Histrelin and its use in Mares Gonadotropin
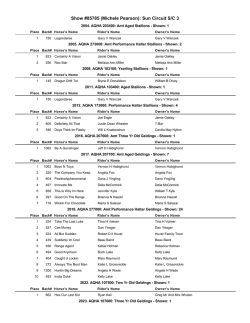
Histrelin and its use in Mares Patrick J. Burns, Ph.D. & Richard M. Gilley B.S. BioRelease Technologies Lexington, KY (www.BioReleasetechnologies.com) Gonadotropin releasing hormone (GnRH) is a naturally occurring decapeptide in mammals, including humans. The biologic action of GnRH (LHRH) is to stimulate the pituitary to release gonadotropins, especially luteinizing hormone (LH), but, also, follicle stimulating hormone (FSH). LH, in concert with FSH, stimulates ovarian follicular development; the LH surge ovulates the follicle. The most sensitive assay for exogenously administered GnRH is the detection of serum changes in LH concentration following GnRH administration. The plasma half-life elimination phase of GnRH in humans has been reported to be 13-27 minutes using radioimmunoassys specific for GnRH. Some analogues of GnRH such as deslorelin and histrelin with substitutions in the 6 and 10 positions have increased and prolonged biological activity due to increased structural and metabolic stability. Histrelin is one such analog which differs from natural GnRH in position 6 where Glycine (GLY) has been replaced with Histidine (Bzl) and in position 10 where glycinamide has been replaced by N-ethylamide as illustrated below. GnRH: pPGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2 Histrelin: pPGlu-His-Trp-Ser-Tyr-His(Bzl)-Leu-Arg-Pro-NHEt In man, the plasma half-life of histrelin was about 8 times that of native GnRH and about double that of deslorelin which is partially responsible for its enhanced potency as illustrated below. Comparative Potency Ranking to GnRH* Potency Estimate to GnRH Dose relative to DA Leuprolide 15 Buserelin 20 Deslorelin 144 1.5 mg Histrelin 210 1.0mg *Ascoli M, Segaloff DL. Adenohypophyseal Hormones and their Hypothalamic releasing factors. In Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds, Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th ed. New York, New York : McGraw Hill 1996 1363-1382 BioRelease Histrelin Clinical Results Because of Histrelin’s enhanced pharmacodynamic properties, BioRelease Technologies has initiated preliminary laboratory studies on a specially formulated BioRelease Histrelin. Results from initial studies suggested that both doses (0.5 mg and 1 mg) of BioRelease Histrelin resulted in ovulation in > 95% of estrus mares treated after follicle size and uterine edema scores met normal farm treatment parameters.1 Because this study (STUDY 1) was conducted after May, additional studies early in the breeding season are also being reported (STUDY 2 & 3). Study 1 – Table 1 Comparison of Deslorelin and BioRelease Histrelin for Induction of Ovulation in Mares 1 Interval to Ovulation Ovulation within 48 h Treatment Dose (n) (days) (% of mares) Deslorelin* 1.5 mg 30 1.97 ± 0.5b 93% b BioRelease Histrelin** 1.0 mg 19 1.63 ± 0.6 b 95%b b Biorelease Histrelin ** 0.5 mg 18 1.83 ± 0.4 100%b * Francks Pharmacy, Ocala, FL ** BioRelease Technologies Lexington, KY USA (Shipped and stored at room temperature) STUDY 2 - Previous studies in our laboratory have suggested that doses of BioRelease Histrelin induce ovulation in >95% of mares after May 1st. The present experiment was designed to compare the effectiveness of a single 1mL injection containing 0.25 mg, 0.5 mg or 1.0 mg of Histrelin for inducing ovulation in cyclic recipient mares prior to April 6th in northwestern Texas. Cyclic recipient mares were chosen from the herd based on 1) the previous detection of ovulation or a functional CL by US exam. Ovulatory induction treatments (Blinded BioRelease Histrelin: BLUE, GREEN OR RED) were used by the investigator after follicle size and uterine edema scores meet normal farm treatment parameters. Following treatment, mares were evaluated until ovulation. Percentage of mares ovulating within 48 hours are presented below. Study 2 - Table 2 Evaluation of BioRelease Histrelin for inducing ovulation in cyclic mares early in the year : Effect of Dose2 Ovulation within 48 h Treatment Dose (n) (% of mares) BioRelease Histrelin* 0.25 mg 19 84.2%a BioRelease Histrelin* 0.5 mg 19 89.5%a BioRelease Histrelin* 1.0 mg 20 85.0%a All Doses 58 86.2% * BioRelease Technologies Lexington, KY USA (Shipped and stored at room temperature) Results from Study 2 confirmed that all three doses of BioRelease Histrelin were equally effective at inducing ovulation early in the year 2 (Feb 25 – April 6th). STUDY 3 - The goal of this study was to compare the efficacy of two dose rates of Histrelin (0.5 and 1.0 mg given IM, Groups 2 and 3, respectively) with hCG (2,500 IU given IV, Group 1) throughout the breeding season in one herd of pastured mares (n=88) under ambient light conditions in southeastern Texas3. There was a non-significant trend for higher ovulation responses with both dose rates of Histrelin compared with hCG treatment (ovulation responses within 2 days of treatment among treatment groups (31/37, 84%; 34/37, 92%; and 33/36, 92% for groups 1-3, respectively). The ovulation rates within 2 days obtained with Histrelin were similar to those obtained in the same herd during the 2010 breeding season when 1.5 mg BioRelease deslorelin was administered (113/128, 88%). Study 3 – Table 3 Comparison of Efficacy of Two Dose Rates of Histrelin to Human Chorionic Gonadotropin for Inducing Ovulation in Broodmares3 Ovulation within 48 h Treatment Dose (n) (% of mares) hCG 2500 IU 37 84%a BioRelease Histrelin* 0.5 mg 37 92%a BioRelease Histrelin * 1.0 mg 36 92%a BioRelease Deslorelin** 1.5 mg 128 88% *BioRelease Technologies Lexington, KY USA (Shipped and stored at room temperature) **Obtained in the same herd during the 2010 breeding season when 1.5-mg BioRelease deslorelin was administered (113/128, 88%). Response rates (ovulation in 2 days) appeared to be similar for both doses of Histrelin, so response rates in each month to both dose rates of Histrelin were compared with those achieved with hCG treatment. Ovulations within 2 days of treatment were 89%, 90%, 96%, and 100% for Histrelin, compared with 75%, 83%, 89%, and 100% for hCG, during the months of February, March, April, and May, respectively (Table 4). Although ovulatory response appeared to improve for both products as the season progressed, no differences were detected between response rates to Histrelin or hCG for any month . Study 3 - Table 4 Comparison of Efficacy of Two Dose Rates of Histrelin to Human Chorionic Gonadotropin for Inducing Ovulation in Broodmares: By Month3 Ovulation within 48 h Treatment Dose (% of mares) FEB a MAR a APR a hCG 2500 IU 75 ( n=8) 83 (n=18) 89 (n=9) BioRelease Histrelin 0.5* mg & 1 mg* 89a (n=18) 90a (n=29) 96a (n=22) *BioRelease Technologies Lexington, KY USA (Shipped and stored at room temperature) MAY 100a (n=2) 100a (n=4) Because eight stallions and different methods of breeding were used, no attempt was made to evaluate effects of treatment on fertility (pregnancy rate). However, pregnancy rates per cycle were > 60% and similar to those obtained in previous years at this farm. REFERENCES 1. Lindholm, AGR., Ferris, RA., D.B. Scofield, DB., McCue, PM (2011) Comparison of deslorelin and histrelin for induction of ovulation in mares. 2011 ESS SYMPOSIUM Abstracts / Journal of Equine Veterinary Science 31 (2011) p 312. 2. Blodgett, Glen (2011) Evaluation of BioRelease Histrelin for inducing ovulation in cyclic mares early in the year : Effect of Dose. Field Study Results 2011. 3. Justin L. Voge DVM, MS, Diplomate ACT, A. Kendrick Sudderth DVM, Steven P. Brinsko DVM, MS, PhD, Diplomate ACT, Patrick J. Burns PhD, Terry L. Blanchard DVM, MS, Diplomate ACT ( 2012) Comparison of Efficacy of Two Dose Rates of Histrelin to Human Chorionic Gonadotropin for Inducing Ovulation in Broodmares. Journal of Equine Veterinary Science ( available at www.j-evs.com, see articles in press).
© Copyright 2026