Molecular Analysis of T-cell Receptor Repertoire in Bone
Molecular Analysis of T-cell Receptor Repertoire in Bone Marrow
Transplant Recipients: Evidence for Oligoclonal T-cell Expansion in
Graft-Versus-Host Disease Lesions
By Xiping Liu, Vera Chesnokova, Stephen J. Forman, and Don J. Diamond
We have analyzed the T-cell receptor (TCR) Vp repertoire
using polymerase chain reaction (PCR) in a cohort of eight
patients receiving allogeneic bone marrow transplantation
(BMT)from related and unrelated
donors at theCity of Hope.
Results of PCR studies from graft-versus-host disease
(GVHD) skin lesions show a bias in the usage of TCR Vp
families, whereas examination of peripheral blood (PB) withdrawn at thesame time did notreveal a similar phenomenon. In one such family, TCR Vp2 is predominantly expressed in 7 of 7 biopsy specimens examined. Vp2 TCR
expression from these patients was analyzed more extensively using a combination of individual TCR gene cloning,
followed by sequence analysis. We found evidence of oligoclonal expansion of single Vp2-bearing TCRs in GVHD leafter
diagsions, and in the PB of some patients
nosis of GVHD. In contrast, GVHD-negative biopsy samples
showed no evidence for clonotypic TCR amplification. Sequence-specific TCR CDR3 region probes were derived from
analysis of the predominant expressed TCR in GVHD lesions,
and used t o probe Southern blots of amplified Vp2 TCR
mRNA from PB and tissue from BMT recipients and their
respective donors. In most cases the probes are highly specific in detecting TCR expression from GVHD lesions alone,
although in several instances expression could be
detected in PB after GVHD diagnosis. These data provide
supporting evidence for the hypothesis that acute GVHD is
associated with expansion of T-cell clones expressing antigen-specific TCRs that may contribute t o the disease pathology.
0 1996 by The American Societyof Hematology.
A
as a lymphocytic infiltrate at the site of disease.”14 A possible mechanism which underlies the development of GVHD
isthat antigenic differences of the BM recipient willbe
subject to immunologic challenge as a result of donor Tcell recognition of recipient processed antigen as foreign.”,”
This may be especially true in the case of MUD transplant
donors selected through the NMDP (National Marrow Donor
Program), because their only genetic similarity to the BMT
recipient may be within a portion of the major histocompatability complex (MHC) class I and class I1 I O C ~ . ~There
. ’ ~ is
still a strong likelihood that mismatches may arise which are
undetectable using conventional approaches for establishing
MHC identity.IR
Confounding the immunologic analysis of the disease is
the paucity of knowledge concerning the putative antigens,
and the specific lymphoid cells involved in inducing GVHD,
although antigen-specific T cells” or natural killer cells2”
are likely contributors to the pathology of GVHD. An exception to our limited knowledge about which antigens stimulate
GVHD are studies involving the the minor histocompatibility
antigens (MiHA). A growing bodyof biochemical studies
has identified new structural information in the identification
of these antigens.”.** Both in vivo clinical data and in vitro
studies strongly suggest that MiHA differences play a role
in the development of GVHD.’.*’
The increased reliance on unrelated or haploidentical donors for BMT recipients makes it crucial to find means to
limit the pathology caused by GVHD.24An approach to limiting the severity of GVHD, especially among NMDP unrelated donors, was developed over a decade ago and it uses
T-cell depletion (TCD) of the marrow graft to eliminate
mature T cells.2s~Z6
This leads to a reduction in both acute
and chronic GVHD,27although studies throughout the 1980s
and into the early 1990s have shown multiple problems with
this approach.**The problems include graft failure in up to
10% of patients, as well as an increased relapse rate, particularly in patients who have chronic myelogenous leukemia
(CML).27Recently, variations of the original protocol including selective in vitro depletion of the CD8’ T-cell subset
for BMT in CML have shown a benefit in terms of reducing
GVHD, but graft failure still remains higher than in patients
LLOGENEIC bone marrow transplantation (ABMT) has
emerged as a successful therapyin the treatment of
otherwise incurable malignant hematologic disorders.’ It has
been most effective in the treatment of acute and chronic
leukemia and many patients enjoy long-term, disease-free
survival as a result of the success of this therapy.’.’ The
patient who is treated with ABMT undergoes a process of
immune reconstitution over a 6- to 12-month period involving all aspects of the immune ~ y s t e m The
. ~ redevelopment
of immunocompetence can be hampered by acute and
chronic graft-versus-host-disease (GVHD).5.6Despite the use
of prophylactic medications for GVHD, approximately 20%
to 30% of patients receiving matched sibling donor (MSD)
allografts and 75% of those patients receiving matched unrelated donor (MUD) allografts develop graft-versus-host reaction that can lead to increased morbid it^.^.'
GVHD is mediated by T cells that are derived from the
BM graft.’ Studies in animal models have shown that specific
T-cell subsets contribute to the pathology of GVHD,’” although there is no consensus as to the predominate subset
implicated in a particular organ involvement.” Although
GVHD is considered as a disease of allorecognition, the
pathologic profile, especially in skin, resembles a number of
autoimmune diseases. Common to these syndromes is the
function of mature T cells in mediating the pathology, such
From the Department of Hematology and Bone Marrow Transplantation and the Division of Immunology, City of Hope Medical
Center and the Beckman Research Instilute, Duarte, CA 91010.
Submitted August 30, 1995; accepted November 14, 1995.
Supported in part by Grants No. P o l - C A 30206 and CA 33572
from the National Cancer Institute, Department of Health and HUman Services, Bethesda, MD.
Address reprint requests to Don J. Diamond, PhD, Department
of Hematology and BMT, City of Hope Medical Center, 1500 E
Duarte Rd, Duarte, CA 91010.
The publication costsof this article were defrayedin part by page
charge payment. This article must therefore be hereby marked
“advertisement” in accordance with 18 U.S.C. section 1734 solely to
indicate this fact.
0 1996 by The American Society of Hematology.
0006-4971/96/8707-0007$3.00/0
3032
Blood, Vol 87,No 7 (April l ) , 1996: pp 3032-3044
3033
MOLECULAR ANALYSIS OF TCR REPERTOIRE IN GVHD
Peripheral Blood
with unmanipulated
More selective T-cell depletion, with emphasis on those subsets that manifest an aggresPatients had blood drawn at the time of admission (20 mL) to
sive phenotype, might address the problems associated with
establish a reference TCR repertoire before total body irradiation
GVHD without disturbing the beneficial effect of the graft
and cytoreduction. BM donors had blood drawn on the day of BM
harvest (day 0) to establish a reference donor TCR repertoire. We
on preventing relapse. Our approach is to characterize the T
also processed a small amount (1 to 5 mL) of BM from the donor
cells that infiltrate the GVHD lesion to evaluate the potential
on the day of the BM harvest for our TCR studies. Subsequent to
for immunotherapeutic intervention as a means to limit tissue
the transplant on days +21, 35, 49, 63, and day 100 PB (20 mL)
destruction.
was drawn from the BMT recipient. In addition, blood was drawn
Because both human and animal studies implicate mature
again at approximately day 180 for patients who were being tapered
T cells in the development of GVHD, we and others have
off pharmacologic immunosuppression. If the patient developed visiconducted research to better define the types of T or other
ble and/or clinical signs of graft-versus-host reaction and a biopsy
lymphoid cells associated with the disease ~ t a t e . ’ ~ .Our
~ ~ - ~ *was performed on the liver, gut, or skin for the diagnosis of GVHD,
previous phenotypic studies have shown that the majority of
a blood sample was also withdrawn for analysis
lymphocytes infiltrating GVHD lesions of BMT recipients
are CD3+/a/?+T-cell receptor (TCR) containing T lymphoBiopsy
cytes.” This is despite the fact that during the early phases
When there was evidence of clinical GVHD a biopsy was taken
of the hematopoietic reconstitution there are large numbers
from the involved area for study. A 4-mm punch biopsy was taken
of CD3’IyS’ T lymphocytes in the p e r i p h e ~ y . ~In
~ . ’this
~
from the affected skin, and divided into two pieces. One piece was
report, we have extended these studies by examining at the
analyzed morphologically for evidence of clinical disease by staff
molecular level the productively rearranged TCR repertoire
pathologists at the City of Hope, and the other portion was frozen
in reconstituting peripheral blood (PB) and GVHD lesions
in liquid nitrogen (LN2t) and made available for our molecular
of BMT patients. We present evidence for specific T-cell
analyses. In the case of gut biopsies, several needle biopsies were
taken from the affected area, and were subdivided as described for
expansion in GVHD lesions of patients, and in certain cases,
skin. The portion to be used in this study was placed immediately
within the PB at the time of development of GVHD.
MATERIALS AND METHODS
Patients
Patients who received allogeneic BMT at the City of Hope were
eligible for this study, and ranged in age between 15 and 56 years,
with a median age of 33 years. Patient accrual for this study was
initiated in January 1992, and at that time PB specimens were obtained from all patients undergoing ABMT at the City of Hope. As
the frequency and degree of seventy of GVHD is far greater in the
MUD versus the MSD group,3s we restricted the accrual to only
individuals undergoing a MUD transplant in 1993 and 1994. The
group of patients who have been enrolled in our study do not statistically differ in GVHD incidence from the ABMT population treated
between 1992 and 1994 at the City of Hope (data not shown). All
patients received their BM grafts from either an HLA-identical sibling, or an HLA-matched unrelated donor. No patient received a
T-cell-manipulated marrow, and all patients received immunosuppression with cyclosporine A (CSA), methotrexate, with or without
prednisone, after BMT.
Patients With GVHD
Biopsy of skin, gut, or liver was taken for the primary purpose
of clinical diagnosis, and secondarily some material was made available for use in our study. We did not rebiopsy any patient for the
purpose of this study after GVHD treatment was initiated. No biopsy
specimens were taken from sites not suspected to be GVHD involved, because the review of our protocol by the Institutional Review Board (IRB) disallowed it. GVHD grade was established by
microscopic evaluation of biopsy samples, performed by members
of the Department of Hematopathology (City of Hope). The clinical
staging’6 and pathologic grading3’ of GVHD were based on published reports. The clinical signs included skin rash, abnormal liver
function tests, or gastrointestinal dysfunction, which led to a biopsy
of the effected organ. In all patients studied in this report, when
GVHD was diagnosed, the therapeutic dose of immunosuppressive
agent(s) was increased (Table 1). The treatment profile and clinical
characteristics of the eight patients we have analyzed for this report
are summarized in Table 1. At the date of occurrence of GVHD,
patients were still receiving oral CSA, 3 to 5 mgkg.
in saline, andthen frozen in LN2 until processing for molecular
studies. Patients who developed chronic GVHD also had biopsies
performed, which were subdivided for use in clinical diagnosis as
described above, and for analysis by molecular methods. Biopsy
material from some of the patients analyzed in this report were also
sectioned, and analyzed morphologically with the aid oflymphocytespecific monoclonal antibodies (MoAbs).”
Molecular Methods
Processing of PB and BM. Ten to 20 mL of heparinized blood
was drawn from donors and patients at various times, diluted 1:1
with phosphate-buffered saline (PBS), and the peripheral blood lymphocytes (PBLs) isolated by centrifugation through Ficoll-HyPaque
(Pharmacia, Piscataway, NJ). In the case of the donor, at the time
ofBM harvesting, we obtained the excess BM cells in which the
mononuclear cells were previously separated by Ficoll-HyPaque
centrifugation. In some cases, we obtained donor BM from the
NMDP. The equivalent of 1 to 5 mL of BM was generally available
for RNA processing.
Preparation of RNA and cDNA. The interface layer containing
PBLs was isolated and washed twice with PBS. RNA was prepared
using a modification of the method of Chomczynski and S a ~ c h i ~ ~
using the TRIzol (Bethesda Research Laboratories [BRL], Gaithersberg, MD) reagent. Total RNA was also isolated from biopsy specimens. Biopsy specimens (skin, gut, or stomach) were placed in
isotonic saline immediately after the procedure, then quickly frozen
in LN2, and stored until used at -80°C. The frozen tissue was placed
in 1 mL of TRIzol (BRL) reagent in a microcentrifuge tube, and then
homogenized in situ for 1 minute using a Tissue-Tearor (Biospec
Products). Total RNA was then isolated using the same method as
for PBLs described above, and the absorbance monitored at 260 and
280 nm. Typically, we recovered 5 to 10 pg of RNA per skin biopsy
specimen, and 20 to 50 pg from gut biopsy samples. cDNA from
both PBLs and tissue was generated with 2 pg RNA using 5 U of
avian myeloblastosis virus (AMV) reverse transcriptase (RT) in a
50-pL reaction at 42°C for 20 minutes, that also contained as a
primer oligo dT12.1x
and deoxyribonucleotides at 250 pmoVL each.
Fifty microliters of ddH20 was added, the sample boiled for 3 minutes, spun out, and the supernatant was used for polymerase chain
3034
LIU ET AL
Table 1. Summary of Patient Characteristics
~
Description
UPN 1
+
Disease
768
CML
789
AML
916
ALL
2081
CML
2085
CML
2093
CML
2094
CML
2098
CMMoL
Donor
Type
HLA Type
Preparative Regimen
A:33, 814, B35.
DR3.
Busulfan (4 mglkg) + CTX
DR4
(60 mglkg)
MSD
A3,
A26,87,835, FTBl
DR1,
1,320 c G +~ VP-16
DRwl5
(60 mglkg)
MSD *A2. A26.87. Bw57.
FTBl
1,320
cGy
+ VP-l6
DR4, DRwll
(60 mglkg)
MUD A l . A3, 88. Bw57.
DR3,
FTBl
1,320
cGy
+ CTX (60
DR7
mg/kg)
A3,
MSD
M U D A3, All, B18,
B35.
DR7,
FTBl
1,320 cGy
DR17
mglkg)
M U D A2, A3, 67, Bw57,
DR7,
FTBl 1,320cGy
DR11
mglkg)
M U D A2, A24, 844, Bw58,
FTBl
1,320
cGy
DR13 DRl1,
mdkg)
M U D A2, A26,
Bw41,
Bw62,
FTBl
1,320
cGy
DR4
mglkg)
Date of
Post-BMT GVHD Prophylaxis Transplant
CsA
+ MTX + MPSE
9/29/92
CSA
+ MTX
1/12/93
CsA
+ MTX + MPSE
10/4/94
GVHD SkinBiopsy (d)
Grade Posttransplant
II
122t
II
371
II
-
CsA
+ anti-Tac + MTX
6/15/94
II
-
+ CTX (60
CsA
+ MTX + MPSE
7/13/94
+ CTX (60
CsA
+ anti-Tac + MTX
9/19/94
CsA
+ MTX + MPSE
+ CTX (60
CsA
+ MTX
32
109
37
74
183 (C)
157
-
35
79 (St)
11/7/94
II
42
11/18/94
-
82
+ MPSE
+ CTX (60
II
~~
Ill
Donors for BMT recipients are either siblings who are matched at major HLA loci (MSD) or unrelated donors selected to be matched as
closely as possible, and identically at HLA A, B, and DR loci (MUD). The pathologic grade of GVHD is noted as determined by the Department
of Hematopathology using standard criteria, with (-) signifying no pathologic evidence of GVHD.“ The days (d) after transplant refers to the
date of the biopsy procedure. The preparative regimen is shown, and for 718 patients included FTBl (fractionated total body irradiation), and/
or busulfan, CTX (cyclophosphamide), and VP-16 (etoposide) at the indicated dosages. Every ABMT patient undergoes prophylaxis for GVHD,
and receives a combination of CsA (50-250 mglkg),MTX (methotrexate), MPSE (methyl-prednisolone).
Abbreviations: C, colon; St, stomach.
* Patient 916 was mismatched and heterozygous (A2IA26 vA2/A2) at the HLA A locus versus the donor.
t Biopsied skin was unavailable for molecular analysis from patients UPN 768 and 789. Confirmed diagnosis of GVHD led to increases in
dosage of CsA and MPSE given to the patient after biopsy was completed.
reaction (PCR) analysis. The quality of the RNAwas evaluated
using an oligonucleotide pair which detects a 661-bp fragment of
the human P-actin cDNA’~(data not shown). If a band could be
detected after 35 cycles, subsequently all 26 V0 gene oligonucleotides were tested (Table 2).
PCR conditions and quanritation. For Vp repertoire studies, 1 of
26 5’ VD-specific primers (Table 2), andan antisense oligonucleotide
(Cpl,2) that is complementary to both Col and Co2 genes,40 were
incubated (5 pmol each) with 5.0 pL cDNA in a 50-pL reaction
volume. To that was added MgCI2to1.5 mmol/L, 200 pmol/L
dXTPs, and 3 U ofthe Stoffel fragment4’ of Taq polymerase in
standard reaction buffer for the enzyme (Perkin Elmer Cetus, Norwalk, CT) . The Vp PCR products were approximately 350 bp (Table
2). As an internal control for Vp gene segment synthesis, each
reaction contained 5’ and 3‘ C a primers which amplify a 591-bp
fragment (CO nucleotides 7 to 598). Theoligonucleotides were synthesized with an Applied Biosystems 391 DNA synthesizer (Applied
Biosystems, Foster City, CA) and purified by solid-phase extraction
on a Sep-PakTMCIRcartridge (Waters, Milford, MA).
Amplification steps for cDNA were performed as follows: “hot
start” at 80°C for 10 minutes, followed by 35 3-step cycles of 94°C
for 25 seconds, 53°C for 25 seconds, and 75°C for 25 seconds. A
final one-step cycle of 72°C for 7 minutes was used. All PCR reactions were performed using the GeneAmp 9600 (ABI-Perkin-Elmer,
Norwalk, CT). For purposes of quantitation, 10pCi of (a-32P)
dCTP+dATP was added to each reaction. Radiolabeled products ( l /
5th of reaction) were separated on 1.3% agarose (Seakem L E FMC
Bioproducts, Rockland, ME) gels, dried under vacuum, and then exposed to a phosphor screen (Molecular Dynamics, Sunnyvale, CA).
Quantitation was accomplished using ImageQuant software after
scanning the screen with the Phosphorimager (Molecular Dynamics).
Quantitative aspects of TCR repertoire analysis using PCR. To
substantiate the reliability and reproducibility of the PCR studies
that are an integral part of this report, we conducted a series of
titration and validation studies of TCR product made from PB of
normal volunteers. Test reactions were performed to determine the
maximum number of PCR cycles that would still cause the linear
incorporation of 32Pnucleotide triphosphate into double-strand DNA
product. Standard reaction conditions as described above were used,
except that the cycle number was varied between 15 and 45repetitive
cycles. Two pairs of oligonucleotides which respectively amplify
the C a (591 bp) fragment and V(D)JC fragment from the rearranged
Vp (360 bp) TCR (vp2,7<pl,z , Table 2) were co-incubated in
our standard reaction mix. Since the incorporation of 32Pnucleotide
triphosphate plateaued at 40 cycles for C a synthesis, we chose to
use 35 cycles in all further PCR. Interestingly, both Vp2 and VD7
synthesis did not plateau, even at 45 cycles (data not shown).
We also determined the amount of oligo-dT primed cDNA that
would give an adequate signal, without saturation of the available
enzyme in the PCR. cDNA was made from PBL RNA as described
above. Two pairs of oligonucleotides were added, as described for
the above experiment, and 35 cycles of synthesis were performed
in the GeneAmp 9600, with the cycles times and temperatures as
described above. Five microliters of cDNA results in a level of
32Pnucleotide triphosphate incorporation in double-stranded TCR
product that is linearly dependent with the amount of input cDNA
(data not shown). That volume of input cDNA has been used in
PCR reactions for our subsequent repertoire studies.
Molecular cloning and sequence analysis. PCR amplification
products from patients and blood donors at various time points were
purified from 1.3% agarose separating gels electrophoresed in TAE
(Tris-Acetate-EDTA, pH 7.5) with the help of glass beads (GeneClean 11; B10 101, La Jolla, CA). The fragments which contain
ragged ends (terminal 3’ dATP addition) as aresult of their synthesis
using modified Taq polymerase (Stoffel Fragment) were cloned into
a modifiedpGEM vector (Promega, Madison, WI) containing 3’
MOLECULAR ANALYSIS
OF
TCRREPERTOIRE IN
3035
GVHD
Table 2. Oligonucleotides UsedTO Detect TCR Va
and Ca Gene Segments
V, Gene
5’ Nucleotide Sequence 3‘
1
GCACAACAGTTCCCTGA
TCATCAACCATGCAAGCC
GTCTCTAGAGAGAAGAAG
ACATATGAGAGTGGATITGT
ATACTTCAGTGAGACACAG
TKCCTAACTATAGCTCTG
AGGCCTGAGGGATCCG
CCTGAATGCCCCAACAG
ATITACITAACAACAACGTT
CCTAAATCTCCAGACAAAG
CTCCAAAAACTCATCCTGT
TCAACAGTCTCCAGAATAA
AAAGGAGAAGTCTCAGAT
CAAGGAGAAGTCCCCAAT
GGTGAGGGTACAACTGC
GTCTCTCGAAAAGAGAAGA
AGTGTCTCTCGACAGGC
AAAGAGTCTMCAGGATGA
CAGATAGTAMTGACTITCA
GATGAGTCAGGAATGCCA
CAATGCCCCAAGAACGC
AGCTCTGAGGTGCCCCAGAA~C~C
TCCAACCTGCAAGGCTTGACGACT
AAGTGATCTTGCGCfGTGTCCCCA
GCAGGGTCCAGGTCAGGACCCCCA
CCCAGITGGAAAGCCAGTGACCC
CACCTCCTTCCCATKAC
AACAAGGTGTTCCCACCCGAGGTCG
CCAGAACCCTGACCCTGCCGTG
ATCATAAATTCGGGTAGGATCC
2
3
4
5.1
5.2
6.1
7
8
9
10
11
12
13.1
13.2
14
15
16
17
18
19
20
W21
W22
w23
w24
CP3.2
C&
Ca5‘
Ca3‘
Fragment (bp)
360
360
360
370
380
350
350
355
390
355
355
350
370
370
380
360
360
380
380
360
310
360
360
360
360
360
59 1
591
PCR primers from 26 V@ families are shown. The oligonucleotides
Vp1-195p and V ~ ~ Z O - W Zare
~ ~ ’modifications from published sequences. c&, c&. Cas’,and Ca3’ were designed by Liu et al.= The
fragment sizes are denoted in nucleotides (bp) based on comparison
with the 100-bp DNA Ladder (GIBCO-BRL).
terminal thymidines at both ends (pGEM-T). Recombinants in
JMI09 bacteria were identified using ,%galactosidase screening on
X-gal plates with isopropyl-0-D-thiogalactoside (IPTG). The plasmids containing inserts were separately grown as 10-mL cultures,
and plasmid DNA prepared from them by the alkaline lysis method
with an added RNAse digestion step (1 p@mL for 60 minutes at
37°C). Sequence analysis was initially performed using complementary primers for the Sp6 or T7 promoter sites flanking the cloning
sites. Further sequence information was obtained using antisense
primers to CB or 17- to 20-bp primers complementary to sequences
unique to individual TCRs. Sequencing was performed using denatured double-stranded plasmid DNA with a deaza-GTP containing
Sequenase Version 2.0 kit (USB, Cleveland, OH) and ”S-dATP
(ICN, Costa Mesa, CA). Gene segments were identified by comparison with the Genbank database using IGSUITE (Intelligenetics, Palo
Alto, CA).
Hybridization of oligomer probes to VB-specific PCR products.
Ten microliters from the 50-pL PCR reaction was size-fractionated
on a 1.3% agarose gel, and the bands localized by staining with
ethidium bromide ( 1 pg/mL). After electrophoresis, the gel was
denatured for 30 minutes in 1.5 m o m NaCV0.5 m o m NaOH, neutralized for 30 minutes in 3.0 mom NaCV0.5 mol& Tris-HCI, pH
7.5, and then transferred to nylon membranes in 2OX SSC (3.0 mol/
L sodium chloride, 0.3 moVL sodium citrate, pH 7.0)by capillary
force for 16 hours. The DNA was fixed to the filter by UV illumina-
tion (Stratalinker; Stratagene, La Jolla, CA). The membrane was
prehybridized for 3 hours at 50°C in a solution containing 1% sodium
dodecyl sulfate (SDS), 6x SSPE (1 X SSPE: 180 mmol/L NaCI, 10
m o Y L NaP04, pH 7.7, 1 mmovL EDTA), and 1OX Denhardt’s
solution (0.1%bovine serum albumin, 0.1 % polyvinylpyrrolidone,
0.1% Ficoll), 50 p g / d denatured salmon sperm DNA, and 20 @g/
mL tRNA.
Oligonucleotides were labeled at the 5’ end by phosphorylation
with T4 polynucleotide kinase and { y-”Pj ATP (see Table 5 ) . The
labeled probe was separated from unreacted ATP by passage over
a NENSORB 20 Nucleic Acid Purification Cartridge (Du Pont-NEN,
Boston, MA). Hybridization was performed in 1.0% SDS and 6x
SSPE at the TM of the DNA-hybrid for 16 hours. The filters were
washed three times for 15 minutes in 6x SSPE and 1.0% SDS at
20”C, followed by a single wash-step in 1X SSPWl.O% SDS at the
TMof the hybrid for 30 minutes. The filters were exposed to Kodak
X-Omat AR film (Eastman Kodak, Rochester, NY). Quantitation
was accomplished using ImageQuant software after scanning the
screen which was exposed to the radioactive filter with the Phosphorimager (Molecular Dynamics).
RESULTS
V0 Family Usage in Eight BMT Recipients
We identified 8 patients for which RNA had beensuccessfully prepared from PB at the appropriate intervals indicated
in Materials and Methods, and in 6 of 8 patients at least one
skin biopsy specimen was available in which RNA had also
been successfully prepared. In addition, RNA was prepared
from a PB sample taken on, or a few days before, a skin or
gut biopsy specimen was taken on suspicion of GVHD (Tables l and 3). Clinical information for these patients is shown
in Table 1. Five of eight patients had MUD donors, 3 had
MSD donors, and in 1 MSD case the recipient was m i s matched at the HLA A locus with respect to the donor (A2/
A26 v A2/A2, Table 1). Inallbut three instances, microscopic evaluation of skin biopsy samples showed them to
beinfiltrated with lymphocytes characteristic of GVHDi9
(and data not shown). We also prepared RNA from colon and
stomach biopsy specimens from patients who had previous
GVHD positive skin biopsies (Table 1).
Results from the Vp repertoire studies from PB are shown
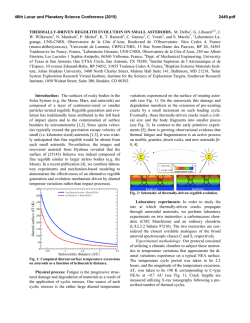
in Fig 1. The nomaiized percentage of expression for each
V,8 family is tabulated and presented underneath an example
of a V 0 TCR profile from one analyzed patient (UPN 916,
d32). Most V@ have expression levels that vary less than a
few fold between patient samples, and there is little evidence
for bias in expression of individual family members within a
sample. The levels of expression of most Vps in the samples
shown in Fig 1 compare well with published analyses and
our determinations from normal individuals4*(and data not
shown). The importance of these results is the contrast they
provide to our observations of V@ family usage in skin biopsies. The results of the repertoire studies in biopsied skin are
shown in Fig 2. A remarkable aspectof the data is the radically
different profile of usagein skin versus P 8 sampledat the
same time (Figs 1 and 2). in contrast to the results with PB,
among 26 VP families examined,only 1 family is consistently
overexpressed in all of the biopsied skin samples tested.
The VD2 family shows predominant expression in biopsied
skin, whether or not it is frompositivelyidentified GVHD
lesions (Fig 2), although the absolute levels of
Vp2 TCR RNA
LIU ET AL
3036
Sample
-
Table 3. Time Points ofPeripheral Blood Samples From the Eight Patients
Discussed
in This Report Analyzed by PCR and Southern Hybridization
UPNl
768
789
916
2081
2085
2093
2094
2098
PER
BM
421-34
X
X
X
X
X
X
635-48
449-62
463-99
d100-dl79
X
X
X
d180-d364
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
1Y
2Y
X
X
Died
Died
Died
X
The numbers following the d(day) refer to days posttransplant. 1Y (lyear) and 2Y (2 year) were samples collected from a single patient (no.
768). Peripheral blood samples were taken pretransplant from the recipient (PER), and a portion of the bone marrow from the donor (BM).
A
I
12
l1 I O
9
eO.1
4.5
6.5
8
I
Vs Family #
7
6
5.2
5.1
4
3
2
1
Flpi
+ d32 1
+
+
6 I 15.8
120811
d157 120851
d74
+ d35
+ d42
eO.1
17.4 eO.1
eO.17.9
6.5
6.3
2.8
5.8
12.4
0.4
eO.1
3.7
6.0
2.3
2.3
1.4
1.7
2.1
16.2 eO.1
eO.1
2.7
3.1
m
m
I
4.1 5.39.6
5.0
0.65.66.1
5.5
3.4
eO.1
I
cO.1
4.8
4.0
6.6
1.7
4.3
2.3
3.5
5.0
3.3
1.8
p.0 cO.1 eO.1
3.1
2.3
4.2
4.3
3.4
1.6
2.6
- d82 12098116.6
~ 0 . 1 7.9
9.0
I
I
I
I
9.4
eO.1
8.7
eO.1 eO.1 11.7
3.8
5.2
8.7
3.6
1.7
1.7
18.0
14.7
B
+
d74
12081]10.1
0.9
2.2
1.1
3.7
1.7
4.8
1.6
4.0
1.5
+
dlS7
m
2.0
2.9
4.0
18.2
2.2
+
11.6
1.5 5.3
4.9
3.1
0.3
0.8
4.4
I
0.9
7.2
17.3
11.8
d35
3.5
0.4
4.5
1.3
0.3
5.8
5.1
22.1
6.1
4.9
3.1
12.0
+ d42
12.3
- d82 12098j10.5
2.2
1.5
0.8
2.0
0.3
0.2
1.926.1
6.5
0.3
0.4
1.8
0.5
3.2
3.3
7.6
CO.l
0.9
0.3
0.7
2.3
14.5
are lower in biopsies uninvolved with GVHD (data notshown).
The Vp2 gene segment is representedin PB atthe level of
7.0% to 14.0%in normal individuals, although in skin there is
no evidence of its preferential usage in a previous study."?Vpl
1
Fig 1. Southern hybridization
analysis of PCR products from
PB. (A) Results using Vpl-12. PB
was analyzed for expression of
Vp TCR familiesonthe
same
date as the biopsy sample was
takenfromtheindicated
patients. The presence of GVHD
wasconfirmedbythe
Department of Pathology, and is indicated by a plus or minus symbol
and the number of days postBMT of occurrence (Table 1).
Techniques of PCR using simultaneous dual amplificationof Vp
and C a fragments are described
in Materials and Methods. Fragments were separated on 1.30/0
agarose gels and transferred by
capillary action t o nylon as described in Materials and Methods. To detect the expression
of the Vp-Cp fragments, 32Plabeled oligonucleotide
CpM
(Table 2) was
hybridized
to
the membrane-bound doublestranded DNA PCR product. The
normalized percentages for each
Vp family are indicated, and for
patient 916, the gel bands are
shown. Data analysis was done
as described in Materials and
Methods. (B) Results using
Vp13.1-24 same as(AI, except
the Vp families examined are
13.1-24. Vp2 as a reference fragment wasgenerated at thesame
time as Vp13.1-24.
is the only other family that showed consistently moderately
high expression in skin biopsies, although its expression is far
less than Vp2. As expected, other Vp families are also expressed in skin, although there is no consistency inthe level
MOLECULAR ANALYSIS OF TCR REPERTOIRE IN GVHD
3037
A
1
9
13.1
101112
Fig 2. Southern hybridization
analysis of PCR products from
skin
biopsy
specimens. (AI
Shown are the results for testing
the expression of Vpl-12 from
skin biopsy R N A using PCR analysis as described in Fig 1. GVHDpositive biopsy samples are distinguished by a plus symbol l+)
and the two GVHD-negative biopsy samples are indicated by a
minus symbol (-1, next to the
U P N number and the days postBMT of occurrence. Methods for
preparation of RNA from biopsy
specimens are described in Materials and Methods. Hybridization to Southern blots waswith
CPM (Table 2). and the visualization and quantitation of radioactive bands was done as
described in Materials and Methods and Fig 1. R N A from each
biopsy specimen was analyzed
for expression of actin mRNA. t o
normalize expression of Vp TCR
families(data not shown). (B1
Analysis of Vp13.1-24: same as
(AI, except that the Vp oligonucleotides used are V/513.1-24. As
a comparison for relative band
intensities, the Vp2 family was
amplified at thesame time (data
not shown).
+ d32
m
+ d74
120811
+ dl57
+ d35
120851
120931
+ d42
m
1 1.0 CO.1
I cO.1
0.3
cO.1
m
m
I
-dl09
-d82
1
cu.1 0.5
cu.1
CO.1
CO.1
cO.1 C0.1
cO.1
1.9
CO.l
CO.1
CO.1
1.0
I
~ 0 . 1 cO.1
23
66.3
6.5
CO.1 CO.1
~ 0 . 1 15.4
CO.1
1
6 2 5.2
3 45.1
7
8
cu.1 CO.1
[cO.1 cO.1
cO.1
4.0
CO.1
I
Vfi Family #
11.3
CO.l
1.1 3.6
0.8
CO.1 CO.1 cO.1
1.1
1.5
CO.l
CO.l
72.3
14.7
2.5 ~ 0 . 1 50.8
9.8
I
1.1
I
57.8
4.0
I
~0.1 cO.1 ~ 0 . 1 cO.1
cO.1
0.8
3.4
0.3
77.5
13.9
CO.1
CO.1
CO.1 CO.1 CO.1 CO.1
CO.1
CO.l
I
1.8
0.4
95.5
2.0
B
21
22
23
24
+ d32
m
+ dl57
120811
120851
+ d35
120931
+ d42
L204
+ d74
I
1 co.1
CO.1 0.2
cO.1
c0.1
cO.1
cO.1
1co.1
CU:I
co.1 co.1
co.1
W
:
I
co.1 co.1
co.1
crrld
19 17
18
Riopsy
The striking overcxpression of the V02 gene segment in
the biopsy samples that we examined led us to determine
whether the mixedpopulationofVD2-bearing
TCRs had
individual members that werespecificallyamplified in the
GVHD lesion.Combinatorialmultiplicationfollowingthe
I
CO.1 CO.1
0.8
0.2
co.1 co.1
co.1
co.1
co.1
co.1
co.1
co.1
co.11
CO.1 ~ 0 . 1CO.1
CO.1
~ 0 . 1 c0.1 29.7
~ 0 . 1~ 0 . 1 CO.1
~ 0 . 1 CO.1
co.1 co.1
co.1
co.1 co.1
cu.1 cu.1 qu.1
co.1
16 13.2
13.1
14
15
C0.1
0.9
cO.1
1.2
~ 0 . 1cO.1
3.9
2.3
of expression of the other VD family members in our cohort
of patient skin biopsy samples. VD4 has significant expression
in some cases. a s do V07 and VD I8 in isolated cases (Fig 2A
and B). Since in none o f the C;ISCS that we have tested was
there a VD limlily that superseded V02 expression in biopsied
skin, we pursued a more detailed look at the individual TCR
composition of the V02 Family in individual patient tissue and
PR samples by sequence analysis.
Seqrrcwcv Atwlysis of' VD2 E.vprcwion iu P R
Sp"'if,lc~rl.s
CO.l
co.1 co.1 co.1
I cO.1
cO.1
I
-dl09
0.3
20
U.Z
co.1 co.1
co.1
0.J
CU.l
co.1
co.1
CU.1
CU.1
co.1 co.1
co.1
CO.1 0.6
CO.1
co.1 co.1
0.3 CO.1
I
I
I
I
co.1
co.1
co.1
rules of association of V, D. and J genesegments and
allowing for the generation of diversity by N nucleotides at
-IO1:
the V-D and D-J joins results in thecalculationof
potentially different VD TCRS.'~"Therefore. examination of
a small number of VD TCR chains by sequence analysis in
PB or the lymphocytic infiltrate of a GVHD lesion without
preferential expansion of a specific T-cell clone(s) should
result in a random assortment of joining sequences without
duplication of any one sequence. We tested that proposition
by subcloning the VP2-containing fragments that were generated by PCR methods from our series of biopsiedskin
lesions. of which tive were positive and two were negative
for GVHD (Fig 2). In addition, WC cloned VD2 PCR fragments from several PB samples from patients 768 and 789
of' whom we did not have a biopsy specimen (Table 3). The
fragments were cloned withoutmoditication into the "T"
3038
LIU ET AL
vector (Promega), individual bacterial clones were picked,
and inserts were partially sequenced so as to identify the Vp
family as Vp2, and to characterize the joining region.
A
Sequence Analysis of Vp2 TCR Joining Segments From
Skin Biopsy Specimens
B
Approximately 1 0 0 bp located upstream of the CO junction with the J segment was sequenced using the dideoxy
technique. This stretch of sequence includes the V(D)J joining segment and enough of the V gene to unambiguously
identify it as V02 (Fig 3 for an illustration and Fig 4).
Sequences were generated from 6 individuals, 5 of whom
developed clinically definable GVHD. In 5 of 7 biopsy samples it was possible to determine a TCR sequence represented
multiple times from independent plasmid clones (Fig 4A and
Tables 4 and 5 ) . Further sequence analysis of each of these
amplified TCRs showed them to be examples of productively
rearranged TCRs with in-frame protein coding sequences
(data not shown). In the two cases where we were unable
to detect multiple copies of a single TCR sequence, there
was a negative diagnosis for GVHD [916 (-) and 2098, Fig
4A and Table 41. In those cases, every independent Vp2
TCR plasmid clone that was sequenced had a unique primary
structure (Table 4). We have recently sequenced multiple
V02 TCRs from three additional biopsy specimens that were
negative for GVHD, and we have not found amplified TCR
sequences (data not shown). Similarly, in those patient samples in which we derived a predominant TCR sequence, all
of the other TCR sequences in the sample were also unique
(Table 4). Despite the presence of multiple TCRs containing
the identical JP in a sample, the joining segment was unique
at multiple nucleotides, precluding the derivation of closely
related TCRs from the same cell. The multiple differences
also made it improbable that errors caused by infidelity of
either the Stoffel fragment of Taq polymerase or the Sequenase I1 version of the "7 polymerase were responsible for
generating the diversity.
Vp2 Sequence Diversity in PB Before and After BMT
Previously, we had conducted PCR analysis of the TCR
VD repertoires from the PB samples indicated in Table 3.
We found little or no difference in the expression levels of
Vp2 as well as the other V@ family members in samples
withdrawn before and after transplant, or simultaneously
with GVHD diagnosis (data not shown). To address whether
clonal diversity or oligoclonality existed in those samples,
an approximately equal number of Vp2 TCRs were sequenced from the BMT donor, the recipient pretransplant
and the day of GVHD diagnosis, as well as on a date postGVHD treatment (day 188 for UPN 789, Fig 4 9 and 2Y for
unique patient number [UPN] 768, see below). All of the
TCRs were of unique sequence for patient 768, except for
a single class of TCRs, which are highlighted in Fig 4B. An
equivalent situation was found with patient 789 on day 35,
although by day 188 in which the GVHD had resolved, we
found no sequences that matched the unique TCR found on
day 35 (Fig 4B). The significance of these results for the
development of GVHD is that they suggest that a small
number, possibly one clone, of T cells became amplified
L V p l L Vp2 LVg3
LVpn
Dpl
Jp1.1-6
n u o n n o o n mmmemne
1
Cp2
/.
I
(N regiondiversification)
RNA Transcrlptlon and Processing
IPrimers
Probe
Jp2.1-7
1mmmmmn
0m / / L L e n t
4
m
C
Dp2
Cpl
x?
4
ISObpl
~~
\VD2 NDpN
I l JB2.3
l I
~
CP2
Fig 3. Diversification of TCRs by combinatorial association of V,
D, and J segments. (A) The chromosomal locus of the TCR p gene is
shown at the top of the figure which depicts the arrangement of
gene segments as they are aligned on the
chromosome?'The arrows
originate fromsegments that werejuxtaposed t o create the TCR that
was found t o be highly expressed during GVHD in patient 768. The
selection of segments could apply t o any of the Vp2 TCRs found in
patient blood or skinlesions described in this report (see Fig 4). (B)
The gene segments which form the mature
TCR become juxtaposed
in chromosomal DNA from individualT cells. Between the ends of the
V (3'), (5') D (3'), and (5') J segments are inserted nonchromosomal
nucleotides (NI that markedly increase the diversity of TCRs. The
ends of the gene segments that receive N nucleotide additionsalso
have deletions of chromosomally
encoded DNA. The rearranged DNA
is transcribed into
nuclear RNA which isprocessed to form the
cytoplasmic mRNA that encodes the productivelyrearranged TCR p protein (see ref 44 for more details). (C) The expanded structure (not t o
scale) of the mature RNA depicted in (B) is shown with references
t o the gene segments and positions of
N nucleotide insertions which
form thecomplete sequence. Cloned PCR products whosesequences
are shown in Fig 4 were generated using the primers shown in (Cl.
The CDR3 sequences (Table 5) derived from data presented in Fig 4
are complementary t o the region of the
TCR underneath the "probe"
designation. PCR fragments from PB and skin (Fig 5A) were made
using the primers depicted
in (C).
within the GVHD lesion, and in some cases the progeny of
this clone as represented by the identical TCR could be
detected in the PB. However, sequencing a small number of
TCR mRNAs is not a sensitive enough technique to preclude
that a given clonotypic TCR is not expressed. That possibility could be approached by sequencing a greater poolof
plasmids, or switching to a different method of analysis that
isuniquely suited to the polymorphism of TCR gene sequences?
Detection of Specijc Vp2 TCR Using
Probe
CI
CDR3 Region
Our sequencing studies served an additional purpose by
providing the primary structure of the conserved TCR sequence in GVHD skin lesions. These data assisted in the
design of a probe that was potentially specific for a single
rearranged TCR clonotypic sequence. We hypothesized that
using sequence information from the most variable portion
of the TCR between the V segment and the J segment would
result in a unique sequence TCR probe.& Therefore, we
synthesized a series of 20- to 23-bp probes that werecomplementary to the unique joining regionofthepredominant
TCR as determined by sequencing studies from 7 of our
ANALYSIS
MOLECULAR
REPERTOIRE OF TCR
IN GVHD
3039
r
UPN # & Skin Biopsy Date
916(-)
dl09
2081
d74
2085
dl57
2093
d35
2094
642
2098
d82
0
0
0
0
0
0
0
0
2
0
3
0
1
3
0
0
0
0
0
0
0
916(+)
d32
W*
0
P
2
0
0
0
8
Y
3
l:?.l"
3
0
0
0
2
0
2
0
1
0
O
T
O
Z
0
0
l
0
0
0
2
1
3
1
1
2
3
0
0
1
2
0
1
1
0
0
1
0
0
l
0
B'
0
1
2
0
1
0
0
1
1
e
3
2
S
1
3
0
1
0
l
l
0
0
0
O
b
2
0
2
1
3
1
3
2
0
5
4
7
4
4
2
5
1
s
Fig 4. TCR Vp2 nucleotide sequences from GVHD skin lesions. (A) Fifty- to 100-ng aliquots of cDNA were selectively amplified using the
Vp2
pair of oligonucleotideprimers into double-strandedfragments. As fully described in Materials and Methods, the 360-bp fragments
(Table 2) were subcloned into the pGEM "T" vector, and individual bacterial colonies sequenced. The nucleotide sequence was determined
between the 5' end of Cp through the last 50 bp of the 3' end of the Vp2 gene segment. Gene segments were identified by comparison with
publishedsequences,"and
those deposited in the GENBANK database accessed through IGSUITE (Intelligenetics Inc). Patient data are
described in Table 1. Patient 916 had two biopsies (see Fig 2 and Table 11. Highlighted and starred numbers represent TCR molecules which
were identical by sequence analysis from independent bacterial clones. All other sequenced TCR molecules had unique primary structures
determined by sequence analysis. (B) Methods for PCR generation of fragments, subcloning, and sequence analysis are the same as in (A).
Samples are indicated at the top of the table, and correspond to those shown in Table 3.
PCR analyzed patients (Fig SA and Table S). To conserve
RNA, we turned to the PCR approach and combined it with
Southern hybridization analysis. Samples of RNA from PB
and biopsy tissue were converted into Vp2 fragments using
a single primer pair as shown in Table 2 (VD2 and C@,,,)
as previously demonstrated in our repertoire studies (Figs 2
and 3). The PCR generated fragments were electrophoresed
onto agarose gels. and the DNA transferred to nylon, followed by UV treatment to covalently attach the DNA.Oligonucleotide probes (Table S) were kinased with (y-?'P} ATP,
andthen hybridized totheboundDNAfrom
a series of
samples of PB and skin biopsies for each of 7 patients who
were diagnosed with GVHD (Fig SA).
The hybridization profiles from patients for which we had
biopsy samples are relevant to our central hypothesis of
the role of TCR amplification and GVHD pathology. The
hybridization profiles of the S patients withbiopsy data
shown in Fig SA are consistent with the findings from our
sequence studies indicating overexpression of a unique TCR
sequence. In S of S patients whose skin biopsy specimens
were shown to have a unique predominant TCR sequence,
there was strong hybridization to the lane containing RNA
from GVHD skin lesions (Fig SA). It is also of interest that
CDR3 sequences determined fromamplified PBL T cells
were also selective in their hybridization to PBL RNA taken
only from timepoints when the patient had GVHD (Fig 5A).
UPN 2081 illustrates the selectivity of the CDR3 probes.
This patient had multiple biopsies, although a defined amplified rearranged TCR was only found in the GVHD positive
skin biopsy sample. The skin biopsy specimen on day 37 as
well as the colon biopsy sample taken on day 183 were both
determined to be negative for GVHD by pathologic study,
which isconsistent with the absence of a hybridization signal
from the probe derived from skin on day 73. It is also noteworthy that nodetectable signal from the probe derived from
the day 73 skin biopsy can be found in PB from the donor,
or the recipient beforeand after transplantation (Fig SA).
One possible conclusion from this exclusivity in expression
is that the T-cell population bearing this TCR expanded at
the site of injury and not in the general peripheralcirculation.
The other 4 patients exhibit patterns that are similar in specificity as the one shown for patient UPN 208 I , yet each have
unique aspects. Patients UPN 916, 2093,and2094
have
strong expression of a unique TCR in the GVHD skin lesion,
and in PB from the recipient withdrawn at the same time or
subsequent to the diagnosis of GVHD (Fig SA). A stomach
biopsy sample takenfrom UPN 2093.which showed no
GVHD pathology upon microscopic analysis, also showed
no hybridization totheday
35 skinprobe,which
further
illustrates the specificity of the CDR3 probes.
Weperformed additional sequence studies of samples
from patient 2093, which are presented in Table 4,and are
informative in comparison with data shown in Fig SA. TCR
sequences from V02 atd3S were all uniquewithoutany
concordance withtheTCRpopulation
in theskinbiopsy
specimen. Sequences determined from the PB at d63 in two
examples are identical to the TCRs whose sequence we determined in the GVHD lesion at day 35. The hybridization
profile for this patient shows a strong signal in the day 35
skin lesion, butnot concurrently in the PBat day 35. In
agreement with our limited sequence data from day 63 is
the presence of a strong hybridization signal from PB initiating at day 63 of the Southern blot profile. This concordance
in the hybridization profileand our limited sequence data
frompatient 2093 suggests that strong hybridizationfrom
skin or blood samples with a unique sequence probe may
be predictive for the occurrence or continuation of the pathology of GVHD.
There were no pre-BMT donor or recipient PB samples
to compare hybridization signals for UPN 916; however, the
intense signal on day 32 in skin and blood disappears from
further PB specimens taken after resolution of GVHD. In
3040
LIU ET AL
Table 4. Summary of the Sequence AnalysisIndicating Semples
That Have TCR Sequences Repeated Two or More Times
UPN and Sample
Description
No. Sequences
Determined
No. Identical
Sequence Repeats
768-PER
768-PED
768-dl25
789-PER
789-PED
789-d35
789-dl88
916 (-) Skin
916 (+) Skin
2081 Skin
2085 Skin
2093-d35
2093-d63
2093 Skin
2094 Skin
2098 Skin
17
17
17
14
15
14
14
11
12
7
15
11
14
14
27
14
0
0
6
0
0
5
0
0
11
2 and 3
5
0
2
14
17
0
~~~
____
Nomenclature for peripheral blood samples is from Table
3. TCR
sequences from skin samples areso noted, next to thepatient's UPN
(see Fig 4 and Table 1). The criteria for an identical sequence repeat
is that the approximate 100 bp between the 3' end of the germline
V02 gene segment and the 5' of the C0 gene does not vary by even
onenucleotide in sequenceidentity. The column that lists unique
sequences is acompilationofalldetermined
TCR nucleotide sequences i n a sample which vary by one or more bps. In most cases,
unless the identical TCR is repeated, the unique sequences tend t o
vary in length and nucleotide identity by5 or more bp in the joining
region (data not shown).
the case of UPN 2094, the PB samples withdrawn pre-BMT
exhibit little or no expression of the unique TCR determined
in skin on day 42 (Fig SA). Patient UPN 2085 has an expression pattern resembling UPN 208 1, in thatlittle or no expression of a unique GVHD skin lesion TCR can be detected in
the PB at any timepoint. Although a small amount of expression can be detected in the BM before transplant, the level
of expression diminishes untilthe development of skin
GVHD, and there is no further expression in the PB at any
timepoint after transplantation that was available for study
(day 180, UPN 2085, Fig 5A). Taken together, data from the
skin biopsy specimens with GVHD as opposed to GVHDnegative biopsy samples show a consistent pattern of expression of oligoclonal TCRs that cannot be satisfactorily explained byPB or BM contamination. The likely, although
still unproven, explanation is that the expression pattern of
these unique TCRs is connected with the pathology of
GVHD in these patients.
Vp2 Expression Is Equivalent in Blood Samples and
Biopsy Samples From GVHD Patients
To rule out a trivial explanation of the highly significant
results reported inFig 5A, all of the Southern blots were
rehybridized with a probe that detects the repertoire of TCRs
that are members of the V02 family (Table S). As expected,
there is very little or no detectable variation in V02 TCR
expression that would alternatively explain the results shown
in Fig SA using unique TCR sequence probes (Fig SB). The
signal from Vp2 TCR expression from samples of PB and
skin or gutis remarkably homogeneous. This provides a
suitable control to demonstrate that the variation in expression of individual Vp2 TCRs shown in Fig 5A is connected
with a specific amplification process, as opposed to differentialRNA degradation or the absence of expression of all
Vp2 family members in the blood or tissue samples.
DISCUSSION
The principal goal of this research has been to establish
whether T-cell amplification occurs in the development of
GVHD in BMT recipients. Our first approach to the problem
was to examine the repertoire of TCRs from unmanipulated
blood samples as a marker for the putative expansion of Tcell clones (Fig I). Based solely on the results from blood
specimens, we were unable to demonstrate that the expression of the majority of TCR Vp families varied significantly
between different individuals we examined in the midst of
an ongoing immune response, namely GVHD (Fig l). The
lack of sensitivity of displaying the PCR generatedTCR
fragments on agarose gels as opposed to sequencing gels
obscures possible variation within a family that isqualitative.
and not q~antitative.~'
Therefore, we later turned tosequenc-
Table 5. The CDR3 Sequence Probes Usedin Southern Hybridization of Peripheral Blood and GVHD Lesions
From Patients Designated by UPNs Discussed in Table l
UPN
768
789
916
2081
2081
2085
2093
2094
All
Probe Name
J02.3D1
J01.4
JP1.5D1
[email protected]
J02.1D1
J02.3D2
JP2.1D2
J02.1D2
VP2P
Sequence of Probe
Length
Detection of Expression
20
Yes
Yes
Yes
No
Yes
Yes
Yes
Yes
Yes
23
20
21
20
20
20
20
25
TCR sequence whose joining region was unique in primary structure. The most polymorphic portion
Each GVHD skin sample had a duplicated
of the joining region was made into an oligonucleotide probe referred to as a "CDR3" sequence for the purposes of Southern hybridization
studies of all available specimens. In one case, it was found that the identical sequence was conserved for two different patients (2093 and
2094). Patients 768 and 789 had CDR3 sequence probes derived from duplicated TCRs from peripheral blood samples withdrawn on the same
date as the biopsy. In thosecases the biopsy sample was not available for molecular analysis.
MOLECULAR ANALYSIS OF TCR REPERTOIRE IN GVHD
3041
A
Fig 5. (AI CDR3 probe analysis ofchronologic PB samples
and skin biopsy specimens. PCR
fragments of Vp2
containing
TCRs were generated as described in Fig 2, and electrophoresed on 1.3% agarose gels,
transferred t o nylon
membranes. and then hybridized with
32P-labeledprobes as shown t o
the right of the autoradiogram
for each patient. A description of
the probes used can be foundin
Table 5. PB sample nomenclature designations can be found
in Table 3. Biopsy samples are
identified with the following letters: S, skin; C, colon; St, stomach. The date of the biopsy inis
dicated as a superscript above
the letter designation. CPM calculated using ImageOuant from
phosphor screen exposures are
shown underneath each lane
( ~ 1 0 ‘ ) .(B) Reprobe of membranes shownin (AI with the
Vp2P probe (Table 51. Blots were
stripped of probes shown in (A),
and rehybridized with “P-labeled Vp2P probe.
F[
109
S’09
100
63
49
44
32
vp 2P
916
vp 2P
2081
l80
2085
157
-
-”.
St’9 S”
96
96 56
61
S”’
63
49
-I
35 2835
--
35
21
PBR
PED
BM
v p 2P
21 PER
PBD
BM
vp 2P
2093
S 42
2094
ing random TCR clones generated from PB at different time
points for a more precise picture of the sequence diversity
(see below).
A significantly different resultwas observed when we
repeated the repertoire analysis, using the GVHD tissue lesion as the source of mRNA (Fig 2). We observed a highly
skewed repertoire that favored the expression of V02 family
containing TCRs in the small sample of patients we ana-
61
59
42
36
PBR
21
PBR
BM
PED
vp 2P
lyzed. This result led us to determine nucleotide sequences of
individual expressed TCR genes from the group of patients
described here (Table 1 andFig 4). Two important points
are addressed by this data: ( I ) random selection of rearranged
TCR gene segments and expression could not account for
the observed usage profile of productively rearranged TCR
V02 genes in skin lesions, and (2) biased usage of amplified
TCRs in GVHD lesions was a disease-specific phenomena
3042
because the equivalent sequence analysis in patients whose
biopsy specimens were negative for GVHD did not show a
comparable amplified TCR usage profile. It remains to be
explained why in every skin biopsy sample we examined,
whether or not it is disease-free, has a bias in the usage of
the VD2 family (Fig 2). Since all of the patients involved in
this study have been treated post-BMT with agents that modify T-cell function regardless of GVHD status, we can hypothesize that the bias in the TCR repertoire of lymphocytes
that infiltrate the skin is related to the unique circumstances
of these patients. Ongoing studies are examining the extent
of this TCR repertoire bias.
Alignment of the sequences from each of the identified
predominant TCRs showed that they arose from in-frame
protein-encoding genes that encode productively rearranged
Vg TCR chains (data not shown). In 6 of 7 cases, the 5‘
ends of rearranged D and J segments were flanked by N
nucleotide insertions and deletions by comparison with nucleotide sequences of the corresponding germline gene segments (Table 5 and data not shown).&Usage of JP segments
was random in skin biopsy or PB samples, although not all
JP segments were selected for a given patient, nor were
some JP segments used in any of the patients (Fig 4). However, apparent bias of usage of particular JP segments was
found as a consequence of the amplification of a predominant
TCR in a skin or PB sample. Results from a larger sample
size of BMT patients may reveal association of JP usage
with the development of GVHD (Liu et al, in preparation),
as has been reported for an example of an autoimmune disease.47
The uniqueness of the TCR joining regions allowed us to
predict probes that would discriminate among a family of
rearranged TCR genes to detect the expression of the TCR
that we determined by sequence analysis (Fig 4 and Table
S). We proved this in one case, when we sequenced rearranged TCR genes that were the product of a Vp2-Jp2.3
rearrangement from a series of samples that were positive
using the unique CDR3 sequence fingerprinting probe (Fig
SA). The identical junctional sequence was found in the
Vp2-Jp2.3 TCR pool, from a sample that was taken 2Y after
the original PB sample on the day of GVHD diagnosis from
UPN 768 (Table 3). The pattern of hybridization from the
Southern blot (Fig 5A) together with the sequencing result
at 2Y (see Results) are in agreement, and also serve to reinforce the correlation between GVHD and the expression of
a unique amplified TCR (Fig 4). Nonetheless, the methods
used in this report are unable to distinguish high expression
of TCR mRNA from a small group of cells, as opposed to
moderate expression of the same RNA by a larger number
of cells. Therefore, use of in situ hybridization methods on
paraffin-embedded GVHD lesions with probes generated
from CDR3 sequences will be more suitable to test whether
greater numbers of an oligoclonal T-cell subset or just limited numbers of TCR over-expressing T cells have infiltrated
the lesion.
The restricted expression of TCRs that are complementary
to the derived CDR3 sequence probe gives support for their
possible association with the development and/or pathology
of GVHD in BMT recipients (Fig 5A). The unique primary
structure of predominant TCRs in individual patients is an
LIU ET AL
argument in favor of their expansion as a result of antigenic
differences between the donor and the recipient. In this small
sample, we only found one instance where a probe from
one patient hybridized to RNA from another patient in a
predictable way (Fig 5A and Table S). The HLA type of
these patients show a single class I and class I1 MHC allele
in common (Table l). We can only speculate whether similarities or differences in HLA alleles will lead to predictable
changes in the T-cell repertoire. A recent report examining
the influence of class I1 HLA alleles on the JP repertoire
suggested that frequencies of usage of particular JP gene
segments could be shaped by the expression of alternative
HLA alleles.47A study of TCR repertoire in GVHD from a
donor and recipient mismatched in the class I1 MHC DR
gene also showed preferential usage of a JP segment.” These
studies establish a precedent that allelic members ofHLA
class I1 genes can cause the altered usage of components of
the TCR repertoire.
Similar approaches as have been described in this report
have been used in several disease states and with BMT pat i e n t ~ .A
’ ~similar approach of examination of the TCR repertoire of BMT patients using different methodologies was
conducted in the Kourilsky laboratory.’” Their recent methodology involving an automated sequencer andImmunoscope (Institute Pasteur, Paris, France) software allows a
precise measurement of the CDR3 rcgion, and the ability to
distinguish different TCR cl ono type^.'^.^^ Yet, there aresome
differences in the expression of V p families in skin samples
reported by Dietrich et al” and in this report. They found a
broader representation of overexpressed Vp families, although the sequencing of consecutive bacterial TCR clones
from a single Vp family was done differently than this study.
The discrepancy from our results is not easily explained,
especially because their one reported case of skin GVHD
arose from an MUD transplant patient whose conditioning
regimen, transplant procedures, and post-GVHD prophylaxis
were similar to what was performed on several individuals
from our group of MUD patients.’” One important difference
in methodologies is that only through actual sequencing of
the V(D)J joins is it possible to establish whether they are in
frame, and either the Immunoscope”~4x
or similarly described
techniques do not directly address whether TCR molecules
are productively reamangedA5
TCR clonotype expansion within a GVHD lesion as studied in this report is reflective of a classic immune response
stimulated by neo-expression of foreign antigen. The donors
of BMT recipients wereall adults inwhichwe
presume
their existent immune system has been thymically educated.
Variation of the HLA allelic complement between the donor
and recipient may either cause presentation of neo-antigenic
epitopes or allorecognition of MHC, events that would be
capable of eliciting an immune response, such as whatis
described in GVHD. An example of a restricted immune
response that was implicated in the development of GVHD
was the cross-reactivity of allorecognition of MHC and an
Epstein-Barr virus e p i t ~ p e .Analogous
~~
to this report, a
dominant cytotoxic T-lymphocyte (CTL) response was demonstrated in which the repertoire of antigen-specific TCR
was highly restricted. Antigenic exposure can shape the Tcell repertoire, andis applicable toBMT, especially with
MOLECULAR ANALYSIS OF TCR REPERTOIRE IN GVHD
respect to MUD transplants.Refinements of theapproach
initiated here, which include useof in situ methods of detection of the CDR3 sequence in lesions, growth of T cells in
vitrofrom PB or lesions, and MoAbblockingstudies of
those cells, mayresultin effective therapeuticalternatives
for BMT patients with acute or chronic GVHD.
ACKNOWLEDGMENT
We thank the dedicated physicians and nurses of the Hematology
and BMT program for their tireless efforts in securing the patient
samples usedinthis
study. Nurse coordinators Pilar Fornbuena,
Barbara Littrell, and Rodrigo Nunez are sincerely thanked for their
skilled organization of accrual of all patient peripheral blood samples. Drs Donald David and Richard Merkreebs are acknowledged
for their help in procuring the gastroenterologic biopsy specimens.
The technical help of Zuzana Valo, Phyllis Hao, and Paul Bienvenue
in the early stages of this study is gratefully acknowledged. The
help of Drs Auayporn Nademanee and Joyce Niland inthe interpretation of clinical parameters and patient characteristics is gratefully
acknowledged. The authors thank DrsPeter Parham and Joshua
Ellenhorn for a critical reading of the manuscript, and for providing
important suggestions for its improvement.
REFERENCES
1. Forman SJ, Blume KG: Allogeneic bone marrow transplantation for acute leukemia. Hematol Oncol Clin North Am 4517, 1990
2. Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL,
Neiman PE, Sale GE, Sanders JE, Singer JW, Shulman H, Storb
R, Weiden PL: Marrow transplantation for acute nonlymphoblastic
leukemia in first remission. N Engl J Med 301597, 1979
3. Thomas ED, Clift RA, Fefer A, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Cheever MMA, Deeg HJ, Doney K, Flournoy N, Greenberg P, Hansen JA, Martin P, McGuffin R, Ramberg
R, Sanders SE, Singer J, Stewart P, Storb R, Sullivan K, Weiden
PL, Witherspoon R: Marrow transplantation for the treatment of
chronic myelogenous leukemia. Ann Intern Med 104:155, 1986
4. Witherspoon RP, Deeg HJ, Lum LC, Ochs HD, Hansen JA,
Thomas ED, Storb R: Immunologic recovery in human marrowgraft
recipients given cyclosporine or methotrexate for the prevention of
graft-versus-host disease. Transplantation 37:456, 1984
5. NoelDR, Witherspoon RP, Storb R, Atkinson K, Doney K,
Mickelson EM, Ochs HD,Warren RP, Weiden PL, Thomas ED:
Does graft-versus-host disease influence the tempo of immunologic
recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood 51:1087, 1978
6. Lum LG: The kinetics of immune reconstitution after human
marrow transplantation. Blood 69:369, 1987
7. Martin PJ: Increased disparity for minor histocompatibility antigens as a potential cause of increased GVHD risk in marrowtransplantation from unrelated donors compared with related donors.
Bone Marrow Transplant 8:217, 1991
8. Beatty PG, Hansen JA, Longton JM: Marrow transplantation
from HLA-matched unrelated donors for treatment of hematologic
malignancies. Transplantation 5 1:443, 1991
9. Ferrara JL, Deeg HJ: Graft-versus-host disease [see comments]. N Engl J Med 324:667, 1991
IO. Sprent J, Komgold R: T cell subsets controlling graft-v-host
disease in mice. Transplant Proc 19:41, 1987
1 1. Sakamoto H, Michaelson J, Jones WK, Bhan AK, Abhyankar
S, Silverstein M, Golan DE, Burakoff SJ, Ferrara JL: Lymphocytes
with a CD4+ CD8- CD3- phenotype are effectors of experimental
cutaneous graft-versus-host disease. Proc NatlAcad
Sci USA
88: 10890, 1991
12. Acha-Orbea H, Steinman L, McDevitt HO: T cell receptors
in murine autoimmune diseases. Annu Rev Immunol 7:371, 1989
3043
13. Oksenberg JR, Panzara MA, and, Steinman L: The Polymerase Chain Reaction and the Analysis of the T Cell Receptor Repertoire. Austin, TX, Landes, 1992
14. Ben-Nun A, Liblau RS, Cohen L, Lehmann D, TournierLasserve E, Rosenzweig A, Zhang JW, Raus JC, BachMA:Restricted T-cell receptor V beta gene usage by myelin basic proteinspecific T-cell clones in multiple sclerosis: Predominant genes vary
in individuals. Proc Natl Acad Sci USA 88:2466, 1991
15. Berger M, Wettstein PJ, Komgold R: T cell subsets involved
in lethal GVHD directed to immunodominant minor hstocompatibility antigens. Transplantation 57:1095, 1994
16. Nishimura M, Akaza T, Mitomi Y, Nieda M, Minami M, Juji
T Establishment of human minor histocompatibility antigen-specific
cytotoxic T cell clones restricted by HLA-DR9. Transplantation
55:1181, 1993
17. Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin MR,
Filipovich A, Gajewski J, Hansen JA, Henslee-Downey J, McCullough J, McGlave P, Perkins HA, Phillips GL, Sanders J, Stroncek
D, Thomas ED, Blume KG: Analysis of 462 transplantations from
unrelated donors facilitated by the National Marrow Donor Program
[see comments]. N Engl J Med 328593, 1993
18. Santamaria P, Reinsmoen NL, Lindstrom AL, Boyce-Jacino
MT, Barbosa JJ, Faras AJ, McGlave PB, Rich SS: Frequent HLA
class I and DP sequence mismatches in serologically (HLA-A, HLAB, HLA-DR) and molecularly (HLA-DRB1, HLA-DQAI, HLADQBI) HLA- identical unrelated bonemarrow transplant pairs.
Blood 83:280, 1994
19. Diamond DJ, Chang KL, Jenkins KA, Forman SJ: Immunohistochemical analysis of T cell phenotypes in patients with graft
versus host disease following allogeneic bone marrow transplantation. Transplantation 59: 1436, 1995
20. Rhoades JL, Cibull ML, Thompson JS, Henslee-Downey PJ,
Jennings CD, Sinn HP, Brown SA, Eichhorn TR, Cave ML, Jezek
DA: Role of natural killer cells in the pathogenesis of human acute
graft- versus-host disease. Transplantation 56: 113, 1993
21. Sekimata M, Griem P, Egawa K, Rammensee HG, Takiguchi
M: Isolation of human minor histocompatibility peptides. Int Immuno1 4:301, 1992
22. den Haan JMM, Sherman NE, Blokland E, Huczko E, Koning
F, Drijfhout JW, Skipper J, Shabanowitz J, Hunt DF, Engelhard
VH, Goulmy E: Identification of a GVHD-associated human minor
histocompatibility antigen. Science 268: 1476, 1995
23. Marijt WA, Veenhof WF, Goulmy E, Willemze R, van Rood
JJ, Falkenburg JH: Minor histocompatibility antigens HA-I-,
-2-, and -4-, and HY-specific cytotoxic T-cell clones inhibit human
hematopoietic progenitor cell growth by a mechanism that is dependent on direct cell-cell contact. Blood 82:3778, 1993
24. Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, Iacucci R, Zei T, Martelli MP, Gambelunghe C: Successful
engraftment of T-cell-depleted haploidentical “three-loci’’ incompatible transplants in leukemia patients by addition of recombinant
human granulocyte colony-stimulating factor-mobilized peripheral
blood progenitor cells tobonemarrow inoculum. Blood 84:3948,
1994
25. Brenner MK, Wimperis JZ, Reittie JE, Patterson J, Asherson
CL, Hoffbrand AV, Prentice HG: Recovery of immunoglobulin isotypes following T-cell depleted allogeneic bone marrow transplantation. Br J Haematol 64:125, 1986
26. O’Reilly RJ, Collins N, Dinsmore R, Keman N, Siena S,
Brochstein J, Kirkpatrick D, Elomenberg N. Shank B, Dupont B, et
al:24 Transplantation of HLA-mismatched marrow depleted of Tcells by lectin agglutination and E-rosette depletion. Tokai J Exp
Clin Med 10:99, 1985
27. Champlin R: T-cell depletion to prevent graft-versus-host disease after bone marrow transplantation. Hematol Oncol Clin North
Am 4:687, 1990
3044
28. KeeverCA,SmallTN,FlomenbergN,HellerG,Pekle
K,
Black P, Pecora A, Gillio A, KernanNA,O’Reilly
RJ: Immune
reconstitution following bone marrow transplantation: Comparison
of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood 73:1340, 1989
29. Champlin R, Giralt S, Przepiorka D, Ho W, Lee K, Gajewski
J, Nimer S, Anderson B, Wallerstein R, Ippolito C: Selective depletion of CD8-positiveT-lymphocytesforallogeneicbonemarrow
transplantation: Engraftment, graft-versus-host disease and graft-versus leukemia. Prog Clin Biol Res 377:385, 1992
30. Dietrich PY, Caignard A, Diu A, Genevee C, Pic0 JL, HenryAmar M. Bosq J, Angevin E, Triebel F, Hercend T: Analysis of Tcell receptorvariability in transplantedpatients with acutegmftversus-host disease. Blood 80:2419, 1992
3 I . Yamanaka K, Kwok WW, Mickelson EM, MasewiczS, Smith
F, Nepom GT: Selective T-cell-receptor gene usage in allorecognition and graft- versus-host disease. Transplantation 55:1167,
1993
32. Dietrich PY, Caignard A,Lim A, Chung V, Pic0 JL, Pannetier
C, Kourilsky P, Hercend T, Even J, Triebel F: In vivo T-cell clonal
amplificationattime
of acutegraft-versus-hostdisease.
Blood
84:28 1 5 , I994
33. Vilmer E, Triebel F, David V, Rabian C, Schumpp M, Leca
G, Degos L, Hercend T, Sigaux F, Bensussan A: Prominent expansion of circulatinglymphocytesbearinggammaT-cellreceptors,
with preferential expression of variable gamma genes after allogeneic bone narrow transplantation. Blood 72:841, 1988
34. van der Harst D, Brand A, van Luxemburg-Heijs SA, KooijWinkelaarYM,Zwaan
FE, Koning F: Selectiveoutgrowth
of
CD45RO+ V gamma 9+/V delta 2+ T-cell receptor gammddelta
T cells early after bone marrow transplantation.
Blood 78: 1875, 1991
35. Sullivan K: Graft-versus-Host Disease, in Forman SJ, Blume
KG, Thomas ED (eds): Bone Marrow Transplantation. Boston, MA.
Blackwell Scientific, 1994, p 339
36. Sale GE, Lemer KG, Barker EA, Shulman HM, Thomas ED:
The skin biopsy in the diagnosis of acute graft versus host disease
i n man. Am J Pathol 89:621, 1977
37. Glucksberg H, Storb R. Fefer A, Buckner CD, Neiman PE,
Clift RA, Lerner KG, Thomas ED: Clinical manifestations of graftversus-hostdisease in humanrecipients of marrowfrom HL-Amatched sibling donors. Transplantation 18:295, 1974
38. Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate- phenol-chloroform extraction.
Anal Biochem 162: 156, 1987
39. Nakajima-Iijima S, Hamada H, Reddy P, Kakunaga T: Molecular structure of the human cytoplasmic beta-actin gene: Interspecies
homology of sequences in theintrons.Proc
Natl Acad Sci USA
82:6133,1985
40. Yoshikai Y, Clark SP, Taylor S, Sohn U, Wilson BI, Minden
MD, Mak TW: Organization and sequences of the variable, joining
and constant region genes of the human T-cell receptor alpha-chain.
Nature 3 I6:837, 1985
LIU ET AL
41.LawyerFC,Stoffel
S, SaikiRK,ChangSY,Landre
PA.
Abramson RD, Gelfand DH: High-level expression, purification, and
enzymatic characterization of full-length Thermus aquaticus DNA
polymerase and a truncated form deficient
in 5’ to 3’ exonuclease
activity. PCR Methods Appl 2:275, 1993
42. Even J, Lim A, Puisieux 1, Ferradini L, Dietrich P-Y. Toubert
A, Hercend T. Triebel F. Pannetier C, Kourilsky P: T-cell repertoires
i n healthy and diseased human tissues analyzed by T-cell receptor
/3-chain CDR3 size determination: Evidence for oligoclonal expansions in tumorsandinflammatorydiseases.ResImmunol146:65.
I995
43. Dunn DA, Gadenne AS. Simha S. LernerEA, Bigby M.
BleicherPA:T-cellreceptor
V betaexpression i n normal human
skin. Proc Natl Acad Sci USA 901267, 1903
44. Davis MM. Bjorkman PJ: T-cell antigen receptor gene\ and
T-cellrecognition[publishederratumappears
in Naturc 335:744.
19881. Nature 334:395. 1988
45. Gorski J. Yassai M, Zhu X, Kissela B, Kissella BB. Keever
C, Flomenberg N, Kissella B: Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed
by
CDR3 size spectratyping. Correlation with immune status. J Immuno1 152:5109,1994
46. Toyonaga B, Yorhikai Y, Vadasz V, Chin B. Mak TW: Organization and sequences of the diversity. joining. and conslant region
genes of the human T-cell receptor beta chain. Proc Nall Acad Sci
USA 82:8624, 1985
47. Waiser-Kuntz DR. Weyand CM. Weaver AJ, O‘Fallon WM.
GoronLy JJ:Mechanismsunderlying
the formation of‘ the T cell
receptor repertoire in rheumatoid arthritis. Immunity 2597, IY95
48. Pannetier C, Even J, Kourilsky P: T-cell repertoire diver\ity
and clonal expansions in normal and clinical samples. Immunol
Today 16: 176, 1995
49.Burrows SR, Khanna R, BurrowsJM,Moss DJ: An alloresponse in humans is dominated by cytotoxic T lymphocytes (C‘rL)
cross-reactive with a single Epstein-Barr virus CTL epitope:
Implications for graft-versus-host disease. J Exp Med 179: I I SS, l994
SO. Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J: Interaction of Staphylococcus aureus toxin “suprrantigens”
with human T cells. Proc Natl Acad Sci USA 86:8941. 1989
S I . Labrecque N. Thibodeau J , Mourad W,Sekaly RP: T cell
rcceptor-major histocompatibility complex class 11 interaction is required for the T cell response to bacterial superantigens. J Exp Med
180:1921. 1994
52. Wilson RK, Lai E, Concannon P, Barth RK, Hood LE: Structure, organization and polymorphism
of murine and human T-cell
receptor alpha and beta chain gene families. lmmunol Rev I O 1 : 149,
I98X
57. Liu X, Chesnokova V. Forman SJ, Diamond DJ: Methods lor
determining expression and oligoclonality of TCRs
in CVHD lesions
from allogeneic BMT recipients, in Oksenberg JR (ed): The Human
Antigen TCellReceptor:SelectedProtocols
and Applications.
Georgetown, TX. Landes (in press)
© Copyright 2026