362 Irreversible electroporation for prostate cancer: In vivo effect
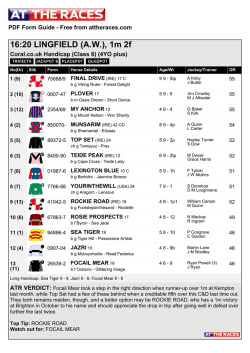
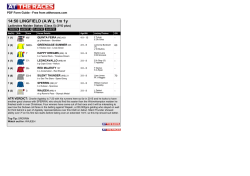
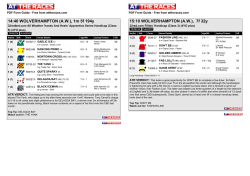
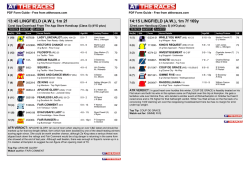
362 Irreversible electroporation for prostate cancer: In vivo effect characterization and numerical simulation Eur Urol Suppl 2014;13;e362 Print! Print! Neal 2nd R.E. 1 , Royce P. 2 , Millar J.L. 3 , Kavnoudias H.1 , Rosenfeldt F.4 , Pham A. 5 , Smith R.3 , Davalos R.V. 6 , Thomson K.R. 1 1 The Alfred Hospital, Dept. of Radiology, Melbourne, Australia, 2 The Alfred Hospital, Dept. of Urology, Melbourne, Australia, 3 The Alfred Hospital, William Buckland Radiotherapy Center, Melbourne, Australia, 4 The Alfred Hospital, Dept. of Surgery, Melbourne, Australia, 5 The Alfred Hospital, Dept. of Anatomical Pathology, Melbourne, Australia, 6 Virginia Tech, School of Biomedical Engineering and Sciences, Melbourne, Australia INTRODUCTION & OBJECTIVES: Irreversible electroporation (IRE) is a targeted ablation tumor therapy that uses needle electrodes placed into the targeted region to deliver a series of brief but intense electric pulses. These pulses destabilize the cellular membranes, killing cells in a manner that does not appear to damage the extracellular matrix or sensitive structures, such as blood vessels and neurovascular bundles. This makes it a promising prostate cancer treatment due to sparing of tissues associated with morbidity risk. IRE procedures exhibit sharp treatment demarcation, rapid lesion creation and resolution, and real-time monitoring of treatment effects. However, clinical IRE applications remain in their infancy, and treatment effects will vary with organ-dependent tissue electrical and biological properties, which will affect IRE treatment outcomes. This study examines resected prostates previously treated with IRE to characterize prostate tissue properties and histology to improve treatment planning and outcome predictions for IRE prostate cancer treatments. MATERIAL & METHODS: Clinically-relevant IRE pulse protocols were delivered through needle electrodes inserted directly into one healthy canine prostate and two human cancerous prostates. Electrical parameters were measured during pulse applications to determine tissue characteristics for creating numerical predictive treatment simulations. The canine prostate was resected five hours post-IRE, while the human prostates were resected during their regularly-scheduled prostatectomies at three and four weeks post-IRE. Histology and pathological lesions were used to evaluate treatment effects, and lesion dimensions were correlated with the numerical simulations to determine an effective prostate lethal IRE electric field threshold. RESULTS: Pulses were delivered to generate a total of five IRE lesions. Tissue electrical conductivity increased due to IRE pulses from 0.284 to 0.927 S/m from the models. Numerical simulations show an average prostate electric field threshold of 1072±119 V/cm, significantly higher than previously examined tissues. Histological findings in the human cases show instances of complete tissue necrosis centrally within the lesions, with additional variable effects, including reactive fibrosis and hemosiderin deposition. CONCLUSIONS: Preliminary IRE experimental trials safely ablated healthy canine and cancerous human prostates while examining treatment effects in the short- and medium-term. IRE-relevant prostate tissue properties are now defined, where it was found that the electric field necessary to kill healthy regions of prostate tissue is substantially higher than previously examined tissues. These findings can now be implemented to optimize future therapeutic prostate cancer IRE treatments.
© Copyright 2026