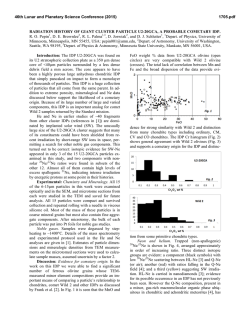
The kinetics of Fe and Ca for the development of
Nuclear Instruments and Methods in Physics Research B 189 (2002) 437–442 www.elsevier.com/locate/nimb The kinetics of Fe and Ca for the development of radiation-induced apoptosis by micro-PIXE imaging S. Harada a,*, Y. Tamakawa a, K. Ishii b, A. Tanaka b, T. Satoh b, S. Matsuyama b, H. Yamazaki b, T. Kamiya c, T. Sakai c, K. Arakawa c, M. Saitoh c, S. Oikawa d, K. Sera e a b Department of Radiology, Iwate Medical University, 19-1 Uchimaru, Morioka, Iwate 020-8505, Japan Department of Quantum Science and Energy Engineering, Tohoku University, Sendai 980-8579, Japan c Takasaki Radiation Chemistry Research Establishment, JAERI, Takasaki, Gunma 370-1292, Japan d Ion Accelerator Corporation, Hakodate 040-0076, Japan e Cyclotron Center, Iwate Medical University, 348-1 Tomegamori, Takizawa, Iwate 020-0173, Japan Abstract To study the interactions between the induction of radiation-induced apoptosis and trace elements kinetics, human leukemia cells were irradiated in vitro by 60 Co c rays, after which the cells were evaluated for the detection of apoptosis and trace element (Fe, Ca, Zn) imaging was carried out. The frequency of apoptosis, i.e. the number of apoptotic bodies per 100 nuclei, was obtained by microscopic assay using TUNEL staining at 400 magnification. The trace element distribution in the cell was determined by micro-PIXE using 2 MeV proton beams. In the early phase of apoptosis, the maximum level of Fe accumulation was observed in the cell stroma. In the mid to end phase, Fe accumulation was diminished, and instead, Ca accumulation increased and Zn decreased in the nucleus. There appear to be two steps for the development of apoptosis: (1) the signaling from cell stroma to nucleus by Fe or an Fe-containing enzyme; and (2) the degeneration of the nucleus by Ca-dependent enzyme, and release of Zn from digested nucleus. Those strong accumulations may be new markers for apoptosis. Ó 2002 Elsevier Science B.V. All rights reserved. PACS: 82.80.Ej; 87.50.Gi Keywords: Radiation; Apoptosis; Micro-PIXE camera; Ca; Fe; Zn 1. Introduction Radiation induced-apoptosis is the suicidal process of the cell initialized by irradiation, which is controlled and executed by a series of genes and * Corresponding author. Tel.: +81-19-651-5111x3660; fax: +81-19-622-1091. E-mail address: [email protected] (S. Harada). protein enzymes [1–4]. In the mechanism of radiation-induced apoptosis, it is well known that there are three steps: (1) the initializing of apoptosis; (2) the decision of apoptosis; and (3) the ‘‘executioner’’ phase of apoptosis. The initializing of radiation-induced apoptosis is performed by irradiation. Then the effectiveness of this initialization is tested by the decision of apoptosis, which is controlled by the gene P 53, Bcl-2/Bax family [5]. When the initialization of apoptosis is determined, 0168-583X/02/$ - see front matter Ó 2002 Elsevier Science B.V. All rights reserved. PII: S 0 1 6 8 - 5 8 3 X ( 0 1 ) 0 1 1 2 1 - 1 438 S. Harada et al. / Nucl. Instr. and Meth. in Phys. Res. B 189 (2002) 437–442 the cell undergoes the executioner phase of apoptosis: the process for the auto-digestion of its nucleus, which is strictly concerned with the kinetics of the trace elements. The executioner phase of apoptosis can be divided into three steps: (1) the depolarization of the mitochondrial membrane [6–10]; (2) the releasing of Cytochrome C from the mitochondria to cytosol [6–10]; (3) activation of Caspase family and/or the DNase c in nucleus [11–17]. In the first step of the executioner phase of apoptosis, the inner membrane of mitochondria is depolarized by inflow of the calcium and/or magnesium ion into the inner membrane of mitochondria [6–10]. After the depolarization of the inner membrane of mitochondria, the mitochondria release cytochrome C to cytosol [6–10], and activate the caspase family, DNase c, and Ca/Mg dependent endonucleases in nucleus [11–17], which digest the DNA in the nucleus and cause cells to die. Of these enzymes, the cytochrome C includes Fe in its chemical structure [18,19]. The caspase family requires Mg, DNase c also requires Ca, and Ca/Mg dependent endonuclease requires Mg and Ca for activation [11–17]. The DNA contains the Zn in its backtyping protein [18,19], which is digested by caspase family, DNase c, and Ca/Mg dependent endonuclease [11–17]. Therefore, it is considered that: the kinetics of Mg, Ca, Fe and Zn may interact with the induction of apoptosis; however this has not been studied extensively. Among the trace elements described above, the interactions of Ca, Fe and Zn were tested for the development of radiation-induced apoptosis, using micro-PIXE. 2. Materials and methods 2.1. Tumor systems The human leukemia cells MOLT-4 and HL-60 were grown in RPMI-1640 supplemented with 10% fetal calf serum, using 9 cm diameter dishes. When the cell covered 70% of the bottom of the dishes, the cells were used for experiments. 24 h before irradiation, the medium was exchanged for a new one containing 10% HEPES (4- (2-hydroxyethyl)-1-piperazineethanesulfonic acid). When the medium was changed, a Mylar film (1 lm in thickness) held in a ring of 2 cm diameter was placed at the bottom of the dish, so that the cells could grow and adhere to the Mylar film. The cells were grown in the same condition for 24 h. Then irradiation was performed. 2.2. Irradiation Irradiation was carried out using 60 Co c rays. The dishes were placed in the square radiation field of dimensions 18 18 cm2 . The dose rate ranged from 34.1 to 35.1 c Gy/min. The backward and side scatter fractions were less than 0.3%. In a preliminary experiment, the radiation was administered incrementally using values of 1, 2, 5, 10, 20 Gy, in order that we could determine a suitable irradiation dose from the observed induction rate of apoptosis as a function of irradiation dose. As a result we chose to measure the frequency of apoptosis at 6 h after irradiation. 2.3. Frequency of apoptosis, induction rate of apoptosis versus the irradiation dose, and selection of a suitable irradiation dose The apoptosis was detected by assaying the apoptotic bodies using the May Giemsa staining. The frequency of apoptosis was expressed as the percentage of apoptotic cells to the whole number of cells. For selecting the suitable irradiation dose, the rate of frequency relative to the radiation dose was calculated using the following formula: F =D (%=Gy), where F was the frequency of apoptosis on 6 h after irradiation and D was the radiation dose. The suitable radiation dose was determined by two criteria: (1) more than 50% of apoptosis is observed in the irradiated cell; and (2) the induction ratio is higher than that given by other doses. 2.4. Preparation of the target for PIXE To study time dependence, the Mylar film was picked up 3, 6, 9, 12 and 24 h after irradiation. It was washed by dipping it in THAM (Tris-hydrooxyaminomethane) buffer five times, and then dipped in isopentane cooled by liquid nitrogen. S. Harada et al. / Nucl. Instr. and Meth. in Phys. Res. B 189 (2002) 437–442 439 Then the Mylar film was freeze dried in vacuum and subjected to micro-PIXE analysis. 2.5. Micro-PIXE analysis The target was irradiated by a 2 MV proton beam, 2 lm in diameter at the point of impact, and induced X-rays were recorded by Si–Li detector. The cell was scanned by the 2 MV micro-proton beam within a 90 90 lm2 area, and the X-ray signals were converted into two-dimensional images. In this way, the distribution of the Ca, Fe and Zn were imaged in the cell. 2.6. Statistics The statistical analysis was carried out using one way ANOVA (analysis of variance). The data were determined significant at the 0.05 probability level. 3. Results 3.1. Optimizing the radiation dose In order to optimize the radiation dose, the alteration of the frequency of apoptosis 6 h after irradiation was plotted against the radiation dose (Fig. 1). The slope of the plotted line below 5 Gy was steeper than that over 5 Gy. Irradiation below the 5 Gy level resulted in a significant greater induction ratio (11:7%=Gy) than that over the 5 Gy level (0:99%=Gy). Below the 5 Gy level, it was only the 5 Gy irradiation that induced more than 50% of apoptosis. Therefore, we determined the suitable irradiation dose to be 5 Gy. In the HL-60 cells, there were no increases of the frequency of apoptosis. 3.2. Alteration of the frequency of apoptosis at 5 Gy irradiation The alteration of the frequency of apoptosis against time is plotted in Fig. 2. In the apoptotic sensitive MOLT-4 cells, the frequency of apoptosis gradually increased, and reached 92:1 2:13% on 24 h after irradiation. Therefore, we have deter- Fig. 1. The alteration of the frequency of apoptosis 6 h after irradiation versus the radiation dose (1, 2, 5, 10, 20 Gy) for HL60 cells (j) and MOLT-4 cells (r). The slope of the plotted line represents the induction of the rate of apoptosis. Note that the slope of the line below 5 Gy dose was steeper than that over 5 Gy in MOLT-4 cells. mined that most MOLT-4 cells underwent the apoptotic pathway under the 5 Gy irradiation. In the HL-60 cells, there were no increases of apoptosis by 5 Gy irradiation. 3.3. Micro-PIXE imaging of Ca, Fe and Zn The images of Ca, Fe and Zn in MOLT-4 cells by micro-PIXE camera are shown in Fig. 3. Before irradiation, there was no high accumulation of Fe in the MOLT-4 cell. The calcium was distributed homogenously in the nucleus of unirradiated MOLT-4 cells. The Zn was mainly present in the nucleus of unirradiated MOLT-4 cells. 6 h after irradiation of MOLT-4 cells, a high accumulation of Fe was observed in the cytosol; 9 h after irradiation, the high accumulation of Fe was enlarged, and a weak accumulation around it was observed; 24 h after irradiation, the high accumulation of Fe was diminished. The Ca began to 440 S. Harada et al. / Nucl. Instr. and Meth. in Phys. Res. B 189 (2002) 437–442 Fig. 2. Alteration of the frequency of apoptosis due to 5 Gy irradiation: The alteration of the frequency of apoptosis against the time courses for HL-60 cells (j) and MOLT-4 cells (r). The MOLT-4 cells show a gradual increase of apoptosis after 5 Gy irradiation, reaching 92:1 2:13% at 24 h after irradiation. accumulate 6 h after irradiation; high accumulation of Ca was observed 9 h after irradiation; that high accumulation was present 24 h after irradiation, when the high accumulation of Fe diminished. As for the Zn, its accumulation decreased from the nucleus 6 h after irradiation. In spite that: there was proximal recovery of Zn accumulation 9 h after irradiation; the Zn in the nucleus decreased 24 h after irradiation. In the apoptotic resistant HL-60 cell, there were no kinetic changes of Ca, Fe and Zn in the microPIXE images. 4. Discussion Previously, several studies have been made concerning the relationships between the induction of apoptosis and trace elements [5–8]. Those elements are mainly Mg, Ca and Zn. The Mg and Ca depolarize the inner membrane of mitochondria, which have been studied using flow cytome- try [5–8]. The Ca and Mg kinetics are correlated with the enzyme activity of DNase c and Ca/Mg dependent endonuclease [14–17], whereas Zn inhibits the activity of those enzymes [14], which have been mainly researched by protein immunoblotting. However, the acting sites of those trace elements have been estimated indirectly by cell fractionation. There are few reports that image the acting site of the trace elements directly. In the kinetics of Fe, it is well known that the cytochrome C contains the Fe [18,19]. However, the kinetics of Fe in the development of apoptosis have not been studied. In this study, we used micro-PIXE imaging for studying the kinetics of Ca, Fe and Zn in the apoptotic cell. We directly imaged the distribution of Ca, Fe and Zn in one apoptotic cell, enabling us to research the localization of those elements in the cell. In this study, the maximum of Fe accumulation was observed in the cytosol of the apoptotic cell 6–9 h after the irradiation. As the only enzyme linked to apoptosis that contains Fe is cytochrome C, the high Fe accumulation may represent the cytochrome C. It is also considered that the Fe or Fe-containing enzyme is not present in the cell in a diffuse manner; but accumulates in the cytosol as a high point, during apoptosis. Accumulation of Ca in the nucleus was observed 9 and 24 h after irradiation, which suggest increased activity of Ca/ Mg dependent endonucleases, and/or the DNase c. The decrease of zinc in the nucleus in the apoptotic cell may represent the digestion of the backtyping protein of DNA. This study presents the kinetics of Fe, Ca and Zn during the induction of apoptosis. However, further molecular study is required. The kinetics of Fe must be tested to ascertain if it correlates with the cytochrome C releasing from mitochondria to cytosol, or not. The correlation between the Ca kinetics in the nucleus and the activity of the Ca related enzymes: (the Ca/Mg dependent endonuclease and the DNase c) remains unclear. The zinc kinetics should be tested to ascertain whether Zn correlates with the DNA fragmentation, or not. In our laboratory, evaluation of the apoptosis related enzyme activity and the kinetics of trace elements is underway. S. Harada et al. / Nucl. Instr. and Meth. in Phys. Res. B 189 (2002) 437–442 441 Fig. 3. The distribution of Ca, Fe and Zn in the apoptotic cells with time. References [1] J.F.R. Kerr, A.H. Wyllie, A.R. Currie, Br. J. Cancer 26 (1972) 239. [2] J.F.R. Kerr, J. Searle, B.B. Harmon, C.J. Bishop, in: C.S. Potten (Ed.), Perspectives on Mammalian Cell Death, Oxford University Press, New York, 1972, p. 93. [3] E. Duchaud, A. Ridet, D. Stoppa-Lyonett, N. Janin, E. Moustacchi, F. Roselli, Cancer. Res. 56 (1997) 1400. [4] S. Harada, R. Sato, R. Nakamura, H. Oikawa, S. Ohgi, Y. Tamkawa, T. Yanagisawa, Int. J. Radiat. Oncol. Biol. Phys. 48 (2000) 1059. [5] J. Yang, X. Liu, K. Bahalla, C. Naekyun Kim, A.M. Ibrado, J. Cai, T.-I. Peng, D.P. Jones, X. Wang, Nature 275 (1997) 1129. [6] Q. Zao, T. Kondo, A. Noda, Y. Fujiwara, Int. J. Radiat. Biol. 75 (1999) 493. [7] M.M. Chien, K.E. Zahradka, M.K. Newell, J.H. Freed, J. Biol. Chem. 274 (1999) 7059. [8] R. Eskes, B. Antonsso, A. Osen-Sand, S. Monteessuit, C. Richter, R. Sadoul, G. Mazzei, A. Nicholas, J.-C. Martinou, J. Cell Biology 143 (1998) 217. [9] G. Fang, B.S. Chang, C.N. Kim, C. Perkins, C.B. Thompson, K.N. Bhalla, Cancer Res. 58 (1998) 3202. 442 S. Harada et al. / Nucl. Instr. and Meth. in Phys. Res. B 189 (2002) 437–442 [10] A. Takahashi, E.S. Alnemri, Y.A. Lazebnk, T. Fernades Alenmri, G. Litwack, R.D. Moir, R.D. Goldman, G.G. Poirier, S.H. Kaufman, W.C. Earnshaw, Proc. Nat. Acad. Sci. USA 93 (1996) 8395. [11] S. Tamura, D. Shiokawa, Biochem. Biophys. Res. commun. 203 (1994) 789. [12] M.J. Arends, R.G. Morris, A.H. Wyllie, Am. J. Pathol. 136 (1990) 593. [13] B. Zhivotovsky, P. Nicotera, G. Bellomo, K. Hanson, S. Orrenius, Exp. Cell Res. 207 (1993) 163. [14] L.V. Nikonova, I.P. Beletsky, S.R. Umansky, Eur. J. Biochem. 215 (1993) 893. [15] W.H. Str€atling, C. Grade, W. H€ orz, J. Biol. Chem. 259 (1984) 5893. [16] T. Hashida, Y. Tanaka, N. Matsunami, K. Yoshihara, T. Kamiya, Y. Tanigawa, S.S. Koide, J. Biol. Chem. 257 (1982) 13114. [17] R. Ishida, H. Akiyoshi, T. Takahashi, Biochem. Biol. Res. commun. 56 (1974) 703. [18] R. Stryer, in: Glycolysis in Biochemistry, Freeman, New york, 1988, p. 409. [19] B. Alberts, D. Brah, J. Lewis, M. Raff, K. Roberts, J.D. Watson, Molecular Biology of the Cell, Garland Publishing Inc., New York, 1989, p. 489.
© Copyright 2026