Administrative costs £1.1 million (H1 2013: £0.8 million),
including one-off costs of change in CEO
Loss for the period £3.0 million (H1 2013: £2.9 million)
Cash and cash equivalents as at 30 June 2014 £6.3 million (30
June 2013: £2.6 million)
R&D tax credit refund of £523,000 received in July 2014
© Sphere Medical plc
25
© Copyright 2026