Hardness based model for determining the kinetics of precipitation
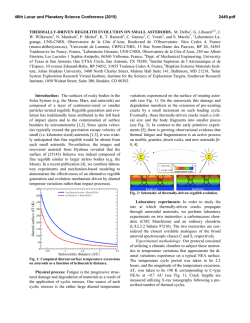
Materials Science and Engineering A 500 (2009) 244–247 Contents lists available at ScienceDirect Materials Science and Engineering A journal homepage: www.elsevier.com/locate/msea Short communication Hardness based model for determining the kinetics of precipitation Joy Mittra ∗ , U.D. Kulkarni, G.K. Dey Materials Science Division, Bhabha Atomic Research Centre, Mumbai 400 085, India a r t i c l e i n f o Article history: Received 22 April 2008 Received in revised form 12 September 2008 Accepted 17 September 2008 Keywords: Precipitation Hardness Kinetics Martensitic steel a b s t r a c t A theoretical model based on Johnson–Mehl–Avrami (JMA) formalism for determining kinetics and activation energy of a precipitation process is derived from the variation in hardness properties. Effectiveness of the model for determining the kinetics of -NiAl precipitation in PH13-8Mo steel and for distinguishing the kinetics between two temperatures is also demonstrated. The activation energy for -precipitation, 244.3 kJ/mol, determined over 808 K to 868 K is in good agreement with that of diffusion of Ni and Al in ␣-iron. © 2008 Elsevier B.V. All rights reserved. 1. Introduction Diffusion controlled phase transformation is a function of temperature where, activation energy helps to comprehend the kinetics of transformation. In the case of diffusion-controlled growth of precipitate through the formation of Guinier–Preston (GP) zone, the strength of the material usually reaches a peak before falling due to the loss of coherency [1,2]. In such cases, indirect determination of activation energies through the measurement of physical properties, such as, strength and hardness becomes easy. In an earlier attempt by Robino et al. [3], the possibility of using hardness data in the Johnson–Mehl–Avrami (JMA) model [4,5] was worked out, assuming the diffusion involving precipitation generates a volume of soft impingement. As pointed out by Guo, Sha and Wilson [2,6], JMA formalism represents a real precipitation process and gives a close approximation in the range of initial stage to high volume fraction and is seen to be successful in describing the precipitation reaction and austenite reversion process during aging. Hence, inability of the model by Robino et al. [3] to describe initial stage of precipitation and questionable conclusion thereof that the JMA equation might not be suitable to quantify the precipitate fraction during the aging of PH13-8Mo steel has been discussed by Guo and Sha [2,6]. Present work re-examines the possibility of using hardness data in the JMA model with significant differences form the earlier approach [3]. ∗ Corresponding author. Tel.: +91 22 25590465; fax: +91 22 25505151. E-mail address: [email protected] (J. Mittra). 0921-5093/$ – see front matter © 2008 Elsevier B.V. All rights reserved. doi:10.1016/j.msea.2008.09.056 The JMA equation of kinetics expresses the relationship between volume fraction transformed, x, and the time of transformation, t, in the following form. x = 1 − e−kt n (1) Where, n and k are constants by which the kinetics are characterized. The equation may also be written in the following linear form. log ln 1 = log k + n log t (1 − x) (2) To incorporate hardness data, we assume that the volume fraction of precipitates, x, is with respect to that of maximum hardness (HF ) and is related to the hardness via flow properties of the material in the following manner [3,7]. If H is the difference in hardness, then, H∝x2/3 . If Ht is the hardness at any time t and H0 is the hardness at t = 0, then, H = Ht − H0 or, x∝(Ht − H0 )3/2 . It is now expressed in the following equation form. x = C0 + C1 (Ht − H0 )3/2 (3) where, C0 and C1 are constants. To find out the constants following conditions may be set; when Ht = HF , x = 1 and when Ht = H0 , x = 0. So, C1 = (HF − H0 )−3/2 and C0 = 0. Hence, x = (Ht − H0 )3/2 /(HF − H0 )3/2 is obtained. If x is substituted in Eq. (2), the following expression is obtained log ln (HF − H0 )3/2 (HF − H0 )3/2 − (Ht − H0 )3/2 = log k + n log t (4) J. Mittra et al. / Materials Science and Engineering A 500 (2009) 244–247 245 Hence, plotting the left hand term of Eq. (4) against log t, values of n and k can be determined. To find out the activation energy, Q, of the diffusion controlled precipitation process, Eq. (1) is differentiated with respect to t and the rate of a fraction transformed, dx/dt, is found out. dx n = nkt n−1 e−kt dt (5) Where, time for a particular fraction transformed is derived from Eq. (1), t = {(ln(1 − x)−1 )/k}1/n . When this is replaced in Eq. (5), dx/dt for a particular fraction transformed is obtained. If T is absolute temperature, then Arrhenius type rate equation is written in the following form, A = dx/dt = A0 exp(−Q/8.314T). Or in the linear form, ln A = ln A0 − Q/8.314T. Hence, Q (J/mol) is calculated from the slope of the ln (dx/dt) vs. 1/T plot. Similar to earlier attempt [3], in this case too, -NiAl precipitation in the PH13-8Mo steel has been chosen to evaluate the above model. In this steel, the ordering of -NiAl precipitate, which is responsible for high strength of the alloy, is fast and is difficult to suppress by conventional heat-treatment. However, the precipitate is highly resistant to overaging, which ensures stability of the alloy in a long term application [8]. Work by Guo et al. [9], using Position Sensitive Atom Probe (PoSAP) in wrought PH13-8Mo alloy has indicated that the process of  precipitation consists of following steps. At first, solute rich cluster or Guinier–Preston zone appears in the matrix, which gradually transforms into finely distributed transition phase coherent with the matrix, which further coarsens to become incoherent precipitates of equilibrium composition. The mechanical properties obtained from hardness measurements and tensile tests indicate changes in the properties during the early stages of aging. In the case of aging at a rather low temperature (783 K), precipitations are detected after 40 min of aging and are of irregular plate morphology. While, in the case of aging at a higher temperature (868 K), precipitates are detected early after 4 min and are of a needle like morphology. During the initial stages of aging, precipitates remain undetected and off stoichiometry, having significant amounts of Fe and Cr atoms in them. Even at this early stage they increase strength and hardness significantly, a behavior similar to that of PH17-4 alloy. The hardening effects observed during early stages of aging are attributed to the GP zone formation involving redistribution of Ni and Al [9]. Apart from lacking in clarity, earlier attempt [3] to study the kinetics of precipitation also appears to lack justification in the consideration of cast variety of PH13-8Mo steel as well as in the consideration of Rockwell hardness values. Regarding the former consideration, the wrought variety appears to be superior in terms of homogenous structure, absence of ␦-ferrite [10] and presence of less amount of retained austenite [10,11] and as a result of these it can achieve higher hardness compared to the cast variety. Regarding the consideration of a hardness scale for establishing the kinetics [3], as seen in Fig. 1, Vicker’s scale appears to maintain a more linear relationship with the tensile strength than Rockwell’s scale. Since, hardness data are incorporated in the model via flow properties, consideration of Vicker’s scale for the kinetics study is justified. Fig. 1. General trend of Vicker’s (HV) and Rockwell (RC) hardness values with tensile strength in the region of present interest [12]. Table 1 k and n values obtained through the linear fitting of data points of Fig. 3 using Eq. (4). T (K) 783 808 840 868 Fitting of all data Fitting of 180 s and 1800 s data k n k n 0.0456 0.1331 0.2886 0.3321 0.408 0.2768 0.2145 0.2572 0.0165 0.076 0.2436 0.3324 0.5795 0.3745 0.2450 0.2572 were taken using the Microhardness Tester having an accuracy of 0.01 m (Model Future Tech Microhardness Tester, Load range 100–1000 g). For hardness measurement, samples were subjected to a load of 500 gf and a dwell time of 15 s. For each sample, an average of the hardness values has been determined and the range has been shown in the plot in terms of error bar. 3. Results Fig. 2 shows the plot of average hardness data with error bar corresponding to samples aged at 783 K, 808 K, 840 K and 868 K for various durations. Plot of Eq. (4) has been generated using hardness data till maximum hardness values for the four aging temperatures and are shown in Fig. 3. For each temperature, two sets of ks and ns have been determined from the data points of Fig. 3. The first set of ks and ns shown in Table 1 are obtained from the linear fitting of all data points in Eq. (2), while the second set of ks and 2. Experimental To generate the hardness profile at various temperatures of aging, small pieces of solutionized wrought PH13-8Mo steel were aged for 180 s, 1800 s, 7200 s and 14400 s at 783 K, 808 K, 840 K and 868 K, respectively. To see the peak in the hardness profile of former two temperatures, samples were aged for durations ranging up to 86,400 s (24 h). After aging, hardness values of all samples Fig. 2. Hardness of PH13-8Mo stainless steel after solutionizing and aging at 783 K, 808 K, 840 K and 868 K for various durations. 246 J. Mittra et al. / Materials Science and Engineering A 500 (2009) 244–247 Fig. 3. Avrami plot produced from the hardness data. Fig. 4. Fit of x vs. t using 2 analysis for 783 K, 808 K, 840 K and 868 K till maximum hardness is shown above. 2 values along with k and n values are also tabulated there. Smooth line plots of the same are shown in the inset, and the lines are drawn at x (0.5, 0.6, 0.7, 0.8 and 0.9) for determining activation energies. ns shown in the last column of Table 1 are obtained from that of first two points, corresponding to 180 s and 1800 s. These ks and ns are only the initial approximation used in the 2 analysis of the results. Fitting of x vs. t, using 2 method has been shown in Fig. 4 and the experimental data are superimposed on the plot. The inset of Fig. 4 is the line plot of itself that reveals the trend of x vs. t. This clearly indicates an out of trend nature of kinetics at 783 K compared to higher temperatures. Plot in the inset also shows lines, which are drawn at x = 0.5, 0.6, 0.7, 0.8 and 0.9, respectively, to calculate various times taken to transform x at various temperatures. Rates of transformation for various fractions transformed at every temperature of aging, which have been derived using Eq. (5) are given in Table 2. These data, which are based upon 2 method are depicted in Fig. 5a for determining Qs. However, similar data have been determined from the kinetics using Eq. (2) and are depicted in Fig. 5b. As in the case of Fig. 4, in Fig. 5 too the data corresponding to Fig. 5. Semi-ln plot of rate of transformation and 1/T is derived in (a) using 2 analysis and in (b) using ks and ns from logarithmic plot as per Eq. (2). In both the figures best linear fit is obtained separately, including and excluding the data from 783 K and Q values measured are given in Table 3. Table 3 Q values derived from Fig. 5a and b, excluding and including data from 783 K. x 0.5 0.6 0.7 0.8 0.9 Q from 2 analysis Q from log–log plot (Eq. (2)) 808–868 K All data 808–868 K All data 233.9 239.1 244.3 249.7 256.5 182.6 166.8 151.5 135.1 115 294 284.9 276 266.5 254.7 230.3 200.3 171.1 140 101.5 783 K deviate from the trend of other temperatures. Hence, activation energies for different x mentioned above have been calculated, including and excluding the data of 783 K and are shown in Fig. 5a (Table 3). Table 2 Time required for the completion of fraction x, (t|x ) and the rate of reaction, dx/dt thereof are calculated using the k and n values obtained through 2 analysis. (K−1 ) T (K) 1 T 783 808 840 868 1.277 × 10−3 1.238 × 10−3 1.191 × 10−3 1.152 × 10−3 t|x=0.5 (s) 709.7 381.5 99.4 29.2 dx dt 0.5 2.0227E−4 2.5445E−4 9.718E−4 2.82E−3 t|x=0.6 (s) 1392.2 1033.4 270.6 69.1 dx dt 0.6 1.0904E−4 9.9343E−5 3.77523E−4 1.16E−3 t|x=0.7 (s) 2691.5 2739.3 720.9 160.3 dx dt 0.7 5.5584E−5 3.6933E−5 1.3965E−4 4.5701E−4 t|x=0.8 (s) 5424.1 7721.1 2042.3 392.2 dx dt 0.8 2.4580E−5 1.1677E−5 4.3931E−5 1.5324E−4 t|x=0.9 (s) 12878.0 27732.3 7381.5 1183.1 dx dt 0.9 7.406E−6 2.3256E−6 8.6947E−6 3.2809E−5 J. Mittra et al. / Materials Science and Engineering A 500 (2009) 244–247 4. Discussion Study of the kinetics of -NiAl precipitation is carried out at standard heat-treatment temperatures. Aging at higher than 868 K is not considered due to the possibility of significant amount of ␣ to ␥ transformation, which essentially involves diffusion of carbon atoms. It is known from the literature that the process of -nucleation in PH13-8Mo steel is fast. Hence, a low hardness value of the solution quenched sample, RC20.5 (VHN 240.8), may be attributed to the suppression of the initiation of -NiAl precipitation, enforced by the fast quenching, as compared to that of RC30, reported in the literature [3]. It is important to note here that the linear axes are used in Fig. 3 and not a log–log one, as used in the earlier work [3], to find out k and n with the help of Eq. (4), since, the intercept for determining the k, can only be obtained in a linear–linear plot. However, obtaining k and n of Eq. (1) through 2 analysis appears better, since, goodness of fit, R2 , of 2 analysis of the present data, are better than that of linear fitting of Eq. (4). Also, fitting of both minimum and maximum hardness values are shown in the 2 method, whereas, number of data points from a particular aging temperature reduces by these two points when Eq. (4) is used. While, Fig. 4 depicts the scatter in experimental data, which are superimposed on the data obtained through 2 analysis, nature of the kinetics may actually be visualized in a smooth plot of Eq. (1), as shown in the inset. It becomes easy to identify here that the trend of 783 K is different from that of 808 K, 840 K and 868 K. This is in support of the PoSAP study reported in the literature [9], where precipitates at 783 K are found to be of irregular plate morphology, whereas, precipitates at 868 K are of needle like morphology. As stated earlier, dx/dt, the slope of x vs. t plot is to be used for determining activation energy and not the ‘1/t’ that is used in the earlier work [3]. Table 2 shows that the time required for the completion of fraction 0.7 transformed, 160 s, is minimum for 868 K aging and is close to the minimum aging time, 180 s, set during experimentation. Hence, there is a minimum extrapolation from experimentation to the modeled value to determine the activation energy for the precipitation at 0.7 fraction-transformed. However, if extrapolation is allowed on the basis of Avrami’s model, then it is possible to obtain the activation energy, more towards the initiation of precipitation. Hence, the time taken for the completion of fraction 0.5 for 868 K appears to be 29.2 s. However, here it should be emphasized that the fraction transformed refers to that of maximum hardness and hence related to the state of precipitate. And at the maximum hardness, precipitate may typically be in a coherent to semi-coherent state. Since, NiAl precipitation in PH13-8Mo is resistant to aging, it appears to be suitable for the study of precipitation using JMA model. Process of evaluating activation energies through 2 analysis and Eq. (4), as shown in Fig. 5, support the superiority of the former method. As identified in Fig. 4, in this case too, data corresponding 247 to 783 K stands out from the rest. Since, the concept of activation energy is applicable when a particular process is valid over a range of temperature, it is intuitive to select 808–868 K temperatures for the calculation of activation energy and hence, data from 783 K is not considered for calculating activation energy. An equivalent comparison of the activation energies, which are obtained from Fig. 5a and b, respectively, shows that the Qs, based on 2 method and in the range of 808–868 K temperature show minimum scatter. The difference from highest Q, at x = 0.9, to lowest Q, at x = 0.5 is 22.6 kJ/mol and is seen to vary linearly with x. The activation energy of NiAl precipitation at 0.7 fraction transformed, 244.3 kJ/mol, appears closely matching with that of diffusion of Ni and Al in ␣-iron, which are 235 kJ/mol and 246 kJ/mol, respectively. 5. Conclusion Suppression of -NiAl precipitation in PH13-8Mo by fast cooling results in a low hardness of the alloy. In this work, a systematic method for determining kinetics of precipitation and activation energy from hardness data has been established using JMA’s model. Obtaining constants of the JMA equation through fitting of data directly using reduced 2 method appears better than fitting linearized JMA equation. The present methodology also helps in comparing the nature of kinetics at different temperatures and in determining activation energy more accurately. The activation energy of the NiAl precipitation appears very close to that of diffusion of Ni and Al in ␣-iron. Acknowledgements Authors would like to thank Mr. Abhishek Chauhan and Mr. Somesh Dutt for various helps during experimentation. Authors are also grateful to Dr. A.K. Suri, Director, Materials Group for extending various facilities to carry out the present work. References [1] A.J. Ardell, Mater. Trans. A 16A (1985) 2131–2165. [2] Z. Guo, W. Sha, Mater. Trans. 43 (2002) 1273–1282. [3] C.V. Robino, P.W. Hochanadel, G.R. Edwards, M.J. Cieslak, Metall. Mater. Trans. A 25A (1994) 697–704. [4] W.A. Jhonson, R.F. Mehl, Trans. Am. Inst. Min. (Metall) Engrs. 135 (1939) 416. [5] M.J. Avrami, Chem. Phys. 8 (1940) 212–224. [6] Z. Guo, W. Sha, E.A. Wilson, Mater. Sci. Technol. 18 (2002) 377–382. [7] J.W. Martin, Precipitation Hardening, 2nd Ed., Butterworth-Heinemann, Oxford, 1998, p. 91. [8] V. Seetharaman, M. Sundararaman, R. Krishnan, Mater. Sci. Eng. 47 (1981) 1–11. [9] Z. Guo, W. Sha, D. Vaumousse, Acta Mater. 51 (2003) 101–116. [10] P.W. Hochanadel, C.V. Robino, G.R. Edwards, M.J. Cieslak, Metall. Mater. Trans. A 25A (1994) 789–798. [11] J. Mittra, G.K. Dey, D. Sen, A.K. Patra, S. Mazumder, P.K. De, Scr. Mater. 51 (2004) 349–353. [12] Internet: http://www.wargamer.org/GVA/background/hardness4.html.
© Copyright 2026