C:\Users\rzellmer\My Documents\My Documents\zellmer\chemistry
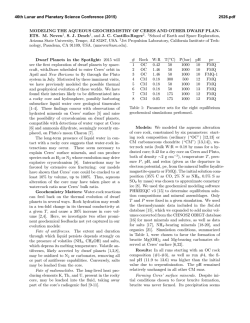
Copyright RJZ 1/30/15 1 no unauthorized use allowed Chemistry 1250 - Sp15 Practice Midterm 1 Copyright RJZ 1/30/15 1. 2. If only one substance is present the material must be uniform throughout. Some pure substances can be decomposed into simpler pure substances. Heterogeneous mixtures may not contain elements. Every compound is a pure substance. A heterogeneous mixture must contain at least two different substances. Choose from the following list those properties that are physical properties of zinc. A. B. C. D. E. a) A 3. no unauthorized use allowed Which of the following statements is INCORRECT? a) b) c) d) e) This material is copyrighted. Any use or reproduction is not allowed except with the expressed written permission of Dr. Zellmer. If you are taking Chem 1250 you are allowed to print one copy for your own use during the semester you are taking Chem 1250 with Dr. Zellmer. You are not allowed to disseminate this material to anyone else during the semester or in the future. 2 It corrodes in moist air. It is a grey metal. It melts at 419EC. It reacts with acid to give hydrogen gas It conducts electricity. b) C c) A, D d) C, E e) B, C, E Do the indicated arithmetic and give the answer to the correct number of significant figures. (14.9 x 0.049) - (3.53 ÷ 0.0840) + 121.600 a) 80.295 4. c) 8.0 x 101 b) 80.29 d) 80 e) 80.3 A crucible is known to weigh 24.3162 g. Three students in the class determine the weight of the crucible by repeated weighings on a simple balance. Which of the conclusions summarizes the data? Student A Student B Student C a) b) c) d) e) trial 1 24.8 24.6 24.5 trial 2 24.9 24.0 24.1 trial 3 24.7 24.2 24.5 trial 4 24.9 24.1 24.1 trial 5 24.8 24.3 24.3 student B has done the most precise work and student C the most accurate student B has done the most precise work and student A the most accurate student C has done the most precise work and student B the most accurate student C has done the most precise work and student A the most accurate student A has done the most precise work and student C the most accurate Copyright RJZ 1/30/15 5. 3 Alka Seltzer tablets contain 2.1 x 10 mg of sodium bicarbonate. How many tablets can be made from 5.5 kg of sodium bicarbonate? a) 2.6 x 105 b) 2.6 x 104 3 2 d) 5.1 x 10 6. Copyright RJZ 1/30/15 8. c) 2.6 x 103 4 no unauthorized use allowed A 27.40-g sample of osmium is placed in a graduated cylinder containing 40.00 mL of water and the water level rises to 41.22 mL. What is the density (in g/cm3) of the sample of osmium? (Look closely at the answers & be careful!) a) 33.43 b) 33.4 c) 22.46 d) 22.5 e) 0.045 e) 5.1 x 10 The lethal dose of the drug strychnine taken orally is 5.0 mg per kilogram of body weight in rats. Calculate the lethal dose in grams for a 140 lb person, assuming that a human functions the way rats do. (1 lb = 453.6 g) a) 0.11 7. no unauthorized use allowed 3 b) 0.32 c) 0.65 d) 1.5 9. Which of the following is the HIGHEST temperature? a) -4EF b) -25EC c) 250 K e) 28 The world's oceans contain approximately 1.4 x 109 km3 of water. What is the volume in liters? a) 1.4 x 1027 b) 1.4 x 1021 d) 1.4 x 1015 e) 1.4 x 1012 c) 1.4 x 1018 10. Select the combination of statements which are CORRECT. 1) The mass number of an atom is the sum of the number of neutrons and protons in the nucleus. 2) The number of neutrons in atom is its atomic number. 3) The number of electrons and protons in a neutral atom are equal. 4) The volume occupied by the nucleus is a small percentage of the total volume of the atom. 5) Isotopes of an element differ only in the number of protons. a) 2, 3 b) 1, 3, 4 c) 1, 2, 3 d) 1, 4, 5 e) 4, 5 Copyright RJZ 1/30/15 11. 5 no unauthorized use allowed Rubidium (atomic weight 85.4678) has two naturally-occurring isotopes, the predominant one being 85Rb Copyright RJZ 1/30/15 14. with isotopic weight 84.9117 and an abundance of 72.15%. Which of the following isotopic weights is the most likely for the other isotope? (Hint: You are solving for the mass of this other isotope.) a) 89.8999 b) 88.9201 c) 87.9124 d) 86.9092 + a) 3 15. 12. Fe2(CO3)3 b) sulfur hexafluoride, SF6 c) diphosphorus tetroxide, P2O4 d) zirconium (IV) iodate, Zr4(IO3) e) zinc bisulfate, Zn(HSO4)2 BiCl3 + a) 11 16. 13. Yttrium nitrate is Y(NO3)3. What are the formulas of yttrium carbonate and yttrium arsenate? (Assume the charge on the yttrium has the same charge in all compounds.) a) Y2(CO3)3, YAsO4 b) YCO3, YAsO4 c) Y3(CO3)2, Y3AsO4 d) Y2(CO3)3, Y2(AsO4)3 e) YCO3, Y2(AsO4)3 NH3 + b) 4 H2O v c) 5 Fe(OH)3 + d) 6 NH4NO3 e) 7 Balance the following equation. What is the sum of the coefficients of the reactants AND products. (If present, don't forget the coefficients of 1.) Which of the following pairs of names and formulas is INCORRECT? a) iron (III) carbonate, no unauthorized use allowed Balance the following equation and choose the answer which is the sum of the coefficients of the REACTANTS. (If present, don't forget the coefficients of 1.) Fe(NO3)3 e) 85.9142 6 NH3 + v H2 O b) 10 Bi(OH)3 + c) 9 NH4Cl d) 7 e) 4 Balance the following equation and choose the quantity which is the sum of the coefficients of REACTANTS AND PRODUCTS. (If present, don't forget the coefficients of 1.) NH3 a) 12 b) 15 + O2 c) 19 v NO d) 20 + H2 O e) 22 Copyright RJZ 1/30/15 17. 18. 7 no unauthorized use allowed Carbonic anhydrase, which catalyzes the interconversion of carbon dioxide and hydrogen carbonate to maintain acid balance in blood, contains one zinc atom per molecule and the zinc is 0.121% by mass. What is the molar mass (g/mole) carbonic anhydrase? (Atomic weight: Zn - 65.38) a) 5.40 x 104 b) 7.91 x 104 d) 5.40 x 102 e) 7.91 x 102 Copyright RJZ 1/30/15 20. c) 1.67 x 104 no unauthorized use allowed A 0.589 g sample of an organic compound containing only carbon, hydrogen and oxygen was burned completely in air to produce 0.733 g of CO2 and 0.299 g of H2O. What is the empirical formula of the compound? (Atomic weights: C = 12.01, H = 1.008, O = 16.00) a) C2H4O2 b) C3H6O5 c) C3H5O2 d) C3H6O2 Cisplatin, an anticancer drug, has the molecular formula Pt(NH3)2Cl2. How many moles of hydrogen atoms are in 2.8 x 10-4 g of cisplatin? (At. wts: Pt = 195.1, H = 1.008, N = 14.01, Cl = 35.45 ; Mol. wt: 300.07) a) 5.6 x 10-6 b) 5.6 x 10-3 d) 2.8 x 10-3 e) 2.8 x 10-6 c) 1.9 x 10-6 21. 19. 8 Which of the following are weak electrolytes? Potassium carbonate has the formula, K2CO3. How many potassium ions are present in 0.10 g of K2CO3? (At. Wts.: C = 12.01, O = 16.00, K = 39.10; Form. Wt.: K2CO3 = 138.21) H3PO4 a) 8.7 x 1020 b) 1.7 x 1025 a) H3PO4, NH3, Ni(NO3)2 d) 4.4 x 1021 e) 8.3 x 1024 c) 4.4 x 1020 HNO3 Ni(NO3)2 b) H3PO4, NH3 c) HNO3, Ni(NO3)2, NaOH d) HNO3, Ni(NO3)2, NH3 e) none are weak electrolytes NaOH NH3 e) C3H6O4 Copyright RJZ 1/30/15 22. 9 no unauthorized use allowed Barium hydroxide reacts with phosphoric acid according to the following equation. Which substance is the limiting reagent when 0.50 mol of Ba(OH)2 reacts with 0.50 mol of H3PO4? How many moles of the excess reagent remain after completion of the reaction? Copyright RJZ 1/30/15 25. 3 Ba(OH)2 (s) + 2 H3PO4 (aq) v Ba3(PO4)2 (aq) + 6 H2O (R) a) H3PO4 ; 0.17 b) H3PO4 ; 0.33 d) Ba(OH)2 ; 0.25 e) Ba(OH)2 ; 0.33 10 no unauthorized use allowed Solutions of lead(II) nitrate and potassium chromate are mixed. Select the correct NET IONIC reaction from those given below. a) No reaction occurs b) Pb2+ + 2 NO3! 6 Pb(NO3)2 c) Ba(OH)2 ; 0.17 c) Pb2+ + CrO42! 6 PbCrO4 d) 2 K+ + 2 NO3! 6 2 KNO3 e) 2 K+ + CrO42! + Pb2+ + 2 NO3! 6 2 K+ + 2 NO3! + PbCrO4 26. What are the expected products of the following reaction? CaCO3 (s) + H2SO4 (aq) 23. v a) Ca(OH)2 + SO3 + CO2 Which of the following is (are) an example(s) of an single-replacement (displacement) reaction (assume all reactons occur to give products)? b) CaSO4 + H2 + CO3 1) Al (s) + Cl2 (g) 6 c) 4 CaO + 4 CO2 + H2S 2) Pb(NO3)2 (aq) + NH4Cl (aq) 6 d) CaCO3H2SO4 3) PCl3 (g) + Cl2 (g) 6 e) CaSO4 + H2O + CO2 4) Zn (s) + HCl (aq) 6 5) HCl (aq) + CaS (aq) 6 a) 2 only 24. b) 3 only c) 4 only d) 1 and 4 e) 2 and 5 Which of the reactions in question 23 is (are) an example(s) of a combination reaction (assume all reactions occur to give products)? a) 1 only b) 3 only c) 4 only d) 1 and 3 e) 2 and 5 27. What volume of 0.141 M H3PO4 is required to neutralize 50.0 mL of 0.0521 M Mg(OH)2 ? 3 Mg(OH)2 + 2 H3PO4 a) 27.1 mL b) 12.3 mL v c) 40.6 mL Mg3(PO4)2 + 6 H2O d) 18.1 mL e) 35.8 mL Copyright RJZ 1/30/15 28. 11 no unauthorized use allowed Copyright RJZ 1/30/15 12 USEFUL INFORMATION Arrange the following phosphorus containing species in order of increasing oxidation number of the phosphorus atom. What compound occupies the intermediate (middle) position? a) P4 b) PH2 & c) HPO3 2& d) P2H4 e) PO4 no unauthorized use allowed 1 in = 2.54 cm 3& 1 mile = 5280 ft 1 lb = 453.6 g 1 qt = 946 mL 1 lb = 16 oz 1 amu = 1.66 x 10-24 g Avogadro's number = 6.02 x 1023 particles/mole 29. -10 1 Å = 1 x 10 Which of the following represent redox reactions? -8 m = 1 x 10 cm 1. Cu(OH)2(s) + 2 HNO3 (aq) 6 Cu(NO3)2 (aq) + 2 H2O(R) 2. Cl2(aq) + 2 NaI (aq) 6 I2 (aq) + 2 NaCl(aq) IA 3. 3 Fe(NO3)2(aq) + 2 Al(s) 6 3 Fe(s) + 2 Al(NO3)3 (aq) a) 1 and 2 b) 2 and 3 c) 1 and 3 IIA IIIB IVB VB VIB VIIB VIIIB IB IIB d) all e) none 2 IVA VA VIA 4 9.012 Be 10.811 12.011 14.007 15.999 18.998 20.179 B C N O F Ne 5 6 7 8 9 10 4 22.990 24.305 Na Mg 12 39.098 K 19 5 Examine the reaction below and the statements concerning the reaction. Select an answer which includes ALL of the CORRECT statements given below. 85.47 Rb 37 6 26.98 Al 40.08 Ca 20 44.96 Sc 21 87.62 Sr 38 13 88.91 Y 39 47.88 Ti 22 81.22 Zr 40 50.94 V 23 92.91 Nb 41 52.00 Cr 24 54.94 Mn 25 95.94 Mo 42 55.85 Fe 26 98 Tc 43 58.93 Co 27 28.09 Si 14 30.974 P 15 32.06 S 16 58.69 63.546 65.38 69.72 72.59 74.92 78.96 79.904 83.80 Ni Cu Zn Ga Ge As Se Br Kr 29 30 31 32 33 34 35 36 101.07 102.91 106.42 107.87 112.41 114.82 118.69 121.75 127.60 126.90 131.39 Ru Rh Pd Ag Cd In Sn Sb Te I Xe 45 46 47 48 49 50 51 52 53 54 44 132.91 137.33 138.91 178.39 180.95 183.85 186.21 190.23 192.22 195.08 196.97 200.59 204.38 207.2 208.98 209 Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 1) 2) 3) 4) 5) + 20 BrF3 v 12 IF5 + 15 O2 + b) 3 c) 2, 3 226.03 227.03 261 Ra Ac Rf 89 104 88 262 Ha 105 263 Sg 106 262 Ns 107 265 Hs 108 266 Mt 109 269 110 272 111 210 At 85 277 112 10 Br2 Br has been oxidized. F is reduced. The oxidation number of Br changed from +3 to 0. O has been oxidized. The oxidation number of F changed from -1 to 0. a) 2, 4 223 Fr 87 35.453 39.948 Cl Ar 18 17 28 55 7 d) 3, 4 VIIIA 4.003 He 11 6 I 2 O5 VIIA 2 6.941 Li 3 3 30. IIIA 1.008 1 H 1 140.12 140.91 144.24 145 Ce Pr Nd Pm 59 60 61 Lanthanide Series 58 Actinide Series 90 150.36 151.96 157.25 158.93 162.50 164.93 167.26 168.93 173.04 173.04 Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 63 64 65 66 67 68 69 70 71 62 232.04 231.04 238.03 237.05 Th Pa U Np Pu 91 92 93 94 Am 95 Cm 96 Bk 97 Cf 98 A PERIODIC CHART OF THE ELEMENTS (Based on 12C) e) 1, 3, 5 Copyright R. J. Zellmer, January 30, 2015 Es 99 Fm 100 Md 101 No 102 Lr 103 222 Rn 86 Copyright RJZ 1/30/15 13 no unauthorized use allowed Copyright RJZ 1/30/15 SOLUBILITY Soluble None nitrates, acetates, chlorates, perchlorates, permanganates Soluble None chlorides, bromides, iodides Soluble Cmpds of Ag+, Hg22+, Pb2+, Hg2+ iodide and Hg2+ bromide sulfates Soluble Cmpds of Sr2+, Ba2+, Hg22+, Pb2+ hydroxides, oxides, sulfides Insoluble Cmpds of alkali metals (grp 1A) , ammonium, Ca2+, Sr2+, Ba2+ sulfites, carbonates, phosphates, chromates Insoluble Cmpds of alkali metals (grp 1A) , ammonium Copyright R. J. Zellmer, January 30, 2015 Answers to Practice Midterm 1 IMPORTANT EXCEPTIONS alkali metal (grp 1A) ammonium no unauthorized use allowed Chemistry 1250 EMPIRICAL RULES FOR THE SOLUBILITY OF IONIC SOLIDS IN H2O COMPOUNDS CONTAINING 14 1) C 11) D 21) B 2) E 12) D 22) C 3) E 13) A 23) C 4) E 14) E 24) D 5) C 15) A 25) C 6) B 16) C 26) E 7) B 17) A 27) B 8) D 18) A 28) A 9) A 19) A 29) B 10) B 20) E 30) D
© Copyright 2026