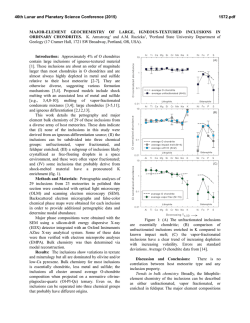
The Kidney Pathology of Fabry Disease
Sixth Congress of Nephrology in Internet – CIN '2011 CIN2011 DIAGNOSTIC APPROACH AND PATHOLOGY OF FABRY NEPHROPATHY. João Paulo Oliveira Departamento de Genética & Unidade de Investigação e Desenvolvimento em Nefrologia Centro Hospitalar de São João & Faculdade de Medicina, Universidade do Porto Porto, Portugal. For many years following the original case reports, the illness independently described in 1898 by William Anderson and Johannes Fabry was regarded as a pure dermatological disorder, descriptively named as „Angiokeratoma Corporis Diffusum’. Although the young males reported by Anderson and Fabry had proteinuria, and despite the recurrent observation of urinary abnormalities in further patients, it was not until the late 1940s and early 1950s, when the first autopsy studies of severely affected males were performed, that the involvement of the kidneys, – as well as of the heart and blood vessels –, were recognized as major pathological features of this disease, providing unequivocal evidence of its systemic nature. In the next two decades, light microscopy, electron microscopy and biochemical studies of kidney tissue in autopsy material and in biopsy samples greatly contributed to the understanding of the biological bases of Fabry disease and of its histological expression. Furthermore, percutaneous kidney biopsy was definitely established as a valuable diagnostic method, particularly in patients lacking the distinctive skin manifestations, in patients without family history of Fabry disease and in females. The purpose of this presentation is to summarize the current approach to the diagnosis of the kidney involvement in Fabry disease – the “Fabry nephropathy” –, giving special emphasis to the value of kidney biopsy as a diagnostic as well as a prognostic tool, and to review in detail the current knowledge about the kidney pathology of Fabry disease. This way, I hope to contribute to raise the awareness about Fabry nephropathy among clinical nephrologists and pathologists, in order to avoid missing or delaying its diagnosis, at a time when early institution of enzyme replacement therapy (ERT), before the occurrence of irreversible lesions, is the most effective treatment available for this rare, but severe and incapacitating disorder. 1 Overview of Fabry disease – biochemical, genetic, clinical and epidemiological aspects CIN2011 Fabry disease is the lysosomal storage disorder that results from an inborn deficiency of the activity of alpha-galactosidase, a glycoside hydrolase involved in the catabolic processing of globotriaosylceramide (Gb3Cer) and of other minor neutral glycosphingolipids that contain terminal, non-reducing alpha-D-galactose residues. Progressive accumulation of Gb 3Cer in vascular endothelia leading to obstruction of the microvasculature and ischemic tissue damage, particularly to the brain, heart and kidneys, has been advocated as the major pathogenic mechanism underlying the systemic complications of the disease. Since the locus of the alpha-galactosidase gene (GLA) is on the X-chromosome, band Xq22.1, affected males are hemizygous for a pathogenic GLA mutation while almost all affected females are heterozygous for the normal allele and a pathogenic mutation. The in vitro demonstration of deficient alpha-galactosidase activity in cell protein extracts (usually from leukocytes) or in biological fluids (usually plasma), utilizing a synthetic fluorogenic substrate, is a straightforward diagnostic test for Fabry disease in males. However, by the effect of random X-chromosome inactivation, females carrying pathogenic GLA mutations in heterozygosity are mosaics of cells, the large majority of which express either the normal or the mutated enzyme. The relative proportion of these two cell populations in specific organs and tissues is a major determinant of the ultimate pathological and clinical phenotype, including of the level of enzyme activity measured in vitro. For this reason, the enzymatic diagnosis of Fabry disease is not reliable in females since carriers of pathogenic GLA mutations may have normal enzyme activity, as conventionally assayed in leukocytes or plasma, and the finding of pathogenic GLA mutations is the gold-standard diagnostic test for Fabry disease in females. In males, the precocity and severity of the clinical manifestations of Fabry disease are related to the degree of alpha-galactosidase deficiency. Patients carrying GLA mutations associated with absent or very low levels (usually ≤1% normal) of residual enzyme activity, who have the so-called „classical phenotype‟, typically present in childhood or adolescence with acroparesthesias, hypohidrosis, abdominal pain, diarrhea, angiokeratomas and cornea verticillata. The early symptoms experienced by the classically affected children and teenagers largely reflect the involvement of the peripheral and autonomic nervous systems but, with advancing age, these patients eventually develop major renal, cardiac and cerebrovascular complications. Indeed, in young- and middle-aged adults, end-stage renal disease (ESRD), arrhythmias, left ventricular hypertrophy and myocardial infarction, transient ischemic attacks and stroke are major causes of the morbidity and premature mortality associated with Fabry disease. The late complications of Fabry disease are thought to result from as yet elusive non-specific secondary pathogenic processes, most likely related to the continuous accumulation of Gb3Cer in those organs. When the residual enzyme activity is higher (usually <5-30% normal), patients present later in life with renal disease and/or cardiac disease, lacking the early, distinctive skin and neuropathic manifestations of the „classical phenotype‟. The expression of renal disease in 2 patients with the „renal variant‟ is clinically indistinguishable from that of the „classical phenotype‟. Although patients with the „renal variant‟ also have cardiac complications, patients with the „cardiac CIN2011 variant‟ usually do not develop ESRD. As the residual enzyme activity in patients with the „cardiac variant‟ is significantly higher than in patients with the „renal variant‟, the alpha-galactosidase activity threshold for progressive kidney disease seems to be lower than for heart disease. Presumably as a beneficial effect of the cellular mosaicism created by X-chromosome inactivation, females commonly have less severe forms of Fabry disease as compared to males carrying the same GLA mutations, although a significant number of heterozygotes may ultimately experience major cardiac, cerebrovascular, or renal clinical events. Because of its rarity, the diagnosis of classical Fabry disease has been frequently overlooked in clinical practice, particularly in patients not previously known to be at genetic risk. The diagnosis of the incomplete, later-onset variants of Fabry disease is even more difficult to recognize, requiring a high index of suspicion. Recently, large-scale newborn screening programs have estimated the population frequency of GLA mutations associated with the severe phenotype as ~1:35.000-40.000; however, the overall allelic frequency of mutations associated with the later-onset phenotypes was estimated as at least >10-fold higher. Until a decade ago, the treatment of patients with Fabry disease was limited to the non-specific supportive management of disease complications. Although the available preventive and symptomatic therapies permitted to alleviate the patients‟ morbidity and improved life expectation, they were of limited success and did not address the underlying metabolic cause of Fabry disease. The recent advent of ERT with genetically engineered human alpha-galactosidases (agalsidase alfa, agalsidase beta) was a major advance, but its clinical efficacy seems to be critically dependent on early initiation, before irreversible lesions occur. Therefore, and despite its rarity, clinicians are now challenged with the new responsibility of having to be more aware of Fabry disease, in order to expedite its diagnosis. Since patients with Fabry disease may seek care from a variety of medical specialties, due to the involvement of multiple organ systems, this applies to many different specialists, including clinical nephrologists. Clinical manifestations of Fabry nephropathy Chronic kidney disease (CKD) with proteinuria and progressive azotemia is one of the prominent features of classical Fabry disease and of the later-onset renal variant. As the renal function deteriorates blood pressure increases in many patients but, at comparable CKD stages, the prevalence of hypertension in patients with Fabry disease is lower than in the general CKD population. Proteinuria is of glomerular origin in almost all cases, albumin representing its major component (≥50%). The prevalence of proteinuria increases with age, being relatively uncommon in children and teenagers. However, by the age of 35 years, ~50% of the affected males are estimated to have proteinuria, and all patients who survive into the 6th decade of life eventually develop proteinuria. Nephrotic range proteinuria occurs in <20% of male Fabry patients with CKD, but the full3 blown presentation of nephrotic syndrome is relatively unusual. Death from ESRD on the 4th or early on the 5th decade of life was a frequent outcome of affected males before the advent of chronic CIN2011 dialysis and kidney transplantation, but since effective renal replacement therapies became available the median age of death has increased by 10-15 years. At early adulthood, most males with Fabry disease have normal glomerular filtration rate (GFR), as estimated from the serum creatinine level (eGFR), but cases of ESRD have exceptionally been reported in teenagers. In affected males, the risk and rate of CKD progression are related to the level of residual alpha-galactosidase activity. Early on their 40s, about half the patients with ≤1% residual enzyme activity already have serum creatinine levels ≥1.5 mg/dl (corresponding to estimated CKD stage 3 or higher), while those with higher enzyme activity levels seldom reach stage 3 CKD before the age of 45 years. The rate of decline of the eGFR is more than twofold higher in patients with advanced CKD (stage 3 or higher) as compared to patients with eGFR >60 ml/min/1.73m2 at baseline. Time of progression from CKD stage 3 to ESRD is quite variable, ranging from ~1 year to more than 12 years. In patients with advanced CKD, typical natural history eGFR average decline rates range between –6.5 to –10.5 ml/min/1.73m2. Higher levels of baseline proteinuria are also positively associated with more rapid progression to ESRD. Virtually all males with the classical phenotype who survive to their mid-50s develop ESRD. Only a minority of patients reach advanced CKD stages without developing overt proteinuria. Heterozygous females have a significantly lower risk of ESRD as compared to the hemizygous males, and in both the European and the United States ESRD registries only ~12% of the patients with the diagnosis of Fabry disease were women. However, females with Fabry disease who have progressive CKD, reach ESRD at similar age ranges than the Fabry male patients. Additional laboratory manifestations of Fabry nephropathy include various defects of tubular function and abnormalities of the urine sediment. The most commonly reported functional tubular defect is an impairment of the urinary concentration mechanisms in the distal tubule, which has been demonstrated even in patients with normal GFR. The urine concentrating defect may lead to isosthenuria, causing symptoms of polyuria, nocturia, and polidipsia. Since early in the natural history of Fabry nephropathy, red blood cells, white blood cells, oval fat bodies, hyaline and granular casts may be present in the urine sediment. About 75% of the exfoliated cells in the urine of classically affected Fabry patients are glycosphingolipid-laden renal tubular cells, which can be recognized as oval fat bodies in the urine sediment. Glycosphingolipid globules may also be seen free in the urine, as fat droplets. Like the oval fat bodies commonly seen in the context of heavy proteinuria, these droplets, as well as the glycosphingolipid inclusions in tubular cells, are birefringent, displaying a „Maltese cross‟ pattern when viewed under polarized light microscopy. However, the oval fat bodies that correspond to glycosphingolipid-laden renal tubular cells in Fabry disease additionally have a characteristic internal lamellar appearance that allows its specific identification. Parapelvic renal cysts are more prevalent among adult males with Fabry disease than among individuals in the general population and their finding the diagnostic workup of a patient with CKD should lead to the consideration of Fabry disease in the differential diagnosis. 4 Diagnosis of Fabry nephropathy Non-invasive diagnostic approach CIN2011 In patients diagnosed with Fabry disease, or in individuals at genetic risk for Fabry disease, proteinuria and progressive CKD are generally assumed to be evidence of Fabry nephropathy. This clinical assumption can be non-invasively corroborated by assaying specific metabolic biomarkers in the urine and/or by targeted urine microscopy examination. Increased urinary excretion of Gb 3Cer and/or the finding of lysoGb 3 (a deacylated metabolite of Gb3Cer) in the urine support the non-invasive diagnosis of Fabry nephropathy in patients both genders, including in children. Urinary Gb 3Cer is consistently elevated in virtually all Fabry patients, including heterozygotes, when measured in the sediment of a 24-hour urine collection, and in most patients when measured in a random sample of whole urine or in urine collected on a filter paper. Urine Gb3Cer levels are highest in patients with null GLA mutations and no residual enzyme activity and lower in patients with significant residual alpha-galactosidase activity and in heterozygotes. In these latter cases, urine Gb3Cer may even be within the normal range when measured in random samples of whole urine. Urinary excretion of Gb 3Cer does not correlate with patient age. The lysoGb3 assay in the urine is technically more demanding than the Gb 3Cer assay but is more specific as a diagnostic test for Fabry nephropathy, because lysoGb 3 is not excreted in the urine of healthy subjects whereas low amounts of Gb 3Cer (≤25 μg/mmol creatinine) are normally found in the urine. In patients who have never received ERT, the lysoGb3/creatinine ratios in the urine are ~6000 and ~1000 times less than the corresponding Gb 3Cer/creatinine ratios, respectively in males and females. The urinary lysoGb3/creatinine and Gb3Cer/creatinine ratios are strongly correlated. Like the urine levels of Gb3Cer, the urinary lysoGb3/creatinine ratio correlates with gender and the type of GLA mutation, which is a surrogate for residual alpha-galactosidase activity. Fresh urine sediment examination under phase-contrast microscopy with polarized light, together with immunocytochemistry for Gb3Cer, have recently been proposed as a cheap, sensitive and specific diagnostic test for Fabry disease. Patients with Fabry nephropathy excrete birefringent oval fat bodies which have characteristic internal lamellation and irregular surface protrusions, ranging in shape from hook-like to spherical. Under polarized light, separate „Maltese crosses‟ are visible in the main body of these particles as well as in the larger protrusions. Such morphological features, which are best appreciated with minor adjustments in focal length, allow to specifically discriminating these particles from the birefringent oval fat bodies commonly found in heavy proteinuric patients. Furthermore, immunocytochemistry of the urine sediment using a standard immunoperoxidase technique with a monoclonal anti-Gb3Cer primary antibody positively stains large vacuolated mononuclear epithelial cells that are reportedly specific for Fabry disease. The lamellated „Maltese cross‟ particles, as well as the Gb3Cer-positive cells, are detectable both in male hemizygotes and female heterozygotes, including before the development of any clinical signs of Fabry nephropathy. 5 Diagnostic kidney biopsy Atypical clinical presentations for Fabry nephropathy, including acute nephritic syndrome, acute onset of severe nephrotic syndrome, severe hypertension early in the course of progressive CKD, rapidly progressive loss of renal function, and macroscopic hematuria, are indications for diagnostic kidney biopsy, even in patients with the diagnosis of Fabry disease. Indeed, minimal change nephropathy, IgA nephropathy, crescentic glomerulonephritis, granulomatous interstitial nephritis and lupus nephritis have been described as coexisting diseases in patients with Fabry nephropathy. Besides to confirm the diagnosis of Fabry nephropathy and to exclude other kidney disorders in patients with atypical presentations, a kidney biopsy is also useful for evaluating the superimposed pathology of concomitant diseases (e.g. diabetes mellitus or malignant hypertension) and for assessing the histological severity of Fabry nephropathy. Kidney biopsy is additionally recommended in adult Fabry patients with GLA mutations associated with a high residual enzyme activity, if they present with low GFR and proteinuria. It is not uncommon that a kidney biopsy reveals Fabry nephropathy in previously undiagnosed patients who are being evaluated for proteinuric CKD, including heterozygous females. Histomorphological evidence of Fabry nephropathy is a very robust clue to the diagnosis of Fabry disease and justifies a thorough molecular genetics diagnostic approach in those cases where a pathogenic GLA mutation is not identified after routine gene sequencing studies. Conversely, the clinical diagnosis of Fabry nephropathy has been excluded by kidney biopsy in a proteinuric female with typical angiokeratomas, who carried a GLA mutation associated with high residual enzyme activity. However, the diagnosis of Fabry disease may be missed in incidental kidney biopsies if only routine light microscopy is done. Identification of glycosphingolipid deposits in kidney tissue requires that the biopsy sample be adequately processed with an appropriate fixative (e.g. glutaraldehyde) for light microscopy and electron microscopy. Under illumination in a stereomicroscope, the glomeruli of patients with Fabry nephropathy, even children with minimal albuminuria, have a striking white color that contrasts with the usual red color of the glomeruli. Therefore, bedside stereomicroscopic inspection of the kidney biopsy material in patients with renal disease of unknown cause may immediately raise the suspicion of Fabry nephropathy and can be useful to guide the histological processing of the biopsy sample. The pathogenesis and the pathology of Fabry nephropathy The kidneys are one of the major sites of glycosphingolipid accumulation in Fabry disease. Globotriaosylceramide and galabiosylceramide (Ga 2Cer) are the glycosphingolipid compounds that are extractable in highest amounts from the kidney tissue of adult Fabry patients, although the latter at threefold lower concentration. The Gb3Cer and the Ga2Cer deposited in the kidney cells of Fabry patients are believed to be locally generated by normal physiologic processes and metabolic pathways. Both Gb3Cer and its major catabolic precursor, globoside, are normal constituents of the human kidney, and the synthase of Gb 3Cer is strongly expressed in the kidneys. Globotriaosylceramide is the membrane antigen CD77, expressed in the kidneys by many different 6 CIN2011 cell types, particularly in the cortical tubular epithelium. CD77 is involved in cellular signaling in CD19mediated cell adhesion and interferon-alpha-induced growth inhibition and apoptosis but also CIN2011 functions as a receptor for the Shiga-like toxins (verotoxins) of Escherichia Coli, which cause the hemolytic-uremic syndrome. Furthermore, the inhibition of GLA gene expression in cultured human tubular epithelial cells (HK2) and primary cultured tubular epithelial cells by RNA interference led to an increase in membrane Gb3Cer expression and to the formation of osmiophilic intracellular lamellar inclusions resembling those observed in patients with Fabry disease. Rudimentary storage of Gb3Cer in the kidney has been noted as early as the second trimester of gestation, a time when only myenteric plexuses cells were also affected. In adults, the concentration of Gb3Cer extracted from kidney tissue is significantly higher in patients with no residual alphagalactosidase activity as compared to patients with detectable residual enzyme activity, indirectly suggesting that the metabolic overload of glycosphingolipids in kidney cells is one of the factors that determine the natural history of Fabry nephropathy. Progressive accumulation of Gb 3Cer in the glomerular and interstitial microvascular endothelia of the kidney, eventually leading to luminal obstruction and tissue ischemia, has been regarded as a prominent factor in the pathogenesis of the nonspecific degenerative glomerular (segmental and global sclerosis/hyalinosis) and tubulointerstitial (tubular atrophy, interstitial fibrosis) changes associated with CKD progression in patients with Fabry disease. For this reason, the clearance of Gb3Cer deposits from the renal microvascular endothelium was selected as the primary efficacy end point in a major randomized, placebo-controlled, doubleblind trial of ERT with agalsidase beta. Another putative mechanism of vascular disease in Fabry nephropathy is the necrosis of severely involved smooth muscle cells in the arteriolar and arterial vessel walls. Lethal injury to Gb3Cer overloaded podocytes, as well as necrosis of mesangial cells, may be additional mechanisms contributing to the pathogenesis of glomerular sclerosis. Because podocytes are highly differentiated, postmitotic cells they generally are not replaced when they are lethally injured. As a result of podocyte loss, parietal epithelial cells of Bowman‟s capsule may gain access to bare areas of the glomerular basement membrane, forming focal adhesions and thereby starting the cascade of pathogenic events that lead to segmental glomerular sclerosis. Moreover, endothelial cells in the vicinity of damaged podocytes are submitted to additional hydraulic forces that may also lead to segmental glomerular collapse and sclerosis. Finally, direct toxic injury to the tubules from the Gb3Cer storage within the tubular epithelial cells may be a factor involved in the pathogenesis of focal tubular atrophy and interstitial fibrosis. Glycosphingolipids consist of a hydrophobic ceramide moiety linked to a hydrophilic carbohydrate. Because of their amphiphilic solubility properties, glycosphingolipids are almost entirely extracted from tissue sections by the non-polar solvents (e.g., ethanol and xylene) that are used in the standard clearing and paraffin-embedding histological procedures for light microscopy examination. For this reason, the most distinctive histopathological feature of Fabry disease on routine light microscopy is the vacuolization of cells in affected tissues. Staining of formalin-fixed paraffin-embedded tissue sections with lipid-soluble dyes is also negative. Glycosphingolipids can be identified in frozen tissue 7 sections by any of the currently available histochemical lipid-staining methods but the staining reactions are not specific for glycosphingolipids and the freezing of tissue leads to loss of fine cellular CIN2011 details. Intracellular glycosphingolipid deposits can be also easily identified as dark-blue or grey-blue inclusions on toluidine blue- or methylene blue-stained semi-thin (0.5–1.0 µm) plastic-embedded electron microscopy scout sections. However, as frozen specimens are not routinely obtained and/or processing of samples for electron microscopy are not performed in many clinical settings, it is imperative that pathologists promptly recognize the light microscopy features of Fabry nephropathy. Indeed, the diagnosis of Fabry disease has been overlooked on kidney biopsies exclusively assessed by standard light microscopy study, while re-examination by electron microscopy readily allowed the recognition of the typical ultrastructural features of Gb3Cer deposits. It has recently been demonstrated that the amount of Gb3Cer remaining in paraffin-embedded kidney tissue sections allows its specific recognition by immunohistochemistry. Therefore, immunohistochemical staining of Gb3Cer is a relatively simple and specific diagnostic method for Fabry disease in routine light microscopy of kidney biopsies, which can be used even for retrospective diagnosis of Fabry nephropathy in archive paraffin blocks, particularly when cell vacuolization is a prominent finding. Furthermore, it allows the differential diagnosis of concurrent kidney disease in patients carrying GLA mutations associated with high residual enzyme activity or with atypical clinical presentations of Fabry nephropathy. Light microscopy The most characteristic finding on routine light microscopy of kidney biopsies of patients with Fabry disease is the vacuolization of the cytoplasm of affected cells. The pattern of cell type involvement and the prevalence of vacuolated cells of each type are roughly dependent on the age and gender of the patient and on the clinical severity of Fabry nephropathy. In children, teenagers and young adult male patients, the cells most prominently affected are the podocytes which are diffusely enlarged, showing abundant cytoplasm filled with numerous clear vacuoles, giving a “honeycomb” appearance to the glomeruli [Fig.1]. Cytoplasm enlargement and vacuolization is also remarkable in the distal tubular epithelial cells, including those of Henle‟s loop and the collecting duct, particularly intercalated cells. The morphological changes are of mild to moderate degree in the epithelial cells of Bowman's capsule, and in the endothelial and smooth muscle cells of the arteries. The glomerular endothelium and the mesangial cells are minimally affected and most proximal tubules look histologically normal. The involvement of the epithelial cells of Bowman‟s capsule, mesangial cells and endothelial cells is more pronounced as the patients get older. With the Figure 1. Extensive vacuolization of the glomerular tuft. Reproduced from: Faria V, Doença de Fabry, tesaurismose rara, Jornal do Médico 1970, LXXII (1423). methenamine silver stain, argyrophilic inclusions may be identified in some affected cells. Heterozygous females have similar morphological abnormalities, but of milder degree as compared to 8 males. Although vacuolization is a light microscopy feature of a number of other kidney diseases, either genetic or non-genetic, the age of the patient at the onset of renal symptoms, the specific CIN2011 clinical phenotype, particularly of the extra-renal manifestations, and the histological pattern of involvement of the kidney cells should allow the differential diagnosis with Fabry nephropathy. Light microscopy examination of toluidine blue- or methylene blue-stained semi-thin sections allows a more accurate identification of the stored glycosphingolipids and can disclose the presence of cytoplasmic Gb3Cer deposits even within cell types which apparently are not vacuolated on the conventional paraffin-embedded sections. At higher magnification (objective: x40–x100), Gb3Cer inclusions appear as small, dark, dense beaded granules, or as larger, more structured, laminated bodies, located either in the cytoplasm around the nucleus or clustering in the peripheral cytoplasm. The laminated inclusions are typically seen in the podocytes and in the epithelium of distal convoluted tubules and collecting ducts. Podocyte nuclei are often eccentrically positioned, pushed aside by the mass of Gb3Cer inclusions. In small capillaries, the cytoplasm of heavily loaded endothelial cells may protrude into the lumen, to the point of causing microvascular obstruction. In studies using this histological technique, glomerular parietal epithelial cells were the most extensively affected in kidney biopsies of middle-aged heterozygous women with CKD stage 2 and no overt proteinuria, and glycosphingolipid inclusions were abundant in the enlarged podocytes of an older male with a cardiac variant of Fabry disease, while the vascular endothelia and smooth muscle cells, and the tubular epithelium looked unremarkable. Immunohistochemistry of paraffin-embedded kidney tissue sections of patients with Fabry nephropathy using an anti-Gb3Cer primary antibody allows the specific identification of residual Gb3Cer in all types of glomerular, tubular, interstitial and vascular kidney cells, although at relatively lower scorings than the corresponding histological scorings on semi-thin plastic embedded sections. The Gb3Cer immunostaining pattern in Fabry nephropathy is distinct from that observed in controls, which have mild Gb3Cer expression limited to tubular cells. Immunofluorescence is important in the differential diagnosis of other kidney diseases that may occur concurrently in patients with Fabry disease. Asymptomatic IgA deposits have been repeatedly identified in kidney biopsies of patients with Fabry disease, with a frequency that seems to be higher than in non-selected autopsy cases. Segmental IgM deposits, typical of focal glomerular sclerosis, may also be identified in some cases. Non-specific changes Non-specific degenerative glomerular and vascular changes are present even before progression to overt proteinuria and decreased glomerular filtration rate. Round deposits of hyaline-like material within the media of arteries are the most prevalent non-specific vascular lesion in kidney biopsies of patients with Fabry disease. These deposits are observed whatever the caliber of the vessels and are especially numerous in the afferent arterioles. They have been described as early as age 11 years, in both affected boys and girls. As the disease progresses, increased mesangial matrix and mesangial widening, segmental and global glomerular sclerosis, vascular intimal and medial thickening, tubular 9 atrophy, interstitial fibrosis and chronic inflammatory cell infiltrates are variably recognized by light microscopy. In a study of children and adolescents with Fabry disease and minimal albuminuria, CIN2011 glomerular hyaline was detected as early as in a 7-year-old boy; incipient signs of focal segmental glomerulosclerosis were present in a 14-year-old girl; and most of the boys aged 16–18 years already had a globally sclerotic glomerulus in the biopsy section. Interstitial fibrosis, of mild degree (~5% of the cut section), was seen exclusively in these latter patients. However, in young adult males aged 25-29 years old, presenting with the classical phenotype of Fabry disease, the degree of tubular atrophy and interstitial fibrosis was moderate to severe and diffuse in all cases. Expansion of the mesangial area, by reducing the surface of glomerular filtration, might contribute to the pathogenesis of segmental glomerular sclerosis. The proportion of segmentally or globally sclerotic glomeruli increases with age in both genders. Most of the progressive non-specific degenerative lesions described in Fabry nephropathy are thought to be related to ischemic damage. Clinicopathological correlations and gender differences In a study of 9 young males with Fabry disease, aged 11–29 years old, with normal creatinine clearance, four of them with overt proteinuria (range: 0.7–2.8 g/day), segmental and/or global glomerulosclerosis were the only histopathological correlates of overt proteinuria. Light microscopy glomerular and tubulointerstitial pathology scores, as well as a glycosphingolipid inclusion score, were computed for kidney biopsies of 25 adult male Fabry patients aged 20–49 years, with residual alpha-galactosidase activity <15% of the control values, as baseline assessment for a double-blind placebo-controlled trial of ERT with agalsidase alfa. Six patients had advanced CKD (stages 3 or 4) and 16 presented overt proteinuria (>300mg/day). The glomerular pathology score was based on the mesangial morphology of each glomerulus, scored into one of the following categories, of increasing histopathological severity: normal; diffuse mesangial widening; segmental sclerosis or solidification; and global sclerosis or glomerular obsolescence. The final glomerular pathology score was the average of the individual glomerular scorings in each biopsy. The tubulointerstitial pathology score was determined as the average of the individual scorings for the degrees of tubular atrophy, interstitial inflammation, interstitial fibrosis, vascular medial thickening and vascular hyalinosis. Glycosphingolipid inclusions were assessed on toluidine blue-stained semi-thin plastic sections, and their amounts rated in glomerular epithelial cells, glomerular endothelial/mesangial cells, proximal tubular epithelial cells, distal tubular epithelial cells, vascular endothelial cells and vascular medial cells. The overall glycosphingolipid inclusion score was the average of the individual cell type scorings. Both the glomerular and the tubulointerstitial pathology scores showed highly significant inverse correlation with GFR at baseline, as estimated by inulin clearance, and direct correlation with proteinuria. Age at kidney biopsy was inversely correlated with inulin clearance and directly correlated with the glomerular pathology score, but did not correlate significantly with the tubulointerstitial pathology score. As it might have been expected, the glomerular and the tubulointerstitial pathology scores were significantly correlated but in stepwise linear regression analysis the glomerular pathology score emerged as the major predictor of both the inulin 10 clearance and the proteinuria levels. Yet, a wide range of variation in individual glomerular pathology scores was noted among patients with inulin clearances below 90 ml/min/1.73m 2. Contrastingly, the CIN2011 glycosphingolipid inclusion score did not correlate with GFR, proteinuria or the other pathology scores. The composite nature of these kidney pathology scorings may have limited their usefulness to recognize additional, particularly cell-type dependent clinicopathological correlations. A different approach was used for the scoring of baseline and post-treatment kidney biopsies obtained from patients enrolled in the phase 3 double-blind, randomized, placebo-controlled trial and open label extension studies of ERT with agalsidase beta. The initial cohort comprised 58 patients (mean age: 30.2 years; range: 16–61 years), including two females, with severe deficiency of alphagalactosidase activity and a serum creatinine level not exceeding 2.2 mg/dl. Mean serum creatinine was 0.8 mg/dl and mean inulin clearance was 89.8 ml/min. Glycosphingolipid accumulation was semiquantitatively scored on semi-thin (1 µm) epoxy-embedded plastic sections, stained with methylene blue/Azure II and examined by light microscopy. Glycosphingolipid inclusion scores were determined for each of the following cell types: peritubular (interstitial) capillary endothelial cells, glomerular endothelial cells, mesangial cells, arterial/arteriolar endothelial cells, vascular smooth muscle cells, interstitial cells (a mixed population of fibroblasts and phagocytic cells), podocytes, and the epithelium of distal convoluted tubules and collecting ducts. In addition, the mesangial matrix of individual glomeruli was also evaluated for pathologic change. The primary endpoint of the trial was the clearance of Gb3Cer deposits from the interstitial capillary endothelial cells. At baseline, glycosphingolipid accumulation was much more concentrated and extensive in the podocytes and the epithelial cells of distal convoluted tubules and collecting ducts than in vascular endothelial and smooth muscle cells, mesangial cells and interstitial cells. Proximal tubular epithelial cells were relatively unaffected. After 20–26 weeks of ERT, highly effective clearance of Gb3Cer deposits from the peritubular capillary, glomerular and arterial/arteriolar endothelial cells, and from the mesangial cells, was robustly demonstrated. Comparable levels of Gb3Cer clearance were reached somewhat later in the interstitial cells. After 22 months of ERT, only moderate Gb3Cer clearance had been obtained from the smooth muscle cells of arterioles and small arteries; clearance in podocytes and in the distal tubular epithelium was much more limited than in the other cell types. In a small number of cases (n = 8) assessed after 48–54 months on ERT, complete Gb3Cer deposits was noted in the distal convoluted tubule and collecting duct cells but significant storage remained within podocytes and in vascular smooth muscle cells. In general, clearance of Gb3Cer storage was the most effective in cell types that have relatively rapid physiological turnover rates, like the endothelial, mesangial and interstitial cells. In contrast, Gb3Cer clearance was much less effective in podocytes, which are terminally differentiated cells with little resting turnover. Although the distal tubular epithelial cells are regularly shed into the urine and replaced, and have a relatively high proliferation index, the delayed Gb3Cer clearance response to ERT observed in the distal convoluted tubules and collecting ducts might be the result of the exposure of these epithelia to high concentrations of Gb3Cer, leading to active or passive uptake. The mesangial matrix was moderately enlarged at baseline and did not 11 change significantly after 20 weeks of ERT. Even in patients treated with agalsidase beta ERT for 11 months, the decrease of the mean score for mesangial matrix widening was negligible. CIN2011 Six out of the 43 patients who had glomeruli available for scoring in both the kidney biopsies obtained at baseline and after 6 months into the open label extension study, had focal segmental glomerulosclerosis or global glomerulosclerosis in ≥50% of the glomeruli. All 3 patients who had a >50% increase in their serum creatinine levels during the first 24-30 months of therapy with agalsidase beta belonged to this group. During the first 48-54 months of treatment with agalsidase beta, the mean rate of decline in eGFR for patients with baseline proteinuria >1 g/day, was significantly higher as compared to patients with lower proteinuria levels (-7.4 vs. -1.0 ml/min per 1.73 m2/year). A similar finding was made for patients with baseline segmental or global glomerular sclerosis in ≥50% of the glomeruli, as compared to those with lesser degrees of glomerulosclerosis (8.9 vs. -1.4 ml/min per 1.73 m2/year). Combined analysis of light microscopy data of the kidney biopsies of 12 heterozygous females (median age: 43.5 years; range: 8–73 years), with eGFR ranging from normal to CKD stage 5, half of them presenting with proteinuria ≥1000 mg/day, demonstrated highly significant correlations between proteinuria and glomerular sclerosis, between CKD stage and glomerular sclerosis and interstitial fibrosis, as well as between glomerular sclerosis and interstitial fibrosis. On stepwise logistic regression, glomerular sclerosis emerged as the most important predictor of proteinuria while interstitial fibrosis was the most important predictor of CKD stage. A disproportionately higher degree of interstitial fibrosis than tubular atrophy was seen in a middle-aged woman with hypertension. Glycosphingolipid inclusions were observed in proximal tubules exclusively in the two patients presenting with overt proteinuria. The International Study Group of Fabry Nephropathy (ISGFN) recently validated a light microscopy scoring system for Fabry nephropathy using standard light microscopy paraffin-embedded sections and epoxy-embedded toluidine blue-stained semi-thin plastic sections. The degrees of interstitial fibrosis and of global and segmental sclerosis could be robustly scored on the paraffin-embedded sections, as well as the podocyte inclusions on the semi-thin sections. The ISGFN scored the kidney biopsies of 59 patients with Fabry nephropathy (age range: 16–70 years), including 35 males (mean age: 36.4 years) and 24 females (men age: 43.9 years); 13 patients, including one female, had advanced CKD (stage 3 or 4); median urine protein to creatinine ratio was 420 mg/g (range: 10–5620 mg/g). As compared to the females, males showed greater podocyte vacuolization scores on standard light microscopy and glycosphingolipid inclusions scores on semi-thin sections. Even in the earliest CKD stages, half of the males had large podocyte inclusions involving>50% of the glomerular tuft. Males also had significantly more proximal tubule, peritubular capillary and vascular intimal inclusions. Segmental and/or global sclerosis and interstitial fibrosis were seen even in patients with CKD stages 1–2 with minimal proteinuria. In patients with eGFR >60 ml/min/1.73 m2, the average proportion of non-sclerotic glomeruli was 75–80%, and 63% of them already had glomerular sclerotic lesions. On 12 trend analyses, statistically significant direct correlations were identified between CKD stage and the proportion of segmentally and/or globally sclerotic glomeruli, the arterial sclerosis score, and the CIN2011 degree of interstitial fibrosis. The proportion of normal glomeruli correlated inversely with the CKD stage. The relationship between CDK stage and interstitial fibrosis appeared to be non-linear, with minimal fibrosis observed in patients with CKD stages 1–2. On regression analyses, the major predictor of proteinuria in males was the proportion of non-sclerotic glomeruli, and in females were the proportion of glomeruli with severe segmental sclerosis and the degree of interstitial fibrosis. A major predictor of severe proteinuria in males, irrespective of CKD stage, was the presence of proximal tubular inclusions. Although clinical disease was milder in the female cohort, there were no gender difference in the scorings of segmental and global glomerulosclerosis, interstitial fibrosis, distal tubular inclusions and vascular medial inclusions. The degree of arteriolar hyalinosis was similar in both genders, but females had significantly more arterial hyalinosis. Electron microscopy On transmission electron microscopy, Gb3Cer deposits typically appear as intralysosomal dense osmiophilic, coarsely lamellated inclusions composed of alternating dark and clear layers. These lamellae can be seen arranged either in concentric (variably named “myelin figures”, ”myeloid bodies” or “onionskin structures”) [Fig.2] or in parallel arrays (“zebra Figure 2. Multiple “myelin figures” within a podocyte (x11,500). Reproduced from: Faria V, Doença de Fabry, tesaurismose rara, Jornal do Médico 1970, LXXII (1423). Figure 3. Zebra body within a podocyte, next to a myelin figure (x27,500). Reproduced from: Faria V, Doença de Fabry, tesaurismose rara, Jornal do Médico 1970, LXXII (1423). bodies”) [Fig.3], and have a periodicity ranging between 3 and 10 nm, when measured using routine plastic thin sections. Smaller, amorphous dense osmiophilic deposits may also be seen in some cells, but are less frequent and not as immediately reminiscent of the diagnosis of Fabry disease as the myelin figures or the zebra bodies. However, at higher magnifications (x50.000–x100.000), these amorphous inclusions also exhibit a regular pattern of lamellation. Even though the ultrastructural features of the Gb3Cer inclusions are not pathognomonic, transmission electron microscopy is the most reliable morphological method to identify glycosphingolipid deposits in tissue specimens of patients with Fabry disease. In kidney biopsies, the finding of myelin figures in podocytes is generally regarded as a diagnostic hallmark of Fabry nephropathy. Electron microscopy study of adult kidney tissue specimens shows lamellar inclusions within all types of glomerular, tubular, vascular and interstitial cells, even when they look unaffected by conventional histology. The involvement of all types of kidney cells and the morphological features of the Gb3Cer deposits are similar in both genders, but in the heterozygous females the pattern of storage has been 13 described as more irregular than in males, consistent with the expected morphological expression of X-chromosome inactivation. The diameters of the myelin figures in kidney cells most commonly range CIN2011 between 0.3–3.0 μm but larger inclusions may be seen in podocytes and in epithelial cells of the Henle‟s loops and distal tubules. The tubular involvement is not homogenous being usually mild in the proximal segments, where it is accompanied by changes of the brush-borders, and more marked and affecting many more cells in Henle‟s loops of distal tubules. In the tubules, normal cells can lay side by side with strikingly enlarged cells, containing giant lamellar inclusions. In the endothelial cells, the glycosphingolipid inclusions are remarkably pleomorphic and may cause the cytoplasm to swollen and protruded into the vascular lumen. Lamellated particles have also been reported in extracellular locations, namely in the Bowman‟s space and in the proximal tubular lumen. Incipient glycosphingolipid inclusions were limited to podocytes on the electron microscopy study of an affected male fetus, aborted at 19 weeks of gestational age, but in children and young teenagers, irrespective of their gender, the pattern of glycosphingolipid storage in kidney cells is already similar to the adult Fabry patients, even in children with normal renal function and no proteinuria. In children, adolescents and young adults with Fabry nephropathy, the largest Gb3Cer inclusions, measuring up to 6–10 μm in diameter, are seen in podocytes and in epithelial cells of the Henle‟s loops and distal tubules. Inclusions are also numerous in the epithelial cells of the Bowman's capsule, but smaller (0.5–3.0 μm) than those in podocytes. Within the glomeruli, the smallest inclusions (0.3–2.0 μm) are seen in mesangial and in endothelial cells. The Gb3Cer deposits in the endothelial cells of peritubular capillaries, pericytes, endothelial and smooth muscle cells of small- or medium-sized arteries are smaller (0.3-3.0 μm) than those in the epithelial distal tubular cells. In this age group, the highest prevalence of Gb3Cer deposits per cell type is seen in the podocytes, followed by the epithelium of the Henle‟s loops and distal tubules, and the vascular smooth muscle cells. Glycosphingolipid storage is also quite prevalent in parietal epithelial cells and interstitial cells (e.g., fibroblasts). At least in middle-aged heterozygous women with Fabry nephropathy, the prevalence of Gb 3Cer deposits in cells of the Bowman‟s capsule may be higher than in podocytes. The electron microcopy study of the kidney biopsy of a 75-year-old hypertensive male with a cardiac variant of Fabry disease (residual enzyme activity estimated as ~8-10% of the normal), who presented with CKD stage 3 (creatinine clearance = 49 ml/min) and severe proteinuria (2.18 g/day), showed abundant Gb3Cer inclusions only within the podocytes. Glycosphingolipid deposition was scanty in the epithelium of Bowman‟s capsules and in atrophic distal tubular segments, rare in mesangial cells, and absent in interstitial, and in vascular endothelial and smooth muscle cells. There was mild expansion of the mesangial matrix and segmental effacement of the podocyte foot processes, involving an estimated 45% of the glomerular basement membrane circumference. A recent quantitative study of the glomerular ultrastructural lesions found in kidney biopsies of young Fabry male and female patients aged 4–19 years, using unbiased stereological methods, demonstrated a significant age-related increase of the volume fraction of glycosphingolipid deposits within podocytes, but not in the mesangial or endothelial cells. Podocyte foot process width also 14 increased significantly with age and correlated directly with the degree of proteinuria. There were highly significantly direct correlations between the volume fraction of glycosphingolipid inclusions per CIN2011 podocyte and both the degree of proteinuria and the width of the podocyte foot processes. Significant direct correlations were also found between the volume fraction of glycosphingolipid inclusions per mesangial cell and the volume fraction of glycosphingolipid deposits within podocytes, the podocyte foot process width and the degree of proteinuria. There was a significant direct correlation between the volume fraction of glycosphingolipid inclusions per mesangial cell and the volume fraction of glycosphingolipid inclusions per endothelial cell, as well as a trend for a direct relationship between the volume fraction of glycosphingolipid inclusions in endothelial cells and in podocytes. However, there was no relationship between the volume fraction of glycosphingolipid inclusions per endothelial cell and proteinuria. The volume fractions of glycosphingolipid inclusions in endothelial cells and in podocytes were significantly greater in the male than in the female patients, this gender difference being particularly striking for the endothelial cells. There was a trend for greater volume fraction of glycosphingolipid inclusions in mesangial cells in the males. Podocyte foot process width was significantly higher in the Fabry male patients than in the healthy controls, but the differences between the male and female Fabry patients and between the Fabry females and the healthy controls were not significant. Non-specific changes Mesangial stalks may be diffusely enlarged, with a marked increase in mesangial matrix. The ultrastructure of the slit diaphragms of the foot processes may be spared despite the evidence of massive glycosphingolipid deposits in the cytoplasm of podocytes. Effacement of the podocyte foot processes correlates with the presence of moderate to nephrotic proteinuria, both in male and in female patients. However, using a stereological quantitative approach to study electron microphotographs of kidney biopsies, segmental effacement of foot processes was noted in all the glomeruli of children and preadolescent boys and girls who had urinary protein excretion rates within the normal range for age, but detachment of podocytes from the glomerular basement membrane was very rare. The ultrastructure of the glomerular basement membrane is usually normal, even alongside heavily affected podocytes, but focal thickening and wrinkling of the glomerular basement membrane and extensive fusion of podocyte foot processes are associated with tortuosity, wrinkling and collapse of the capillary walls. The basement membranes of atrophic tubules also appear thickened and wrinkled. Extracellular deposits of striated membranous structures intermingled with finely granular ground material have been described in the walls of interstitial arteries, in areas of focal and segmental hyalinosis, in association with glomerular basement membrane duplications and in the mesangium. The origin of these deposits, some of which contain residues of dense laminated bodies, is not known but they might represent remnants of damaged vascular smooth muscle cells or of endothelial, and/or residual glycosphingolipids from ruptured cells. Intravascular platelets and platelet aggregates, platelet adhesion to endothelial cells and platelet adhesion to the basement membrane in sites of 15 capillary endothelial microlesions are frequently observed in glomerular and interstitial vessels are possible morphological features of the prothrombotic state described in Fabry disease. CIN2011 Electron-microscopy phenocopies of Fabry nephropathy Cytoplasmic inclusions with ultrastructural features identical to those of Fabry disease have been described on kidney biopsies of patients with silicosis or with drug-induced phospholipidosis, a nephrotoxic condition associated with the therapeutic use of some drugs, including amiodarone, antimalarial agents (chloroquin) and aminoglycosides. The pattern of cell involvement in amiodaroneand chloroquin-induced renal phospholipidosis, with lamellated inclusions visible in virtually all types of kidney cells and dominant involvement of podocytes and epithelial cells of distal tubules, may lead to the erroneous diagnosis of Fabry nephropathy, if the iatrogenic phospholipidosis is not considered in the differential diagnosis. In the case of aminoglycoside-induced renal phospholipidosis, the lamellar deposits are typically restricted to epithelial tubular cells, a pattern of distribution that does not mimic Fabry nephropathy. Discussion, conclusions and future perspectives In patients with Fabry disease, kidney biopsy provides important information that is not available from routine assessment of kidney function and proteinuria. Like in other progressive kidney diseases, nonspecific glomerular sclerosis and interstitial fibrosis, rather than the direct (i.e., glycosphingolipid inclusions) and indirect (i.e., cell vacuolization) signs of glycosphingolipid storage, are the major histopathological correlates of CKD stage and degree of proteinuria. However, the pathogenesis of the glomerular and interstitial degenerative lesions underlying progressive CKD in patients with Fabry disease is not well understood and taking into consideration the recent data from systematic histological and ultrastructural studies of kidney biopsies of Fabry patients, mechanisms other than the ischemic tissue damage due to microvascular endothelial involvement will have to be additionally considered. In contrast to patients with Fabry disease, alpha-galactosidase deficient mice do not manifest significant proteinuria or progressive CKD, even at advanced ages. Histologically, they accumulate Gb3Cer in kidney cells with a similar pattern to that observed in Fabry patients but do not develop glomerular sclerosis or interstitial fibrosis. The biological mechanisms underlying this major difference between the animal model and human Fabry disease are not known and their elucidation might give some clues to the pathogenesis of Fabry nephropathy. Glycosphingolipids accumulated in the kidney cells of patients with Fabry disease are probably of endogenous production within the kidney, and the varied storage loads in the different kidney cell types, as assessed by light microscopy and electron microscopy techniques, most likely reflect the balance between the local synthesis and residual catabolism of Gb3Cer, the turnover rate of the different kidney cell populations and the exposure to high concentrations of Gb3Cer in the urine. Histological studies of repeat kidney biopsies in patients receiving long-term ERT suggest that its therapeutic success, in addition to or rather than Gb3Cer clearance from affected cells, might be due to the physiological replacement of these cells by new cells that are prevented from accumulating 16 abnormal amounts of glycosphingolipids by ERT. Podocytes are the kidney cells most vulnerable to glycosphingolipid accumulation in Fabry disease, showing extensive storage even in patients with CIN2011 residual levels of alpha-galactosidase activity that are high enough to prevent significant Gb3Cer storage in other cell types. Low grade podocyte loss into the urine is a frequent finding in urine samples of patients with Fabry disease, whether or not they have clinical Fabry nephropathy or are treated with ERT. Like in other progressive glomerular diseases, podocyte shedding into the urine may be involved in the development of progressive glomerular sclerosis also in Fabry nephropathy. The parietal epithelial cells of the Bowman‟s capsule have the slowest turnover rate of all kidney cell populations, which could be the explanation for their increasingly extensive pathological involvement with age. However, the pathogenic implications of Gb3Cer storage in the Bowman‟s capsule have so far been ignored. The reasons for the difference in susceptibility to glycosphingolipid accumulation between the proximal and the distal tubular epithelia are not known and the finding that Gb3Cer accumulation within proximal tubular cells is related to higher levels of protein excretion rates in the urine is worth investigating in more detail, as it may help to elucidate the pathophysiology of proteinuria in Fabry nephropathy. Although Gb3Cer accumulation within kidney cells is regarded as the critical pathogenic event of Fabry nephropathy, the biological links between intralysosomal Gb3Cer storage and cellular dysfunction are mostly unknown. On the basis of available electron microscopy data, lethal injury to cells following mechanical disruption of Gb3Cer overloaded lysosomes might be a factor in some cells. Many secondary biochemical processes have been identified in recent years that might be involved in the pathogenesis of Fabry disease. These include lysoGb3-induced vascular smooth muscle cells proliferation, altered lipid composition of membranes causing abnormalities in the trafficking and sorting of rafts-associated proteins, and compromised cellular energy metabolism. The latter two mechanisms, for example, might provide a pathophysiologic link between Gb3Cer storage in proximal tubular cells and increased urinary protein excretion rates, by interfering with the reabsorption of proteins by the proximal tubules. Despite the major progresses made in recent years in the understanding and treatment of Fabry disease, the elucidation of the biological mechanisms underlying progressive CKD in Fabry nephropathy should remain in the investigation agenda as a priority as it may provide important clues to novel, additional therapeutic approaches. 17 Further reading: 1. Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol 2002; 13: S134–138. 2. Askari H, Kaneski CR, Semino-Mora C et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch 2007; 451: 823–834. 3. Branton MH, Schiffmann R, Sabnis SG et al. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 2002; 81: 122–138. 4. Desnick RJ, Ioannou YA, Eng CM. Alpha-Galactosidase A deficiency: Fabry disease. In Scriver C, Beaudet A, Sly W, Valle D (eds), The metabolic bases of inherited disease, 8th edn. McGraw-Hill, New York, 2001; pp 3733–3774. 5. Desnick RJ, Wasserstein MP, Banikazemi M. Fabry disease (alpha-galactosidase A deficiency): renal involvement and enzyme replacement therapy. Contrib Nephrol 2001; 136: 174–192. 6. Desnick RJ, Brady R, Barranger J et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 2003; 138: 338–346. 7. Eng CM, Guffon N, Wilcox WR et al. Safety and efficacy of recombinant human alphagalactosidase A–replacement therapy in Fabry‟s disease. N Engl J Med 2001; 345: 9–16. 8. Faraggiana T, Churg J, Grishman E et al. Light- and electron-microscopic histochemistry of Fabry's Disease. Am J Pathol 1981; 103: 247–262. 9. Fischer EG, Moore MJ, Lager DJ. Fabry disease: a morphologic study of 11 cases. Mod Pathol 2006; 19: 1295–1301. 10. Fogo AB, Bostad L, Svarstad E et al. Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN) Nephrol Dial Transplant 2010; 25: 2168–2177. 11. Germain DP, Waldek S, Banikazemi M et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 2007; 18: 1547–1557. 12. Gubler MC, Lenoir G, Grunfeld JP et al. Early renal changes in hemizygous and heterozygous patients with Fabry‟s disease. Kidney Int 1978; 13: 223–235. 13. Meehan SM, Junsanto T, Rydel JJ, Desnick RJ. Fabry disease: renal involvement limited to podocyte pathology and proteinuria in a septuagenarian cardiac variant. Pathologic and therapeutic implications. Am J Kidney Dis 2004; 43: 164–171. 18 CIN2011 14. Najafian B, Svarstad E, Bostad L et al. Progressive podocyte injury and globotriaosylceramide (GL-3) accumulation in young patients with Fabry disease. Kidney Int 2011; 79: 663–670. 15. Nakao S, Kodama C, Takenaka T et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a "renal variant" phenotype. Kidney Int 2003; 64: 801–807. 16. Oqvist B, Brenner BM, Oliveira JP et al. Nephropathy in Fabry disease: the importance of early diagnosis and testing in high-risk populations. Nephrol Dial Transplant 2009; 24: 1736–1743. 17. Ortiz A, Oliveira JP, Wanner C et al. Recommendations and guidelines for the diagnosis and treatment of Fabry nephropathy in adults. Nat Clin Pract Nephrol 2008; 4: 327–336. 18. Ortiz A, Cianciaruso B, Cizmarik M et al. End-stage renal disease in patients with Fabry disease: natural history data from the Fabry Registry. Nephrol Dial Transplant 2010; 25: 769–775. 19. Schiffmann R, Kopp JB, Austin HA 3rd et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 2001; 285: 2743–2749. 20. Schiffmann R, Warnock DG, Banikazemi M et al. Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant 2009; 24: 2102–2111. 21. Schiffmann R, Waldek S, Benigni A, Auray-Blais C. Biomarkers of Fabry Disease Nephropathy. Clin J Am Soc Nephrol 2010; 5: 360–364. 22. Selvarajah M, Nicholls K, Hewitson TD, Becker GJ. Targeted urine microscopy in Anderson-Fabry Disease: a cheap, sensitive and specific diagnostic technique. Nephrol Dial Transplant 2011; Mar 7 [Epub ahead of print]. 23. Sessa A, Meroni M, Battini G et al. Renal involvement in Anderson-Fabry disease. J Nephrol 2003; 16: 310–313. 24. Sessa A, Meroni M, Battini G et al. Evolution of renal pathology in Fabry disease. Acta Paediatr Suppl 2003; 92(443): 6–8. 25. Thomaidis T , Relle M , Golbas M et al. Downregulation of -galactosidase A upregulates CD77: functional impact for Fabry nephropathy. Kidney Int 2009; 75: 399–407. 26. Thurberg BL, Rennke H, Colvin RB et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int 2002; 62: 1933–1946. 19 CIN2011 27. Tøndel C, Bostad L, Hirth A, Svarstad E. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis 2008; 51: CIN2011 767–776. 28. Tosoni A, Nebuloni M, Zerbi P et al. Ultrastructural study of renal involvement in two females with Anderson-Fabry disease. Ultrastruct Pathol 2005; 29: 203–207. 29. Valbuena C, Carvalho E, Bustorff M et al. Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch 2008; 453: 329–338. 30. Valbuena C, Oliveira JP, Carneiro F et al. Kidney histologic alterations in αgalactosidase-deficient mice. Virchows Arch 2011; 458: 477–486. 31. Wanner C, Oliveira JP, Ortiz A et al. Prognostic indicators of renal disease progression in adults with Fabry disease: natural history data from the Fabry Registry. Clin J Am Soc Nephrol 2010; 5: 2220–2228. 32. Warnock DG. Fabry disease: diagnosis and management, with emphasis on the renal manifestations. Curr Opin Nephrol Hypertens 2005; 14: 87–95. 33. Warnock DG, Valbuena C, West M, Oliveira JP. Renal manifestations of Fabry Disease. In Elstein D, Altarescu G, Beck M (eds), Fabry Disease, 1 st edn. Springer, Dordrecht, 2010; pp 211–243. 34. Warnock DG, Ortiz A, Mauer M et al. Renal outcomes of agalsidase beta treatment for Fabry disease: role of proteinuria and timing of treatment initiation. Nephrol Dial Transplant 2011; Jul 29 [Epub ahead of print]. 35. Wilcox WR, Banikazemi M, Guffon N et al. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 2004; 75: 65–74. 20 Acknowledgements The illustrations included in this presentation were reproduced from an article published in “Jornal do Médico”, a historical Portuguese medical journal that has ceased its publication a long time ago. The author of that article, Vítor Faria, was the pioneer of histological and ultrastructural studies of the pathology of Fabry disease in Portugal. 21 CIN2011
© Copyright 2026