highly siderophile element geochemistry of impact
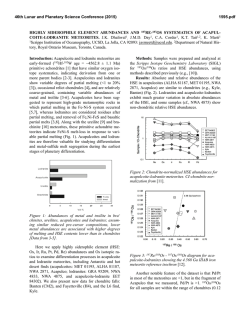
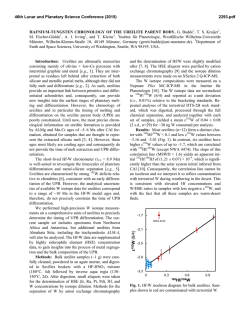
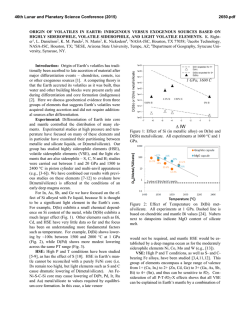
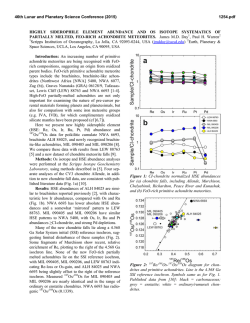
46th Lunar and Planetary Science Conference (2015) 1963.pdf HIGHLY SIDEROPHILE ELEMENT GEOCHEMISTRY OF IMPACT-RELATED GLASSES AND TARGET ROCKS FROM THE ZHAMANSHIN IMPACT STRUCTURE, KAZAKHSTAN. L. Ackerman1, K. Žák1, Š. Jonášová1, J. Ďurišová1, R. Skála1, T. Magna2 and A. Deutsch3, 1Institute of Geology, The Czech Academy of Sciences, v.v.i., Rozvojová 269, Prague 6, 165 00, Czech Republic. [email protected], 2Czech Geological Survey, Klárov 3, Prague 1, 118 21, Czech Republic, 3Institut für Planetologie, Universität Münster, WilhelmKlemm-Str. 10, Münster, D-48149, Germany. Introduction: Tektites are natural glasses, representing high-velocity distal impact ejecta genetically related to impacts of extraterrestrial bodies on the Earth’s surface while impact-related glasses originated as a result of extensive melting of target lithologies without ballistic transport. Origin and composition of both glass types is a result of complex processes which include composition of target rocks, character of the projectile and physical attributes of the elements [1-3]. Due to generally large differences in elemental concentrations between the Earth and extraterrestrial materials, distribution of highly siderophile elements (HSE – Os, Ir, Ru, Pt, Pd, Re) coupled with 187Os/188Os isotopic systematics represents a powerful tool for the discovery of meteorite impact structures, identification and estimation of amount of the extraterrestrial components in impact-related rocks and sometimes the classification of the projectile type [4-6]. Samples: The Zhamanshin Impact Structure (~1 Ma), Kazakhstan, has an estimated diameter of ~13–14 km and complex target lithology includes Paleogene sands, clays and locally sandstones which cover platform Cretaceous sandstones and marls. The lowermost part of the target is represented by Neoproterozoic to Upper/Lower Paleozoic volcanosedimentary series with conglomerates, sandstones and andesitic–basaltic volcanic rocks. Impact-related rocks can be subdivided into three different types [7,8]: (i) irghizite splashforms, SiO2-rich (~73 wt. %) glass bodies with complex shapes commonly formed by coalescence of <1 mm glass droplets (Fig. 1), (ii) basic (~54 wt. % SiO2) splash-forms with rather uniform tear-drop shapes or their fragments and (iii) impact glasses (zhamanshinite) which occur as irregular objects, including large blocks and their fragments with a wide range in chemical compositions, external shapes and internal structures. The basic splash-forms are chemically akin to impact glasses having similar SiO2 and trace element contents. In contrast, the irghizites are chemically and morphologically unique showing slightly elevated to very high contents of moderately siderophile elements Ni, Co and, in part, also Cr (up to ~2100 ppm, 115 ppm and 410 ppm, respectively). Content of nickel, and possibly also other siderophile elements, is not invariable thorough entire samples, nickel has been found concentrated along thin zones at rims of droplets forming the splash-forms, instead. In one sample, these rims contain elevated concentration of chromium as well. Methods and Results: We analyzed eight irghizites, four basic splash-forms, three impact glasses and five rocks from the target area (schist, quartzite, diabase, peridotite). HSE concentrations and 187Os/188Os isotopic ratios were obtained at the laboratories of the Institute of Geology CAS and Czech Geological Survey following protocol described in [9] modified by using desilification procedure [10]. Nickel, Co and Cr contents in splash-forms and target rocks were determined by laser ablation ICP-MS and solution nebulization ICP-MS, respectively. Nickel and Cr contents were monitored by EPMA. Fig. 1. Photograph of typical SiO2-rich irghizite of the splash-form type, Zhamanshin Impact Structure, Kazakhstan. With the exception of two samples (IZ-3 and IR-7) the HSE contents of irghizites are generally low with the lowest values found for I-PGE (Os–Ir–Ru) with the ranges 0.001–0.011 ppb Os, 0.030–0.16 ppb Ir and 0.26–0.57 ppb Ru. These values are similar to the estimates for the upper continental crust (UCC) [11]. In contrast, Pt and Pd contents reach up to 5.3 and 11.7 ppb, respectively, much higher than UCC, but similar to previous data [4]. Therefore, all these samples show similar, smooth HSE distributions with overall enrichments in Pt, Pd and Re over I-PGE (Fig. 2). Further, 46th Lunar and Planetary Science Conference (2015) the I-PGEs are strongly fractionated with OsN/IrN ranging from 0.01 to 0.26. The sample IZ-3 has similar HSE distribution, yet with much higher HSE levels. In contrast, the sample IR-7 significantly differs from other irghizites showing almost flat HSE distribution from Ir to Cr with the absence of I-PGE fractionation. The basic splash-forms exhibit highly fractionated patterns (Fig. 2) with common Ir enrichments over Os and Ru (OsN/IrN <0.02 and RuN/IrN = 0.04–0.7). One sample (IR-13) differs in being significantly enriched in all HSE and with almost no HSE fractionation. These features may relate to the occurrence of small inclusions of Fe- and Ti-bearing oxides (spinel-group phase), which make this sample specific. All basic splash-forms are characterized by enrichment in Co (Fig. 2). The 187Os/188Os isotope ratios of irghizites and basic splash-forms overlap and range from subchondritic to superchondritic values of 0.115–0.151. Fig. 2. C1-normalized HSE patterns of irghizites and basic splash-forms. C1 values from [12,13] The impact glasses have HSE distributions similar to irghizites, but at much lower contents. However, the most striking difference is much lower Ni concentrations in comparison to irghizites. They are also characterized by much higher, superchondritic 187Os/188Os 1963.pdf between 0.463 and 0.673. All analyzed target rocks with the exception of a peridotite have similar or even lower HSE and Ni–Co–Cr concentrations as UCC. The peridotite exhibits HSE distribution similar to that of the primitive upper mantle [14]. Discussion: Very low HSE contents detected in basic splash-forms and impact glasses suggest no or very limited addition of extraterrestrial material to these rocks. On the contrary, the irghizites exhibit complex HSE, Ni, Co and Cr systematics. Ruthenium, Pd, Pt and Ni levels are much higher than those in target rocks and average UCC strongly supporting the idea of their addition from projectile. In contrast, very low Os contents and non-chondritic HSE distribution require either projectile with fractionated HSE (e.g., some irons [15]) or, most likely, post impact HSE fractionation. The separation of Fe, Ni, Co and Cr from HSE elements would require a complex process including selective evaporation of part of the impactor matter followed by condensation supporting earlier hypothesis for irghizite formation [8,16]. This is also supported by our detailed microprobe study of irghizite droplets showing highly variable Ni and Cr contents, with rims of individual droplets strongly enriched in these elements and also in Fe. The irghizite IR-7 with flat HSE distribution is characterized by presence of abundant solid inclusions including among others almost completely dissolved particles of Ni-Fe sulfides less than 500 nm in diameter which may represent fragments of Ni-Fe sulfidic melts enriched in HSE not so strongly fractionated during evaporation and condensation processes. Acknowledgements: This research was supported by the Grant Agency of the Czech Republic (project no. 13-22351S). References: [1] Koeberl C. 2014, Treatise in Geochemistry, Volume 2, 73-117. [2] Magna T. et al. 2011, GCA, 75, 2137-2158. [3] French B.M. and Koeberl C. 2010, Earth Sci. Rev., 98, 123-170. [4] Palme H. et al. 1981, GCA, 45, 2417-2424. [5] Koeberl C. and Shirey S.B. 1993, Science, 261, 595-598. [6] Lee S.R. et al. 2006, Met. Planet. Sci. Lett., 41, 819-833. [7] Florenskii P.V. 1977, Chem. Erde, 36, 83-95. [8] Mizera Z. et al. 2012, J. Radioanal. Nucl. Chem., 293, 359-376. [9] Mundl A. et al. 2015 Geology, 43, 39-42. [10] Ishikawa A. et al. 2014, Chem. Geol., 384, 27-46. [11] Rudnick R.L. and Gao S. 2014, Treatise in Geochemistry, Volume 4, 1-51. [12] Jochum K.P. 1996, GCA, 60, 3353-3357. [13] Anders E. and Grevesse N. 1989, GCA, 53, 197-214. [14] Becker H. et al. 2006, GCA, 70, 4528-4550. [15] Pernicka E. and Wasson J.T. 1987, GCA, 51, 1717-1726. [16] Florenskii P.V. and Dabizha A.I. 1980, Nauka. Moscow, 1-128,
© Copyright 2026