Alchemia Shareholder Update
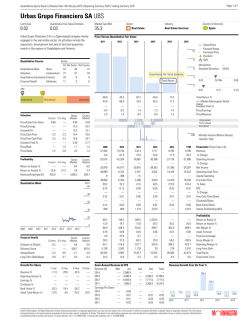
Alchemia Announces Investor Update Company to Target Fondaparinux Dividend Brisbane, Australia - 29 January, 2015: Australian drug development company Alchemia Limited (ASX:ACL) announced today an update on its ongoing corporate and clinical review which commenced in October 2014 following the Phase III trial results for HA-Irinotecan in metastatic colorectal cancer (mCRC). Over the course of the past three months, the Company has been conducting an internal evaluation of all programs and assets including a post-hoc subset analysis to explore why the Phase III trial of HA-Irinotecan when used in the FOLFIRI regimen did not reach its primary endpoint. The findings from this initiative produced the following key strategic objectives: 1. Target a payment of a dividend to shareholders in the second half of calendar year 2015. When this dividend is formally declared, the Company intends to pay out to shareholders all revenues from fondaparinux, less the costs of running the core business functions of a listed company, via the payment of dividends. Given the substantial tax losses that Alchemia has amassed, it is expected that these future dividends will not have any franking (imputation) credits attached. 2. Further investigate the possible sale of fondaparinux. In the event of a sale, it is the intention of the Company that the net sale proceeds will be returned to shareholders. 3. Target the execution of a corporate transaction or partnership for HyACT for further clinical development. Discussions with potential partners are underway to gauge interest. The transaction specifics currently being investigated include a variety of structures, for example, a sale, partnership, or a demerged oncology focused entity. Any additional capital that may be required to support the future development of HyACT is intended to be raised in an entity outside of the Alchemia Group (eg. through a demerged entity) so that it does not affect the ability of the Company to pay future dividends to its shareholders. 4. Target the execution of a corporate transaction for the VAST technology by June 30, 2015. Alchemia has engaged Evolution Life Sciences to assist in this process. The Company is in discussions with potential international and domestic partners. The objective of Alchemia in these negotiations is to maximise the value of the technology for the Company's shareholders and ensure there are no further internal capital commitments to this platform technology beyond June 30, 2015. 5. Continue to cut costs with the target being the minimum needed for the Company to continue to meet its corporate obligations and maximise its opportunities. 6. Continue to manage the availability of the Company's tax losses in any transaction that the Company undertakes. While a number of uncertainties remain that could impact decisions as to Alchemia's future strategic direction, the Board believes the primary objectives listed above will have the maximum impact on generating shareholder value going forward. Posted below, is a detailed summary covering all aspects of the strategic review. Page 1 of 16 www.alchemia.com.au Results of further detailed analysis of the Phase III trial of HAIrinotecan (HA-I) when used in the FOLFIRI regimen for the treatment of second- and third-line metastatic colorectal cancer (mCRC) Phase III: Results Summary With respect to the Phase III trial analysis, as previously disclosed, the trial failed to reach its primary endpoint of a statistically significant improvement in progression-free-survival (PFS) and also did not meet its secondary endpoint of an improvement in overall-survival (OS). Rather the results for the FOLF(HA)IRI arm were virtually identical to those of the control arm (Table 1). However, for both endpoints the control arm (FOLFIRI) performed significantly better than has been observed in any previously reported clinical trial. ALL (n=415) FOLF(HA)IRI (n=207) FOLFIRI (n=208) Difference p-value HR Median PFS and OS (months)* Independent Review of PFS Overall-survival (OS) (351 events) 5.5 (95% CI: 4.2, 5.9) (175e, 29c) 5.5 (276 events) 14.3 (95% CI: 12.4, 16.8) (130e, 77c) 14.4 (95% CI: 4.3, 6.7) (176e, 31c) (95% CI: 12.4, 15.7) (146e, 62c) 0.0 months 0.785 0.97 -0.1 months 0.408 0.90 (95% CI: 0.78, 1.20) *CRO generated data Key: e=event; c= censored CI = confidence interval Table 1: Phase III top-line results of median OS and PFS. (95% CI: 0.71, 1.15) Phase III: Post-hoc Exploratory Subset Analyses Since the announcement of the above top line results, Alchemia has further analysed the data in an attempt to determine potential reasons why the control arm on this study out-performed the historical clinical experience with this drug regimen. As part of this further analysis, the original Statistical Analysis Plan was expanded to include extensive post-hoc analyses of various subsets of patients. The purpose of these post-hoc analyses was to increase the Company's understanding of factors that may have contributed to the out-performance of the FOLFIRI control arm compared to historical experience and to determine whether there is still enough of a signal for proof-of-concept to warrant ongoing investment in additional clinical development of HA-I. Some of the subset analyses have revealed unexpected results, which the Company and its consultants continue to seek to understand further. This has required substantial further analysis, data collection and experimentation, as well as consulting a range of relevant clinical, regulatory and industry experts. The results of these analyses suggest that it is unlikely that these study results will support regulatory approval from the United States Food and Drug Administration (FDA) for HA-I in the mCRC indication. However, the Company intends to request a meeting with the FDA to discuss the results of the trial and possible next steps, however there is a risk that the FDA may not grant a meeting. Furthermore, Page 2 of 16 www.alchemia.com.au it should be noted that, even if the FDA grants a meeting, the Company's advisors have indicated that trials that have previously failed to meet the primary endpoint in the intention-to-treat analysis, under similar circumstances, have not succeeded in obtaining regulatory approval from the FDA. Summary of findings of post-hoc exploratory subset analyses 1. The Phase III results have been carefully reviewed to identify potential reasons for the observed FOLFIRI control arm median PFS and OS results being higher than prior historical literature reports for FOLFIRI in second- and third-line treatment of mCRC. The subset analysis suggests differences in outcome compared across geographic regions. 2. In Russia, which accrued 39.3% of the total trial population, the median PFS and OS in both study arms are higher than the results for patients enrolled in each arm from the study sites in the rest of the world. In particular, the median PFS and OS values in the Russian FOLFIRI control group patients are statistically significantly higher than the results observed in the control arm patients in the rest of the world. 3. An exploratory analysis after removal of the Russian patients reveals a positive trend in the FOLF(HA)IRI arm compared to the control arm median PFS (PFS = 5.3 vs 4.3 months, p=0.530) and a statistically significant improvement in median OS (OS = 14.0 vs 11.4 months, p=0.019). 4. Subset analysis was also conducted to explore possible gender effects (for all countries). In the FOLF(HA)IRI arm of the trial, the analysis revealed a statistically significantly better outcome for females in both median PFS (6.0 months vs 4.9 months, p=0.047) and median OS (17.2 months vs 13.3 months, p=0.016), than the standard FOLFIRI treatment. 5. Conversely, when looking at all countries, in males there was a trend towards the FOLF(HA)IRI arm providing a worse outcome than control arm FOLFIRI treatment for both median PFS and median OS (PFS 4.3 months vs 5.7 months, p=0.181, OS 13.1 months vs 15.3 months, p=0.292). In a further substudy analysis excluding Russia, no difference in PFS outcome between arms was observed for males, with a positive trend in median OS (median PFS 4.3 months vs 4.3 months, p=0.816, OS 13.3 months vs 11.9 months, p=0.747). Warning: It must be emphasised that these analyses are very exploratory as they are derived from subsets of subsets of patients. 6. One possible explanation for the observed differences in the subset analysis by gender is related to the possibility that the deposition of HA-I at the tumour site blocks the access of other anticancer drugs to the tumour for a period of time. The last drug administered in both the FOLF(HA)IRI and the FOLFIRI regimens is 5-Fluorouracil, which is metabolised significantly faster in men than in women. Thus, the order of administration of HA-I in the FOLFIRI regimen appears to be of importance. Warning: It should be noted that this is a working hypothesis only and remains to be tested. 7. The Company has recently filed a patent application in the United States to seek to protect this novel and unexpected outcome from the trial as regards the importance of the order of drug administration related to HA-I's mechanism of action. Page 3 of 16 www.alchemia.com.au 8. In certain subsets of the data, the magnitude of the improvement in OS in the treatment arm was substantially greater than the magnitude of the improvement in PFS. 9. Alchemia believes that these observed differences, with a more noteworthy improvement in OS than PFS, could relate to the mechanism of action of HA-I. As HA-I binds to a protein (CD44), which is highly expressed on the surface of cancer stem cells, the HA-I may be eliminating these stem cells, which slows the rate of the tumour regrowth and ultimately the time it takes for the cancer to reach a lethal burden. Warning: It should be noted that this is a working hypothesis only and remains to be tested. Cautionary Note to Readers It should be noted that these findings are the result of post-hoc exploratory subset analyses and as such, should be viewed with caution, as there is a very high risk of generating a false positive outcome in any particular subgroup analysis. If one analyses enough subgroups then random chance alone makes it more likely that one or more subgroups will be found in which a seemingly significant p-value is observed. Phase III: Current Status At the time of the primary analysis for progression-free-survival, an interim analysis of overall-survival was conducted. At that point 276 deaths had been observed, less than the 300 deaths specified for the final OS analysis in the protocol. To date, there have been 297 deaths reported, 1 patient is still on treatment and 65 patients are in follow-up for survival. Next Steps for HA-Irinotecan and the HyACT platform The future of HA-I and the HyACT platform remains uncertain at this time and, as noted above, the results of the subset analyses suggest that it is unlikely that these study results will support FDA regulatory approval for HA-I in the mCRC indication. However, the Company intends to request a meeting with the FDA to discuss the results of the trial and possible next steps. It should be noted there is a risk the FDA may not grant a meeting. Furthermore, even if the FDA grants a meeting, the Company's advisors have indicated that trials that have previously failed to meet the primary endpoint in the intention-to-treat analysis, under similar circumstances, have not succeeded in obtaining regulatory approval from the FDA. The Board is of the view that any potential FDA regulatory approval of HA-I would require additional clinical trial(s) and that any such trial(s) would require a partner versus being funded by Alchemia shareholders. Not only would it be extremely difficult to raise the capital required with the current Alchemia share price, but the dilutive impact of doing so would substantially reduce the intended dividend yield of those shareholders whose interest in the Company is the dividends that it will return. As a consequence of these factors, if the Company's further investigations and consultations confirm that there is significant value in HA-I and/or the HyACT platform, then one potential avenue to best maximise value for shareholders will be the execution of a corporate transaction, such as a broad partnership, sale, or a spin out of the Alchemia oncology business. However, even if the Company's Page 4 of 16 www.alchemia.com.au current investigations and consultations conclude that there is value in the HyACT platform, there is no guarantee that a suitable partner will be found or that the terms of a transaction can be agreed with any such partner on terms acceptable to the Company. The Company has already commenced confidential discussions with potential partners to discuss such a transaction. Strategic Review: Status and Update Since the results of the Phase III trial were received in late October 2014 the Company's management and Board have considered at length the strategic options for Alchemia going forward. These discussions have been informed by external expert assessments of the Phase III data, discussions with various shareholders (both institutional and retail), and ongoing discussions with market participants and potential partners. Fondaparinux In the context of significant analysis and consideration, the Board believes that a dividend policy related to the fondaparinux revenues is the optimal strategy going forward and will allow the market to more appropriately reflect the underlying value of the Company. • • • • • Therefore, the Board has resolved an intention of returning all fondaparinux revenues to shareholders, less the costs of running the core business functions of a listed company, via the payment of dividends. Given the substantial tax losses that Alchemia has amassed, it is expected that these dividends will not have any franking (imputation) credits attached. Given the costs of closing out the Phase III trial, and other financial commitments associated with existing business development activities, the Company expects to be in a position to pay its first dividend in the second half of calendar year 2015 and to pay dividends on a regular basis thereafter until its patents or cashflow on fondaparinux expire. In the meantime, in concert with its commitment to explore all options in this period of change for the Company, Alchemia continues to revisit the possible sale of fondaparinux. The Company is working with the same advisor previously retained to look at options concerning the possible monetisation of fondaparinux and has commenced discussions with potentially interested parties. A transaction will only be entered into if the Company believes the price achieved reflects appropriate value of fondaparinux for Alchemia shareholders at that time. In the event of a sale, it is the intention of the Company that the net proceeds, will be returned to shareholders. In the event that a sale of fondaparinux does not occur, the Board aims to formally resolve to commence paying dividends from fondaparinux revenues on the basis noted above before the end of calendar year 2015. Shareholders should be aware that there is some uncertainty surrounding future revenues from fondaparinux. The market for injectable anticoagulants is going through a period of substantial change. There is greater competition from novel oral drugs and the potential emergence of new entrants in the injectable market with another generic manufacturer having filed with the FDA for approval. At the same time Mylan has purchased the US rights for the original drug and its authorised generic. It is still too early to predict what impact this latter factor might have on the dynamics in the Page 5 of 16 www.alchemia.com.au US market, but the Company is monitoring these emerging market influences carefully with our commercial partner, Dr Reddy's. HA-Irinotecan and the HyACT Platform In addition to the Phase III clinical trial for HA-I in mCRC, the Company has two other ongoing HA trials - the CHIME study and a small cell lung cancer trial. HA-Irinotecan in combination, Erbitux (cetuximab) in mCRC patients (CHIME study) Alchemia and Merck Serono are in collaboration to support an investigator-led Phase II clinical trial. The trial evaluates Alchemia's HA-Irinotecan used in the FOLFIRI regimen, combined with Merck Serono's leading therapeutic antibody, Erbitux (cetuximab), in patients with mCRC. The goal of the trial is to demonstrate that HA-Irinotecan has an acceptable safety profile in combination with Erbitux when administered as part of the FOLFIRI regimen in the treatment of mCRC. The trial was intended to enrol approximately 45-50 patients receiving second-line treatment for mCRC. The first patient was recruited to the study in June 2014. The trial currently has five patients enrolled, and the safety experience to date suggests that the addition of cetuximab to the FOLF(HA)IRI regimen is well tolerated. The CHIME trial was placed on hold following the announcement of our Phase III study. It is anticipated that a decision on the CHIME trial will be made after receipt of feedback from the FDA regarding the Phase III trial. HA-Irinotecan for Small Cell Lung Cancer This investigator-led Phase II clinical trial of HA-Irinotecan is being conducted in first- and second-line extensive small cell lung cancer (SCLC) and is investigating the safety and efficacy benefits of HAIrinotecan/carboplatin compared with irinotecan/carboplatin. A secondary objective of this study is to evaluate the direct effect of HA-Irinotecan on cancer stem cells and provide clinical evidence that CD44positive tumours respond preferentially to HyACT drugs, an objective that has direct relevance in explaining some of the prolonged survival data observed in the ACO-002 Phase III HA-Irinotecan treatment arm when patients did not receive follow-on therapy. To date, 35 patients have been enrolled (88% of total recruitment target) and preliminary experience suggests that HA-Irinotecan in combination with carboplatin is well-tolerated with clinical activity demonstrated in both first- and second-line SCLC patients. Preliminary investigations indicate that patients with CD44positive tumours have superior response rates that demonstrate a longer time to tumour progression. The investigators aim to recruit the remaining 10% of patients within 2015, and the investigator intends to publish the study results in an oncology journal with the objective of providing the first-in-man data demonstrating the specificity of the HyACT technology for CD44positive tumours. These data could support the potential therapeutic use of HA-Irinotecan in SCLC. The cash outlay to complete the SCLC study is expected to be approximately $100,000. The additional cost to the Company of retaining a core capability for the HyACT platform until the Company can execute on a corporate transaction or restructure, compared to ceasing any further activity on the HyACT platform, is expected to be approximately $160,000 per month, or approximately $90,000 per month after accounting for the receipt of potential R&D Tax Credit refunds Page 6 of 16 www.alchemia.com.au in the future. This includes maintaining the existing HyACT patent portfolio. This does not include clinical trial costs mentioned above. VAST Although the VAST technology has been under development for many years, it is still at a very early stage in terms of its drug discovery potential. The current collaboration with AstraZeneca is continuing to progress as planned and it is hoped this will provide a valuable proof-of-concept. Nevertheless, it will take a substantial sum of money and many years before there is any prospect of a VAST derived drug entering the market. Even then there are no guarantees of success. The Board has come to the decision that the best way forward for VAST is to seek external funding for the technology. As previously advised to the market, Evolution Life Science Partners has been appointed to assist the Company in this process. Confidential discussions are underway with a number of parties. Our primary objective for VAST is the execution of transaction that will maximise the value from this asset to our shareholders. The close of any potential transaction is targeted prior to the end of June 2015, but we will continue to assess the viability of this process on a monthly basis and are prepared to terminate it earlier should there be no identifiable corporate transaction to consummate. The operational cost of the VAST division is expected to be approximately $160,000 per month going forward through to 30 June, 2015. This amount is reduced to approximately $66,000 per month after accounting for corresponding grant revenues received to date (in advance) and potential R&D Tax Credits which are expected to be received in the future. After taking into consideration all contractual fixed costs associated with VAST such as rent, the total incremental cost to continue to invest in the VAST technology through to 30 June, 2015, compared to ceasing all activity on the VAST program effective from 28 February, 2015, is expected to be approximately $170,000 in total, and approximately $75,000 after accounting for the receipt of additional grant funding and potential related R&D Tax Credit refunds in the future. Cash Burn and Cost Reductions The Company reported an average monthly operating cash burn (before receipt of revenues) of $1.4 million for the quarter ended 30 September, 2014, just prior to the Phase III results in October 2014. As at 30 September, the Company had estimated that the contracted costs yet to be incurred for completing the Phase III trial and for the establishment of plant and equipment for commercial drug manufacture and product presentation were approximately $4.0 million and $2.3 million respectively. In addition, as at 30 September, 2014, the Company had liabilities outstanding of $1.3 million for the manufacture of product which had yet to be paid, taking the amount owing for manufacturing to an estimated $3.6 million. Of the $1.3 million of recorded liabilities at 30 September, 2014, $567k was reported in the year ending 30 June, 2014, annual report. The Company expects that its ongoing operational monthly cash burn to be approximately $620,000, excluding the Phase III trial and associated manufacturing liabilities mentioned above. This cash burn amount is before allowing for any potential R&D Tax Credit Refunds and other revenues (eg: fondaparinux profit share revenue and interest). Page 7 of 16 www.alchemia.com.au Since the release of the Phase III trial results, a number of staff have been made redundant and others have been placed on notice and are serving out their contracted notice period. Other overheads have been cut where possible. The Company has also been in negotiations aimed at limiting its exposure for the remaining trial costs. At the time of writing, the costs owing on the Phase III trial have been reduced from the previously estimated approximately $4.0 million to approximately $2.0 million. However, we caution that this amount includes estimates and the actual final Phase III liabilities may vary. The total estimated manufacturing liability owing at 30 September, 2014, of $3.6 million, has now been finalised and confirmed and remains outstanding as a liability as at 31 December, 2014. Going forward, as noted above, the Company is aiming to spin out the VAST technology and obtain alternative sources of finance for HyACT, either through partnering or demerging into a separate entity capable of raising further capital. This will mean a further substantial reduction in the Company's operations and ongoing costs. The Board is also very mindful of the need to substantially reduce governance overhead. While the full skills of the current Board are essential to the completion of the strategic review and corporate restructuring that the Company has embarked upon, Alchemia will continue to assess the composition and size of the Board to ensure it is appropriate given the Company's position and adequately meets the needs of the Company going forward. It is anticipated that any changes to the Board composition will occur during the second half of calendar year 2015. Taking all of the above factors into consideration, the Company is targeting to further reduce its monthly expenses by 70% by the end of calendar year 2015. This target is made on the basis of maintaining an ASX listed entity whose only operational activities will be to receipt fondaparinux revenues and pay dividends to its shareholders. (Refer to the appendix for a detailed discussion of the Phase III Trial Results). Page 8 of 16 www.alchemia.com.au APPENDIX: Detailed Discussion of Phase III Trial Results As previously acknowledged the top line outcome of the Phase III trial showed no efficacy benefit for HA-I in the FOLFIRI regimen as compared to standard FOLFIRI. Since that time the Company's staff and statistical consultants have conducted many hundreds of analyses seeking to explain the trial results. These subset analyses have revealed some novel and unexpected outcomes. Baseline Characteristics The two study arms were generally well balanced in terms of baseline characteristics. The two arms were closely matched in median age, race, geographic region, weight, BSA, smoking history, performance status, number of metastatic sites and time since diagnosis. Males comprised 54.1% and 49.5% of the FOLF(HA)IRI and FOLFIRI subjects, respectively. Patient Disposition The intention-to-treat (ITT) population was comprised of 415 study subjects, 207 on the FOLF(HA)IRI arm and 208 on the FOLFIRI arm. Reasons for patients coming off study were generally balanced between the two arms. The most frequent reason was disease progression, reported in 61.8% and 63.5% of FOLF(HA)IRI and FOLFIRI subjects, respectively. Primary Endpoint: Progression-Free-Survival (PFS) PFS as assessed by a blinded, independent radiologic review was the primary endpoint of the study. The protocol specified that at least 350 PFS events would be required for the primary analysis. In the primary analysis 351 events had been observed. The median PFS values with 95% confidence intervals are shown in the table below: PFS Median, months (95% CI) Hazard Ratio (95% CI) p-value (stratified log-rank test) FOLF(HA)IRI FOLFIRI 5.5 (4.2, 5.9) 5.5 (4.3, 6.7) 0.97 (0.78, 1.2) 0.785 Key: CI = confidence interval Table 2: Primary endpoint: progression-free-survival (PFS). The stratified log-rank test was pre-specified as the primary analysis for the PFS endpoint. The p-value did not reach statistical significance, indicating the study did not demonstrate a statistically significant difference in PFS between the two treatment arms. Page 9 of 16 www.alchemia.com.au Secondary Endpoint: Overall-Survival (OS) Overall-survival was analysed at the time of the primary analysis. At this time, 276 deaths had been observed, less than the 300 deaths specified in the protocol. For this reason, the overall-survival data should be considered immature. The current analysis can be considered interim. The medians, hazard ratio and p-value are shown in the following table: FOLF(HA)IRI OS Median, months (95% CI) Hazard Ratio (95% CI) p-value (stratified log-rank test) FOLFIRI 14.3 (12.4, 16.8) 14.4 (12.4, 15.7) 0.90 (0.71, 1.15) 0.408 Key: CI = confidence interval Table 3: Secondary endpoint: overall-survival (OS). Unprecedented Performance of the Control Arm The performance of the FOLFIRI control arm in our trial was higher than any previously published values for second- and third-line treatment of mCRC. The most recent study was presented at the ASCO GI conference in January 2015 and reported values of 4.5 months for median PFS and 11.7 months for median OS (RAISE study1), as compared to the median PFS of 5.5 months and median OS of 14.4 months observed for the control arm in our study. A geographic breakdown (Table 4) of the results has revealed that this unprecedented control arm outcome was likely due to exceptionally strong PFS and OS outcomes for patients treated in Russia (Table 5). As seen in Table 5 the control-arm Russian patients when compared to the control-arm patients in the rest of the world performed significantly better in both PFS (p=0.04) and OS (p<0.001) highlighting the unexpected and unprecedented efficacy of FOLFIRI in this geographic territory. These patients accounted for 39.3% of the total patient population. Country Australia Bulgaria UK Poland Russia Serbia Ukraine FOLF(HA)IRI (n=207) 13 3 22 14 81 22 52 FOLFIRI (n=208) 22 6 23 12 82 25 38 TOTAL 35 (8.4%) 9 (2.2%) 45 (10.8%) 26 (6.3%) 163 (39.3%) 47 (11.3%) 90 (21.7%) Table 4: Phase III clinical trial patient distribution by country. Page 10 of 16 www.alchemia.com.au Median OS and PFS comparing Russia with ROW (months)* FOLFIRI Independent Review of PFS Russia (n=82) ROW (n=126) Difference 6.8 17.6 (95% CI: 5.6, 7.6) (68e, 14c) (95% CI: 15.4, 22.3) (49e, 33c) 4.3 Russia (n=81) ROW (n=126) Difference 11.4 (95% CI: 3.9, 5.6) (108e, 17c) (95% CI: 9.0, 14.0) (97e, 29c) +2.5 months +6.2 months 0.040 <0.001 p-value FOLF(HA)IRI OS 5.7 15.1 (95% CI: 4.2, 7.5) (64e, 15c) (95% CI: 11.1, 18.2) (51e, 30c) 5.3 14.0 (95% CI: 4.1, 5.7) (111e, 14c) (95% CI: 12.4, 16.9) (79e, 47c) +0.4 months +1.1 months 0.593 0.871 p-value *CRO generated data. Note: p-values calculated in-house at Alchemia on un-stratified data (to be verified by CRO). Key: e=event; c= censored; CI = confidence interval Table 5: Median PFS and OS in Russia and Rest Of World (ROW). The differences between the trial outcomes for patients treated in Russia and those in the rest of the world are shown below (Tables 6 and 7): Country Russia ROW All Countries Summary of PFS comparing Russia with ROW – ITT population* PFS per ARM in months Log Difference Rank HR FOLF(HA)IRI FOLFIRI p-value 5.7 6.8 -1.1 1.05 0.782 (95% CI: 4.2, 7.5) (95% CI: 5.6, 7.6) (95% CI:0.74,1.50) months (n=79: 64e, 15c) (n=82: 68e, 14c) 5.3 (95% CI: 4.1, 5.7) (n=125: 111e, 14c) 5.5 4.3 (95% CI: 3.9, 5.6) (n=125: 108e, 17c) 5.5 +1.0 months 0.0 (95% CI: 4.2, 5.9) (95% CI: 4.3, 6.7) months (n=107: 175e, 29c) (n=208: 176e, 31c) *CRO generated data. Key: e=event; c= censored; CI = confidence interval Table 6: Summary of PFS in Russia compared to the rest of the world. Country Russia ROW All Countries 0.785 0.92 (95% CI:0.69,1.21) 0.97 (95% CI:0.78,1.20) Summary of OS comparing Russia with ROW – ITT population* PFS per ARM in months Log Difference Rank HR FOLF(HA)IRI FOLFIRI p-value 15.1 17.6 -2.5 1.28 0.242 (95% CI: 11.1, 18.2) (95% CI: 15.3, 22.3) (95% CI:0.85,1.92) months (n=81: 51e, 30c) (n=82: 49e, 33c) 14.0 (95% CI: 12.4, 16.9) (n=126: 79e, 47c) 14.3 11.4 (95% CI: 9.0, 14.0) (n=126: 97e, 29c) 14.4 +2.6 months -0.1 (95% CI: 12.4, 16.8) (95% CI: 12.4, 15.7) months (n=207: 130e, 77c) (n=208: 146e, 62c) *CRO generated data. Key: e=event; c= censored; CI = confidence interval Table 7: Summary of OS in Russia compared to the rest of the world. Page 11 of 16 0.530 0.019 0.408 0.70 (95% CI:0.51,0.94) 0.90 (95% CI:0.71,1.15) www.alchemia.com.au To date, Alchemia has investigated a broad range of factors that might explain the Russian results for the control arm patients. However, none of these factors adequately explain the Russian results, but further investigation is ongoing. It was necessary for Alchemia with its Contract Research Organisation (CRO) to conduct this trial using Eastern European sites because of the difficulties associated with recruitment of patients in a commercially-relevant timeframe in most Western countries. After performing extensive recruitment feasibility studies, the major factors that would have made it impractical to conduct this study only in Western European and the USA were: • • Not using an antibody in the regimen. In the US and the majority of Western Europe FOLFIRI is used in combination with an antibody therapeutic (such as cetuximab or bevacizumab). This would have added another variable into the study that would have decreased the probability of clinical trial success. Time taken to conduct the study. We estimate the study would have taken at least four times longer to conduct due to competing studies in the US and Western Europe, which would have increased the cost substantially while significantly minimising the patent life, market-life and commercial viability of the drug. Alchemia implemented stringent monitoring and quality control processes in the conduct of this trial, including: • • • • • Double approval process for patient inclusion. Alchemia implemented a double approval process whereby Alchemia's US-based Chief Medical Officer had to review all patients and agree that they qualified for the study, as well as, the Medical Monitor. This risk mitigation process yielded valuable outcomes, as Alchemia's screen failure rate was high at about 30% (compared to the expected 10%). Double review and approval that patients had progressed in their disease. Alchemia implemented a system whereby all patient scans had to be reviewed by two site radiologists and they both had to agree that the patient had progressed. In addition, a US-based independent imaging group using two independent radiologists assessed patient disease progression. Double review and approval of drug preparation. To ensure that the drug was being prepared correctly, the Alchemia team trained and certified each pharmacy and implemented a system whereby a second pharmacist witnessed the preparation of each drug dose. This process was introduced to eliminate the chance of the wrong drug, or dosage, being administered to patients. Analysis of all drug doses. Every bag of drug prepared for use , other than those prepared by a central compounding facility, had a small sample withdrawn, which was sent to Alchemia for analysis to ensure that the drug had been prepared correctly. After unblinding of the database this will enable Alchemia to verify that patients received the correct drug and dosage of drug. Prior and continual auditing of clinical vendors and trial sites. Alchemia engaged independent, US-based auditors to conduct quality audits on all key trial vendors prior to commencing the study. In addition, when a site demonstrated a high recruitment rate the Page 12 of 16 www.alchemia.com.au sites were audited to ensure that they were conducting the study thoroughly and as per Good Clinical Practice (GCP) industry standards. Significance of Gender Unlike the Phase III trial where HA-I was part of a three-drug regimen (FOLFIRI), the HA-I Phase II trial was a comparison to assess the efficacy of monotherapy HA-I against standard monotherapy irinotecan. That trial found no difference between outcomes for male and female patients, although admittedly it was a small trial with 76 patients. Similarly there is no reference in the scientific literature to irinotecan having any gender related differences in its effectiveness. However, as the table below shows, certain subset analyses in the Phase III trial revealed that there were marked differences in this Phase III outcome between females and males (Table 8). The Phase III trial was not powered to show a statistically significant improvement in OS in the ITT analysis. The post-hoc subset analysis revealed improvement in median OS for females of 3.9 months that reached statistical significance. Median OS and PFS comparing gender differences (months)* ALL (n=415) Independent Review of PFS FOLF(HA)IRI (n=207) FOLFIRI (n=208) Difference p-value FEMALE (n=200) HR FOLF(HA)IRI (n=95) FOLFIRI (n=105) Difference p-value MALE (n=215) HR FOLF(HA)IRI (n=112) FOLFIRI (n=103) Difference p-value HR 5.5 (95% CI: 4.2, 5.9) (175e, 29c) 5.5 OS 14.3 (95% CI: 12.4, 16.8) (130e, 77c) 14.4 (95% CI: 4.3, 6.7) (167e, 31c) (95% CI: 12.4, 15.7) (146e, 63c) 0.0 months -0.1 months 0.785 0.408 0.97 (0.78, 1.20) 0.90 (0.71, 1.15) 6.0 (95% CI: 4.2, 7.4) (75e, 21c) 4.9 17.2 (95% CI: 12.9, 22.7) (54e, 41c) 13.3 (95% CI: 3.3, 6.7) (90e, 13c) (95% CI: 10.6, 15.4) (76e, 29c) +1.1 months +3.9 months 0.047 0.016 0.72 (0.52, 1.00) 0.64 (0.45, 0.92) 4.3 (95% CI: 3.2, 5.47) (102e, 9c) 5.7 13.1 (95% CI: 11.1, 15.4) (76e, 36c) 15.3 (95% CI: 4.4, 6.9) (86e, 17c) (95% CI: 11.8, 17.4) (70e, 33c) -1.4 months -2.2 months 0.181 0.292 1.23 (0.91, 1.66) 1.20 (0.85, 1.69) *CRO generated data Key: e=event; c= censored; CI = confidence interval Table 8: Median OS and PFS comparing gender differences. The PFS and OS Kaplan-Meier curves for females in the trial are shown below (Figure 1): Page 13 of 16 www.alchemia.com.au Females: Progression Free Survival and Overall Survival PFS (p=0.047; HR=0.72) OS (p=0.016; HR=0.64) FOLF(HA)IRI (75e,21c) Median 6.0 months (4.4; 7.0) FOLFIRI (90e,13c) Median 4.9 months (3.7; 6.1) FOLF(HA)IRI (54e, 41c) Median 17.2 months (12.9; 22.7) FOLFIRI (76e,29c) Median 13.3 months (10.6; 15.4) Figure 1: PFS and OS Kaplan-Meier curves for females in the Phase III trial. It should be noted that the magnitude of the improvement in median PFS for females, at just 1.1 months, was below the hoped for outcome based on the Phase II results (i.e. the trial was designed to have sufficient patients to provide a statistical significance for the entire population for a PFS improvement of ≥ 6 weeks with a hazard ratio of 0.73) and below the level of improvement that would be considered clinically meaningful. Conversely, the subset analyses suggest that males may have had a worse outcome on FOLF(HA)IRI than FOLFIRI. While the results were not statistically significant, the median PFS observed for the FOLF(HA)IRI arm was 1.4 months lower and 2.2 months lower for median OS. Further analysis revealed that this negative trend in males was likely driven by geographic factors in Russia. A substudy analysis excluding Russia yields no difference in median PFS and a slight positive trend in median OS for male patients. Accepting the limitations of post-hoc subset analyses and further sub-setting of subsets, after the removal of the Russian data set, there remains a statistically significant difference in performance between males and females on the study. The Phase III trial was conducted using the standard of care FOLFIRI regimen. In this regimen, irinotecan and leucovorin are administered intravenously, over a 90 minute period. After administration of these two drugs, a large dose of intravenous 5-Fluorouracil is administered to quickly raise the level of that drug in the body followed by a 48 hour infusion of a lower dose of 5-Fluorouracil. As stated above, there is nothing in the scientific literature to suggest any gender impact on the effectiveness of irinotecan as a chemotherapeutic agent. Similarly there is no evidence of any gender effect on the efficacy of leucovorin. However, there is evidence that there is a gender-based difference in the rate at which 5-Fluorouracil is metabolised, with the drug being metabolised significantly faster in men than in women. Page 14 of 16 www.alchemia.com.au Alchemia's working hypothesis to explain the unexpected outcomes in certain subsets relative to gender In preclinical studies HA-I has been shown to form a depot at the tumour site which persists for over 18 hours. It is believed that the formation of this depot enables the prolonged and increased uptake of irinotecan into the tumour. While Alchemia currently has no preclinical evidence that the formation of such depots might block access to tumours for other drugs given in combination with HA-I, based on the evidence from the Phase III trial, this may explain the observed gender differences seen in our trial. So, for example, in the FOLFIRI regimen, the HA-I is given first and builds up a depot at the tumour site during and after the infusion period. Our working hypothesis is that HA-I has at least ninety minutes to form a depot at the tumour site before the major infusion of 5-Fluorouracil begins. The higher clearance of the 5-Fluorouracil in men would suggest that, to the extent that the depot starts to break down over time and provide greater access to the tumour site, the remaining level of 5-Fluorouracil in the blood stream may be well below the desired therapeutic level. With women clearing the drug at a slower rate it is postulated that their levels of the drug would be higher as the HA depot breaks down and hence the 5-Fluorouracil would have a greater beneficial impact. If this hypothesis is correct, the beneficial impacts of the HA-I are being offset by the 5-Fluorouracil being less effective than would normally be the case. Further the impact of the reduced effectiveness of the 5-Fluorouracil would be significantly greater for males than for females. This may explain why females gain a statistically significant benefit from the incorporation of HA-I in the FOLFIRI regimen. This hypothesis would also suggest that the order of administration is important when HA-I is given as part of a multi-drug regimen. Alchemia has filed a patent application to protect this novel and unexpected finding. If granted, this will extend the Company's patent protection out to 2036. The Company's current patent protection expires in 2025. Further analysis of clinical samples from the Phase III study and preclinical studies investigating the effects of order of administration are being undertaken, which may provide a better understanding of the reasons behind the observed gender differences. It should be noted that this is a working hypothesis only and remains to be tested. Alchemia's working hypothesis to explain superior overall-survival outcomes in certain subsets relative to progression-free-survival: “The Tumor Stem Cell Hypothesis” Pre-clinical work by Alchemia suggests that HA binds to CD44 on the surface of cancer stem cells and hence that HA-formulated drugs would attack cancer stem cells at the tumour site and potentially also the cancer stem cells circulating in the bloodstream. By targeting and reducing the number of cancer stem cells (normally <2% of any tumour population) any short-term changes in the tumour would not necessarily be evident as the HA-I would have been eliminating at most an additional 2% of cells, a change that may not be detected by scanning of the patient tumour (how PFS is measured). The strong clinical benefit of killing cancer stem cells is always evident in the time it takes for a tumour to “regrow or re-populate” and this becomes evident in the overall-survival of the patient. Whilst the data Page 15 of 16 www.alchemia.com.au generated from the HA-I Phase III study are supportive of this hypothesis, it should be noted that this is a working hypothesis only and remains to be tested. As is evident from many of the post-hoc subset analyses presented here, the Phase III trial appeared to provide an overall-survival benefit, which was not predicted by the PFS. The subset analysis generated statistically significant outcomes even though the sample size was small and the trial was not designed to demonstrate such a clinical benefit in these subsets. Footnotes: 1. RAISE: A randomized, double-blind, multicenter phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab (RAM) or placebo (PBO) in patients (pts) with metastatic colorectal carcinoma (CRC) progressive during or following first-line combination therapy with bevacizumab (bev), oxaliplatin (ox), and a fluoropyrimidine (fp). Taberner0 J., et al. J Clin Oncol 33, 2015 (suppl 3; abstr 512) About Alchemia Limited Alchemia is a drug discovery and development company marketing fondaparinux, an FDA approved injectable antithrombotic, in the US and other markets, via partner Dr. Reddy's Laboratories. The Company has an oncology pipeline with several ongoing preclinical and development programs through its proprietary HyACT drug delivery platform, which targets anti-cancer drugs to solid tumours. HA-Irinotecan is in two Phase II investigatorsponsored trials, one of which is in collaboration with Merck Serono combining HA-Irinotecan with Erbitux® (cetuximab). In October 2014, Alchemia announced that its Phase III trial of HA-Irinotecan in Metastatic Colorectal Cancer did not meet its primary endpoint of statistically significant improvement in progression-freesurvival. Alchemia is also exploring additional small molecule drug discovery targets via an internal discovery platform VAST, based on the Company's deep chemistry expertise. The VAST technology is being developed in collaboration with leading academic institutions and is partnered with AstraZeneca AB. In December 2014, Alchemia announced the appointment of Evolution Life Science Partners to execute a strategic transaction by way of securing an investment and/or partner to accelerate the development of the VAST technology. Erbitux® is a trademark of Merck KGaA. Contacts and Website www.alchemia.com.au Alchemia Limited Tracie Ramsdale Executive Director Alchemia Limited Tel: +61 7 3340 0200 Investor Relations USA Candice Knoll Blueprint Life Science Group Tel: +1 415 375 3340 Ext. 105 [email protected] Page 16 of 16 www.alchemia.com.au
© Copyright 2026