2275
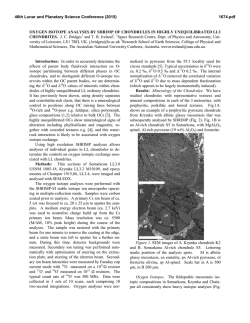
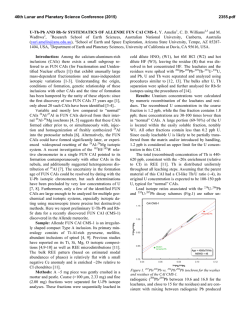
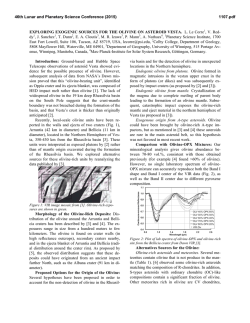
46th Lunar and Planetary Science Conference (2015) 2275.pdf A SILICON ISOTOPIC STUDY OF A FUN-LIKE FORSTERITE-BEARING INCLUSION FROM ALLENDE. K. Fukuda1, H. Hiyagon1, N. Takahata2, Y. Sano2, and A. Hashimoto3, 1Department of Earth and Planetary Science, Graduate School of Science, The University of Tokyo, Tokyo 113-0033, Japan ([email protected]), 2Atmosphere and Ocean Research Institute, The University of Tokyo, Chiba 277-8564, Japan, 3 Depertment of Cosmoscience, Hokkaido University, Hokkaido 060-0810, Japan. Introduction: There is a minor group of CalciumAluminum-rich Inclusions (CAIs), so called FUN (Fractionation and Unknown Nuclear effects) CAIs, which are characterized by (i) large mass-dependent fractionation in O, Mg, and Si, (ii) relatively large mass-independent fractionation in Ca, Ti, Cr, and other elements, and (iii) little or no excess 26Mg from the decay of 26Al [e.g., 1, 2, 3, 4]. The origin of FUN CAIs is still not well understood, but they may have information about an earliest stage of the solar system evolution. Recently, [5] conducted evaporation experiments for Mg- and Si-rich melts simulating precursor materials of FUN CAIs, and tried to estimate the chemical compositions of the precursor materials of some FUN CAIs based on the observed massdependent isotopic fractionation of O, Mg, and Si and changes in chemical compositions of the melts. To better understand the chemical compositions of precursor materials of FUN CAIs, we conducted electron microprobe analyses and in-situ Si isotopic measurements of a FUN-like inclusion from Allende, called AL1B-F, which shows large mass-dependent fractionation in O and Mg [6]. Sample and Methods: AL1B-F is a forsteritebearing inclusion which shows large mass dependent fractionation in O and Mg [6]. The central part of this inclusion is composed of coarse-grained forsteritic olivine, spinel, and Al-Ti-rich pyroxene. The outer part is mainly composed of spinel with lath-shaped hibonite and corundum. Between forsterite-rich core and spinelrich outer part are filled with abundant secondary minerals (e.g., sodalite and nepheline). A polished section of AL1B-F was mapped in Mg, Ca, Al, Ti, Si, Na, Cl, K, S, Cr, Fe, and Ni x-rays with a focused electron beam, 15keV, 200nA beam current, 5s per pixel acquisition time, and resolution of 1 x 1µm per pixel using the JEOL JXA-8530F field-emission gun electron probe microanalyzer (FEG-EPMA), the University of Tokyo. Quantitative analyses were also conducted with a ~2µm beam, 15keV, and 12nA beam current .A ZAF matrix correction method was applied. Silicon isotopic analyses were made using the NanoSIMS 50 at AORI, the University of Tokyo. A primary beam of Cs+ with a diameter of 1µm and an intensity of 10pA was used for the analyses. Primary beam was rastered by 5 x 5µm during the analysis to stabilize the secondary ion intensities. Before each analysis, the sample was pre-sputtered for 3-5 minutes. A mass resolving power was set to ~3500, which is sufficient to separate 28SiH- and 29Si- peaks. Secondary ions, 28Si-, 29Si- and 30Si-, were detected using an electron multiplier (EM) (Tr5) by a peak-jumping mode, and 18O- was detected simultaneously with 28Si- using another EM (Tr2). The measured 29Si-/28Si- and 30Si/28Si- were normalized to the literature value of 0.050633 and 0.033474, respectively [7], and those of the samples were also normalized to the average of the measured San Carlos olivine data or the Traversella Al-Ti-rich pyroxene data. Results and Discussion: Silicon isotopic mesurements were peformed on 9 forsteritic olivines and 3 Al-Ti-rich pyroxenes in AL1B-F. Figure 1 shows the δ30Si-δ29Si diagram of several olivine and pyroxene grains. These minerals show a large mass-dependent fractionation of up to ~22‰/amu. Al-Ti-rich pyroxene data show rather homogeneous compositions with an average of +39‰ in δ30Si, while olivine data show a large range of fractionation with δ30Si from +12 to +31‰. These results suggest that Al-Ti-rich pyroxene formed only at the last stage of the evaporation event, while olivine probably crystallized at various stages of the evaporation event. Furthermore, all the data for olivine and Al-Ti-rich pyroxene are located slightly above the mass-dependent fractionation line. Under the decribed analytical condition, however, the contribution of 28SiH- to 29Si- is estimated to be at most ~0.1‰. Hence, the reason of the offset is not clear, but one possiblility is a mass-independent isotopic fractionation effect in excess 29Si (or a corresponding deficiency of 28Si or 30Si), because a small but similar effect was observed in other FUN CAIs [8, 9]. The relationship between the mass-dependent fractionation degree of Si and that of Mg and O [6] in olivine are similar to those of experimental resuslts by [5]. If we assume that mass-dependent fractionations in olivine were resulted from a simple one-stage evaporation event and that a precursor composition of AL1B-F was similar to the FUN 1 composition (53.14 wt% MgO, 41.27 wt% SiO2, 2.97 wt% Al2O3, and 2.36 wt% CaO) [5], ~78% of Mg and ~75% of Si must have been lost (evaporated) from the molten precursor of AL1B-F based on the experimentally determined isotopic fractionation factors [5]. Because of the presence of abundant secondary minerals, it is not possible to precisely 46th Lunar and Planetary Science Conference (2015) 2275.pdf determine the bulk chemical composition of AL1B-F. If we assume that secondary minerals in AL1B-F are alteration products of primaly melilites with Ak mole% of 23 or 89, which are compositions of melilites in the Vigarano forsterite-bearing FUN CAI 1623-5 [10, 11], the estimated precursor composition for AL1B-F would be 34.0 wt% MgO, 42.7 wt% SiO2, 12.8 wt% Al2O3, and 10.6 wt% CaO for Ak mole% of 23, and 37.0 wt% MgO, 48.5 wt% SiO2, 5.6 wt% Al2O3, and 9.1 wt% CaO for Ak mole% of 89, respectively. These results suggest that the precursor of AL1B-F also have a Mg- and Si-rich composition like C1, 1623-5, and CMS-1 FUN CAIs [5, 12]. Further isotopic studies, (e.g., Ca and Ti isotopes) may be required to to better understand formation processes and a relationship between AL1B-F and other FUN CAIs. References: [1] Clayton R. N. and Mayeda T. K. (1977) GRL, 4, 295–298. [2] Wasserburg G. J. et al. (1977) GRL, 4, 299–302. [3] Clayton R. N. et al. (1988) Philos. Trans. R. Soc. London., 325, 483–501. [4] Krot A. N. et al. (2014) GCA, 145, 206–247. [5] Mendybaev R. A. et al. (2013) GCA, 123, 368–384. [6] Hiyagon H. and Hashimoto A. (2008) Meteoritics & Planet. Sci., 43, #5128. [7] Zinner E. K. (1989) U.S. Geol. Surv. B., 1890, 145–162. [8] Clayton R. N. et al. (1984) GCA, 48, 535–548. [9] Molini-Velsco C. et al. (1983) LPS XIV, 509–510. [10] Davis A. M. et al. (1991) GCA, 55, 621–637. [11] McKeegan K. D. (2005) LPS XXXVI, Abstract #2077. [12] Williams C. D. et al. (2014) LPS XXXXV, Abstract #2146. [13] Knight K. B. et al. (2009) GCA, 73, 6390–6401. 30 δ29Si (‰) 25 20 15 10 olivine Al-Ti-rich pyroxene MF line 5 0 0 10 20 30 40 50 60 δ30Si (‰) Figure 1: The δ30Si-δ29Si plot for olivine and Al-Tirich pyroxene in AL1B-F. The blue line represents the Mass-Fractionation (MF) line with the parameters recommended by [13]. Errors are 2σ. Figure 2: (a) A back-scattered electron image of AL1B-F. (b) A combined X-ray elemental map in Mg (red): Ca (green): Al (blue) of AL1B-F. Abbrevations; Fo = forsterite, Sp = spinel, Px = Al-Ti-rich pyroxene.
© Copyright 2026