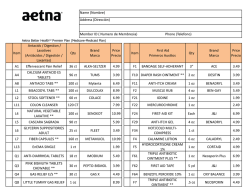
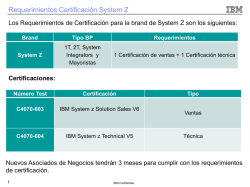
Preferred Drug List - UHCCommunityPlan.com
Preferred Drug List (PDL) Lista de Medicamentos Preferidos (PDL) Arizona Effective Date/Vigencia: 10/1/16 © 2016 United Healthcare Services, Inc. All rights reserved. Introduction UnitedHealthcare Community Plan is pleased to provide this Preferred Drug List (PDL) to be used when prescribing for patients covered by the pharmacy benefit plan offered by UnitedHealthcare Community Plan. The drugs listed in this PDL are intended to provide sufficient options to treat patients who require treatment with a drug from that pharmacologic or therapeutic class. The drugs listed in the UnitedHealthcare Community Plan PDL have been reviewed and approved by the Pharmacy and Therapeutics Committee. The drugs have been selected to provide the most clinically appropriate and cost-effective medications for patients who have their drug benefit administered through UnitedHealthcare Community Plan. It is also recognized there may be occasions where an unlisted drug is desired for proper medical management of a specific patient. In those infrequent instances, the unlisted medication may be requested through the prior authorization process. The drugs represented have been reviewed by the Pharmacy and Therapeutics (P&T) Committee and are approved for inclusion. The PDL is reflective of current medical practice as of the date of review. This edition incorporates drugs added to the PDL since the last edition as well as numerous revisions to the prescribing information based on changes in pharmacotherapy. Comments and suggestions from practicing physicians have also been incorporated to ensure that the UnitedHealthcare Community Plan PDL is reflective of current medical practice. Notice The information contained in this PDL and its appendices is provided by UnitedHealthcare Community Plan, solely for the convenience of medical providers. UnitedHealthcare Community Plan does not warrant or assure accuracy of such information nor is it intended to be comprehensive in nature. This PDL is not intended to be a substitute for the knowledge, expertise, skill and judgment of the medical provider in their choice of prescription drugs. i UnitedHealthcare Community Plan assumes no responsibility for the actions or omissions of any medical provider based upon reliance, in whole or in part, on the information contained herein. The medical provider should consult the drug manufacturer’s product literature or standard references for more detailed information. National guidelines can be found on the Web sites listed in the Web site section or go to the National Guideline Clearinghouse site at http://www.guideline.gov. Preface The UnitedHealthcare Community Plan PDL is organized by sections. Each section includes therapeutic groups identified by either a drug class or disease state. Products are listed by generic name. Brand names are included as a reference to assist in product recognition. Unless exceptions are noted, generally all applicable dosage forms and strengths of the drug cited are included in the PDL. Generics should be considered the first line of prescribing. The UnitedHealthcare Community Plan PDL covers selected over-the-counter (OTC) products. You are encouraged to prescribe OTC medications when clinically appropriate. Pharmacy and Therapeutics (P&T) Committee The P&T Committee includes physicians and pharmacists who are not employees or agents of UnitedHealthcare Community Plan or its affiliates. They must adhere to the Ethics Policy standards of the P&T Committee. UnitedHealthcare Community Plan medical directors and pharmacists also participate in the P&T Committee. The P&T Committee meets quarterly to discuss a variety of issues. Those issues pertaining to pharmaceutical selection and pharmacy program management are communicated quarterly. This newsletter is distributed to all participating physicians who have received the PDL. PDL decisions are also communicated quarterly on the UnitedHealthcare Community Plan internet site. Outpatient Prescription Drug BenefitCovered Medications Medically necessary outpatient prescription drugs are covered when prescribed by a provider licensed to prescribe federal legend drugs or medicines. Some items are covered only with prior authorization. Eligibility for Outpatient Prescription Drug Benefits and copays are based on the individual member’s benefit plan. Product Selection Criteria The P&T Committee considers clinical information on new-to-market drugs that are typically included in an outpatient pharmacy benefit. The evaluation includes all or part of the following: •Safety •Efficacy PDL Product Descriptions To assist in understanding which specific strengths and dosage forms are covered on the PDL, examples are noted below. The general principles shown in the examples can then usually be extended to other entries in the book. Any exceptions are noted in the drug list. There may also be a statement associated with a drug list that gives additional information about which specific products or dosage forms are covered. Products covered include all strengths associated with the dosage form of the cited brand name product. carvedilol Coreg All strengths of Coreg would be covered by this listing. •Comparison studies Extended-release and delayed-release products require their own entry. •Approved indications •Adverse effects •Contraindications/Warnings/Precautions •Pharmacokinetics •Patient administration/compliance considerations •Medical outcome and pharmacoeconomic studies When a new drug is considered for PDL inclusion, it will be reviewed relative to similar drugs currently included in the UnitedHealthcare Community Plan PDL. This review process may result in deletion of drug(s) in a particular therapeutic class in an effort to continually promote the most clinically useful and cost-effective agents. All the information in the PDL is provided as a reference for drug therapy selection. Specific drug selection for an individual patient rests solely with the prescriber. diltiazem sustained release Cardizem SR Dosage forms covered will be consistent with the category and use where listed. Neomycin/polymixin B/Cortisporin Hydrocortisone As listed in the OTIC section, this is limited to the otic solution and suspension. From this entry, the ophthalmic solution and ointment, and the topical cream, cannot be assumed to be on the list unless there are entries for these products in the OPHTHALMIC and DERMATOLOGY sections of the PDL. When a strength or dosage form is specified, only the specified strength and dosage form is on the PDL. Other strengths/dosage forms of the reference product are not. citalopram 40mg tabs Celexa tabs ii Generic Substitution The UnitedHealthcare Community Plan PDL requires generic substitution on the majority of products when a generic equivalent is available. Generic substitution is a pharmacy action whereby a generic equivalent is dispensed rather than the brand name product. The PDL indicates generic availability in the “Covered Drug” column. If a brand name drug is medically necessary, please submit a prior authorization request. The UnitedHealthcare Community Plan MAC list sets a ceiling price for the reimbursement of certain multisource prescription drugs. This price will typically cover the acquisition of most generics but not branded versions of the same drug. The products selected for inclusion on the MAC list are commonly prescribed and dispensed and have usually gone through the FDA’s review and approval process. An important consideration for generic substitution is the knowledge that all approvals of generic drugs by the FDA since 1984, and many generic approvals prior to 1984, have a showing of bioequivalence between the generic versions and the reference brand product. To gain FDA approval: 1. The generic drug must contain the same active ingredient(s), be the same strength and the same dosage form as the brand name product. 2. The FDA has given the generic an “A” rating compared to the branded product indicating bioequivalence, and has determined the generic is therapeutically equivalent to the reference brand. The ratings of generic drugs are available by referring to the FDA reference, Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book). iii When the above two criteria are met, a generic can be substituted with the full expectation that the substituted product will produce the same clinical effect and safety profile as the prescribed product. Drug products that have a narrow therapeutic index (NTI) can also be guided by these principles. It is not necessary for the health care provider to approach any one therapeutic class of drug products (e.g., NTI drugs) differently from any other class, when there has been a determination of therapeutic equivalence by the FDA for the drug products under consideration. Also, additional clinical tests or examinations by the physician are not needed when a therapeutically equivalent generic drug product is substituted for the brand name product. There are now many brand name products that are repackaged or distributed under a generic label. The generic label version should always be considered therapeutically equivalent and substitutable for the source branded product. Drug Efficacy Study Implementation (DESI) Drugs Drugs first marketed between 1938 and 1962 were approved as safe but required no showing of effectiveness for FDA approval. Beginning in 1962, all new drugs were required to be both safe and effective before they could be marketed. This legislation also applied retroactively to all drugs approved as safe from 1938-1962. The DESI program was established by the FDA to review the effectiveness of these pre-1962 drugs for their labeled indications, and a determination of “fully effective” was made for most of these products and they remain in the marketplace. A few DESI products remain classified as “less than fully effective” while awaiting final administrative disposition. Also, classified as DESI are many products listed as identical, similar, or related to actual DESI products. UnitedHealthcare Community Plan’s PDL does not cover DESI “less than fully effective” drug products. Plan Exclusions The following drug categories are excluded from coverage under the outpatient pharmacy benefit and are not part of the UnitedHealthcare Community Plan PDL. •DESI drugs •Antiobesity agents •Experimental / research drugs •Cosmetic drugs •Immunization agents •Nutritional / diet supplements •Blood and blood plasma products •Agents used to promote fertility •Agents used for erectile dysfunction •Agents used for cosmetic hair growth •Drugs from manufacturers that do not participate in the FFS Medicaid Drug Rebate Program •Diagnostic products •Medical supplies and DME except as listed: syringes, needles, lancets, alcohol swabs, spacers, preferred diabetes test strips, peak flow meters (Astech, Assess, Peak Air brands, max two per year), vaporizer (limit of one per three years), humidifier (limit of one per three years) •Mirena Days Supply Dispensing Limitations UnitedHealthcare Community Plan members may receive up to a one- month supply of a specific medication per prescription order or prescription refill. A medication may be reordered or refilled when seventy-five percent (75%) of the medication has been utilized. If a claim is submitted before 75% of the medication has been used, based on the original day supply submitted on the claim, the claim will reject with a “refill too soon” message. Mandatory Generic Substitution The UnitedHealthcare Community Plan PDL requires mandatory generic substitution on the vast majority of products when a generic equivalent is available; however, brand name drugs may be covered in certain situations by requesting a prior authorization. The UnitedHealthcare Community Plan PDL prior authorization (PA) list does not include branded items where a generic equivalent is covered. Prior Authorization of Non-PDL Medications The drugs in the UnitedHealthcare Community Plan PDL have been selected to provide the most clinically appropriate and cost-effective medications for patients who have their drug benefit administered through UnitedHealthcare Community Plan. It is also recognized that there may be occasions where an unlisted drug is desired for the proper medical management of a specific patient. In those infrequent instances, the prior authorization process reviews requests for unlisted medications the physician may consider medically necessary for patient management. Requests for these exceptions should be either made in writing by the physician and faxed or called in to: UnitedHealthcare Community Plan Pharmacy Services Department Fax 1-866-940-7328 Phone 1-800-310-6826 iv A prior authorization request form is available in the UnitedHealthcare Community Plan provider manual and should be used for all prior authorization requests if possible. Appropriate documentation must be provided to support the medical necessity of the non-PDL request. The UnitedHealthcare Pharmacy Department will respond to all requests in accordance with state requirements. Physicians are requested to adhere to this PDL when prescribing for patients covered by their pharmacy benefit plan offered by UnitedHealthcare Community Plan. If a pharmacist receives a prescription for a non-PDL drug, the pharmacist should contact the prescribing physician and request that the prescription be changed to a medication included in this PDL. If a PDL alternative is not appropriate, the physician should then be instructed to contact the Plan for a prior authorization. Please contact the UnitedHealthcare Community Plan Pharmacy Prior Notification Service at 1-800-310-6826 with questions concerning the prior authorization process. Non-PDL Drugs Five-Day Temporary Supply Overrides To ensure the use of PDL drugs, all non-PDL drugs should be discussed with the prescribing physician. If you cannot speak to the physician immediately, and there is an immediate need for the medication, the claim processing system will accept an override to permit a onetime dispensing of a five-day supply of the newly prescribed non-PDL drug. The pharmacy should submit a claim for a five day supply, with a PA Type of 8 and Prior Authorization number of “00000000120”. Please note that non-preferred drugs are available for a five-day supply, however, availability is subject to the benefit design. For assistance, pharmacies may call 1-800-310-6826. v The pharmacy should contact the physician to discuss a PDL drug or if a prior authorization request is warranted. If the prescribing physician feels a drug is medically necessary, the physician may fax a request for prior authorization to UnitedHealthcare Community Plan at 1-866-940-7328. Quantity Limitations (QL) Prescriptions for monthly quantities greater than the indicated limit require a prior authorization request. Quantity Limits Based on Efficient Medication Dosing The Efficient Medication Dosing Program is designed to consolidate medication dosage to the most efficient daily quantity to increase adherence to therapy and also promote the efficient use of health care dollars. The limits for the program are established based on FDA approval for dosing and the availability of the total daily dose in the least amount of tablets or capsules daily. Quantity Limits in the prescription claims processing system will limit the dispensing to consolidate dosing. The pharmacy claims processing system will prompt the pharmacist to request a new prescription order from the physician. Additions to the QL program drug list will be made from time to time and providers notified accordingly. As always, we recognize that a number of patientspecific variables must be taken into consideration when drug therapy is prescribed and therefore overrides will be available through the medical exception (prior authorization) process. Please contact the UnitedHealthcare Community Plan Pharmacy Prior Notification Service at 1-800-310-6826 with questions. Controlled Substances You may fill any FOUR medications from the following classes in a 30-day period: •opiate analgesics •benzodiazepines •sedative hypnotic agents •barbiturates •select muscle relaxants Additional fills will require prior authorization. Medications in these classes may also be subject to individual quantity limits. Step Therapy (ST) The following PDL drugs are routinely covered only after a sufficient trial of an indicated first-line agent has been adequately tried and failed. These medications may also be requested through the Prior authorization process. While lower cost PDL alternatives may be appropriate in many instances, other non-PDL alternatives are available with prior authorization (PA). STEP Drug First-Line Agent(s) Advair (1) 30 day trial of one inhaled corticosteroid (e.g. Asmanex Twisthaler, Flovent HFA, QVAR) OR (2) 60 day trial of a long acting beta2- agonist (e.g. Arcapta, Striverdi) OR 60 day trial of an orally inhaled anticholinergic agent (e.g. Atrovent, Spiriva). Amerge Trial at a minimum dose of 50mg of sumatriptan tablets. Aricept 23mg 90-day trial of Aricept 10mg daily. calcipotriene cream & oint 0.005% Trial of two topical corticosteroids. The UnitedHealthcare Pharmacy Department will review and respond to all requests in accordance with state requirements, and if authorized for payment, UnitedHealthcare Community Plan will coordinate the delivery of the product to the member or provider. calcitriol 3mcg/gm Trial of two topical corticosteroids. Ditropan XL 30-day trial of oxybutynin immediate release. Step Therapy only applies to members less than 65 years of age. Drugs that are part of this program and are on the PDL are identified in this booklet by the designation “SP”. DPP4 Inhibitors At least a 90-day trial of (Tradjenta, 1500mg/day of metformin. Januvia, Janumet, Janumet XR, Jentadueto) Specialty Pharmaceutical Management Program UnitedHealthcare Community Plan is continuously looking for ways to provide high-quality costeffective care for Plan members. The Specialty Pharmaceutical Management Program helps UnitedHealthcare Community Plan to achieve these goals. Injectable medications that are part of this program require plan authorization and are not available through the retail pharmacy network. To obtain authorization, the provider must submit the appropriate Prior Authorization form to the UnitedHealthcare Community Plan Pharmacy Department via fax at 1-866-940-7328. Prior Authorization request forms can be requested by calling the UnitedHealthcare Community Plan Pharmacy Department at 1-800-310-6826. vi vii STEP Drug First-Line Agent(s) STEP Drug First-Line Agent(s) Dulera (1) 30 day trial of one inhaled corticosteroid (e.g. Asmanex Twisthaler, Flovent HFA, QVAR). Symbicort Elidel Trial of two different topical corticosteroids. Step therapy only applies to members 12 years of age and older. fenofibrate Fill of a statin or 90 days of gemfibrozil within the previous 180 days. (1) 30 day trial of one inhaled corticosteroid (e.g. Asmanex Twisthaler, Flovent HFA, QVAR) OR (2) 60 day trial of a long acting beta2- agonist (e.g. Arcapta, Striverdi) OR 60 day trial of an orally inhaled anticholinergic agent (e.g. Incruse Ellipta, Atrovent, Combivent, Anoro Ellipta). tacrolimus 0.03% Trial of two different topical corticosteroids. Step therapy only applies to members 12 years of age and older. tacrolimus 0.1% Minimum age of 16. Trial of two different topical corticosteroids. tolterodine 30 day trial of oxybutynin immediate release. Step Therapy only applies to members less than 65 years of age. trospium 30 day trial of oxybutynin immediate release. Step Therapy only applies to members less than 65 years of age. TZD’s (ActosPlusMet, ActoPlusMet XR, Duetact) At least a 90-day trial of 1500mg/day of metformin. Uloric 8-week trial of up to 600mg of allopurinol required first. GLP-1 Agonists (Victoza) At least a 90-day trial of 1500mg/day of metformin. Midodrine Tablet Trial of fludrocortisone. Optivar 14 day trial of ketotifen within previous 90 days required first. oxymorphone ER (non-crush resistant) 30-day trial of both Fentanyl patch and morphine sulfate ER at least 200mg per day. Ranexa Trial of one drug from the following classes: beta blockers, calcium channel blockers, long acting nitrates. Renvela 8-week trial of calcium acetate. SGLT-2 Inhibitors (Jardiance, Invokana, Invokamet, Synjardy) At least a 90-day trial of 1500mg/day of metformin. STEP Drug First-Line Agent(s) Vancocin One fill of metronidazole tabs or caps. Xopenex Respules 30-day trial of Albuterol .083% or .5% respules. Zohydro ER 30-day trial of both Fentanyl patch and morphine sulfate ER at least 200mg per day. PDL Suggestions Providers who wish to propose PDL suggestions should forward the information to the UnitedHealthcare Community Plan Director of Pharmacy Services by either mail or fax. Attn: Director of Pharmacy Services UnitedHealthcare Community Plan 1001 Brinton Road Pittsburgh, PA 15221 Fax: 1-866-940-7328 Providers should furnish adequate documentation, such as clinical studies from the medical literature, in order for the request to be considered for PDL addition. This literature should include information documenting clinical necessity as well as therapeutic advantages over current PDL products. Suggestions received by UnitedHealthcare Community Plan will be reviewed by the Pharmacy and Therapeutics Committee at the subsequent P&T Committee meeting. Editor Your comments and suggestions regarding the UnitedHealthcare Community Plan PDL are encouraged. Your input is vital to this PDL’s continued success. All responses will be reviewed and considered. Please send your comments to: UnitedHealthcare Community Plan 1001 Brinton Road Pittsburgh, PA 15221 Fax: 1-866-940-7328 viii Notice Legend ix # Only the dosage forms/strengths of the brand name products noted are on the PDL OTC over-the-counter delayed-rel delayed-release (also known as enteric coated) EC enteric-coated ext-rel extended-release (also known as sustained-release) PA Prior Authorization required QL Quantity Limits apply ST Step Therapy, see pages viii - ix for details SP Specialty Pharmaceuticals; see page vii for details The information contained in this document is proprietary information. The information may not be copied in whole or in part without the written permission of UnitedHealthcare Community Plan. All rights reserved. The drug names listed here are the registered and/or unregistered trademarks of third-party pharmaceutical companies unrelated to and unaffiliated with UnitedHealthcare Community Plan. These trademarked brand names are included here for informational purposes only and are not intended to imply or suggest any affiliation between UnitedHealthcare Community Plan and such third-party pharmaceutical companies. If viewing this PDL via the Internet, please be advised that the PDL is updated periodically and changes may appear prior to their effective date to allow for notification. Introducción Aviso UnitedHealthcare Community Plan se complace en ofrecer esta Lista de medicamentos preferidos (Preferred Drug List, PDL) que se utilizará al realizar recetas para los pacientes que tienen cobertura del plan de beneficios de farmacia ofrecido por UnitedHealthcare Community Plan. Los medicamentos incluidos en esta PDL tienen como finalidad ofrecer opciones suficientes para tratar a los pacientes que necesitan tratamiento con un medicamento de dicha clase farmacológica o terapéutica. Los medicamentos incluidos en la PDL de UnitedHealthcare Community Plan han sido revisados y aprobados por el Comité de Farmacia y Terapéutica. Los medicamentos se han seleccionado para ofrecer los medicamentos más apropiados desde el punto de vista clínico y más asequibles para los pacientes que tienen su beneficio de medicamentos administrado a través de UnitedHealthcare Community Plan. También se reconoce que puede haber ocasiones en que un medicamento no incluido en la lista se requiere para el control médico adecuado de un paciente específico. En estas instancias poco frecuentes, los medicamentos que no estén incluidos pueden ser requeridos a través del proceso de autorización previa. La información incluida en esta PDL y sus apéndices es provista por UnitedHealthcare Community Plan, exclusivamente para la comodidad de los proveedores médicos. UnitedHealthcare Community Plan no garantiza ni asegura la precisión de dicha información ni pretende ser integral por naturaleza. Los medicamentos representados han sido revisados por el Comité de Farmacia y Terapéutica (Pharmacy and Therapeutics, P&T) y están aprobados para su inclusión. La PDL refleja la práctica médica actual desde la fecha de la revisión. Prólogo Esta edición incorpora medicamentos agregados a la PDL desde la última edición así como numerosas revisiones para la información de prescripción basada en los cambios en la farmacoterapia. También se han incorporado comentarios y sugerencias de médicos practicantes para garantizar que la PDL de UnitedHealthcare Community Plan refleje la práctica médica actual. Esta PDL no tiene la finalidad de sustituir el conocimiento, la pericia, las habilidades ni el criterio del proveedor médico en su elección de medicamentos recetados. UnitedHealthcare Community Plan no asume ninguna responsabilidad por las acciones u omisiones de los proveedores médicos sobre la base de la confianza, total o parcial, de la información incluida aquí. El proveedor médico debe consultar la información del producto del fabricante del medicamento o las referencias estándar para obtener información detallada. Las pautas nacionales pueden encontrarse en los sitios web que se enumeran en la sección del sitio web, o bien, visite el sitio del Centro de Intercambio de Información de Pautas Nacionales en http://www.guideline.gov. La PDL de UnitedHealthcare Community Plan está organizada por secciones. Cada sección incluye grupos terapéuticos identificados por una clase de medicamento o estado de la enfermedad. Los productos están enumerados por nombre genérico. Las marcas están incluidas como una referencia para ayudarlo a reconocer el producto. A menos que se incluyan excepciones, por lo general todas las formas de dosificación y concentraciones aplicables del medicamento citado están incluidas en la PDL. Los medicamentos genéricos deben ser considerados como medicamentos recetados de primera línea. x La PDL de UnitedHealthcare Community Plan cubre algunos productos de venta libre (over-the-counter, OTC). Lo alentamos a que recete medicamento OTC cuando sea clínicamente apropiado. Comité de Farmacia y Terapéutica (P&T) El Comité de P&T incluye médicos y farmacéuticos que no son empleados ni agentes de UnitedHealthcare Community Plan o sus afiliadas. Deben respetar los estándares de la Política sobre ética del Comité de P&T. Los directores médicos de UnitedHealthcare Community Plan y los farmacéuticos también participan en el Comité de P&T. El Comité de P&T se reúne trimestralmente para analizar diversos temas. Los temas pertinentes a la selección farmacéutica y la administración del programa de farmacia se comunican trimestralmente. Este boletín informativo se distribuye a todos los médicos participantes que hayan recibido la PDL. Las decisiones de PDL también son comunicadas trimestralmente en el sitio de Internet de UnitedHealthcare Community Plan. Beneficio de Medicamentos Recetados Para Pacientes Ambulatorios - Medicamentos Cubiertos Los medicamentos recetados para pacientes ambulatorios médicamente necesarios están cubiertos cuando son recetados por un proveedor autorizado para recetar medicamentos o fármacos con leyenda federales. Algunos artículos solo se cubren con autorización previa. La elegibilidad para los beneficios de medicamentos recetados para pacientes ambulatorios y los copagos están basados en el plan de beneficios del miembro individual. xi Criterios de Selección de Productos El Comité de P&T considera la información clínica en los medicamentos nuevos para el mercado que por lo general se incluyen en el beneficio de farmacia para pacientes ambulatorios. La evaluación incluye todo o parte de lo siguiente: •Seguridad •Eficacia •Estudios de comparación •Indicaciones aprobadas •Efectos adversos •Contraindicaciones/Advertencias/ Precauciones •Farmacocinética •Administración de pacientes/consideraciones de cumplimiento •Resultados médicos y estudios farmacoeconómicos Cuando un medicamento nuevo se considera para su inclusión en la PDL, se revisará en relación a los medicamentos similares que se incluyen actualmente en la PDL de UnitedHealthcare Community Plan. Este proceso de revisión puede derivar en la supresión de medicamentos en una clase terapéutica en particular con el fin de promover continuamente los agentes más económicos y útiles desde el punto de vista clínico. Toda la información que se incluye en la PDL se proporciona como referencia para la selección de tratamientos con medicamentos. La selección de medicamentos específicos para un paciente individual la realiza exclusivamente el profesional autorizado para recetar medicamentos. Descripciones de los Productos Incluidos en la PDL A fin de brindar ayuda para entender qué concentraciones específicas y formas de dosificación están cubiertas en la PDL, a continuación se incluyen ejemplos: Los principios generales que se muestran en los ejemplos generalmente luego pueden extenderse a otras entradas del libro. Las excepciones se indican en la lista de medicamentos. También puede haber una declaración relacionada con una lista de medicamentos que ofrece información adicional acerca de cuáles son los productos específicos o formas de dosificación que se cubren. Los productos cubiertos incluyen todas las concentraciones asociadas con la forma de dosificación del producto de marca citado. carvedilol Coreg Todas las concentraciones de Coreg estarían cubiertas según esta lista. Los productos de liberación prolongada y de liberación retardada requieren su propia entrada. diltiazem de liberación Cardizem SR Las formas de dosificación cubiertas serán consistentes con la categoría y el uso en los casos que se incluyan en la lista. Neomicina/polimixina B/Cortisporin Hidrocortisona Según lo enumerado en la sección de productos ÓTICOS, se limita a la solución y suspensión ótica. En esta entrada, no puede suponerse que la solución oftálmica, el ungüento y la crema tópica estén incluidos en la lista a menos que existan entradas para estos productos en las secciones de productos OFTÁLMICOS y DERMATOLÓGICOS de la PDL. En los casos en que se especifique la concentración y la forma de dosificación, solo la concentración especificada y la forma de dosificación se encuentran incluidas en la PDL. Otras concentraciones o formas de dosificación del producto de referencia no son. los comprimidos de citalopram 40 mg Celexa tabs Sustitución por Genéricos La PDL de UnitedHealthcare Community Plan requiere la sustitución por genéricos en la mayoría de los productos cuando se encuentra disponible un equivalente del medicamento genérico. La sustitución por genéricos es una medida que toma la farmacia en los casos en que un equivalente de genérico se dispense en lugar del producto de marca. El PDL indica la disponibilidad de genéricos en la columna de “Medicamentos cubiertos”. Si un medicamento de marca es médicamente necesario, por favor envíe una solicitud de autorización previa. La lista del Consejo de Apelaciones de Medicare (Medicare Appeals Council, MAC) de UnitedHealthcare Community Plan establece un precio máximo para el reembolso de ciertos medicamentos recetados de múltiples fuentes. Este precio por lo general cubrirá la adquisición de la mayoría de los medicamentos genéricos pero no las versiones de marca del mismo medicamento. Los productos seleccionados para su inclusión en la lista del MAC son recetados y dispensados comúnmente, y por lo general han pasado por el proceso de revisión y aprobación de la Administración de Alimentos y Medicamentos (FDA). xii Una consideración importante para la sustitución por genéricos es el conocimiento de que todas las aprobaciones de medicamentos genéricos por parte de la FDA desde el año 1984, y muchas aprobaciones de medicamentos genéricos antes de este año, demuestran una equivalencia biológica entre las versiones genéricas y el producto de marca de referencia. Para obtener la aprobación de la FDA: 1. El medicamento genérico debe incluir los mismos ingredientes activos y tener la misma concentración y forma de dosificación que el producto de marca. 2. La FDA ha otorgado a los medicamentos genéricos la calificación “A” en comparación con los productos de marca que indican la equivalencia biológica; además, ha determinado que, desde el punto de vista terapéutico, el medicamento genérico es equivalente al medicamento de marca. Las calificaciones de los medicamentos genéricos están disponibles al consultar la referencia de la FDA, Productos farmacéuticos aprobados con evaluaciones de equivalencia terapéutica (Libro naranja) En los casos en que se cumpla con los dos criterios mencionados, un medicamento genérico puede sustituirse con la total expectativa de que el producto sustituido producirá el mismo efecto clínico y tendrá el mismo perfil de seguridad que el producto recetado. Los productos farmacéuticos que tengan un índice terapéutico estrecho (NTI) también pueden ser guiados por estos principios. No es necesario que el proveedor de atención médica se aproxime a cualquier clase terapéutica de los productos farmacéuticos (por ejemplo, medicamentos con NTI) de forma diferente a la de cualquier otra clase, cuando la FDA ha determinado la equivalencia terapéutica de los productos farmacéuticos en cuestión. Además, no es necesario que los médicos realicen pruebas clínicas o exámenes adicionales cuando un producto farmacológico genérico equivalente desde el punto de vista terapéutico se sustituye por el producto de marca. xiii Actualmente, hay muchos productos de marca que cuentan con un envase nuevo o son distribuidos con etiquetas de medicamento genérico. La versión con etiqueta de medicamento genérico siempre debe considerarse como un equivalente desde el punto de vista terapéutico y sustituible por el producto de marca original. Medicamentos del Programa Implementación del Estudio Sobre Eficacia de Medicamentos (DESI) Los medicamentos que se comercializaron por primera vez entre 1938 y 1962 fueron aprobados por ser seguros pero no requerían demostración de eficacia para la aprobación de la FDA. A partir de 1962, todos los medicamentos nuevos debían ser seguros y eficaces antes de que pudieran ser comercializados. Esta legislación también se aplicó de forma retroactiva a todos los medicamentos aprobados por su seguridad entre los años 1938 y 1962. El programa DESI fue establecido por la FDA para revisar la eficacia de estos medicamentos anteriores a 1962 para las indicaciones de sus etiquetas, y se realizó una determinación de eficacia total para la mayoría de estos productos, y permanecen en el mercado. Unos pocos productos del programa DESI permanecen clasificados como “menos que totalmente eficaces” mientras se espera la disposición administrativa final. Además, muchos productos incluidos como idénticos, similares o relacionados con los productos verdaderos del programa DESI están clasificados como DESI. La PDL de UnitedHealthcare Community Plan no cubre los productos farmacéuticos “menos que totalmente eficaces” de DESI. Exclusiones del Plan Las siguientes categorías de medicamentos están excluidas de la cobertura conforme al beneficio de farmacia para pacientes ambulatorios y no son parte de la PDL de UnitedHealthcare Community Plan. •Medicamentos del programa DESI •Agentes contra la obesidad •Medicamentos experimentales o en investigación •Medicamentos usados para fines cosméticos •Agentes de vacunación Limitaciones en la Provisión de Suministros de Días Los miembros de UnitedHealthcare Community Plan pueden recibir hasta un suministro de un mes de un medicamento específico por pedido de receta o reposición de medicamentos recetados. Un medicamento puede volver a pedirse o reponerse cuando se ha utilizado el setenta y cinco por ciento (75%) del medicamento. Si se presenta una reclamación antes de haberse utilizado el 75% del medicamento, según los días de suministro original presentado en la reclamación, esta será rechazada con un mensaje de “reposición prematura”. •Suplementos nutricionales/dietéticos Sustitución por Genéricos Obligatoria •Productos de sangre o plasma sanguíneo La PDL de UnitedHealthcare Community Plan PDL requiere de la sustitución por genéricos obligatoria en gran parte de los productos cuando se encuentra disponible un equivalente genérico; no obstante, los medicamentos de marca pueden estar cubiertos en determinadas situaciones al solicitar una autorización previa. La lista de autorización previa (PA) de la PDL de UnitedHealthcare Community Plan no incluye artículos de marca en los casos en que el equivalente genérico está cubierto. •Medicamentos usados para promover la fertilidad •Agentes usados para la disfunción eréctil •Agentes usados con fines cosméticos para el crecimiento del cabello •Medicamentos de fabricantes que no participan en el Programa de descuentos en medicamentos de Medicaid de FFS •Productos de diagnóstico •Suministros médicos y equipo médico duradero (durable medical equipment, DME) excepto según se menciona: jeringas, agujas, lancetas, toallitas con alcohol, espaciadores, tiras reactivas para medir la glucosa, medidores de flujo máximo (marcas Astech, Assess, Peak Air, máx. dos por año), vaporizador (límite de 1 por cada 3 años), humidificador (límite de 1 por cada 3 años) •Mirena Autorización Previa de Medicamentos No Incluidos en la PDL Los medicamentos incluidos en la PDL de UnitedHealthcare Community Plan PDL han sido seleccionados para ofrecer los medicamentos más apropiados desde el punto de vista clínico y más asequibles para los pacientes que tienen su beneficio de medicamentos administrado a través de UnitedHealthcare Community Plan. También se reconoce que puede haber ocasiones en que un medicamento no incluido en la lista se requiere para el control médico adecuado de un paciente xiv específico. En estos casos poco frecuentes, el proceso de autorización previa revisa las solicitudes para los medicamentos no incluidos en la lista que el médico puede considerar médicamente necesario para el control del paciente. El médico debe realizar las solicitudes de estas excepciones por escrito y enviarlas por fax, o bien, debe llamar a: UnitedHealthcare Community Plan Pharmacy Services Department Fax 1-866-940-7328 Teléfono 1-800-310-6826 En el manual de proveedores de UnitedHealthcare Community Plan se encuentra disponible un formulario de solicitud de autorización previa y, si es posible, debe utilizarse para todas las solicitudes de autorización previa. La documentación correspondiente debe proporcionarse para respaldar la necesidad médica de la solicitud de medicamentos no incluidos en la PDL. El Servicio de Farmacia de UnitedHealthcare responderá a todas las solicitudes de acuerdo con los requisitos del estado. Los médicos deben respetar esta PDL al realizar recetas para los pacientes que tienen cobertura mediante su plan de beneficios de farmacia ofrecido por UnitedHealthcare Community Plan. Si un farmacéutico recibe una receta para un medicamento que no está incluido en la PDL, debe comunicarse con el médico que realizó la receta y solicitarle que cambie el medicamento por uno que esté incluido en la PDL. Si una alternativa de la PDL no es adecuada, debe indicarse al médico que se comunique con el plan para solicitar una autorización previa. xv Comuníquese con el Servicio de Notificación Previa de Farmacia de UnitedHealthcare Community Plan al 1-800-310-6826 si tiene preguntas relacionadas con el proceso de autorización previa. Sustituciones de Suministros Temporales de 5 Días de Medicamentos Que No Están Incluidos en la PDL Para garantizar el uso de medicamentos incluidos en la PDL, debe consultar al médico que realiza la receta acerca de todos los medicamentos que no están incluidos en la PDL. Si no puede hablar con el médico de inmediato y necesita el medicamento de forma urgente, el sistema de procesamiento de reclamaciones aceptará una sustitución para permitir una provisión por única vez de un suministro de 5 días del medicamento recientemente recetado que no está incluido en la PDL. La farmacia debe enviar una reclamación para un suministro de 5 días, con el tipo 8 de PA y el número de autorización previa “00000000120”. Tenga en cuenta que los medicamentos no preferidos están disponibles para un suministro de 5 días, no obstante, la disponibilidad está sujeta al esquema de beneficios. Para obtener ayuda, las farmacias pueden llamar al 1-800-310-6826. La farmacia debe comunicarse con el médico para analizar el medicamento de la PDL o si se justifica la solicitud de una autorización previa. Si el médico que realiza la receta considera que un medicamento es médicamente necesario, el médico puede enviar por fax una solicitud de autorización previa a UnitedHealthcare Community Plan al 1-866-940-7328. Limitaciones de Cantidad (QL) •agentes sedantes hipnóticos Las recetas para cantidades mensuales que superen el límite indicado requieren de una solicitud de autorización previa. •barbitúricos Límites de cantidad basados en la dosificación de medicamentos eficaces El Programa de dosificación de medicamentos eficaces está diseñado para consolidar la dosificación del medicamento a la cantidad diaria más eficaz, para aumentar el seguimiento del tratamiento y también promover el uso eficaz del dinero invertido en la atención médica. Los límites del programa se establecen conforme a la aprobación de la FDA en cuanto a la dosificación y la disponibilidad de la dosis diaria total con la menor cantidad de comprimidos o cápsulas diarias. Los límites de cantidad en el sistema de procesamiento de reclamaciones de recetas limitará la provisión para consolidar la dosificación. El sistema de procesamiento de reclamaciones de farmacia indicará al farmacéutico que solicite un nuevo pedido de receta del médico. Las adiciones a la lista de medicamentos del programa de nivel de cantidad (QL) se realizarán de vez en cuando y se notificará a los proveedores al respecto. Como siempre, reconocemos que deben tenerse en cuenta diversas variables específicas del paciente cuando se indica un tratamiento con medicamentos y, por consiguiente, las sustituciones estarán disponibles a través del proceso de excepción médica (PA). Comuníquese con el Servicio de Notificación Previa de Farmacia de UnitedHealthcare Community Plan al 1-800-310-6826 si tiene preguntas. Sustancias controladas Puede surtirse con cualquiera de los CUATRO medicamentos de las siguientes clases en un período de 30 días: •analgésicos opioides •algunos relajantes musculares. Los surtidos adicionales requieren de autorización previa. Los medicamentos de estas clases también pueden estar sujetos a los límites de cantidad individuales. Programa de administración de productos farmacéuticos especiales UnitedHealthcare Community Plan busca continuamente formas de ofrecer una atención asequible de alta calidad para los miembros del plan. El Programa de administración de productos farmacéuticos especiales ayuda a UnitedHealthcare Community Plan a lograr estos objetivos. Los medicamentos inyectables que forman parte de este programa requieren de la autorización del plan y no están disponibles a través de la red de farmacias minoristas. Para obtener la autorización, el proveedor debe enviar por fax el formulario de autorización previa correspondiente al Departamento de Farmacia de UnitedHealthcare Community Plan al 1-866-940-7328. El Servicio de Farmacia de UnitedHealthcare revisará y responderá a todas las solicitudes de acuerdo con los requisitos del estado, y si se autoriza el pago, UnitedHealthcare Community Plan coordinará la entrega del producto al miembro o proveedor. Los medicamentos que forman parte de este programa y están incluidos en la PDL están identificados en este folleto mediante la designación “SP”. Los formularios de solicitud de autorización previa pueden solicitarse llamando al Departamento de Farmacia de UnitedHealthcare Community Plan al 1-800-310-6826. •benzodiazepinas xvi Terapia Escalonada (Step Therapy, ST) Los siguientes medicamentos de la PDL se cubren rutinariamente solo después de un estudio suficiente de un agente de primera línea indicado que se haya estudiado adecuadamente y se haya desaprobado. Estos medicamentos también pueden solicitarse a través del proceso de autorización previa. Si bien las alternativas de menor costo que se incluyen en la PDL pueden ser apropiadas en muchos casos, otras alternativas que no se incluyen en la PDL se encuentran disponibles con autorización previa (prior authorization, PA). Medicamento para TERAPIA ESCALONADA Agentes de primera línea Advair (1) Un estudio de 30 días de un corticoesteroide inhalado (por ejemplo Asmanex Twisthaler, Flovent HFA, QVAR) O (2) un estudio de 60 días de un agonista beta2 de acción prolongada (por ejemplo Arcapta, Striverdi) O un estudio de 60 días de un agente anticolinérgico inhalado por vía oral (por ejemplo, Atrovent, Spiriva). Amerge Estudio con dosis mínima de 50 mg de comprimidos de sumatriptán. Aricept 23mg Estudio de 90 días de Aricept de 10 mg diario. calcipotriene crema y ungüento 0.005% Estudio de dos corticosteroides tópicos. calcitriol 3mcg/gm Estudio de dos corticosteroides tópicos. xvii Medicamento para TERAPIA ESCALONADA Agentes de primera línea Ditropan XL Estudio de 30 días de oxibutinin de liberación inmediata. La terapia escalonada se aplica solamente a los miembros menores de 65 años de edad. Inhibidores de DPP4 (Tradjenta, Januvia, Janumet, Janumet XR, Jentadueto) Estudio de al menos 90 días de 1500 mg/día de metformina. Dulera (1) Un estudio de 30 días de un corticoesteroide inhalado (por ejemplo Asmanex Twisthaler, Flovent HFA, QVAR). Elidel Estudio de dos corticosteroides tópicos diferentes. La terapia escalonada solo se aplica a los miembros mayores de 12 años. fenofibrato Surtido de una estatina o 90 días de Gemfibrozil dentro de los 180 días previos. Agonistas GLP-1 (Victoza) Estudio de por lo menos 90 días de 1500mg/día de metformina. Tableta Midodrina Prueba de fludrocortisona. Optivar Se requiere primero un estudio de 14 días de ketotifen dentro de los 90 días anteriores. Medicamento para TERAPIA ESCALONADA Agentes de primera línea Medicamento para TERAPIA ESCALONADA Agentes de primera línea oxymorphone ER (no resistente al aplastamiento) Un estudio de 30 días de los dos, parches de Fentanyl y sulfato de morfina ER de por lo menos 200mg al día. tacrolimus 0.1% Edad mínimo de 16. Estudio de dos corticosteroides tópicos diferentes. tolterodine Estudio de 30 días de oxibutinina de liberación inmediata. La terapia escalonada solo se aplica a los miembros menores de 65 años. trospium Estudio de 30 días de oxibutinin de liberación inmediato. La terapia escalonada solamente se aplica a los miembros menores de 65 años de edad. TZD’s (ActosPlusMet, ActoPlusMet XR, Duetact) Un estudio de por lo menos 90 días de 1500mg/día de metformina. Uloric Primero se requiere un estudio de 8 semanas de hasta 600 mg de alopurinol. Vancocin Un surtido de comprimidos o cápsulas de metronidazol. Ranexa Estudio de un medicamento de las siguientes categorías: bloqueadores beta, antagonistas del calcio, nitratos de acción prolongada. Renvela Estudio de 8 semanas de acetato de calcio. Inhibidores Estudio de por lo menos SGLT-2 90 días de 1500mg/día (Jardiance, Invokana, de metformina. Invokamet, Synjardy) Symbicort tacrolimus 0.03% (1) Un estudio de 30 días de un corticoesteroide inhalado (por ejemplo Asmanex Twisthaler, Flovent HFA, QVAR) O (2) un estudio de 60 días de un agonista beta2 de acción prolongada (por ejemplo Arcapta, Striverdi) O un estudio de 60 días de un agente anticolinérgico inhalado por vía oral (por ejemplo, Incruse Ellipta, Atrovent, Combivent, Anoro Ellipta). Estudio de dos corticosteroides tópicos diferentes. La terapia escalonada solo se aplica a los miembros de 12 años de edad y mayores. Xopenex Respules Estudio de 30 días de Albuterol 0.83% o 0.5% de Respules. Zohydro ER Estudio de 30 días de los dos, parches de Fentanyl y sulfato de morfina de por lo menos 200mg al día. xviii Sugerencias Sobre la PDL Los proveedores que deseen hacer sugerencias sobre la PDL deben enviar la información por correo o fax al Director de Servicios de Farmacia de UnitedHealthcare Community Plan. Attn: Director of Pharmacy Services UnitedHealthcare Community Plan 1001 Brinton Road Pittsburgh, PA 15221 Fax: 1-866-940-7328 Los proveedores deben proporcionar la documentación adecuada, como los estudios clínicos de la literatura médica, para que la solicitud sea considerada para la inclusión en la PDL. Esta literatura debe incluir información que documente la necesidad clínica así como las ventajas terapéuticas por sobre los productos actuales incluidos en la PDL. Las sugerencias recibidas por UnitedHealthcare Community Plan serán revisadas por el Comité de Farmacia y Terapéutica en la reunión subsiguiente del comité. Editor Se alienta a que realice sus comentarios y sugerencias relacionados con la PDL de UnitedHealthcare Community Plan. Su comentario es muy importante para el éxito continuo de la PDL. Todas las respuestas serán revisadas y tomadas en cuenta. Envíe sus comentarios a: UnitedHealthcare Community Plan 1001 Brinton Road Pittsburgh, PA 15221 Fax: 1-866-940-7328 xix Leyenda # Solo las concentraciones o formas de dosificación de los productos de marca indicados están incluidas en la PDL. OTC de venta libre delayed-rel liberación ret liberación retardada (también conocido como recubrimiento entérico) EC recubrimiento entérico ext-rel liberación prolongada (también conocida como liberación sostenida) PA Autorización previa requerida QL Se aplican límites de cantidad ST Terapia escalonada, ver páginas xviii - xx para obtener detalles SP Productos farmacéuticos especiales, ver página xvii para obtener detalles Aviso La información incluida en este documento es privada. La información no puede ser copiada total o parcialmente sin el permiso escrito de UnitedHealthcare Community Plan. Todos los derechos reservados. Los nombres de los medicamentos incluidos aquí son marcas comerciales registradas y no registradas de compañías farmacéuticas de terceros no relacionadas ni afiliadas a UnitedHealthcare Community Plan. Estas marcas comerciales registradas se incluyen aquí con fines informativos solamente y no tienen la finalidad de denotar ni sugerir afiliación entre Evercare y dichas compañías farmacéuticas de terceros. Si ve esta PDL por Internet, tenga en cuenta que la misma se actualiza periódicamente y es posible que se incluyan cambios antes de la fecha de vigencia para permitir su notificación. xx 10/16 © 2016 United Healthcare Services, Inc. All rights reserved. Table of Contents Antineoplastics & Immunosuppressants . . . 4 Antineoplastic Agents . . . . . . . . . . . . . . . . . . . . . . 4 Hormonal Antineoplastic Agents . . . . . . . . . . . . . 5 Immunomodulators . . . . . . . . . . . . . . . . . . . . . . . . 5 Immunosuppressants . . . . . . . . . . . . . . . . . . . . . . 6 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 Dermatology . . . . . . . . . . . . . . . . . . . . . . . . . 17 Acne Vulgaris . . . . . . . . . . . . . . . . . . . . . . . . . . . 17 Bacterial Infections . . . . . . . . . . . . . . . . . . . . . . . 18 Corticosteroids . . . . . . . . . . . . . . . . . . . . . . . . . . 19 Fungal Infections . . . . . . . . . . . . . . . . . . . . . . . . . 20 Psoriasis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 Rosacea . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 Scabies and Pediculosis . . . . . . . . . . . . . . . . . . 21 Viral Infections . . . . . . . . . . . . . . . . . . . . . . . . . . . 21 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 21 Blood Modifiers - Anticoagulants . . . . . . . . . 7 Anticoagulants . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 Blood Cell Formation . . . . . . . . . . . . . . . . . . . . . . . 7 Platelet Inhibitors . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 Ear, Nose & Throat . . . . . . . . . . . . . . . . . . . . 22 Ear . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22 Nose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23 Throat and Mouth . . . . . . . . . . . . . . . . . . . . . . . . 24 Cardiovascular Agents . . . . . . . . . . . . . . . . . 8 Ace Inhibitors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 Ace Inhibitor/Diuretic Combinations . . . . . . . . . . 8 Adrenolytics, Central . . . . . . . . . . . . . . . . . . . . . . . 8 Alpha Blockers . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 Angiotensin II Receptor Blockers (Antagonists) . 8 Angiotensin II Receptor Blocker Combinations . 9 Antiarrhythmics and Cardiac Glycosides . . . . . . 9 Beta Blockers and Beta Blocker/Diuretic Combinations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 Calcium Channel Blockers . . . . . . . . . . . . . . . . 10 Diuretics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 Lipid Lowering Agents . . . . . . . . . . . . . . . . . . . . 11 Nitrates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11 Potassium-Removing Agents . . . . . . . . . . . . . . 12 Pulmonary Arterial Hypertension . . . . . . . . . . 12 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 12 Endocrinology . . . . . . . . . . . . . . . . . . . . . . . 25 Adrenal Corticosteroids . . . . . . . . . . . . . . . . . . . 25 Androgens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25 Diabetes Mellitus . . . . . . . . . . . . . . . . . . . . . . . . . 25 Growth Stimulating Agents . . . . . . . . . . . . . . . . 27 Metabolic Modifiers . . . . . . . . . . . . . . . . . . . . . . 27 Osteoporosis . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27 Thyroid Disease . . . . . . . . . . . . . . . . . . . . . . . . . 27 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 27 Gastrointestinal . . . . . . . . . . . . . . . . . . . . . . 28 Diarrhea . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Emesis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Gastroesophageal Reflux Disease (Gerd)/ Peptic Ulcers . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Gastrointestinal Spasm . . . . . . . . . . . . . . . . . . . 29 Inflammatory Bowel Disease . . . . . . . . . . . . . . . 29 Laxatives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30 Pancreatic Enzymes . . . . . . . . . . . . . . . . . . . . . . 30 Probiotic Supplementation . . . . . . . . . . . . . . . . 30 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 31 Central Nervous System . . . . . . . . . . . . . . . 13 Alzheimer’s Disease . . . . . . . . . . . . . . . . . . . . . . 13 Analgesics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13 Migraine Acute Therapy . . . . . . . . . . . . . . . . . . . 15 Migraine Prophylactic Therapy . . . . . . . . . . . . . 15 Multiple Sclerosis . . . . . . . . . . . . . . . . . . . . . . . . 15 Myasthenia Gravis . . . . . . . . . . . . . . . . . . . . . . . 15 Parkinson’s Disease . . . . . . . . . . . . . . . . . . . . . . 15 Seizures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 17 Home Infusion Drugs . . . . . . . . . . . . . . . . . . 31 Analgesics - NSAIDS . . . . . . . . . . . . . . . . . . . . . 31 Analgesics - OPIOD . . . . . . . . . . . . . . . . . . . . . . 31 Antibiotics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31 Antihistamines . . . . . . . . . . . . . . . . . . . . . . . . . . . 32 2 Diuretics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33 Electrolyte Mixtures . . . . . . . . . . . . . . . . . . . . . . . 33 Genitourinary Irrigants . . . . . . . . . . . . . . . . . . . . 33 Minerals & Electrolytes . . . . . . . . . . . . . . . . . . . . 33 Nutrients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33 Spasticity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33 Vitamins . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 49 Respiratory Drugs . . . . . . . . . . . . . . . . . . . . 49 Antitussives, Decongestants, Expectorants and Combinations . . . . . . . . . . . . . . . . . . . . . . . . . . . 49 Asthma/COPD . . . . . . . . . . . . . . . . . . . . . . . . . . 55 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 56 Urological . . . . . . . . . . . . . . . . . . . . . . . . . . . 56 Symptomatic Benign Prostatic Hypertrophy . . 56 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 56 Infectious Diseases . . . . . . . . . . . . . . . . . . . 33 Antibacterials . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33 Antifungals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35 Antihelmintics . . . . . . . . . . . . . . . . . . . . . . . . . . . 35 Antivirals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 37 Vitamins and Minerals . . . . . . . . . . . . . . . . . 57 Potassium . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . 61 Anaphylaxis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61 Cystic Fibrosis . . . . . . . . . . . . . . . . . . . . . . . . . . . 61 Detoxification Agents . . . . . . . . . . . . . . . . . . . . . 61 Hereditary Angioedema . . . . . . . . . . . . . . . . . . . 61 Hyperphosphatemia . . . . . . . . . . . . . . . . . . . . . . 61 Idiopathic Pulmonary Fibrosis (IPF) . . . . . . . . . 62 Immune Thrombocytopenic Purpura . . . . . . . . 62 Medical Devices . . . . . . . . . . . . . . . . . . . . . . . . . 62 Vaccine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62 Musculoskeletal . . . . . . . . . . . . . . . . . . . . . . 38 Arthritis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38 Gout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40 Skeletal Muscle Relaxants . . . . . . . . . . . . . . . . . 40 OB-GYN . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40 Contraceptives . . . . . . . . . . . . . . . . . . . . . . . . . . 40 Endometriosis . . . . . . . . . . . . . . . . . . . . . . . . . . . 41 Hormone Therapy/Menopause . . . . . . . . . . . . 41 Vaginal Infections . . . . . . . . . . . . . . . . . . . . . . . . 42 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 43 OTC MEDICATIONS . . . . . . . . . . . . . . . . . . . 64 Acne . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64 Antifungals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64 Atopic Dermatitis . . . . . . . . . . . . . . . . . . . . . . . . . 64 Cough/Cold Allergy . . . . . . . . . . . . . . . . . . . . . . 64 Diabetes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65 Earwax Removal Products . . . . . . . . . . . . . . . . 65 Family Planning . . . . . . . . . . . . . . . . . . . . . . . . . . 65 First Aid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65 Gastrointestinal . . . . . . . . . . . . . . . . . . . . . . . . . . 66 Lice Products . . . . . . . . . . . . . . . . . . . . . . . . . . . 66 Motion Sickness . . . . . . . . . . . . . . . . . . . . . . . . . 66 Ophthalmics . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66 Pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66 Smoking Cessation Products . . . . . . . . . . . . . . . 67 Vitamins/Minerals . . . . . . . . . . . . . . . . . . . . . . . . . 67 Warts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67 Ophthalmic . . . . . . . . . . . . . . . . . . . . . . . . . . 43 Allergy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43 Anti-Inflammatories . . . . . . . . . . . . . . . . . . . . . . . 43 Glaucoma . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44 Infections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45 Miscellaneous . . . . . . . . . . . . . . . . . . . . . . . . . . . 45 Psychiatric . . . . . . . . . . . . . . . . . . . . . . . . . . 45 Alcohol Deterrents . . . . . . . . . . . . . . . . . . . . . . . 45 Anxiety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46 Attention Deficit Hyperactivity Disorder (ADHD) . . . . . . . . . . . . . . . . . . . . . . . . 46 Bipolar Disorder . . . . . . . . . . . . . . . . . . . . . . . . . 47 Depression . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47 Insomnia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48 Narcotic Antagonists . . . . . . . . . . . . . . . . . . . . . 48 Psychoses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48 Smoking Cessation . . . . . . . . . . . . . . . . . . . . . . 49 Index of Covered Drugs . . . . . . . . . . . . . . . . 68 3 Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits Antineoplastics & Immunosuppressants Antineoplastic Agents Alkylating Agents altretamine busulfan chlorambucil cyclophosphamide estramustine phosphate sodium lomustine melphalan temozolomide HEXALEN MYLERAN LEUKERAN CYTOXAN GLEOSTINE ALKERAN TEMODAR brand brand generic capecitabine mercaptopurine mercaptopurine susp thioguanine trifluridine/tipiracil XELODA PURINETHOL PURIXAN TABLOID LONSURF generic generic brand brand brand QL PA, SP panobinostat vorinostat FARYDAK ZOLINZA brand PA, SP afatinib alectinib axitinib bosutinib cabozantinib ceritinib cobimetinib crizotinib dabrafenib dasatinib erlotinib GILOTRIF ALECENSA INLYTA BOSULIF COMETRIQ ZYKADIA COTELLIC XALKORI TAFINLAR SPRYCEL TARCEVA AFINITOR brand brand brand brand brand brand brand brand brand brand brand PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP brand PA, SP brand brand brand generic brand brand brand PA, SP PA, SP PA, SP PA, QL, SP PA, SP PA, SP PA, SP Antimetabolites brand brand brand generic EMCYT brand Histone Deacetylase Inhibitors Kinase Inhibitor everolimus gefitinib ibrutinib idelalisib imatinib mesylate lapatinib ditosylate lenvatinib nilotinib AFINITOR DISPERZ IRESSA IMBRUVICA ZYDELIG GLEEVEC TYKERB LENVIMA TASIGNA OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 4 PA, SP SP Covered Drug brand brand brand brand brand brand brand brand brand brand Generic Drug Name Brand Drug Name palbociclib pazopanib ponatinib regorafenib ruxolitinib sorafenib sunitinib trametinib vandetanib vemurafenib IBRANCE VOTRIENT ICLUSIG STIVARGA JAKAFI NEXAVAR SUTENT MEKINIST CAPRELSA ZELBORAF ixazomib NINLARO brand PA, SP abiraterone ZYTIGA brand PA, QL, SP bicalutamide flutamide CASODEX EULEXIN generic generic QL QL tamoxifen toremifene NOLVADEX FARESTON generic brand QL QL anastrozole exemestane letrozole ARIMIDEX AROMASIN FEMARA generic generic generic QL QL QL leuprolide LUPRON LUPRON DEPOT generic PA, QL, SP leuprolide LUPRON DEPOT 6-MONTH brand PA, QL, SP Proteasome Inhibitors Hormonal Antineoplastic Agents Androgen Biosynthesis Inhibitors Antiandrogens Antiestrogens Aromatase Inhibitors Gonadotropin Releasing Hormone Analog Progestin Requirements & Limits PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP LUPRON DEPOT-PED megestrol acetate MEGACE interferon alfa-2b peginterferon alfa-2b INTRON A SYLATRON brand brand PA, SP PA, SP lenalidomide pomalidomide thalidomide REVLIMID POMALYST THALOMID brand brand brand PA, SP PA, SP PA, QL, SP Immunomodulators Interferons Miscellaneous generic OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 5 Generic Drug Name Immunosuppressants Antimetabolites azathioprine mycophenolate mofetil mycophenolate sodium Calcineurin Inhibitors cyclosporine cyclosporine cyclosporine cyclosporine, modified tacrolimus Rapamycin Derivative Brand Drug Name Covered Drug IMURAN CELLCEPT MYFORTIC generic generic generic GENGRAF generic NEORAL SOLUTION SANDIMMUNE SANDIMMUME SOLUTION NEORAL brand oral soln 100 mg/ml generic GENGRAF HECORIA generic PROGRAF RAPAMUNE RAPAMUNE generic brand everolimus ZORTRESS brand alitretinoin 1% gel bexarotene caps bexarotene topical gel cysteamine bitartrate etoposide hydroxyurea hydroxyurea mesna mitotane octreotide olaparib panobinostat pasireotide procarbazine sonidegib topotecan tretinoin vismodegib PANRETIN TARGRETIN TARGRETIN CYSTAGON VEPESID DROXIA HYDREA MESNEX LYSODREN SANDOSTATIN LYNPARZA FARYDAK SIGNIFOR MATULANE ODOMZO HYCAMTIN VESANOID ERIVEDGE Miscellaneous modified oral soln 100 mg/ml generic sirolimus sirolimus Miscellaneous Requirements & Limits brand generic brand brand generic brand generic brand brand generic brand brand brand brand brand brand generic brand OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 6 tabs soln PA PA, SP PA, SP SP tabs SP PA, SP PA, SP PA, SP SP PA, SP PA, SP SP, caps PA, SP Generic Drug Name Brand Drug Name Covered Drug Requirements & Limits Blood Modifiers - Anticoagulants Anticoagulants apixaban dabigatran etexilate mesylate edoxaban ELIQUIS brand QL PRADAXA brand capsule, PA SAVAYSA brand enoxaparin LOVENOX generic heparin rivaroxaban warfarin HEPARIN XARELTO COUMADIN generic brand generic QL PA, QL PA only applies for quantities greater than 14 days darbepoetin alfa ARANESP EPOGEN Blood Cell Formation epoetin alfa filgrastim oprelvekin pegfilgrastim plerixafor sargramostim TBO-filgrastim Platelet Inhibitors anagrelide aspirin cilostazol clopidogrel dipyridamole ticagrelor Miscellaneous aminocaproic acid pentoxifylline extended-release PROCRIT NEUPOGEN ZARXIO NEUMEGA NEULASTA MOZOBIL LEUKINE GRANIX AGRYLIN BAYER QL QL brand PA, SP brand PA, SP brand PA, SP brand brand brand brand brand PA, SP PA, SP PA, SP PA, SP PA, SP generic generic ECOTRIN PLETAL PLAVIX PERSANTINE BRILINTA generic generic generic brand AMICAR brand TRENTAL generic OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 7 OTC QL PA tabs & syrup only Brand Drug Name Covered Drug benazepril captopril captopril enalapril LOTENSIN CAPOTEN CAPTOPRIL POWDER VASOTEC generic generic generic generic enalapril oral soln EPANED fosinopril lisinopril moexipril perindopril quinapril ramipril trandolapril MONOPRIL ZESTRIL UNIVASC ACEON ACCUPRIL ALTACE MAVIK generic generic generic generic generic generic generic LOTENSIN HCT generic CAPOZIDE generic VASERETIC generic MONOPRIL-HCT generic QL ZESTORETIC generic QL UNIRETIC generic ACCURETIC generic QL clonidine clonidine transdermal guanfacine CATAPRES CATAPRES-TTS TENEX generic generic generic PA doxazosin prazosin terazosin CARDURA MINIPRESS HYTRIN generic generic generic irbesartan losartan valsartan AVAPRO COZAAR DIOVAN generic generic generic Generic Drug Name Cardiovascular Agents Ace Inhibitors brand Ace Inhibitor/Diuretic Combinations benazepril/ hydrochlorothiazide captopril/ hydrochlorothiazide enalapril/ hydrochlorothiazide fosinopril/ hydrochlorothiazide lisinopril/ hydrochlorothiazide moexipril/ hydrochlorothiazide quinapril/ hydrochlorothiazide Adrenolytics, Central Alpha Blockers Angiotensin II Receptor Blockers (Antagonists) OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit Requirements & Limits (bulk) powder Members ≥ 8 years of age will require prior authorization. QL QL ST = Step Therapy SP = Specialty Pharmacy 8 QL QL Generic Drug Name Brand Drug Name Angiotensin II Receptor Blocker Combinations Covered Drug Requirements & Limits QL PA losartan/HCTZ sacubitril/valsartan valsartan/ hydrochlorothiazide HYZAAR ENTRESTO generic brand DIOVAN HCT generic amiodarone amiodarone tabs digoxin disopyramide disopyramide extended-release dofetilide dronedarone flecainide mexiletine propafenone quinidine gluconate extended-release quinidine sulfate quinidine sulfate extended-release PACERONE CORDARONE LANOXIN NORPACE generic generic generic generic NORPACE CR brand TIKOSYN MULTAQ TAMBOCOR MEXITIL RYTHMOL QUINIDINE GLUCONATE EXT-REL QUINIDINE SULFATE QUINIDINE SULFATE EXT-REL generic brand generic generic generic acebutalol atenolol atenolol/chlorthalidone bisoprolol bisoprolol/ hydrochlorothiazide carvedilol labetalol metoprolol metoprolol succinate nadolol pindolol propranolol propranolol propranolol extended-release propranolol/HCTZ sotalol sotalol (AFIB/AFL) timolol maleate SECTRAL TENORMIN TENORETIC ZEBETA generic generic generic generic ZIAC generic COREG TRANDATE LOPRESSOR TOPROL XL CORGARD PINDOLOL HEMANGEOL INDERAL generic generic generic generic generic generic brand generic INDERAL LA generic INDERIDE BETAPACE BETAPACE AF generic generic generic generic Antiarrhythmics and Cardiac Glycosides 200 mg and 400 mg PA IR only generic generic generic Beta Blockers and Beta Blocker/Diuretic Combinations OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 9 QL IR only tablets Generic Drug Name Brand Drug Name Calcium Channel Blockers Dihydropyridines Covered Drug Requirements & Limits amlodipine felodipine extended-release isradipine nicardipine nifedipine nifedipine extended-release nimodipine nimodipine oral soln nisoldipine NORVASC generic QL PLENDIL generic QL DYNACIRC CARDENE PROCARDIA ADALAT CC generic generic generic diltiazem CARDIZEM CARDIZEM LA Nondihydropyridines PROCARDIA XL NIMOTOP NYMALIZE SULAR generic QL generic brand generic QL sustained-release generic generic sustained-release coated beads, QL sustained-release, QL generic QL generic QL CARDIZEM SR generic QL CALAN generic verapamil VERALAN PM generic verapamil extended-release sustained-release caps, (100 mg, 200 mg and 300 mg only) CALAN SR generic QL MIDAMOR generic MODURETIC generic BUMEX CHLORTHALIDONE DIURIL DIURIL ORAL SUSPENSION LASIX HYDROCHLOROTHIAZIDE MICROZIDE generic generic generic diltiazem diltiazem diltiazem extended-release diltiazem extended-release diltiazem sustained-release verapamil Diuretics amiloride amiloride/ hydrochlorothiazide bumetanide chlorthalidone chlorothiazide chlorothiazide furosemide hydrochlorothiazide hydrochlorothiazide generic MATXIM LA CARDIZEM SR CARDIZEM CD DILACOR XR TIAZAC OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit brand QL generic generic generic soln, tabs 12.5 mg caps ST = Step Therapy SP = Specialty Pharmacy 10 Generic Drug Name Brand Drug Name indapamide metolazone spironolactone spironolactone spironolactone/ hydrochlorothiazide torsemide triamterene/ hydrochlorothiazide LOZOL ZAROXOLYN ALDACTONE SPIRONOLACTONE Covered Drug generic generic generic generic ALDACTAZIDE generic DEMADEX DYAZIDE generic Lipid Lowering Agents Bile Acid Resin cholestyramine coliestipol Fibrates QUESTRAN generic QUESTRAN-LIGHT COLESTID generic LOFIBRA generic gemfibrozil TRIGLIDE LOPID generic atorvastatin lovastatin pravastatin simvastatin LIPITOR MEVACOR PRAVACHOL ZOCOR generic generic generic generic niacin niacin extended-release NIACOR NIASPAN generic generic alirocumab ezetimibe PRALUENT ZETIA isosorbide dinitrate ISORDIL DILATRATE-SR isosorbide dinitrate extended-release ISOCHRON HMG-CoA Reductase Inhibitors and Combinations Miscellaneous Nitrates Oral isosorbide mononitrate Only the bulk products are covered (cans). Individual packets are not covered. (not packets) ANTARA TRICOR Niacins powder generic MAXZIDE LIPOFEN fenofibrate Requirements & Limits brand brand generic generic ISOSORBIDE DINITRATE ER ISMO OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit caps, tabs and micronized caps, ST generic ST = Step Therapy SP = Specialty Pharmacy 11 QL QL QL PA, QL, SP PA Generic Drug Name isosorbide mononitrate extended-release nitroglycerin extended-release Sublingual isosorbide dinitrate nitroglycerin nitroglycerin Transdermal nitroglycerin nitroglycerin Brand Drug Name Covered Drug IMDUR generic Requirements & Limits generic ISORDIL S.L. NITROLINGUAL NITROSTAT generic generic brand NITREK NITRO-DUR NITRO-BID Potassium-Removing Agents generic transdermal, QL generic oint generic susp (susp only) sodium polysterene sulfonate KAYEXALATE ambrisentan bosentan macitentan riociguat sildenafil tadalafil (PAH) LETAIRIS TRACLEER OPSUMIT ADEMPAS REVATIO ADCIRCA brand brand brand brand generic brand PA, SP PA, SP PA, SP PA, SP PA, SP PA, SP eplerenone INSPRA generic epoprostenol FLOLAN brand guanabenz hydralazine WYTENSIN APRESOLINE PA SP, Coverable through Medical Benefit iloprost VENTAVIS methyldopa methyldopa/HCTZ midodrine minoxidil ranolazine ALDOMET ALDORIL PROAMATINE LONITEN RANEXA generic generic generic generic brand treprostinil REMODULIN brand treprostinil TYVASO brand Pulmonary Arterial Hypertension Miscellaneous OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit generic generic brand SP, Coverable through Medical Benefit ST ST SP, Coverable through Medical Benefit SP, Coverable through Medical Benefit ST = Step Therapy SP = Specialty Pharmacy 12 Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits Central Nervous System Alzheimer’s Disease donepezil donepezil galantamine ARICEPT ARICEPT RAZADYNE generic generic generic memantine NAMENDA generic memantine SR rivastigmine NAMENDA XR EXELON brand generic 5 mg and 10 mg, QL 23 mg, ST QL QL, Members <18 years of age will require prior authorization PA, QL QL generic generic QL QL generic QL generic QL TYLENOL generic OTC EXCEDRIN MIGRAINE generic OTC ULTRAM generic QL VOLTAREN generic VOLTAREN XR LODINE NALFON NALFON ANSAID generic generic brand generic generic ibuprofen ADVIL generic ibuprofen MOTRIN generic indomethacin ketoprofen ketoprofen SR ketorolac tromethamine meloxicam nabumetone naproxen INDOCIN ORUDIS ORUDIS TORADOL MOBIC RELAFEN NAPROSYN generic generic generic generic generic generic generic Analgesics Barbiturate Non-Narcotic Analgesics butalbital/acetaminophen PHRENILIN butalbital/acetaminophen SEDAPAP ESGIC butalbital/acetaminophen/ FIORICET caffeine ZEBUTAL butalbital/aspirin/caffeine FIORINAL Non-Narcotic Analgesics acetaminophen aspirin/acetaminophen/ caffeine tramadol NSAIDS diclofenac sodium delayed-release diclofenac sodium SR etodolac fenoprofen fenoprofen flurbiprofen OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 13 QL IR Only 400 mg 600 mg tabs, chew tabs and susp, OTC tabs, chew tabs and susp IR only cap, 200 mg QL QL Generic Drug Name naproxen delayed release naproxen sodium oxaprozin piroxicam sulindac Covered Drug Brand Drug Name ENTERIC COATEDNAPROSYN ANAPROX DAYPRO FELDENE CLINORIL generic generic generic generic generic Opioids - Narcotic Analgesics butalbital/apap/caff/cod butalbital/asa/caff/cod butorphanol codeine/acetaminophen codeine sulfate fentanyl transdermal Requirements & Limits FIORICET W/CODEINE FIORINAL W/CODEINE STADOL TYLENOL W/CODEINE generic generic generic generic generic generic QL, 50-325-40-30 mg QL nasal spray, QL QL QL QL generic QL VICOPROFEN ZOHYDRO ER DILAUDID DEMEROL DOLOPHINE MSIR RMS brand generic generic generic generic generic QL, ST QL QL QL QL QL MS CONTIN generic QL OXYFAST ROXICODONE OXYCONTIN OXYCONTIN MAGNACET generic generic brand generic soln, QL QL PA, QL, 15 mg, 30 mg, 60 mg PA, QL generic QL brand QL generic generic QL QL, ST, non-crush resistant DURAGESIC LORCET LORTAB hydrocodone/ acetaminophen LORTAB ELIXIR NORCO VICODIN VICODIN ES hydrocodone ER hydromorphone meperidine methadone morphine morphine morphine extended-release oxycodone oxycodone oxycodone ER oxycodone ER oxycodone/ acetaminophen oxycodone/ acetaminophen CR oxycodone/aspirin oxymorphone ER PERCOCET XARTEMIS XR PERCODAN OXYMORPHONE ER OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 14 Generic Drug Name Migraine Acute Therapy Ergotamine Derivatives Covered Drug Brand Drug Name Requirements & Limits dihydroergotamine dihydroergotamine ergotamine/caffeine ergotamine tartrate/ caffeine D.H.E. 45 MIGRANAL CAFERGOT MIGERGOT SUPPOSITORIES generic generic generic inj, QL brand QL naratriptan rizatriptan sumatriptan AMERGE MAXALT/MAXALT MLT IMITREX generic generic generic ST QL QL amitriptyline divalproex sodium cap sprinkle divalproex sodium delayed-release propranolol verapamil ELAVIL generic DEPAKOTE SPRINKLE generic DEPAKOTE generic Minimum age 2 INDERAL CALAN generic generic IR only dimethyl fumarate fingolimod glatiramer acetate TECFIDERA GILENYA COPAXONE COPAXONE brand brand brand PA, QL, SP PA, QL, SP PA, QL, SP (40mg) generic PA, QL, SP (20mg) brand brand QL, SP SP brand PA, SP brand PA, SP tabs syrup Selective Serotonin Agonists Migraine Prophylactic Therapy Multiple Sclerosis glatiramer acetate interferon beta-1a interferon beta-1a interferon beta-1b teriflunomide Myasthenia Gravis GLATOPA AVONEX/AVONEX PEN REBIF BETASERON EXTAVIA AUBAGIO pyridostigmine pyridostigmine pyridostigmine extended-release MESTINON MESTINON generic brand MESTINON TIMESPAN generic amantadine benztropine bromocriptine carbidopa/levodopa carbidopa/levodopa extended-release SYMMETREL COGENTIN PARLODEL SINEMET generic generic generic generic SINEMET CR generic Parkinson’s Disease OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 15 except tabs Covered Drug generic generic Generic Drug Name Brand Drug Name entacapone pramipexole pramipexole dihydrochloride SR ropinirole ropinirole hydrochloride SR selegiline tolcapone trihexyphenidyl COMTAN MIRAPEX REQUIP REQUIP XL ELDEPRYL TASMAR ARTANE generic generic generic generic generic carbamazepine carbamazepine extended-release TEGRETOL CARBATROL generic Seizures carbamazepine SR clobazam clobazam suspension clonazepam clonazepam diazepam diazepam divalproex sodium cap sprinkle divalproex sodium delayed-release ethosuximide exogabine felbamate gabapentin lacosamide lacosamide solution lamotrigine lamotrigine chew dispersable tab lamotrigine dispersible tab lamotrigine ODT lamotrigine SR lamotrigine SR ODT lamotrigine starter kit lamotrigine titration kit levetiracetam levetiracetam SR methsuximide MIRAPEX ER brand 24hr tab 24hr tab tabs generic TEGRETOL-XR EPITOL brand EQUATRO ONFI ONFI SUSPENSION KLONOPIN KLONOPIN DIASTAT ACUDIAL DIASTAT PEDIATRIC brand brand generic generic brand brand DEPAKOTE SPRINKLE generic DEPAKOTE generic ZARONTIN POTIGA FELBATOL NEURONTIN VIMPAT VIMPAT LAMICTAL generic brand generic generic brand brand generic LAMICTAL CD CHEW TAB generic LAMICTAL ODT KIT LAMICTAL ODT LAMICTAL XR LAMICTAL XR ODT KIT LAMICTAL STARTER KIT LAMICTAL XR KIT KEPPRA KEPPRA XR CELONTIN brand brand generic brand brand brand generic generic brand OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit Requirements & Limits PA tabs ODT tabs rectal gel, QL Minimum age 2 Age Limits Apply caps and tabs only Age Limits Apply PA QL ST = Step Therapy SP = Specialty Pharmacy 16 PA PA tab PA PA QL Covered Drug generic generic generic Generic Drug Name Brand Drug Name oxcarbazepine phenobarbital phenytoin phenytoin sodium extended pregabalin pregabalin primidone rufinamide rufinamide suspension TRILEPTAL PHENOBARBITAL DILANTIN INFATABS DILANTIN tiagabine GABITRIL generic tiagabine GABITRIL brand topiramate topiramate sprinkle caps valproic acid vigabatrin oral solution zonisamide TOPAMAX TOPAMAX SPRINKLE DEPAKENE SABRIL SOLUTION ZONEGRAN tetrabenazine XENAZINE brand PA, SP isotretinoin ACCUTANE generic Max Age of 25, PA azelaic acid benzoyl peroxide benzoyl peroxide benzoyl peroxide benzoyl peroxide benzoyl peroxide benzoyl peroxide benzoyl peroxide benzoyl peroxide benzoyl peroxide bar 10% benzoyl peroxide creamy wash 8% & benzoyl peroxide bar 5% k FINACEA BENZAC AC BENZEFOAM BREVOXYL DELOS NEOBENZ MICR LIQ OC8 PANOXYL CREAM OTC TRIAZ CLOTHS PANOXYL BAR OTC brand generic generic generic generic generic brand generic generic brand gel BPO CREAMY KIT generic Miscellaneous PHENYTEK LYRICA LYRICA SOLUTION MYSOLINE BANZEL BANZEL SUSPENSION Requirements & Limits QL generic brand brand generic brand brand PA oral solution, PA tablets only, QL PA Age Limits Apply, 2mg & 4mg Age Limits Apply, 12mg & 16mg QL QL generic generic generic brand generic PA, SP QL Dermatology Acne Vulgaris Oral Topical OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit foam 5.3% & 9.8% gel 4%, 8% cleanser 3.5%, OTC liquid 7% gel 7%, OTC cream 2.5%, OTC cloth 3%, 6%, 9% OTC ST = Step Therapy SP = Specialty Pharmacy 17 Generic Drug Name Covered Drug Requirements & Limits brand 2.75%, 5.25% BREVOXYL-4 OTC generic OTC CLEOCIN T CLEOCIN T CLEOCIN T ERYGEL T-STAT CLEAN & CLEAR GEL generic generic generic generic generic gel lotion soln gel 2% soln generic gel 2%, OTC brand gel 3, 6% generic pad 0.5%, 1&, 2%, OTC generic liquid 2%, OTC generic generic lotion 6% lotion generic lotion 10% Brand Drug Name BENZIQ LS benzoyl peroxide gel benzoyl peroxide wash 4% & benzoyl peroxide bar 5% kit clindamycin clindamycin clindamycin erythromycin erythromycin BENZIQ BREVOXYL salicylic acid NEUTROGENA RAPID CLEAR GEL salicylic acid EXUVIANCE KERALYT STRI-DEX salicylic acid salicylic acid salicylic acid sulfacetamide/sulfur sulfacetamide sodium AVEENO CLEAR PADS OTC NEUTROGENA OIL FREE ACNE WASH SALEX SULFACET-R KLARON LOTION sulfacetamide/sulfur SEBIZON PLEXION ATRALIN tretinoin AVITA Bacterial Infections bacitracin bacitracin-polymyxinneomycin HC gentamicin mupirocin neomycin/polymyxin B/ bacitracin silver sulfadiazine generic generic RETIN-A BACITRACIN generic OTC brand oint 1% GENTAK BACTROBAN generic generic ointment, 22 gram tube only NEOSPORIN generic OTC SILVADENE generic CORTISPORIN OINTMENT OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 18 Generic Drug Name Corticosteroids Low Potency alclometasone fluocinolone acetonide fluocinolone acetonide fluocinolone acetonide hydrocortisone hydrocortisone hydrocortisone hydrocortisone hydrocortisone/aloe Medium Potency betamethasone val fluocinolone acetonide flurandrenolide fluticasone propionate fluticasone propionate hydrocortisone butyrate hydrocortisone valerate mometasone furoate prednicarbate triamcinolone acetonide triamcinolone acetonide triamcinolone acetonide High Potency betamethasone augmented dip betamethasone augmented dip betamethasone dipropionate diflorasone diacetate diflorasone diacetate fluocinonide fluocinonide emulsified base triamcinolone acetonide Very High Potency betamethasone dip augmented Covered Drug Requirements & Limits ACLOVATE CAPEX SHAMOO DERMA-SMOOTHE OIL/FS SYNALAR CORTAID SOLUTION OTC CORTIZONE HYTONE HYTONE CORTIZONE-10 INTENSIVE HEALING generic brand 0.05% crm/oint shampoo 0.01% generic oil 0.01% generic generic generic generic generic soln/crm 0.01% soln 1% OTC crm, oint, lot OTC lotion 1% & 2.5% crm 0.5%, 1%, & 2.5% generic crm 0.5% & 1%, OTC BETA-VAL SYNALAR CORDRAN CUTIVATE CUTIVATE LOTION LOCOID WESTCORT ELOCON DERMATOP KENALOG KENALOG TRIANEX generic generic brand generic generic generic generic generic generic generic generic brand crm/oint/lotion 0.1% crm, oint 0.025% tape crm 0.05%, oint 0.005% lotion 0.05% crm/lotion/oint 0.1% crm/oint 0.2% crm/oint/soln 0.1% crm 0.1% crm/lot/oint 0.025% crm/oint/lotion 0.1% oint 0.05% DIPROLENE generic lotion 0.05% DIPROLENE AF generic crm 0.05% DIPROSONE generic crm/lotion/oint 0.05% PSORCON CREAM PSORCON OINTMENT LIDEX generic generic generic cream 0.05% oint 0.05% crm/oint/gel/soln 0.05% LIDEX E generic crm 0.05% KENALOG generic crm 0.5% DIPROLENE generic gel 0.05% Brand Drug Name OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 19 Generic Drug Name betamethasone dip augmented clobetasol propionate halobetasol Fungal Infections ciclopirox clotrimazole clotrimazole clotrimazole crystals clotrimazole with betamethasone ketoconazole ketoconazole foam ketoconazole gel ketoconazole gel 2% & hydrocortisone gel 1% kit ketoconazole gel 2% & pyrithione zinc shampoo 1% kit ketoconazole shampoo miconazole miconazole miconazole miconazole nitrate kit miconazole nitrate lotion Brand Drug Name Covered Drug Requirements & Limits DIPROLENE generic oint 0.05% TEMOVATE ULTRAVATE generic generic crm/gel/oint/soln 0.05% cream PENLAC SOLUTION 8% LOTRIMIN AF MYCELEX CLOTRIMAZOLE generic generic generic brand LOTRISONE generic NIZORAL EXTINA XOLEGEL generic generic brand XOLEGEL KIT brand XOLEGEL DUO KIT brand OTC 2% 2% brand generic generic generic brand brand 1% OTC 2% OTC OTC brand 2% OTC nystatin terbinafine terbinafine gel terbinafine HCL tolnaftate NIZORAL A-D OTC DESENEX MICATIN MONISTAT-DERM FUNGOID TINCTURE KIT ZEASORB-AF AZOLEN TINCTURE SOLUTION OTC MYCOSTATIN LAMISIL AT LAMISIL GEL LAMISIL SOLUTION TINACTIN generic generic brand brand generic OTC 1% soln 1% OTC acitretin calcipotriene calcipotriene calcitriol methoxsalen salicylic acid SORIATANE DOVONEX DOVONEX VECTICAL OXSORALEN-ULTRA SCALPICIN generic generic generic generic generic generic oral caps, PA crm/oint, ST soln ST brimonidine MIRVASO brand PA miconazole nitrate soln Psoriasis Rosacea OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 20 2% 2% OTC liquid 3% Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits METROCREAM metronidazole METROGEL generic METROLOTION Scabies and Pediculosis crotamiton ivermectin malathion permethrin permethrin pyrethrins/piperonyl but. spinosad Viral Infections podofilox salicylic acid 17%/ collodion EURAX SKLICE OVIDE ELIMITE NIX CREME RINSE RID SHAMPOO/ BUTOXIDE SHAMPOO NATROBA brand brand generic generic generic hydrocortisone hydrocortisone acetate hydrocortisone acetate hydrocortisone acetate w/pramoxine imiquimod 5% cream ketoconazole 5%, QL 1%, OTC generic 4% OTC brand susp 0.9%, PA CONDYLOX SOL generic sol DUOFILM generic OTC DRYSOL OTC brand generic soln/cream, OTC soln 12%, OTC HYPERCARE 20% generic LAC-HYDRIN LACTINOL REGRANEX generic generic brand brand brand generic generic brand brand generic Miscellaneous aluminum acetate aluminum chloride aluminum chloride hexahydrate ammonium lactate ammonium lactate becaplermin gel calamine collagenase oint fluorouracil fluorouracil fluorouracil hexachlorophene hydrocortisone lotion 0.5%, PA SANTYL CARAC EFUDEX FLUOROPLEX PHISOHEX PROCTO-PAK CREAM PROCTOSOL HC CREAM 2.5% PROCTOZONE CREAM-HC 2.5% ANUSOL HC 2.5% CORTIFOAM AEROSOL TUCKS OINTMENT OTC crm 12%, lotion 5% & 12% lotion 10% PA lotion/ointment, OTC QL 0.5% cream 1% cream rectal cream 1% generic brand generic rectal foam 90 mg rectal ointment PROCTOFOAM HC FOAM generic rectal foam 1-1% ALDARA NIZORAL SHAMPOO generic generic PA shampoo 2% OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 21 Covered Drug generic generic generic generic brand generic generic brand Generic Drug Name Brand Drug Name lidocaine lidocaine lidocaine lidocaine lidocaine patch lidocaine/prilocaine lidocaine-prilocaine nitroglycerin LIDAMANTEL LMX-4 XYLOCAINE XYLOCAINE LIDODERM PATCH EMLA EMLA RECTIV pimecrolimus ELIDEL brand salicylic acid brand generic shampoo 2%, 3% OTC salicylic acid salicylic acid salicylic acid salicylic acid selenium sulfide CALICYLIC P & S SHAMPOO SELSUN BLUE DHS SAL SHAMPOO OTC SALAC OTC SALACYN SALEX SALVAX SELSUN 3% cream 4% cream (15 gm tubes), QL jelly 2% oint 5% 5%, PA 2.5% cream kit 2.5-2.5% 0.4% rectal ointment, PA cream QL, ST, Step therapy is not required for members ages 2-11; not covered for members less than 2 years of age cream 10% brand generic generic generic generic tacrolimus PROTOPIC 0.03% generic tacrolimus PROTOPIC 0.1% generic urea 40% UREA 40% LOTION generic foam 2%, OTC 6% cream, lotion shampoo 6% foam 6% lotion 2.5% ointment 0.03% ST, Step therapy is not required for members ages 2-11; not covered for members less than 2 years of age ointment 0.1%, ST (minimum age 16) lotion acetic acid acetic acid/ aluminum acetate acetic acid/ hydrocortisone VOSOL OTIC generic otic DOMEBORO OTIC generic VOSOL HC OTIC generic antipyrine-benzocaine AURALGAN generic benzocaine/antipyrine carbamide peroxide ciprofloxacin/ dexamethasone BENZOTIC DEBROX generic generic CIPRODEX brand salicylic acid Requirements & Limits Ear, Nose & Throat Ear OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit otic soln 55-14 mg/mL (5.5-1.4%) ST = Step Therapy SP = Specialty Pharmacy 22 6.5%, OTC PA Generic Drug Name neomycin/polymyxin B/ hydrocortisone ofloxacin Brand Drug Name Covered Drug Requirements & Limits CORTISPORIN OTIC generic otic FLOXIN OTIC generic Nose Antihistamines - First Generation, Sedating J-TAN PD RESPA-BR BROVEX CT CHLOR-TRIMETON ALLERGY CHLOR-TRIMETON chlorpheniramine maleate SYRUP chlorpheniramine maleate CHLORPHENIRAMINE chlorpheniramine maleate CHLORPHENIRAMINE chlorpheniramine tannate CHLORPHENIRAMINE chlorpheniramine tannate CHLORPHENIRAMINE clemastine CLEMASTINE cyproheptadine CYPROHEPTADINE dexchlorpheniramine malePOLARAMINE ate diphenhydramine diphenhydramine BENADRYL diphenhydramine BENADRYL diphenhydramine TRIAMINIC hydroxyzine HCL ATARAX hydroxyzine pamoate VISTARIL brompheniramine maleate brompheniramine maleate brompheniramine tannate chlorpheniramine extended-release Antihistamines - Second Generation, Nonsedating cetirizine cetirizine chew tab fexofenadine levocetirizine loratadine ZYRTEC ZYRTEC CHEWABLE TABLET ALLEGRA XYZAL ALAVERT loratadine loratadine CLARITIN CLARITIN CHEW CLARITIN RDT azelastine ASTELIN brand brand generic drops 1 mg/mL tab SR 12hr 11 mg chew tab 12 mg generic 12 mg, OTC generic 2 mg/5 ml, OTC generic generic generic generic generic generic drops 2 mg/mL tabs 4 mg susp 2 mg/mL tabs generic syrup 2 mg/5ml generic generic generic brand generic generic generic OTC generic OTC generic generic tabs tabs generic OTC brand brand chew tab 5 mg ODT 5 mg generic spray Antihistamines - Others Antihistamine/Decongestant Combinations Antihistamine/Decongestant Combinations - First Generation OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 23 OTC tab disp 12.5 mg oral strips Generic Drug Name brompheniramine/ pseudoephedrine chlorpheniramine/ phenylephrine/ pyrilamine chlorpheniramine/ pseudoephedrine Brand Drug Name LODRANE D Covered Drug Requirements & Limits brand cap 4-60 mg TRIOTANN generic ACTIFED generic OTC Antihistamine/Decongestant Combinations - Second Generation cetirizine hydrochloride/ pseudoephedrine ZYRTEC-D hydrochloride 12 hours extended-release fexofenadine/ ALLEGRA-D pseudoephedrine ALAVERT-D loratadine/ ALAVERT ALRG pseudoephedrine TAB/SINUS extended-release ALLERGY/CONG generic 5 mg-120 mg tablet generic SR tabs generic OTC FLONASE NASACORT AQ NASACORT ALLERGY 24 HOUR generic generic PA brand OTC cromolyn sodium ipratropium bromide saline nasal spray 0.65% NASALCROM ATROVENT NS OCEAN NASAL SPRAY generic generic generic OTC oxymetazoline AFRIN NEO-SYNEPHRINE generic OTC phenylephrine DIMEATAPP DRO DECONGES generic OTC APHTHASOL PERIOGARD PERIDEX XYLOCAINE SALAGEN KENALOG IN ORABASE brand generic generic generic generic generic oral paste 5% Nasal Steroids fluticasone triamcinolone acetonide triamcinolone nasal spray Miscellaneous Nasal Miscellaneous Nasal Decongestants Throat and Mouth amlexanox chlorhexidine chlorhexidine gluconate lidocaine viscous pilocarpine triamcinolone OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 24 OTC paste Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits Endocrinology Adrenal Corticosteroids cortisone acetate dexamethasone fludrocortisone hydrocortisone hydrocortisone inj methylprednisolone methylprednisolone methylprednisolone inj prednisolone prednisolone prednisolone sodium phosphate prednisone prednisone prednisone triamcinolone inj Androgens generic generic generic generic generic generic brand generic DECADRON FLORINEF CORTEF SOLU-CORTEF MEDROL MEDROL SOLU-MEDROL PRELONE ORAPRED 4mg, 8mg, 16mg, 32mg 2mg generic syrup generic PEDIAPRED DELTASONE PREDNISONE RAYOS KENALOG-40 generic generic generic generic fluoxymesterone testosterone testosterone testosterone cypionate ANDROXY ANDRODERM ANDROGEL 1.62% DEPO-TESTOSTERONE generic brand brand generic testosterone enanthate DELATESTRYL generic testosterone gel testosterone gel topical tube, packet, and pump bottle testotesterone TESTIM conc 5 mg/mL tabs delayed-release PA patch 2 mg and 4 mg, PA gel, PA brand Vials only. Disposable syringes not covered. PA generic PA ANDROGEL 1% brand gel (not pump), PA glucagon, human recombinant GLUCAGON brand QL insulin detemir insulin detemir insulin glargine insulin glargine insulin isophane insulin lisopro pro/lispro insulin lisopro prot/lispro LEVEMIR LEVEMIR FLEXPEN LANTUS LANTUS SOLOSTAR HUMULIN N HUMALOG MIX 50/50 HUMALOG MIX 75/25 brand brand brand brand brand brand brand PA, QL, vials PA, QL QL, vials PA, QL OTC, QL, vials QL, vials QL, vials Diabetes Mellitus Glucose Elevating Agents Insulins TESTOSTERONE 1% TOPICAL GEL OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 25 Generic Drug Name Brand Drug Name insulin lispro prot 50%/lispro 50% insulin lispro prot 75%/lispro 25% insulin isophane/regular insulin lispro insulin lispro insulin regular HUMALOG MIX 50/50 KWIKPEN HUMALOG MIX 72/25 KWIKPEN HUMULIN 70/30 HUMALOG HUMALOG KWIKPEN HUMULIN R HUMULIN R 500 U/ML KWIKPEN insulin regular (human) Covered Drug Requirements & Limits brand PA, QL brand PA, QL brand brand brand brand OTC, QL, vials QL, vials 100 U/ml only, PA, QL OTC, QL, vials brand PA Monitoring - Strips and Kits/Diabetic Supplies ONE TOUCH SYSTEMS (ULTRA 2, ULTRAMINI, VERIO, VERIO FLEX, VERIO IQ, VERIO SYNC) brand ONE TOUCH TEST STRIPS (ULTRA, VERIO) brand Oral Agents acarbose canagliflozin canagliflozin/metformin chlorpropamide empagliflozin empagliflozin/metformin glimepiride glipizide glipizide extended-release glipizide/metformin glyburide glyburide, micronized linagliptin linagliptin/metformin metformin metformin ER metformin/glyburide nateglinide pioglitazone pioglitazone/glimepiride pioglitazone/metformin pioglitazone/metformin ER repaglinide sitagliptan sitagliptan/metformin PRECOSE INVOKANA INVOKAMET DIABINESE JARDIANCE SYNJARDY AMARYL GLUCOTROL GLUCOTROL XL METAGLIP MICRONASE GLYNASE TRADJENTA JENTADUETO GLUCOPHAGE GLUCOPHAGE ER GLUCOVANCE STARLIX ACTOS DUETACT ACTOPLUSMET ACTOPLUS MET XR PRANDIN JANUVIA JANUMET OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit QL for insulin dependent or pregnant members: allow testing up to 6 times per day QL for non-insulin dependent members: allow once daily testing generic brand brand generic brand brand generic generic generic generic generic generic brand brand generic generic generic generic generic generic generic brand generic brand brand ST = Step Therapy SP = Specialty Pharmacy 26 QL QL, ST QL, ST QL QL, ST QL, ST QL QL QL QL QL QL QL, ST QL, ST QL QL QL QL QL QL, ST QL, ST QL, ST QL QL, ST QL, ST Covered Drug brand generic generic Generic Drug Name Brand Drug Name sitagliptan/metformin ER tolazamide tolbutamide JANUMET XR TOLINASE TOLBUTAMIDE albiglutide exenatide liraglutide pramlintide TANZEUM BYETTA VICTOZA SYMLIN brand brand brand brand ST PA ST PA mecasermin INCRELEX GENOTROPIN brand PA, SP somatropin NORDITROPIN brand PA, SP brand brand brand brand PA, SP PA, SP PA, SP PA, SP generic PA, SP brand PA, SP QL nasal spray, QL nasal spray, QL Miscellaneous Antidiabetic Agents Growth Stimulating Agents Metabolic Modifiers Requirements & Limits QL, ST QL QL NUTROPIN AQ NUSPIN carglumic acid glycerol phenylbutyrate idursulfase imiglucerase sodium phenylbutyrate oral powder sodium phenylbutyrate tab CARBAGLU RAVICTI ELAPRASE CEREZYME BUPHENYL ORAL POWDER BUPHENYL alendronate calcitonin-salmon calcitonin-salmon etidronate raloxifene teriparatide inj FOSAMAX MIACALCIN FORTICAL DIDRONEL EVISTA FORTEO generic generic brand generic generic brand levothyroxine levothyroxine liothyronine liotrix methimazole propylthiouracil thyroid tab uridine LEVOXYL SYNTHROID CYTOMEL THYROLAR TAPAZOLE PROPYLTHIOURACIL ARMOUR THYROID VISTOGARD generic generic generic brand generic generic brand brand QL SP agalsidase alglucerase alglucosidase asfotase alfa FABRAZYME CEREDASE LUMIZYME STRENSIQ brand brand brand brand PA, SP PA, SP PA, SP PA, SP Osteoporosis Thyroid Disease Miscellaneous OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 27 PA, SP Generic Drug Name betaine powder for oral solution bromocriptine cabergoline cholic acid desmopressin laronidase methylergonovine mifepristone pegvisomant sapropterin Brand Drug Name CYSTADANE Covered Drug Requirements & Limits brand SP PARLODEL DOSTINEX CHOLBAM DDAVP ALDURAZYME METHERGINE KORLYM SOMAVERT KUVAN KUVAN POWDER FOR SOLUTION ELELYSO VPRIV generic generic brand generic brand generic brand brand brand diphenoxylate/atropine loperamide loperamide loperamide loperamide LOMOTIL IMODIUM A-D IMODIUM A-D CHW IMODIUM A-D LIQ LOPERAMIDE aprepitant EMEND dolasetron dronabinol granisetron granisetron meclizine metoclopramide ANZEMET MARINOL KYTRIL KYTRIL ANTIVERT REGLAN ZOFRAN sapropterin powder taliglucerase velaglucerase alfa PA, SP QL PA, SP PA, SP PA, SP PA, SP brand PA, SP brand brand PA, SP PA, SP generic brand brand generic generic OTC chew 2 mg liq 1 mg/7.5ml Gastrointestinal Diarrhea Emesis ondansetron prochlorperazine promethazine trimethobenzamide brand brand generic generic generic generic generic QL applies to 40 mg, 80 mg and 80-125 mg PA PA PA SP oral soln PA generic QL generic generic generic 300 mg caps brand OTC MAALOX generic OTC MYLANTA generic OTC ZOFRAN ODT COMPAZINE PHENERGAN TIGAN Gastroesophageal Reflux Disease (Gerd)/Peptic Ulcers alginic acid/sodium bicarbonate alumina/magnesia alumina/magnesia/ simethicone OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 28 Covered Drug generic brand Generic Drug Name Brand Drug Name cimetidine esomeprazole TAGAMET NEXIUM 24 HR OTC esomeprazole granules NEXIUM DELAYEDRELEASE PACKET Requirements & Limits famotidine lansoprazole lansoprazole delayed-release lansoprazole susp omeprazole delayed-release omeprazole delayed-release pantoprazole pantoprazole delayed-release ranitidine ranitidine effer ranitidine syrup sucralfate PEPCID AZ CHW PREVACID brand generic PA Members ≥ 2 years of age will require prior authorization. OTC Pepcid AC 10 mg and 20 mg also covered/ encouraged with written prescription. chew PA PREVACID SOLUTAB generic orally disintegrating tabs, QL sulcralfate famotidine Gastrointestinal Spasm dicyclomine glycopyrrolate hyoscyamine sulfate hyoscyamine sulfate extended-release PEPCID generic PEPCID AC FIRST-LANSOPRAZOLE PRILOSEC PRILOSEC POWDER PROTONIX brand generic Capsules only, QL brand susp packet generic PROTONIX PAK brand susp packet ZANTAC ZANTAC ZANTAC CARAFATE generic brand generic generic 150 mg tabs tab 25 mg CARAFATE SUSPENSION generic BENTYL ROBINUL generic suspension, Members 10 years of age up to 65 years of age will require prior authorization. generic ROBINUL FORTE LEVSIN CYSTOSPAZ generic LEVBID generic LEVSINEX Inflammatory Bowel Disease balsalazide budesonide hydrocortisone mesalamine brand COLAZAL ENTOCORT EC COLOCORT ROWASA OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit generic generic generic generic ST = Step Therapy SP = Specialty Pharmacy 29 PA enema enema only Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits APRISO mesalamine extended-release mesalamine extendedrelease mesalamine supp olsalazine sodium sulfasalazine sulfasalazine delayed-release DELZICOL brand LIALDA PENTASA ASACOL HD generic CANASA DIPENTUM AZULFIDINE brand brand generic QL AZULFIDINE EN-TABS generic QL generic OTC COLACE GLYCERIN SUPPOSITORY ENULOSE KRISTALOSE COLYTE generic generic generic generic generic brand generic OTC OTC OTC suppository, OTC NULYTELY generic TRILYTE generic MIRALAX SENOKOT generic generic Laxatives casanthranol-docusate sodium docusate calcium plus docusate potasssium docusate sodium glycerin lactulose lactulose peg 3350/electrolytes peg 3350/sodium bicarbonate/sodium chloride/potassium chloride peg 3350/sodium bicarbonate/sodium chloride polyethylene glycol 3350 sennosides Pancreatic Enzymes oral packet 8.6 mg tab, OTC CREON pancrelipase CREON 3000 UNIT brand pancrelipase pancrealipase DR pancrealipase DR sacrosidase ZENPEP PANCREAZE ZENPEP DR CREON DR SUCRAID brand brand brand brand QL 25000-85000-136000 unit 36000-114000-180000 unit soln 8500 unit/ml, PA, SP acidophilus acidophilus ACIDOPHILUS XTRA ACIDOPHILUS brand brand OTC caps and tabs, OTC Probiotic Supplementation OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 30 Generic Drug Name acidophilus/bifidus acidophilus/citrus pectin acidophilus/pectin lactobacillus probiotic product Miscellaneous Brand Drug Name ACIDOPHILUS/BIFIDUS WAFER ACIDOPHILUS/CITRUS PECTIN ACIDOPHILUS/PECTIN FLORANEX PROBIOTIC FORMULA Covered Drug Requirements & Limits generic OTC generic tabs, OTC generic generic brand caps, OTC chewable tabs, OTC caps, OTC atropine sulfate linaclotide misoprostol naloxegol teduglutide SAL-TROPINE LINZESS CYTOTEC MOVANTIK GATTEX ACTIGALL brand brand generic brand brand ursodiol URSO generic PA PA PA, SP URSO FORTE Home Infusion Drugs Analgesics - NSAIDS ketorolac tromethamine im inj ketorolac tromethamine inj KETOROLAC INJ brand KETOROLAC INJ brand fentanyl citrate inj FENTANYL CIT INJ brand morphine sulfate inj ASTRAMOR INJ brand morphine sulfate inj DURAMORPH INJ brand morphine sulfate inj MORPHINE SUL INJ brand morphine sulfate inj MORPHINE SUL INJ brand morphine sulfate iv soln MORPHINE SUL INJ brand amikacin sulfate inj AMIKACIN INJ brand cefazolin sodium for inj ANCEF INJ brand Analgesics - OPIOD Antibiotics OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit 30 mg/ml, Limited to 14 days supply every 30 days. 30 mg/ml, Limited to 14 days supply every 30 days. 0.05 mg/ml, Limited to 14 days supply every 30 days. 4 mg/ml, Limited to 14 days supply every 30 days. 10 mg/ml, Limited to 14 days supply every 30 days. 2 mg/ml, Limited to 14 days supply every 30 days. 15 mg/ml, Limited to 14 days supply every 30 days. 50 mg/ml, Limited to 14 days supply every 30 days. 250 mg/ml, Limited to 14 days supply every 30 days. 1 gm, Limited to 14 days supply every 30 days. ST = Step Therapy SP = Specialty Pharmacy 31 Generic Drug Name Brand Drug Name Covered Drug ceftriaxone sodium for inj CEFTRIAXONE INJ brand ceftriaxone sodium for inj CEFTRIAXONE INJ brand ceftriaxone sodium for inj CEFTRIAXONE INJ brand CEFTRIAX/DEX INJ brand CEFTRIAXONE/ INJ DEX brand ceftriaxone sodium for iv soln 1 gm and dextrose 3.74% ceftriaxone sodium in dextrose inj ciprofloxacin 0.2% in d5w CIPRO I.V. SOL ciprofloxacin iv soln 1% brand CIPRO I.V. INJ brand clindamycin phosphate inj CLEOCIN PHOS INJ brand imipenem-cilastatin intravenous for soln brand IMIPENEM/CIL INJ levofloxacin in d5w iv soln levofloxacin iv soln brand LEVAQUIN INJ brand piperacillin sodtazobactam sod in dex ZOSYN SOL iv sol piperacillin sodiumtazobactam sodium for PIPER/TAZOBA INJ inj piperacillin sodiumtazobactam sodium for PIPER/TAZOBA INJ inj brand Limited to 14 days supply every 30 days. 20 mg/ml, Limited to 14 days supply every 30 days. Limited to 14 days supply every 30 days. Limited to 14 days supply every 30 days. 150 mg/ml, Limited to 14 days supply every 30 days. 500 mg, Limited to 14 days supply every 30 days. 5 mg/ml, Limited to 14 days supply every 30 days. 25 mg/ml, Limited to 14 days supply every 30 days. 3-0.375 gm/50 ml, Limited to 14 days supply every 30 days. 3-0.375 gm, Limited to 14 days supply every 30 days. brand 4-0.5 gm, Limited to 14 days supply every 30 days. VANCOCIN HCL INJ brand vancomycin hcl for inj VANCOCIN HCL INJ brand vancomycin hcl in dextrose inj VANCOCIN/DEX INJ brand BENA-D-50 INJ brand Antihistamines OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit 1 gm, Limited to 14 days supply every 30 days. 2 gm, Limited to 14 days supply every 30 days. 500 mg, Limited to 14 days supply every 30 days. brand vancomycin hcl for inj diphenhydramine hcl inj Requirements & Limits 500 mg, Limited to 14 days supply every 30 days. 1000 mg, Limited to 14 days supply every 30 days. 500 mg/100 ml, Limited to 14 days supply every 30 days. 50 mg/ml, Limited to 14 days supply every 30 days. ST = Step Therapy SP = Specialty Pharmacy 32 Generic Drug Name Brand Drug Name Covered Drug Requirements & Limits promethazine hcl inj ANERGAN 25 INJ brand 25 mg/ml, Limited to 14 days supply every 30 days. DIAQUA-2 INJ brand 10 mg/ml, Limited to 14 days supply every 30 days. D5W/NACL INJ brand Limited to 14 days supply every 30 days. brand Limited to 14 days supply every 30 days. Diuretics furosemide inj Electrolyte Mixtures dextrose 5% w/ sodium chloride 0.45% Genitourinary Irrigants sodium chloride irrigation soln 0.9% Minerals & Electrolytes sodium chloride inj BD POSIFLUSH INJ brand sodium chloride inj SOD CHLORIDE INJ brand sodium chloride iv soln SOD CHLORIDE INJ brand dextrose inj DEXTROSE INJ brand dextrose inj DEXTROSE INJ brand Nutrients Spasticity diazepam VALIUM AQUA-MEPHYTO INJ 5%, Limited to 14 days supply every 30 days. 50%, Limited to 14 days supply every 30 days. generic inj 5 mg/ml, QL, Limited to 14 days supply every 30 days. brand 10 mg/ml, Limited to 14 days supply every 30 days. Vitamins phytonadione inj 0.9%, Limited to 14 days supply every 30 days. 0.45%, Limited to 14 days supply every 30 days. 0.9%, Limited to 14 days supply every 30 days. Infectious Diseases Antibacterials Antituberculosis Agents aminosalicyclic acid cycloserine ethambutol ethionamide isoniazid pyrazinamide rifabutin rifampin rifapentine PASER SEROMYCIN MYAMBUTOL TRECATOR ISONIAZID PYRAZINAMIDE MYCOBUTIN RIFADIN PRIFTIN OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit brand generic generic brand generic generic generic generic brand ST = Step Therapy SP = Specialty Pharmacy 33 Generic Drug Name Covered Drug Brand Drug Name Cephalosporins - First Generation Requirements & Limits cefadroxil cephalexin DURICEF KEFLEX generic generic tabs are not covered cefaclor cefprozil cefuroxime axetil cefuroxime axetil CECLOR CEFZIL CEFTIN CEFTIN generic generic generic brand tabs suspension cefdinir cefixime cefixime cefpodoxime OMNICEF SUPRAX SUPRAX VANTIN generic generic generic generic ciprofloxacin levofloxacin ofloxacin CIPRO LEVAQUIN FLOXIN generic generic generic azithromycin clarithromycin clarithromycin ER erythromycin delayed-release erythromycin delayed-release erythromycin ethylsuccinate erythromycin stearate erythromycin/sulfisoxazole ZITHROMAX BIAXIN BIAXIN XL generic generic generic Cephalosporins - Second Generation Cephalosporins - Third Generation Fluoroquinolones Macrolides Penicillins amoxicillin amoxicillin amoxicillin amoxicillin/clavulanate amoxicillin/clavulanate ampicillin dicloxacillin penicillin VK Sulfonamides sulfadiazine sulfamethoxazole/ trimethoprim, DS ERY-TAB tablets only tabs QL brand ERYC generic E.E.S. generic ERYTHROCIN PEDIAZOLE generic generic AMOXICILLIN CAPSULES AND CHEWABLES AMOXIL AMOXIL SUSP AUGMENTIN AUGMENTIN ES-600 PRINCIPEN DICLOXACILLIN VEETIDS generic generic generic generic brand generic generic generic Except 500 mg and 875 mg film-coated tabs. tabs, 500 mg & 875 mg suspension generic BACTRIM generic BACTRIM DS OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit QL susp ST = Step Therapy SP = Specialty Pharmacy 34 Brand Drug Name Covered Drug Requirements & Limits DECLOMYCIN generic PA VIBRAMYCIN generic generic minocycline minocycline SR tetracycline VIBRAMYCIN DOXYCYCLINE MONOHYDRATE MINOCIN MINOCIN SUMYCIN generic brand generic caps and tabs 24hr vancomycin HCl VANCOCIN HCL generic cap, ST clotrimazole fluconazole flucytosine griseofulvin microsize griseofulvin ultramicrosize itraconazole itraconazole itraconazole ketoconazole miconazole buccal nystatin posaconazole susp terbinafine terbinafine voriconazole MYCELEX DIFLUCAN ANCOBAN GRIFULVIN V GRIS-PEG ONMEL SPORANOX SPORANOX NIZORAL MICONAZOLE MYCOSTATIN NOXAFIL LAMISIL LAMISIL VFEND generic generic generic generic generic brand generic brand generic brand generic brand brand generic generic troches QL PA albendazole ivermectin praziquantel ALBENZA STROMECTOL BILTRICIDE brand brand brand cidofovir VISTIDE brand foscarnet sodium FOSCAVIR generic ganciclovir valganciclovir valganciclovir CYTOVENE VALCYTE VALCYTE generic brand generic enfuvirtide FUZEON Generic Drug Name Tetracyclines demeclocycline doxycycline delayed-release doxycycline hyclate doxycycline monohydrate Miscellaneous Antifungals Antihelmintics generic Antivirals Cytomegalovirus Treatment Entry/Fusion Inhibitors OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit tab 200 mg, PA caps, PA, QL soln, PA, QL tab 50 mg (mouth-throat) PA oral granules QL tabs, PA Coverable through Medical Benefit Coverable through Medical Benefit brand ST = Step Therapy SP = Specialty Pharmacy 35 soln PA PA Brand Drug Name Covered Drug Requirements & Limits adefovir boceprevir daclatasvir elbasvir/grazoprevir entecavir interferon alfa-2b lamivudine lamivudine ledipasvir/sofosbuvir ombitasvir/paritaprevir/ ritonavir ombitasvir/paritaprevir/ritonavir/dasabuvir peginterferon alfa-2a peginterferon alfa-2a peginterferon alfa-2b HEPSERA VICTRELIS DAKLINZA ZEPATIER BARACLUDE INTRON A EPIVIR HBV EPIVIR HBV HARVONI generic brand brand brand brand brand brand generic brand QL, SP PA, SP PA, QL, SP PA QL, SP PA, SP SP, solution QL, SP, tablets PA, QL, SP TECHNIVIE brand PA VIEKIRA PAK brand PA PEGASYS PEGASYS PROCLICK PEGINTRON brand brand brand ribavirin REBETOL/COPEGUS generic sofosbuvir telaprevir telbivudine SOVALDI INCIVEK TYZEKA PA, QL, SP PA, QL, SP PA, QL, SP 200 mg Ribavirin caps and tabs only, PA, SP PA, QL, SP PA, QL, SP PA, QL, SP acyclovir acyclovir acyclovir acyclovir buccal docosanol famciclovir penciclovir valacyclovir ZOVIRAX ZOVIRAX CREAM ZOVIRAX OINTMENT SITAVIG ABREVA OTC FAMVIR DENAVIR VALTREX generic brand generic brand brand generic brand generic amantadine oseltamivir rimantadine zanamivir SYMMETREL TAMIFLU FLUMADINE RELENZA generic brand generic brand delavirdine efavirenz nevirapine nevirapine ER rilpivirine RESCRIPTOR SUSTIVA VIRAMUNE VIRAMUNE XR EDURANT brand brand generic brand brand Generic Drug Name Hepatitis Treatment Herpes Treatment Influenza Treatment brand brand brand Non-Nucleoside Reverse Transcriptase Inhibitors OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 36 cream 5% oint 5% cream 10%, OTC PA cream 1% except tabs QL QL Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits Nucleoside Analogues Nucleoside Reverse - Transcriptase Inhibitors/and Combinations abacavir abacavir/lamivudine abacavir/lamivudine/ zidovudine didanosine didanosine delayed-release emtricitabine emtricitabine/rilpivirine/ tenofovir lamivudine lamivudine/zidovudine stavudine zidovudine ZIAGEN EPZICOM generic brand TRIZIVIR generic VIDEX brand VIDEX EC generic EMTRIVA brand COMPLERA brand EPIVIR COMBIVIR ZERIT RETROVIR generic generic generic generic Nucleoside/Nucleotide Reverse - Transcriptase Inhibitor Combination cobicistat/elvitegravir/ emSTRIBILD tricitabine/tenofovir efavirenz/emtricitabine/ ATRIPLA tenofovir emtricitabine/tenofovir TRUVADA brand PA brand brand Nucleotide Analogues Nucleotide Reverse-Transcriptase Inhibitor tenofovir VIREAD brand atazanavir REYATAZ REYATAZ POWDER PACKET PREZISTA LEXIVA CRIXIVAN KALETRA VIRACEPT NORVIR INVIRASE APTIVUS brand Protease Inhibitors atazanavir darunavir fosamprenavir indinavir lopinavir/ritonavir nelfinavir ritonavir saquinavir mesylate tipranavir Miscellaneous brand brand brand brand brand brand brand brand brand elvitegravir/cobicistat/ emtricitabine/tenofovir GENVOYA alafenamide fumarate brand Miscellaneous abacavir/dolutegravir/ lamivudine artemether-lumefantrine TRIUMEQ brand COARTEM brand OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 37 PA Covered Drug generic generic brand generic generic generic brand brand brand brand brand brand generic brand generic brand generic generic brand brand Generic Drug Name Brand Drug Name atovaquone atovaquone/proguanil bedaquiline chloroquine phosphate clindamycin clindamycin cobicistat dapsone darunavir/cobicistat dolutegravir etravirine etravirine hydroxychloroquine interferon gamma-1b linezolid maraviroc mefloquine metronidazole neomycin sulfate neomycin sulfate soln MEPRON MALARONE SIRTURO ARALEN CLEOCIN CLEOCIN TYBOST DAPSONE PREZCOBIX TIVICAY INTELENCE INTELENCE PLAQUENIL ACTIMMUNE ZYVOX SELZENTRY LARIAM FLAGYL nitazoxanide suspension ALINIA SUSPENSION brand nitazoxanide tablet nitrofurantoin extended-release nitrofurantoin macrocrystals ALINIA brand nitrofurantoin susp palivizumab paromomycin povidone-iodine primaquine pyrimethamine quinine sulfate raltegravir raltegravir trimethoprim NEO-FRADIN MACROBID Requirements & Limits PA cap, 75 mg tabs tab 25 mg QL PA, SP PA tabs only Members ≥ 8 years of age will require prior authorization. PA generic MACRODANTIN MACRODANTIN 25 MG FURADANTIN SUSP 25 MG/5 ML SYNAGIS HUMATIN DARAPRIM QUALAQUIN ISENTRESS ISENTRESS CHEWABLE TRIMETHOPRIM generic generic brand generic generic generic brand generic brand brand generic Members ≥ 8 years of age will require prior authorization. PA, SP OTC PA, SP 324 mg caps only chewable tablet tabs only Musculoskeletal Arthritis Disease Modifying Anti-Rheumatic Drugs adalimumab HUMIRA OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit brand ST = Step Therapy SP = Specialty Pharmacy 38 PA, QL, SP Generic Drug Name Brand Drug Name auranofin azathioprine etanercept hydroxychloroquine leflunomide methotrexate penicillamine penicillamine sulfasalazine sulfasalazine delayed-release RIDAURA IMURAN ENBREL PLAQUENIL ARAVA METHOTREXATE DEPEN TITRATABLE CUPRIMINE AZULFIDINE Covered Drug brand generic brand generic generic generic brand brand generic AZULFIDINE EN-TABS generic QL acetaminophen TYLENOL BAYER generic OTC generic OTC brand tablet DR ASCRIPTIN brand tab BUFFERIN generic brand generic OTC PA, QL NSAIDs and Other Analgesics aspirin aspirin aspirin-al hydro-mg hydro-ca carb aspirin buffered capsaicin celecoxib diclofenac 1% gel diclofenac potassium diclofenac sodium delayed-release diclofenac sodium extended-release diflunisal etodolac fenoprofen ECOTRIN HALFPRIN CELEBREX VOLTAREN 1% TOPICAL GEL CATAFLAM generic generic VOLTAREN XR generic DOLOBID LODINE NALFON generic generic generic ibuprofen ADVIL generic ibuprofen indomethacin ketoprofen meloxicam naproxen MOTRIN INDOCIN ORUDIS MOBIC NAPROSYN ENTERIC COATEDNAPROSYN ANAPROX DAYPRO FELDENE generic generic generic generic generic naproxen sodium oxaprozin piroxicam OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit QL QL PA, QL, SP QL QL QL PA, QL, SP SP QL generic VOLTAREN naproxen delayed release Requirements & Limits IR only 600 mg tabs, chew tabs and susp, OTC tabs, chew tabs and susp generic generic generic generic ST = Step Therapy SP = Specialty Pharmacy 39 IR only QL Generic Drug Name Brand Drug Name sulindac CLINORIL Covered Drug generic allopurinol colchicine colchicine febuxostat probenecid ZYLOPRIM COLCRYS MITIGARE ULORIC PROBENECID generic generic brand brand generic chlorzoxazone cyclobenzaprine methocarbamol orphenadrine extended-release PARAFON FORTE DSC FLEXERIL ROBAXIN generic generic generic NORFLEX generic baclofen dantrolene diazepam tizanidine BACLOFEN DANTRIUM VALIUM ZANAFLEX generic generic generic generic QL tabs only, QL desogestrel/EE norethindrone/EE MIRCETTE ORTHO-NOVUM 10/11 generic generic QL QL levonorgestrel levonorgestrel PLAN B PLAN B ONE STEP generic generic QL 1.5 mg tab medroxyprogesterone acetate DEPO-PROVERA generic QL Gout Skeletal Muscle Relaxants Muscle Spasm Spasticity Requirements & Limits PA ST OB-GYN Contraceptives Biphasic Emergency Contraception Injectable Intravaginal etonogestrel/EE NUVARING ORTHO COIL brand ring, QL ortho diaphragm ORTHO FLAT brand QL generic generic 0.1/20, QL 1/20, QL generic 1/20, QL ORTHO FLEX Monophasic - 20 mcg Estrogen levonorgestrel/EE ALESSE norethindrone acetate/EE LOESTRIN 1/20 norethindrone acetate/EE/ LOESTRIN FE 1/20 iron OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 40 Generic Drug Name Brand Drug Name Monophasic - 30 mcg Estrogen Covered Drug Requirements & Limits desogestrel/EE levonorgestrel/EE norethindrone acetate/EE norethindrone acetate/EE/ iron norgestrel/EE ORTHO-CEPT NORDETTE LOESTRIN 1.5/30 generic generic generic 0.15/30, QL 0.15/30, QL 1.5/30, QL LOESTRIN FE 1.5/30 generic 1.5/30, QL LO/OVRAL generic 0.3/30, QL ethynodiol diacetate/EE norethindrone/EE norethindrone/EE norethindrone/EE norgestimate/EE ZOVIA 1/35 BALZIVA MODICON ORTHO-NOVUM 1/35 ORTHO-CYCLEN generic generic generic generic generic 1/35, QL 0.4/35, QL 0.5/35, QL 1/35, QL 0.25/35, QL ethynodiol diacetate/EE norethindrone/EE norethindrone/ME norgestrel/EE ZOVIA 1/50 OVCON 50 ORTHO-NOVUM 1/50 OVRAL generic generic generic generic 1/50, QL 1/50, QL 1/50, QL 0.5/50, QL norethindrone norgestrel ORTHO MICRONOR OVRETTE generic generic norelgestromin/EE ORTHO EVRA generic Monophasic - 35 mcg Estrogen Monophasic - 50 mcg Estrogen Progestin Transdermal Triphasic desogestrel/EE levonorgestrel/EE norethindrone acetate/EE/iron norethindrone/EE norethindrone/EE norgestimate/EE Endometriosis danazol CAZIANT CESIA generic QL VELIVET TRIVORA generic QL ESTROSTEP FE generic QL ORTHO-NOVUM 7/7/7 TRI-NORINYL ORTHO TRI-CYCLEN generic generic generic QL QL QL DANOCRINE generic Gender edits apply: for female patients only. CYCLESSA Hormone Therapy/Menopause Estrogens - Intravaginal estradiol estradiol estradiol ESTRACE CRM ESTRING VAGIFEM OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit brand brand brand ST = Step Therapy SP = Specialty Pharmacy 41 vaginal ring vaginal tablet Generic Drug Name Brand Drug Name estradiol acetate estrogens, conjugated FEMRING PREMARIN Covered Drug brand brand esterified estrogens estradiol estrogens, conjugated estrogens, conjugated, synthetic A estrogens, conjugated, synthetic B estropipate MENEST ESTRACE PREMARIN brand generic brand CENESTIN brand ENJUVIA brand OGEN generic estradiol estradiol CLIMARA DIVIGEL ELESTRIN generic brand QL gel brand gel pump 0.06% brand brand brand brand generic emulsion spray patch weekly 14 mcg/24hr patch biweekly Estrogens - Oral Estrogens - Transdermal estradiol estradiol estradiol estradiol estradiol estrogen patch Estrogen/Progestin ESTROGEL ESTRASORB EVAMIST MENOSTAR VIVELLE-DOT ESTRADIOL TDS estrogens, conjugated/me- PREMPHASE droxyprogesterone PREMPRO Requirements & Limits vaginal ring, PA crm brand Progestins medroxyprogesterone acetate norethindrone acetate progesterone micronized PROVERA generic AYGESTIN PROMETRIUM generic generic fluconazole metronidazole DIFLUCAN FLAGYL generic generic QL tabs clotrimazole clindamycin clindamycin GYNE-LOTRIMIN CLEOCIN CLEOCIN METROGEL-VAGINAL generic brand generic OTC supp crm Vaginal Infections Oral Vaginal metronidazole miconazole miconazole miconazole nitrate generic METROGEL 1% MONISTAT MONISTAT 3 MICONAZOLE KIT OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit generic generic generic OTC vaginal supp & cream kit ST = Step Therapy SP = Specialty Pharmacy 42 Generic Drug Name Brand Drug Name sulfanilamide terconazole AVC VAGINAL TERAZOL 3/7 Miscellaneous conjugated estrogen/ bazedoxifene methylergonovine tranexamic acid Covered Drug brand generic DUAVEE Requirements & Limits vaginal cream crm brand METHERGINE LYSTEDA generic generic OPTIVAR CROLOM ALAWAY OTC generic generic brand brand PA Ophthalmic Allergy azelastine cromolyn sodium ketotifen naphazoline/antazoline naphazoline/glycerin naphazoline HCL naphazoline w/pheniramine naphazoline/zinc sulfate tetrahydrozoline /zinc sulfate CLEAR EYES REDNESS RELIEF VASOCLEAR NAPHCON A OPCON A VASOCLEAR A ST QL generic generic soln 0.02% generic ophth soln 0.027-0.315% generic VISINE-AC generic Anti-Inflammatories Anti-Infective/Anti-Inflammatory Combinations gentamicin/prednisolone acetate neomycin/polymyxin B/ dexamethasone neomycin/polymyxin B/ hydrocortisone sulfacetamide/pred. phos. sulfacetamide sodiumprednisolone tobramycin/ dexamethasone tobramycindexamethasone Nonsteroidal diclofenac sodium flurbiprofen ketorolac PRED-G brand MAXITROL generic CORTISPORIN generic VASOCIDIN BLEPHAMIDE generic 10%/0.25% generic ophth susp 10-0.2% VASOCIDIN TOBRADEX generic TOBRADEX ST brand VOLTAREN OCUFEN ACULAR/ACULAR LS OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ophth susp technology 0.30.05% generic generic generic ST = Step Therapy SP = Specialty Pharmacy 43 Generic Drug Name Steroidal Brand Drug Name Covered Drug Requirements & Limits ophth suspension 0.1% dexamethasone dexamethasone sodium phosphate fluorometholone fluorometholone fluorometholone prednisolone acetate prednisolone acetate prednisolone phosphate rimexolone MAXIDEX brand DEXASOL generic FML FML FORTE FML LIQUIFILM PRED FORTE PRED MILD INFLAMASE FORTE VEXOL brand brand generic generic brand generic brand oint 0.1% susp 0.25% susp 0.1% 1% 0.12% 1% betaxolol carteolol levobunolol metipranolol timolol timolol maleate BETOPTIC -S ophth suspension 0.25% BETAGAN OPTIPRANOLOL TIMOPTIC XE TIMOPTIC brand generic generic generic generic generic brinzolamide dorzolamide AZOPT TRUSOPT brand generic Glaucoma Beta-Blockers Carbonic Anhydrase Inhibitors Carbonic Anhydrase Inhibitor/Beta-Blocker Combination dorzolamide HCL-timolol maleate dorzolamide/ timolol maleate Cholinesterase Inhibitor COSOPT PF brand COSOPT PHOSPHOLINE IODINE brand atropine cyclopentolate homatropine scopolamine ISOPTO ATROPINE CYCLOGYL ISOPTO HOMATROPINE ISOPTO HYOSCINE generic generic generic brand acetazolamide acetazolamide extended-release methazolamide ACETAZOLAMIDE generic DIAMOX SEQUELS generic NEPTAZANE generic latanoprost tafluprost travoprost XALATAN ZIOPTAN TRAVATAN Z generic brand brand Oral Prostaglandins OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit PA ophth solution preservative free generic ecothiophate Mydriatics ophthalmic solution 0.3% ophthalmic solution gel forming solution QL ophth soln 0.0015%. PA ophth soln 0.004%, PA ST = Step Therapy SP = Specialty Pharmacy 44 Generic Drug Name Brand Drug Name Covered Drug Requirements & Limits travoprost TRAVATAN Z PF generic ophth soln 0.004% (benzalkonium free), PA Topical - Parasympathomimetics pilocarpine pilocarpine ISOPTO CARPINE PILOPINE HS GEL generic brand brimonidine brimonidine brimonidine ALPHAGAN P ALPHAGAN P ALPHAGAN brand generic generic CILOXAN ERYTHROMYCIN ZYMAR GENTAK VIGAMOX generic generic generic brand generic brand NEOSPORIN generic OCUFLOX POLYSPORIN POLYTRIM BLEPH-10 generic generic generic generic Topical - Sympathomimetics Infections Bacterial bacitracin ciprofloxacin erythromycin gatifloxin gentamicin moxifloxacin HCl neomycin/polymyxin B/ gramicidin ofloxacin polymyxin B/bacitracin polymyxin B/trimethoprim sulfacetamide sulfacetamide/ phenylephrine tobramycin VASOSULF 0.1% 0.15% 0.2% PA ophth solution 0.5% oint/soln brand TOBREX generic trifluridine VIROPTIC generic natamycin NATACYN brand ophth suspension 5% cyclosporine cysteamine 0.44% ophthalmic solution sodium chloride hypertonic RESTASIS brand (ophth) emulsion 0.05%, PA CYSTARAN brand PA, SP MURO 128 generic soln 5% CAMPRAL ANTABUSE REVIA brand generic generic Fungal Viral Miscellaneous Psychiatric Alcohol Deterrents acamprosate disulfiram naltrexone OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 45 Brand Drug Name Covered Drug Requirements & Limits alprazolam alprazolam alprazolam chlordiazepoxide clonazepam clonazepam ODT clorazepate diazepam diazepam diazepam conc lorazepam lorazepam lorazepam conc oxazepam XANAX XANAX XANAX XR LIBRIUM KLONOPIN KLONOPIN WAFERS TRANXENE VALIUM VALIUM DIAZEPAM INTENSOL ATIVAN ATIVAN LORAZEPAM INTENSOL SERAX generic generic generic generic generic generic generic generic generic generic generic generic generic generic QL, IR only solution tablet SR, QL QL not wafers, QL ODT tabs, QL QL QL solution, QL QL QL solution, QL QL QL buspirone clomipramine fluvoxamine fluvoxamine SR BUSPAR ANAFRANIL LUVOX LUVOX CR generic generic generic generic QL ADDERALL generic QL brand QL STRATTERA DEXEDRINE DEXTROSTAT brand generic brand PA, QL QL QL DEXEDRINE SPANSULE generic QL INTUNIV VYVANSE METHYLIN SOLUTION RITALIN generic brand generic generic Age Limits Apply, QL solution, QL tabs only, QL CONCERTA generic QL Generic Drug Name Anxiety Benzodiazepines Miscellaneous Attention Deficit Hyperactivity Disorder (ADHD) amphetamine/ dextroamphetamine mixed salts amphetamine/ dextroamphetamine mixed salts extended-release atomoxetine dextroamphetamine dextroamphetamine dextroamphetamine extended-release guanfacine ER lisdexamfetamine methylphenidate methylphenidate methylphenidate extended-release ADDERALL XR (BRAND ADDERALL XR IS PREFERRED) OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 46 Generic Drug Name methylphenidate extended-release Bipolar Disorder divalproex sodium cap sprinkle divalproex sodium delayed-release lithium carbonate lithium carbonate extended-release Brand Drug Name Covered Drug Requirements & Limits generic QL METADATE ER RITALIN-SR RITALIN LA DEPAKOTE SPRINKLE generic DEPAKOTE generic LITHIUM CARBONATE ESKALITH CR generic LITHOBID Depression Monoamine Oxidase Inhibitor (MAOI) Minimum age 2 generic tranylcypromine PARNATE generic citalopram escitalopram CELEXA LEXAPRO generic generic fluoxetine PROZAC generic paroxetine paroxetine paroxetine SR sertraline PAXIL PAXIL ORAL SUSP PAXIL CR ZOLOFT generic generic generic generic 10 mg and 20 mg caps and 20 mg soln only tablets oral susp, QL tablets, QL tablets, QL duloxetine venlafaxine venlafaxine XR CYMBALTA EFFEXOR EFFEXOR XR generic generic generic QL QL QL amitriptyline amoxapine desipramine doxepin imipramine HCL nortriptyline protriptyline ELAVIL generic generic generic generic generic generic generic tablets Selective Serotonin Reuptake Inhibitor (SSRIs) Serotonin Norepinephrine Reuptake Inhibitors (SNRIs) Tricyclic Antidepressants (TCAs) NORPRAMIN SINEQUAN TOFRANIL PAMELOR VIVACTIL Tricyclic Antidepressant/Phenothiazine Combination amitriptyline/perphenazine TRIAVIL Miscellaneous Agents bupropion bupropion extended-release generic FORFIVO XL generic ST = Step Therapy SP = Specialty Pharmacy 47 tablets generic WELLBUTRIN OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit QL 450 mg Brand Drug Name Covered Drug Requirements & Limits WELLBUTRIN SR generic QL WELLBUTRIN XL generic 150 mg and 300 mg LUDIOMIL REMERON REMERON ODT DESYREL generic generic generic generic tabs (not soltabs) odt tablets 150 mg only estazolam flurazepam temazepam triazolam PROSOM DALMANE RESTORIL HALCION generic generic generic generic QL QL 15 mg and 30 mg only, QL QL chloral hydrate diphenhydramine ramelteon zaleplon zolpidem CHLORAL HYDRATE NYTOL QUICK CAPS ROZEREM SONATA AMBIEN generic generic brand generic generic OTC QL, PA QL QL buprenorphine/naloxone SUBOXONE naloxone naloxone naltrexone naltrexone for IM extended release susp NALOXONE INJ NARCAN NASAL SPRAY REVIA VIVITROL brand aripiprazole ABILIFY TABLETS brand aripiprazole ER injection clozapine clozapine (ODT formulation) olanzapine olanzapine ODT paliperidone paliperidone quetiapine quetiapine XR risperidone ABILIFY MAINTENA CLOZARIL Generic Drug Name bupropion extended-release bupropion extended-release maprotiline mirtazapine mirtazapine trazodone Insomnia Benzodiazepines Non-Benzodiazepines Narcotic Antagonists brand generic brand generic Psychoses Atypicals FAZACLO ZYPREXA ZYPREXA ZYDIS INVEGA SUSTENNA INVEGA TRINZA SEROQUEL SEROQUEL XR RISPERDAL OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit 2 mg and 8 mg film only, QL QL QL PA brand generic Age Limit Applies, tablets, PA, QL Age Limit Applies, PA, QL Age Limit Applies, QL brand Age Limit Applies, QL generic generic brand brand generic brand generic Age Limit Applies, QL Age Limit Applies, QL Age Limit Applies, PA, QL PA Age Limit Applies, QL Age Limit Applies, PA, QL Age Limit Applies, QL ST = Step Therapy SP = Specialty Pharmacy 48 RISPERDAL CONSTA RISPERDAL M-TAB RISPERDAL SOLUTION GEODON Covered Drug brand generic generic generic Age Limit Applies, PA, QL Age Limit Applies, QL Age Limit Applies, QL Age Limit Applies, QL bupropion nicotine nicotine inhaler system nicotine polacrilex gum nicotine polacrilex lozenge varenicline ZYBAN NICODERM NICOTROL NICORETTE COMMITT CHANTIX brand generic brand generic generic brand smoking deterrent, QL TD patch, QL QL 2 mg & 4 mg, QL 2 mg & 4 mg, QL QL chlorpromazine dextromethorphan/ quinidine fluphenazine fluphenazine decanoate haloperidol haloperidol decanoate loxapine perphenazine pimozide thioridazine thiothixene trifluoperazine THORAZINE generic NUEDEXTA brand Generic Drug Name Brand Drug Name risperidone risperidone m-tab risperidone oral soln ziprasidone Smoking Cessation Miscellaneous PROLIXIN PROLIXIN DECANOATE HALDOL HALDOL DECANOATE LOXITANE TRILAFON ORAP MELLARIL NAVANE STELAZINE Requirements & Limits PA generic generic generic generic generic generic generic generic generic generic Respiratory Drugs Antitussives, Decongestants, Expectorants and Combinations benzonatate brompheniramine & phenylephrine brompheniramine/ pseudoephedrine brompheniramine/ pseudoephedrine brompheniramine/ pseudoephedrine brompheniramine/ pseudoephedrine TESSALON DIMETAPP CLD ELX/ ALLERGY ACCUHIST DROPS generic generic RESPERAL-DM ANDEHIST generic liquid brand syrup 4-60 mg/5 ml BROMFENEX PD CAP DIMETAPP ELX CLD/ALLE generic UNI-HIST DRO CARDEC SYP BROVEX PSB OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit generic ST = Step Therapy SP = Specialty Pharmacy 49 liq 4-20 mg/5 ml Generic Drug Name brompheniramine/ pseudoephedrine brompheniramine/ pseudoephedrine brompheniramine/ pseudoephedrine/ dextromethorphan brompheniramine/ pseudoephedrine/ dextromethorphan brompheniramine/ pseudoephedrine/ dextromethorphan brompheniramine/ pseudoephedrine/ dextromethorphan/ guaifenesin carbetapentane/ chlorpheniramine/ ephedrine/ phenylephrine carbinoxamine/ pseudoephedrine chlorphen-PEmethscopolamine chlorphen tan/ carbetapentane tan chlorphen tan/ pseudoeph tan chlorphen tan/pyrilamine tan/PE tan Brand Drug Name Covered Drug Requirements & Limits J-TAN D PD generic drops 1-7.5 mg/ml RONDEC SYRUP generic syrup ACCUHIST PDX DROPS generic liquid BROMALINE DM generic elixir generic syrup HISTACOL DM generic syrup RYNATUSS generic RONDEC DROPS generic liquid generic syrup TUSSI-12 S generic susp TANAFED generic susp TRIOTANN PEDIATRIC SUSP generic susp BROMFED DM DALLERGY DM CARBOFED NEO DM ANAPLEX DM DURADRYL QV-ALLERGY R-TANNAMINE OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 50 Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits ROBITUSSIN PED LIQ CGH/COLD chlorpheniramine/ dextromethorphan ROBITUSSIN LIQ CGH/CLD generic DIMETAPP SYP CGH/CLD CORICIDIN TAB CGH/CLD chlorpheniramine maleate ED A-HIST TABLETS AND phenylephrine HCL LIQUID RONDEC DROPS chlorpheniramine/ phenylephrine CARDEC DRO RONDEC SYRUP chlorpheniramine/ phenylephrine CARDEC SYP HISTEX chlorpheniramine/ pseudoephedrine CPM/PSE chlorpheniramine tan/ RYNATAN PEDIATRIC SUSP phenylephrine tan codeine/ DIHISTINE DH chlorpheniramine/ PHENYLHIST LIQ DH pseudoephedrine CHERATUSSIN AC codeine/guaifenesin GUIATUSS/AC generic generic liquid generic syrup generic generic susp generic generic M-CLEAR WC QL MYTUSSIN AC codeine/guaifenesin/pseuGUIATUSS DAC doephedrine PROMETHAZINE codeine/promethazine W/CODEINE codeine/promethazine/ PROMETHAZINE VC phenylephrine W/CODEINE dextromethorphan/ brompheniramine/ BROMETANE DX pseudoephedrine dextromethorphan/ carbinoxamine/ CARDEC-DM pseudoephedrine dextromethorphanDURATUSS DM ELX guaifenesin OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit generic generic QL generic QL generic generic drops generic soln 25-225 mg/5 ml ST = Step Therapy SP = Specialty Pharmacy 51 Generic Drug Name Brand Drug Name Covered Drug Requirements & Limits generic OTC generic liq 10-200 mg/ 5 ml generic syrup brand OTC GG/DM CR dextromethorphan/ guaifenesin dextromethorphanguaifenesin dextromethorphan HBR dextromethorphan polistirex extended-release dextromethorphan/ promethazine guaifenesin guaifenesin guaifenesin guaifenesin guaifenesin extended-release guaifenesin/ pseudoephedrine guaifenesin/ pseudoephedrine/ dextromethorphan guaifenesin/ pseudoephedrine extended-release guaifenesin/ pseudoephedrine extended-release hydrocodone/ chlorpheniramine/ phenylephrine hydrocodone/ guaifenesin MUCINEX DM ROBITUSSIN DM TUSSIN DM ROBITUSSIN LIQ CGH/ CONG ROBITUSSIN SYP MAX-ST ROBITUSSIN PED SYP DELSYM PHENERGAN DM PROMETHAZINE SYP DM MUCINEX KIDS ROBITUSSIN ROBITUSSIN SYP CHST CNG TRIACTIN CHEST CONGESTION MUCINEX ROBITUSSIN PE PSE/GG generic brand generic granules packet OTC generic syrup 100 mg/5 ml brand soln 50 mg/5 mL generic OTC generic syrup, OTC ROBITUSSIN CF generic GUAIFED generic GUAIFEN PSE MUCINEX D generic GG/PSE CR HISTUSSIN HC generic VITUSSIN HYDROCODO/GG SYP OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit generic ST = Step Therapy SP = Specialty Pharmacy 52 OTC Generic Drug Name Covered Drug Brand Drug Name Requirements & Limits HYCODAN hydrocodone/ homatropine loratadine & pseudoephedrine SR 24hr phenyleph/bromphen/ dextromethorphan/ guaifenesin phenylephrine/ brompheniramine/ dextromethorphan phenylephrine/ brompheniramine-DM phenylephrine/ brompheniramine-DM phenylephrine/ brompheniramine-DM phenylephrine/ brompheniramine-DM phenylephrine/ chlorpheniramine phenylephrine/ chlorpheniramine/ dextromethorphan phenylephrine/ chlorpheniramine/ dextromethorphan phenylephrine/ chlorpheniramine/ dextromethorphan phenylephrine/ chlorpheniramine/ dihydrocodeine phenylephrine/ dextromethorphan HYDROMET SYP generic HYDROCODONE/ TAB HOMATROP ALLERGY/CONG TAB RELIEF generic ALLANHIST SYP PDX generic tab 10-240 mg generic OTC ALAHIST DM generic liq 7.5-4-15 mg/5 ml DIMETAPP DM generic liq 2.5-1-5 mg/5 ml LOHIST-DM generic syrup 5-2-10 mg/5 ml PRESGEN B generic liq 10-4-20 mg/5 ml QUAL-TUSSIN SYP DC generic ATUSS DR DE-CHLOR DM generic syrup generic syrup generic liquid RONDEC DM STATUSS DM SYP CARDEC DM SYP MINUTUSS DR SYP RONDEC DM DROPS CARDEC DM DRO ROBITUSSIN LIQ CGH/ALRG DIHYDRO-PE SYP generic DIMETAPP DRO DCON/CGH generic OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 53 Generic Drug Name phenylephrine/ dextromethorphan/ guaifenesin phenylephrine/ ephed/CPM w/carbetapentane phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/guaifenesin phenylephrine/ hydrocodone/ guaifenesin phenylephrine/pyrilamine/ dextromethorphan phenylephrine/pyrilamine w/hydrocodone phenylephrine tan/ pyrilamine tan/ carbeta tan promethazine & phenylephrine pseudoephedrine pseudoephedrine/ acetaminophen/ dextromethorphan pseudoephedrine/ chlorpheniramine/ dextromethorphan Brand Drug Name Covered Drug ROBITUSSIN PE generic RYNATUSS PEDIATRIC SUSP generic susp generic brand generic brand brand generic generic brand generic syrup 5-200 mg/5 ml cap 10-390 mg tab 10-380 mg liq 10-100 mg/5 ml tab 10-388 mg tab 5-200 mg tab 10-400 mg liq 2.5-100 mg/5 ml liq 7.5-200 mg/5 ml DECONEX DECONEX IR ENTEX LQ LIQ GILPHEX TR LIQUIBID PD MEDIFIN PE MUCINEX COLD LIQ /KIDS NARIZ ROBITUSSIN LIQ HD/CHST SUPRESS-PE PEDIATRIC DROPS TRIAMINIC LIQ CHST/NAS TRIAMINIC SYR CHST/NAS Requirements & Limits generic brand drops 2.5-50 mg/ml brand brand liq 2.5-50 mg/5 ml syrup 2.5-50 mg/5 ml QUAL-TUSSIN SYP DC generic CODAL-DM generic CODIMAL DH generic syrup TUSSI 12D S generic susp generic syrup 6.25-5 mg/ 5 mg PROMETH VC SYP 6.25-5/5 SUDAFED generic MAPAP COLD TAB generic PEDIACARE LIQ MULTI-SY generic ROBITUSSIN LIQ PED NGHT OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 54 Generic Drug Name Brand Drug Name Covered Drug pseudoephedrine/ dextromethorphan/ guaifenesin MULTI SYMPTOM TAB COLD RLF generic pseudoephedrine/ iburprofen pseudoephedrine tan/ dexchlorphen tan/ DM tan pyrilamine tan/phenyleph tan tripolidine/ pseudoephedrine Asthma/COPD Inhalers - Beta Agonists CHILD IBUPRO SUS COLD Requirements & Limits generic IBUOROFEN TAB COLD/SIN TANAFED DMX SUSPENSION generic susp generic susp TRI-FED X RYNA-12 S TRIPROL/PSE SYP generic APHEDRID TAB albuterol sulfate formoterol indacaterol olodaterol salmeterol xinafoate VENTOLIN HFA FORADIL AEROLIZER ARCAPTA NEOHALER STRIVERDI RESPIMAT SEREVENT DISKUS brand brand brand brand brand PA, QL beclomethasone fluticasone HFA mometasone QVAR FLOVENT HFA ASMANEX TWISTHALER brand brand brand QL QL QL budesonide/formoterol fluticasone/salmeterol mometasone/formoterol SYMBICORT ADVAIR DISKUS DULERA brand brand brand ST QL, ST ST cromolyn ipratropium HFA ipratropium/albuterol nedocromil omalizumab tiotropium tiotropium respimat INTAL ATROVENT HFA COMBIVENT RESPIMAT TILADE XOLAIR SPIRIVA SPIRIVA RESPIMAT brand brand brand brand brand brand brand QL albuterol ACCUNEB generic albuterol budesonide PROVENTIL PULMICORT RESPULES generic brand Inhalers - Corticosteroids Inhalers - Corticosteroid/Beta Agonist Combinations Inhalers - Others Inhalers for Nebulization OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit QL PA inhaler PA, SP 0.63 mg/3 ml and 1.25 mg /3 ml soln 0.083%, 0.5% susp, QL ST = Step Therapy SP = Specialty Pharmacy 55 Generic Drug Name Brand Drug Name cromolyn ipratropium ipratropium/albuterol levalbuterol HCl INTAL ATROVENT DUONEB XOPENEX RESPULES Covered Drug generic generic generic generic albuterol SR tabs metaproterenol metaproterenol terbutaline VOSPIRE ER ALUPENT METAPROTERENOL SYRUP BRETHINE generic generic generic generic montelukast zafirlukast SINGULAIR ACCOLATE generic generic QL tabs theophylline theophylline extended-release theophylline extended-release theophylline extended-release theophylline extended-release THEOPHYLLINE generic liquid brand caps THEOCHRON generic tabs THEOPHYLLINE EXT-REL generic caps (12 hr) UNIPHYL generic tabs Oral Agents - Beta Agonists Oral Agents - Leukotriene Modifiers Oral Agents - Theophylline THEO-24 Miscellaneous alpha1-proteinase inhibitor (human) alpha1-proteinase inhibitor (human) ARALAST NP brand PROLASTIN brand Requirements & Limits soln, QL soln, QL soln QL, ST tabs SP, Coverable through Medical Benefit SP, Coverable through Medical Benefit Urological Symptomatic Benign Prostatic Hypertrophy UROXATRAL CARDURA CARDURA XL PROSCAR FLOMAX HYTRIN generic generic brand generic generic generic bethanechol URECHOLINE hyoscyamine, methenamine, phenyl salicylate, soUTIRA C dium phosphate monobasic, methylene blue generic alfuzosin ER doxazosin doxazosin SR finasteride tamsulosin terazosin Miscellaneous OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit brand ST = Step Therapy SP = Specialty Pharmacy 56 Generic Drug Name methenamine hippurate oxybutynin chloride oxybutynin IR pentosan phenazopyridine potassium citrate propantheline sodium citrate/citric acid tolterodine tolterodine SR trospium Covered Drug Brand Drug Name HIPREX Requirements & Limits generic UREX DITROPAN XL DITROPAN ELMIRON PYRIDIUM UROCIT-K BICITRA DETROL DETROL LA SANCTURA generic generic brand generic generic generic generic generic brand generic calcitriol ROCALTROL generic calcitriol oral soln ROCALTROL SOLUTION generic calcium cholecalciferol generic brand cholecalciferol cholecalciferol cholecalciferol cholecalciferol cholecalciferol cholecalciferol OS-CAL BIO-D DRO-MULSION BIO-D-MULSIO DRO FORTE D3-50 CAP VITAMIN D CAP 400 UNIT VITAMIN D CAP 2000 UNIT VITAMIN D TAB 400 UNIT VITAMIN D TAB 1000 UNIT VITAMIN D TAB 2000 UNIT cyanocobalamin VITAMIN B-12 generic electrolyte PEDIALYTE generic ergocalciferol (D2) DRISDOL generic QL, ST PA ST ST ST Vitamins and Minerals cholecalciferol OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit brand brand brand brand brand brand brand Members ≥ 8 years of age will require prior authorization. OTC drops 400 unit/0.03 ml, OTC drops 2000 unit/0.03 ml, OTC cap 50000 unit, OTC cap 400 unit, OTC cap 2000 unit, OTC tab 400 unit, OTC tab 1000 unit, OTC tab 2000 unit, OTC inj, Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. soln, oral, OTC Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. ST = Step Therapy SP = Specialty Pharmacy 57 Generic Drug Name ferrous bisglycinate/ polysaccarides iron ferrous sulfate Brand Drug Name Covered Drug Requirements & Limits NIFEREX generic caps, OTC FEOSOL GEL-KAM generic OTC Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. LURIDE fluoride LURIDE LOZI-TABS generic PREVIDENT folic acid PHOS-FLUR FOLIC ACID generic magnesium oxide MAG-OX generic multivitamins/ fluoride/±iron POLY-VI-FLOR generic multivitamins/minerals CENTRUM generic SUPER OMEGA omega-3 fatty acids ESKIMO-3 OTC, Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. OTC, Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. generic capsule HM FISH OIL generic delayed-release capsule GNP FISH OIL MEPHYTON brand SEA-OMEGA PRO NUTRIENT omega-3 fatty acids phytonadione polysaccharide iron complex NIFEREX OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit generic ST = Step Therapy SP = Specialty Pharmacy 58 elixir, OTC Generic Drug Name prenat-FE Bis-FE prot succ-FA-CA & omega 3 prenat-FE Bis-FE prot succ-FA-CA & omega 3 prenat-FE Bis-FE prot succ-FA-CA & omega 3 prenat w/o A w/fecbn-fegl-DSS-FA & DHA prenatal vit w/FE bisglyc-FE prot succ-FA prenatal vit w/FE bisglycinate chelate-FA prenatal vit w/FE bisglycinate chelate-FA prenatal vit w/FE bisglycinate chelate-FA prenatal vit w/FE polysac cmplx-FA prenatal vit w/iron carbonyl-FA prenatal w/o A w/ FE carbonyl-FE gluc-DSS-FA prenatal vit w/o vit a w/fe bisglycinate-fa tab 32-1 mg Covered Drug Brand Drug Name COMPLETE NATALCARE PAK DHA brand PRUET DHA PAK SETONET PAK brand TRUST NATALCARE PAK DHA brand FOLTABS PAK PLUS DHA RE OB + DHA PAK brand VINATE III brand GENTEX ADE 28-1 MG brand VINATE AZ EX brand VITAPHIL brand EDGE OB CHW brand ATABEX PRENATAL brand FOLTABS PRENATAL brand TRI RX OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit Requirements & Limits generic ST = Step Therapy SP = Specialty Pharmacy 59 Generic Drug Name Brand Drug Name Covered Drug Requirements & Limits generic QL PRENATAL VITAMINS W/ FOLIC ACID prenatal vitamins w/folic acid CENOGEN OB/ULTRA MATERNA NATALCARE NESTABSCBF/FA/RX renat-FE bis-FE prot succFA-CA & omega DR vitamin A NIFEREX-PN FORTE PRUET DHAEC PAK brand generic vitamin ADC/fluoride/±iron TRI-VI-FLOR drops generic vitamin B complex/ vitamin C/folic acid generic NEPHROCAPS vitamin B-1 vitamin B-6 vitamin C vitamins pediatric generic generic generic TRI-VI-SOL generic OTC Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. OTC OTC OTC members <3 years old, OTC, Coverage for these medications will be determined by the member’s individual benefits. Please refer to individual plan documents for coverage information. OTC zinc generic phosphorus K-PHOS NEUTRAL potassium acid phosphate K-PHOS ORIGINAL potassium bicarbonate/ potassium citrate K-LYTE effervescent generic brand tabs generic tabs Potassium OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 60 Generic Drug Name Brand Drug Name potassium chloride potassium chloride K-LOR POTASSIUM CHLORIDE K-DUR 10 potassium chloride extended-release K-DUR 20 potassium chloride extended-release potassium iodide KLOR-CON 8 Covered Drug generic generic Requirements & Limits powder liquid generic tabs generic caps KLOR-CON 10 MICRO-K 10 PIMA brand Miscellaneous Anaphylaxis epinephrine epinephrine Cystic Fibrosis acetylcysteine dornase alfa ivacaftor ivacaftor lumacaftor/ivacaftor tobramycin neb soln Detoxification Agents deferasirox EPINEPHRINE AUTO-INJECT BY IMPAX LABS EPIPEN generic QL brand QL generic brand brand brand brand PA, SP PA, SP PA, SP PA, SP brand PA, SP brand PA, SP brand PA, SP brand brand PA, SP PA, SP generic 667 mg tablet only, PA PHOSLYRA brand PA SENSIPAR FOSRENOL RENVELA RENAGEL brand brand generic brand PA PA ST tablet, PA EPIPEN JR. MUCOMYST PULMOZYME KALYDECO KALYDECO GRANULES ORKAMBI BETHKIS KITABIS EXJADE deferiprone JADENU FERRIPROX C1 Inhibitor, Human icatibant BERINERT FIRAZYR Hereditary Angioedema Hyperphosphatemia calcium acetate calcium acetate oral solution cinacalcet lanthanum carbonate sevelamer sevelamer hcl OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 61 Generic Drug Name Brand Drug Name Idiopathic Pulmonary Fibrosis (IPF) Covered Drug Requirements & Limits nintedanib pirfenidone capsule OFEV ESBRIET brand brand PA, SP PA, SP eltrombopag PROMACTA brand PA, SP Immune Thrombocytopenic Purpura Medical Devices Spacers QL Vaccine diphtheria-tetanus tox adsorbed (dt) im hepatitis a vaccine susp DIP/TET PED INJ VAQTA HAVRIX hepatitis b vaccine (recom- ENGERIX-B binant) RECOMBIVAX HB human papillomavirus (hpv) 9-valent recomb GARDASIL 9 vac human papillomavirus (hpv) bival (type 16, 18) CERVARIX recmb vac human papillomavirus (hpv) quadrivalent GARDASIL recombinant vac influenza virus vaccine recombinant hemag- FLUBLOK glutinin (ha) AFLURIA influenza virus vaccine split FLUZONE SPLT influenza virus vaccine split FLUZONE HD PF high-dose pf influenza virus vaccine split AFLURIA PF pf FLUARIX QUAD influenza virus vaccine split FLULAVAL QUAD quadrivalent FLUZONE QUAD OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit brand QL brand QL brand QL brand QL brand QL brand QL brand QL, Age Limits Apply brand QL, Age Limits Apply brand QL, Age Limits Apply brand QL, Age Limits Apply brand QL, Age Limits Apply ST = Step Therapy SP = Specialty Pharmacy 62 Generic Drug Name influenza virus vaccine tisscult subunit influenza virus vaccine types a&b surface antigen measles, mumps & rubella virus vaccines for inj meningococcal (a, c, y, and w-135) meningococcal (a, c, y, and w-135) conjugate vaccine meningococcal (a, c, y, and w-135) oligo conj vac for inj pneumococcal 13-valent conjugate pneumococcal vaccine polyvalent tet tox-diph-acell pertuss ad tetanus-diphtheria toxoids (td) tetanus immune globulin (human) typhoid vaccine varicella virus vac live for subcutaneous zoster vaccine live Covered Drug Requirements & Limits FLUCELVAX brand QL, Age Limits Apply FLUVIRIN brand QL, Age Limits Apply M-M-R II brand QL MENOMUNE brand QL MENACTRA brand QL MENVEO brand QL PREVNAR 13 brand QL brand QL brand QL brand QL HYPERTET S/D brand QL VIVOTIF BERNA brand capsules VARIVAX brand QL ZOSTAVAX brand QL, Age Limits Apply Brand Drug Name PNEUMOVAX PNEUMOVAX 23 ADACEL BOOSTRIX TENIVAC TET/DIP TOX INJ OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 63 Generic Drug Name Brand Drug Name Examples OTC MEDICATIONS The following is a list of OTC products on the PDL. Some OTC products are listed on the drug list. OTC products covered are restricted to generics when available. Brand names are provided as reference only. Acne BREVOXYL-4 CLEARASIL benzoyl peroxide crm, gel, lotion, cleanser, bar, foam, DELOS cloth, wash OC8 PANOXYL BAR PANOXYL CREAM Antifungals clotrimazole MICATIN miconazole crm, soln LOTRIMIN AF tolnaftate TINACTIN MONISTAT vaginal products GYNE-LOTRIMIN Atopic Dermatitis BETACARE CREAM AND LOTION CETAPHIL CREAM AND LOTION DERMAPHOR OINTMENT emollients E-OINTMENT GLYCERIN TOPICAL Cough/Cold Allergy Antihistamine CHLOR-TRIMETON BENADRYL CLARITIN antihistamines ALAVERT ZYRTEC OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 64 Generic Drug Name Brand Drug Name Examples Antihistamine/Decongestant Combinations brompheniramine/pseudoephedrine cetirizine/pseudoephedrine OTC chlorpheniramine/pseudoephedrine DIMETAPP ZYRTEC D ACTIFED ALAVERT ALRG TAB/SINUS loratadine/pseudoephedrine ALAVERT D ALLERGY/CONG Cough/Cold ROBITUSSIN ROBITUSSIN DM antitussives Age edit applied. Not covered for members under ROBITUSSIN PE the age 2. ROBITUSSIN CF DELSYM NEO-SYNEPHRINE AFRIN nasal sprays DIMETAPP DRO DECONGES Diabetes alcohol swabs glucose oral tablets insulin (vials only) CURITY ALCOHOL PADS carbamide peroxide DEBROX condoms contraceptive foam contraceptive gel condoms - female TROJAN DELFEN GYNOL II CONDOMS OTC Burow’s soln, wet dressings dermatological baths hydrocortisone crm, oint DOMEBORO COLLOIDAL OATMEAL BATHS CORTAID NEOSPORIN topical antibacterials MYCITRACIN HUMULIN Earwax Removal Products Family Planning First Aid BACITRACIN OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 65 Generic Drug Name Brand Drug Name Examples Gastrointestinal MYLANTA LIQUID MAALOX LIQUID antacids liquids, chew tabs TUMS IMODIUM A-D antidiarrheals KAOPECTATE PEDIALYTE PEPCID AC FLEET ENEMA DULCOLAX electrolyte rehydrating soln famotidine laxative enemas laxatives psyllium rectal crm, suppositories simethicone stool softeners sugar+orthophosphoric acid FLEET PHOSPHO-SODA METAMUCIL PREPARATION H MYLICON COLACE EMETROL permethrin piperonyl butoxide gel, liquid shampoo NIX PIPERONYL BUTOXIDE dimenhydrinate meclizine DRAMAMINE BONINE allergic conjunctivitis artificial tears ALAWAY HYPOTEARS VISINE decongestants MURINE Lice Products Motion Sickness Ophthalmics NAPHCON A Pain acetaminophen tabs, liquid, drops, suppositories TYLENOL BAYER aspirin tabs, EC. tabs ECOTRIN aspirin with buffers tabs ADVIL ibuprofen tabs, chew tabs and susp OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit MOTRIN IB ST = Step Therapy SP = Specialty Pharmacy 66 Generic Drug Name Brand Drug Name Examples Smoking Cessation Products COMMIT LOZENGES (QUANTITY LIMIT) NICODERM CQ (QUANTITY LIMIT) nicotine NICOTINE GUM (QUANTITY LIMIT) NICOTROL (QUANTITY LIMIT) Vitamins/Minerals OS-CAL CALTRATE calcium TUMS iron ferrous fumarate, ferrous, gluconate, errous sulfate, ferrous bis-glycinate chelate and polysaccharide iron caps iron polysaccharides magnesium oxide vitamin D 400 IU FERGON FEOSOL NIFEREX MAG-OX VITAMIN D 400 IU VI-DAYLIN vitamins pediatric members <3 years old POLY-VI-SOL vitamins prenatal TRI-VI-SOL STUART PRENATAL Warts salicylic acid strip 40% salicylic acid topical patch 15% OTC DUOFILM MOSCO CALLUS/CORN REMOVER OTC DUOPLANT GEL, COMPOUND W, SALPLANT OTC CORN/CALLUS REMOVER PAD, CLEAR AWAY, ONE STEP OTC MEDIPLAST MOSCO CALLUS/CORN LIQUID REMOVER OTC COMPOUND W STRIPS TRANS-VER-SAL PATCH fluoride dental rinse PHOS-FLUR salicylic acid 17%/collodion salicylic acid film forming liquid 27.5% OTC salicylic acid gel 17% OTC salicylic acid pad 40% OTC salicylic acid plaster 40% salicylic acid soln OTC Miscellaneous OTC = Over the Counter PA = Prior Authorization required QL = Quantity Limit ST = Step Therapy SP = Specialty Pharmacy 67 Index of of Covered CoveredDrugs Drugs A abacavir . . . . . . . . . . . . 37 abacavir/dolutegravir/ lamivudine . . . . . . . . . 37 abacavir/lamivudine . . . . . 37 abacavir/lamivudine/ zidovudine . . . . . . . . . 37 ABILIFY MAINTENA . . . . . . 48 ABILIFY TABLETS . . . . . . . 48 abiraterone . . . . . . . . . . . 5 ABREVA OTC . . . . . . . . . 36 acamprosate . . . . . . . . . 45 acarbose . . . . . . . . . . . . 26 ACCOLATE . . . . . . . . . . . 56 ACCUHIST DROPS . . . . . . 49 ACCUHIST PDX DROPS . . . 50 ACCUNEB . . . . . . . . . . . 55 ACCUPRIL . . . . . . . . . . . .8 ACCURETIC . . . . . . . . . . .8 ACCUTANE . . . . . . . . . . 17 acebutalol . . . . . . . . . . . . 9 ACEON . . . . . . . . . . . . . .8 acetaminophen . . . . 13, 39, 66 acetazolamide . . . . . . . . . 44 ACETAZOLAMIDE . . . . . . . 44 acetazolamide extended-release . . . . . 44 acetic acid . . . . . . . . . . . 22 acetic acid/aluminum acetate22 acetic acid/hydrocortisone . . 22 acetylcysteine . . . . . . . . . 61 acidophilus . . . . . . . . . . 30 ACIDOPHILUS . . . . . . . . . 30 acidophilus/bifidus . . . . . . 31 ACIDOPHILUS/ BIFIDUS WAFER . . . . . 31 acidophilus/citrus pectin . . . 31 ACIDOPHILUS/ CITRUS PECTIN . . . . . . 31 acidophilus/pectin . . . . . . 31 ACIDOPHILUS/PECTIN . . . . 31 ACIDOPHILUS XTRA . . . . . 30 acitretin . . . . . . . . . . . . 20 ACLOVATE . . . . . . . . . . . 19 ACTIFED . . . . . . . . . . 24, 65 ACTIGALL . . . . . . . . . . . 31 ACTIMMUNE . . . . . . . . . . 38 ACTOPLUSMET . . . . . . . . 26 ACTOPLUS MET XR . . . . . 26 ACTOS . . . . . . . . . . . . . 26 ACULAR/ACULAR LS . . . . 43 acyclovir . . . . . . . . . . . . 36 acyclovir buccal . . . . . . . . 36 ADACEL . . . . . . . . . . . . 63 ADALAT CC . . . . . . . . . . 10 adalimumab . . . . . . . . . . 38 ADCIRCA . . . . . . . . . . . . 12 ADDERALL . . . . . . . . . . . 46 ADDERALL XR . . . . . . . . . 46 adefovir . . . . . . . . . . . . 36 ADEMPAS . . . . . . . . . . . 12 ADVAIR DISKUS . . . . . . . . 55 ADVIL . . . . . . . . . 13, 39, 66 afatinib . . . . . . . . . . . . . 4 AFINITOR . . . . . . . . . . . . .4 AFINITOR DISPERZ . . . . . . .4 AFLURIA . . . . . . . . . . . . 62 AFLURIA PF . . . . . . . . . . 62 AFRIN . . . . . . . . . . . 24, 65 agalsidase . . . . . . . . . . . 27 AGRYLIN . . . . . . . . . . . . .7 ALAHIST DM . . . . . . . . . . 53 ALAVERT . . . . . . . . . 23, 64 ALAVERT ALRG TAB/SINUS . . . . . . 24, 65 ALAVERT-D . . . . . . . . 24, 65 ALAWAY . . . . . . . . . . . . 66 ALAWAY OTC . . . . . . . . . 43 albendazole . . . . . . . . . . 35 ALBENZA . . . . . . . . . . . . 35 albiglutide . . . . . . . . . . . 27 albuterol . . . . . . . . . . . . 55 albuterol SR tabs . . . . . . . 56 albuterol sulfate . . . . . . . . 55 alclometasone . . . . . . . . 19 alcohol swabs . . . . . . . . . 65 ALDACTAZIDE . . . . . . . . . 11 ALDACTONE . . . . . . . . . . 11 ALDARA . . . . . . . . . . . . 21 ALL CAPS = Brand-name drug lower case = Generic drug 68 ALDOMET . . . . . . . . . . . 12 ALDORIL . . . . . . . . . . . . 12 ALDURAZYME . . . . . . . . . 28 ALECENSA . . . . . . . . . . . .4 alectinib . . . . . . . . . . . . . 4 alendronate . . . . . . . . . . 27 ALESSE . . . . . . . . . . . . . 40 alfuzosin ER . . . . . . . . . . 56 alginic acid/sodium bicarbonate . . . . . . . . 28 alglucerase . . . . . . . . . . 27 alglucosidase . . . . . . . . . 27 ALINIA . . . . . . . . . . . . . 38 ALINIA SUSPENSION . . . . . 38 alirocumab . . . . . . . . . . 11 alitretinoin 1% gel . . . . . . . 6 ALKERAN . . . . . . . . . . . . 4 ALLANHIST SYP PDX . . . . . 53 ALLEGRA . . . . . . . . . . . . 23 ALLEGRA-D . . . . . . . . . . 24 allergic conjunctivitis . . . . . 66 ALLERGY/CONG . . . . 24, 65 ALLERGY/CONG TAB RELIEF . . . . . . . . 53 allopurinol . . . . . . . . . . . 40 alpha1-proteinase inhibitor . . 56 ALPHAGAN . . . . . . . . . . 45 ALPHAGAN P . . . . . . . . . 45 alprazolam . . . . . . . . . . . 46 ALTACE . . . . . . . . . . . . . .8 altretamine . . . . . . . . . . . 4 alumina/magnesia . . . . . . 28 alumina/magnesia/ simethicone . . . . . . . . 28 aluminum acetate . . . . . . . 21 aluminum chloride . . . . . . 21 aluminum chloride hexahydrate . . . . . . . . 21 ALUPENT . . . . . . . . . . . . 56 amantadine . . . . . . . . 15, 36 AMARYL . . . . . . . . . . . . 26 AMBIEN . . . . . . . . . . . . 48 ambrisentan . . . . . . . . . . 12 AMERGE . . . . . . . . . . . . 15 Index of Covered Drugs AMICAR . . . . . . . . . . . . . 7 AMIKACIN INJ . . . . . . . . . 31 amikacin sulfate inj . . . . . . 31 amiloride . . . . . . . . . . . . 10 amiloride/hydrochlorothiazide10 aminocaproic acid . . . . . . . 7 aminosalicyclic acid . . . . . 33 amiodarone . . . . . . . . . . . 9 amitriptyline . . . . . . . . 15, 47 amitriptyline/perphenazine . 47 amlexanox . . . . . . . . . . . 24 amlodipine . . . . . . . . . . 10 ammonium lactate . . . . . . 21 amoxapine . . . . . . . . . . . 47 amoxicillin . . . . . . . . . . . 34 AMOXICILLIN CAPSULES AND CHEWABLES . . . . . . . 34 amoxicillin/clavulanate . . . . 34 AMOXIL . . . . . . . . . . . . . 34 AMOXIL SUSP . . . . . . . . . 34 amphetamine/dextroamphetamine mixed salts . . . . 46 amphetamine/dextroamphetamine mixed salts extended-release . . . . . 46 ampicillin . . . . . . . . . . . 34 ANAFRANIL . . . . . . . . . . 46 anagrelide . . . . . . . . . . . 7 ANAPLEX DM . . . . . . . . . 50 ANAPROX . . . . . . . . . 14, 39 anastrozole . . . . . . . . . . . 5 ANCEF INJ . . . . . . . . . . . 31 ANCOBAN . . . . . . . . . . . 35 ANDEHIST . . . . . . . . . . . 49 ANDRODERM . . . . . . . . . 25 ANDROGEL 1% . . . . . . . . 25 ANDROGEL 1.62% . . . . . . 25 ANDROXY . . . . . . . . . . . 25 ANERGAN . . . . . . . . . . . 33 ANSAID . . . . . . . . . . . . . 13 ANTABUSE . . . . . . . . . . . 45 antacids liquids, chew tabs . 66 ANTARA . . . . . . . . . . . . 11 antidiarrheals . . . . . . . . . 66 antihistamines . . . . . . . . . 64 antipyrine-benzocaine . . . . 22 antitussives . . . . . . . . . . 65 ANTIVERT . . . . . . . . . . . 28 ANUSOL HC 2.5% . . . . . . 21 ANZEMET . . . . . . . . . . . 28 APHEDRID TAB . . . . . . . . 55 APHTHASOL . . . . . . . . . . 24 apixaban . . . . . . . . . . . . 7 aprepitant . . . . . . . . . . . 28 APRESOLINE . . . . . . . . . 12 APRISO . . . . . . . . . . . . . 30 APTIVUS . . . . . . . . . . . . 37 AQUA-MEPHYTO INJ . . . . . 33 ARALAST NP . . . . . . . . . 56 ARALEN . . . . . . . . . . . . 38 ARANESP . . . . . . . . . . . . 7 ARAVA . . . . . . . . . . . . . 39 ARCAPTA NEOHALER . . . . 55 ARICEPT . . . . . . . . . . . . 13 ARIMIDEX . . . . . . . . . . . . 5 aripiprazole . . . . . . . . . . 48 aripiprazole ER injection . . . 48 ARMOUR THYROID . . . . . . 27 AROMASIN . . . . . . . . . . . .5 ARTANE . . . . . . . . . . . . 16 artemether-lumefantrine . . . 37 artificial tears . . . . . . . . . 66 ASACOL HD . . . . . . . . . . 30 ASCRIPTIN . . . . . . . . . . . 39 asfotase alfa . . . . . . . . . . 27 ASMANEX TWISTHALER . . . 55 aspirin . . . . . . . . . . . . 7, 39 aspirin/acetaminophen/ caffeine . . . . . . . . . . 13 aspirin-al hydro-mg hydro-ca carb . . . . . . . 39 aspirin buffered . . . . . . . . 39 aspirin tabs, EC. tabs . . . . . 66 aspirin with buffers tabs . . . 66 ASTELIN . . . . . . . . . . . . 23 ASTRAMOR INJ . . . . . . . . 31 ATABEX PRENATAL . . . . . . 59 ATARAX . . . . . . . . . . . . . 23 ALL CAPS = Brand-name drug lower case = Generic drug 69 atazanavir . . . . . . . . . . . 37 atenolol . . . . . . . . . . . . . 9 atenolol/chlorthalidone . . . . 9 ATIVAN . . . . . . . . . . . . . 46 ATIVAN . . . . . . . . . . . . . 46 atomoxetine . . . . . . . . . . 46 atorvastatin . . . . . . . . . . 11 atovaquone . . . . . . . . . . 38 atovaquone/proguanil . . . . 38 ATRALIN . . . . . . . . . . . . 18 ATRIPLA . . . . . . . . . . . . 37 atropine . . . . . . . . . . . . 44 atropine sulfate . . . . . . . . 31 ATROVENT . . . . . . . . . . . 56 ATROVENT HFA . . . . . . . . 55 ATROVENT NS . . . . . . . . . 24 ATUSS DR . . . . . . . . . . . 53 AUBAGIO . . . . . . . . . . . . 15 AUGMENTIN . . . . . . . . . . 34 AURALGAN . . . . . . . . . . 22 auranofin . . . . . . . . . . . 39 AVAPRO . . . . . . . . . . . . . 8 AVC VAGINAL . . . . . . . . . 43 AVEENO CLEAR PADS OTC . 18 AVITA . . . . . . . . . . . . . . 18 AVONEX/AVONEX PEN . . . 15 axitinib . . . . . . . . . . . . . 4 AYGESTIN . . . . . . . . . . . 42 azathioprine . . . . . . . . 6, 39 azelaic acid . . . . . . . . . . 17 azelastine . . . . . . . . . 23, 43 azithromycin . . . . . . . . . . 34 AZOLEN TINCTURE SOLUTION OTC . . . . . . 20 AZOPT . . . . . . . . . . . . . 44 AZULFIDINE . . . . . . . 30, 39 AZULFIDINE EN-TABS . . 30, 39 B bacitracin . . . . . . . . . 18, 45 BACITRACIN . . . . . . . 18, 65 bacitracin-polymyxinneomycin HC . . . . . . . 18 baclofen . . . . . . . . . . . . 40 Index of Covered Drugs BACLOFEN . . . . . . . . . . . 40 BACTRIM . . . . . . . . . . . . 34 BACTRIM DS . . . . . . . . . . 34 BACTROBAN . . . . . . . . . 18 balsalazide . . . . . . . . . . 29 BALZIVA . . . . . . . . . . . . 41 BANZEL . . . . . . . . . . . . 17 BANZEL SUSPENSION . . . . 17 BARACLUDE . . . . . . . . . . 36 BAYER . . . . . . . . . 7, 39, 66 BD POSIFLUSH INJ . . . . . . 33 becaplermin gel . . . . . . . . 21 beclomethasone . . . . . . . 55 bedaquiline . . . . . . . . . . 38 BENA-D-50 INJ . . . . . . . . 32 BENADRYL . . . . . . . . 23, 64 benazepril . . . . . . . . . . . .8 benazepril/hydrochlorothiazide8 BENTYL . . . . . . . . . . . . 29 BENZAC AC . . . . . . . . . . 17 BENZEFOAM . . . . . . . . . 17 BENZIQ . . . . . . . . . . . . . 18 BENZIQ LS . . . . . . . . . . . 18 benzocaine/antipyrine . . . . 22 benzonatate . . . . . . . . . . 49 BENZOTIC . . . . . . . . . . . 22 benzoyl peroxide . . . . . . . 17 benzoyl peroxide bar 10% . . 17 benzoyl peroxide creamy wash 8% & benzoyl peroxide bar 5% k . . . . . . . . . . . . 17 benzoyl peroxide crm, gel, lotion, cleanser, bar, foam, cloth, wash . . . . . . . . 64 benzoyl peroxide gel . . . . . 18 benzoyl peroxide wash 4% & benzoyl peroxide bar 5% kit . . . . . . . . . 18 benztropine . . . . . . . . . . 15 BERINERT . . . . . . . . . . . 61 BETACARE CREAM AND LOTION . . . . . . . . . . . 64 BETAGAN . . . . . . . . . . . 44 betaine powder for oral solution . . . . . . . . . . 28 betamethasone augmented dip . . . . 19, 20 betamethasone dipropionate 19 betamethasone val . . . . . . 19 BETAPACE . . . . . . . . . . . .9 BETAPACE AF . . . . . . . . . .9 BETASERON . . . . . . . . . . 15 BETA-VAL . . . . . . . . . . . . 19 betaxolol . . . . . . . . . . . . 44 bethanechol . . . . . . . . . . 56 BETHKIS . . . . . . . . . . . . 61 BETOPTIC -S . . . . . . . . . . 44 bexarotene caps . . . . . . . . 6 bexarotene topical gel . . . . . 6 BIAXIN . . . . . . . . . . . . . 34 BIAXIN XL . . . . . . . . . . . 34 bicalutamide . . . . . . . . . . 5 BICITRA . . . . . . . . . . . . 57 BILTRICIDE . . . . . . . . . . . 35 BIO-D DRO-MULSION . . . . 57 BIO-D-MULSIO DRO FORTE . 57 bisoprolol . . . . . . . . . . . . 9 bisoprolol/hydrochlorothiazide 9 BLEPH-10 . . . . . . . . . . . 45 BLEPHAMIDE . . . . . . . . . 43 boceprevir . . . . . . . . . . . 36 BONINE . . . . . . . . . . . . 66 BOOSTRIX . . . . . . . . . . . 63 bosentan . . . . . . . . . . . 12 BOSULIF . . . . . . . . . . . . .4 bosutinib . . . . . . . . . . . . 4 BPO CREAMY KIT . . . . . . 17 BRETHINE . . . . . . . . . . . 56 BREVOXYL . . . . . . . . 17, 18 BREVOXYL-4 . . . . . . . . . . 64 BREVOXYL-4 OTC . . . . . . . 18 BRILINTA . . . . . . . . . . . . .7 brimonidine . . . . . . . . 20, 45 brinzolamide . . . . . . . . . 44 BROMALINE DM . . . . . . . 50 BROMETANE DX . . . . . . . 51 BROMFED DM . . . . . . . . . 50 BROMFENEX PD CAP . . . . 49 ALL CAPS = Brand-name drug lower case = Generic drug 70 bromocriptine . . . . . . 15, 28 brompheniramine maleate . . 23 brompheniramine & phenylephrine . . . . . . . 49 brompheniramine/pseudoephedrine . . . 24, 49, 50, 65 brompheniramine/ pseudoephedrine/ dextromethorphan . . . . 50 brompheniramine/ pseudoephedrine/dextromethorphan/guaifenesin 50 brompheniramine tannate . . 23 BROVEX CT . . . . . . . . . . 23 BROVEX PSB . . . . . . . . . 49 budesonide . . . . . . . . 29, 55 budesonide/formoterol . . . . 55 BUFFERIN . . . . . . . . . . . 39 bumetanide . . . . . . . . . . 10 BUMEX . . . . . . . . . . . . . 10 BUPHENYL . . . . . . . . . . 27 BUPHENYL ORAL POWDER 27 buprenorphine/naloxone . . . 48 bupropion . . . . . . . . . 47, 49 bupropion extended-release . . . 47, 48 Burow’s soln, wet dressings . 65 BUSPAR . . . . . . . . . . . . 46 buspirone . . . . . . . . . . . 46 busulfan . . . . . . . . . . . . . 4 butalbital/acetaminophen . . 13 butalbital/acetaminophen/ caffeine . . . . . . . . . . 13 butalbital/apap/caff/cod . . . 14 butalbital/asa/caff/cod . . . . 14 butalbital/aspirin/caffeine . . 13 butorphanol . . . . . . . . . . 14 BYETTA . . . . . . . . . . . . . 27 C C1 Inhibitor, Human . . . . . 61 cabergoline . . . . . . . . . . 28 cabozantinib . . . . . . . . . . 4 CAFERGOT . . . . . . . . . . 15 Index of Covered Drugs calamine . . . . . . . . . . . . 21 CALAN . . . . . . . . . . 10, 15 CALAN SR . . . . . . . . . . . 10 calcipotriene . . . . . . . . . 20 calcitonin-salmon . . . . . . . 27 calcitriol . . . . . . . . . . 20, 57 calcium . . . . . . . . . . 57, 67 calcium acetate . . . . . . . . 61 CALICYLIC . . . . . . . . . . . 22 CALTRATE . . . . . . . . . . . 67 CAMPRAL . . . . . . . . . . . 45 canagliflozin . . . . . . . . . . 26 canagliflozin/metformin . . . 26 CANASA . . . . . . . . . . . . 30 capecitabine . . . . . . . . . . 4 CAPEX SHAMOO . . . . . . . 19 CAPOTEN . . . . . . . . . . . . 8 CAPOZIDE . . . . . . . . . . . .8 CAPRELSA . . . . . . . . . . . .5 capsaicin . . . . . . . . . . . 39 captopril . . . . . . . . . . . . .8 captopril/hydrochlorothiazide . 8 CAPTOPRIL POWDER . . . . . 8 CARAC . . . . . . . . . . . . . 21 CARAFATE . . . . . . . . . . . 29 CARAFATE SUSPENSION . . 29 CARBAGLU . . . . . . . . . . 27 carbamazepine . . . . . . . . 16 carbamazepine extended-release . . . . . 16 carbamazepine SR . . . . . . 16 carbamide peroxide . . . 22, 65 CARBATROL . . . . . . . . . 16 carbetapentane/chlorpheniramine/ephedrine/ phenylephrine . . . . . . . 50 carbidopa/levodopa . . . . . 15 carbidopa/levodopa extended-release . . . . . 15 carbinoxamine/ pseudoephedrine . . . . . 50 CARBOFED . . . . . . . . . . 50 CARDEC-DM . . . . . . . . . . 51 CARDEC DM DRO . . . . . . 53 CARDEC DM SYP . . . . . . . 53 CARDEC DRO . . . . . . . . . 51 CARDEC SYP . . . . . . . 49, 51 CARDENE . . . . . . . . . . . 10 CARDIZEM . . . . . . . . . . . 10 CARDIZEM CD . . . . . . . . . 10 CARDIZEM LA . . . . . . . . . 10 CARDIZEM SR . . . . . . . . . 10 CARDURA . . . . . . . . . 8, 56 CARDURA XL . . . . . . . . . 56 carglumic acid . . . . . . . . 27 carteolol . . . . . . . . . . . . 44 carvedilol . . . . . . . . . . . . 9 casanthranol-docusate sodium . . . . . . . . . . 30 CASODEX . . . . . . . . . . . . 5 CATAFLAM . . . . . . . . . . . 39 CATAPRES . . . . . . . . . . . .8 CATAPRES-TTS . . . . . . . . . 8 CAZIANT . . . . . . . . . . . . 41 CECLOR . . . . . . . . . . . . 34 cefaclor . . . . . . . . . . . . 34 cefadroxil . . . . . . . . . . . 34 cefazolin sodium for inj . . . . 31 cefdinir . . . . . . . . . . . . . 34 cefixime . . . . . . . . . . . . 34 cefpodoxime . . . . . . . . . 34 cefprozil . . . . . . . . . . . . 34 CEFTIN . . . . . . . . . . . . . 34 CEFTRIAX/DEX INJ . . . . . . 32 CEFTRIAXONE INJ . . . . . . 32 CEFTRIAXONE/ INJ DEX . . . 32 ceftriaxone sodium for inj . . 32 ceftriaxone sodium for iv soln 1 gm and dextrose 3.74% . 32 ceftriaxone sodium in dextrose inj . . . . . . . . 32 cefuroxime axetil . . . . . . . 34 CEFZIL . . . . . . . . . . . . . 34 CELEBREX . . . . . . . . . . . 39 celecoxib . . . . . . . . . . . 39 CELEXA . . . . . . . . . . . . 47 CELLCEPT . . . . . . . . . . . .6 CELONTIN . . . . . . . . . . . 16 ALL CAPS = Brand-name drug lower case = Generic drug 71 CENESTIN . . . . . . . . . . . 42 CENOGEN OB/ULTRA . . . . 60 CENTRUM . . . . . . . . . . . 58 cephalexin . . . . . . . . . . . 34 CEREDASE . . . . . . . . . . . 27 CEREZYME . . . . . . . . . . 27 ceritinib . . . . . . . . . . . . . 4 CERVARIX . . . . . . . . . . . 62 CESIA . . . . . . . . . . . . . . 41 CETAPHIL CREAM AND LOTION . . . . . . . . . . . 64 cetirizine . . . . . . . . . . . . 23 cetirizine chew tab . . . . . . 23 cetirizine hydrochloride/pseudoephedrine hydrochloride 12 hours extended-release . . 24 cetirizine/pseudoephedrine OTC . . . . . . . . . . . . 65 CHANTIX . . . . . . . . . . . . 49 CHERATUSSIN AC . . . . . . 51 CHILD IBUPRO SUS COLD . 55 chloral hydrate . . . . . . . . 48 CHLORAL HYDRATE . . . . . 48 chlorambucil . . . . . . . . . . 4 chlordiazepoxide . . . . . . . 46 chlorhexidine . . . . . . . . . 24 chlorhexidine gluconate . . . 24 chloroquine phosphate . . . . 38 chlorothiazide . . . . . . . . . 10 chlorothiazide . . . . . . . . . 10 CHLORPHENIRAMINE . . . . 23 chlorpheniramine/ dextromethorphan . . . . 51 chlorpheniramine extended-release . . . . . 23 chlorpheniramine maleate . . 23 chlorpheniramine maleate phenylephrine HCL . . . . 51 chlorpheniramine/ phenylephrine . . . . . . . 51 chlorpheniramine/phenylephrine/pyrilamine . . . . . . 24 chlorpheniramine/ pseudoephedrine 24, 51, 65 Index of Covered Drugs chlorpheniramine tannate . . 23 chlorpheniramine tan/ phenylephrine tan . . . . 51 chlorphen-PEmethscopolamine . . . . 50 chlorphen tan/ carbetapentane tan . . . . 50 chlorphen tan/ pseudoeph tan . . . . . . 50 chlorphen tan/pyrilamine tan/ PE tan . . . . . . . . . . . 50 chlorpromazine . . . . . . . . 49 chlorpropamide . . . . . . . . 26 chlorthalidone . . . . . . . . . 10 CHLORTHALIDONE . . . . . . 10 CHLOR-TRIMETON . . . . . . 64 CHLOR-TRIMETON ALLERGY . . . . . . . . . 23 CHLOR-TRIMETON SYRUP . 23 chlorzoxazone . . . . . . . . . 40 CHOLBAM . . . . . . . . . . . 28 cholecalciferol . . . . . . . . . 57 cholestyramine . . . . . . . . 11 cholic acid . . . . . . . . . . . 28 ciclopirox . . . . . . . . . . . 20 cidofovir . . . . . . . . . . . . 35 cilostazol . . . . . . . . . . . . 7 CILOXAN . . . . . . . . . . . . 45 cimetidine . . . . . . . . . . . 29 cinacalcet . . . . . . . . . . . 61 CIPRO . . . . . . . . . . . . . 34 CIPRODEX . . . . . . . . . . . 22 ciprofloxacin . . . . . . . 34, 45 ciprofloxacin 0.2% in d5w . . 32 ciprofloxacin/dexamethasone22 ciprofloxacin iv soln 1% . . . 32 CIPRO I.V. INJ . . . . . . . . . 32 CIPRO I.V. SOL . . . . . . . . . 32 citalopram . . . . . . . . . . . 47 clarithromycin . . . . . . . . . 34 clarithromycin ER . . . . . . . 34 CLARITIN . . . . . . . . . 23, 64 CLARITIN CHEW . . . . . . . 23 CLARITIN RDT . . . . . . . . . 23 CLEAN & CLEAR GEL . . . . 18 CLEARASIL . . . . . . . . . . 64 CLEAR EYES REDNESS RELIEF . . . . . . . . . . . 43 clemastine . . . . . . . . . . . 23 CLEMASTINE . . . . . . . . . 23 CLEOCIN . . . . . . . . . 38, 42 CLEOCIN PHOS INJ . . . . . 32 CLEOCIN T . . . . . . . . . . . 18 CLIMARA . . . . . . . . . . . . 42 clindamycin . . . . . . 18, 38, 42 clindamycin phosphate inj . . 32 CLINORIL . . . . . . . . . 14, 40 clobazam . . . . . . . . . . . 16 clobazam suspension . . . . 16 clobetasol propionate . . . . 20 clomipramine . . . . . . . . . 46 clonazepam . . . . . . . 16, 46 clonazepam ODT . . . . . . . 46 clonidine . . . . . . . . . . . . 8 clonidine transdermal . . . . . 8 clopidogrel . . . . . . . . . . . 7 clorazepate . . . . . . . . . . 46 clotrimazole . . . .20, 35, 42, 64 CLOTRIMAZOLE . . . . . . . . 20 clotrimazole crystals . . . . . 20 clotrimazole with betamethasone . . . . . . 20 clozapine . . . . . . . . . . . 48 CLOZARIL . . . . . . . . . . . 48 COARTEM . . . . . . . . . . . 37 cobicistat . . . . . . . . . . . 38 cobicistat/elvitegravir/ emtricitabine/tenofovir . . 37 cobimetinib . . . . . . . . . . . 4 CODAL-DM . . . . . . . . . . . 54 codeine/acetaminophen . . . 14 codeine/chlorpheniramine/ pseudoephedrine . . . . . 51 codeine/guaifenesin . . . . . 51 codeine/guaifenesin/ pseudoephedrine . . . . . 51 codeine/promethazine . . . . 51 ALL CAPS = Brand-name drug lower case = Generic drug 72 codeine/promethazine/ phenylephrine . . . . . . . 51 codeine sulfate . . . . . . . . 14 CODIMAL DH . . . . . . . . . 54 COGENTIN . . . . . . . . . . . 15 COLACE . . . . . . . . . 30, 66 COLAZAL . . . . . . . . . . . 29 colchicine . . . . . . . . . . . 40 COLCRYS . . . . . . . . . . . 40 COLESTID . . . . . . . . . . . 11 coliestipol . . . . . . . . . . . 11 collagenase oint . . . . . . . 21 COLLOIDAL OATMEAL BATHS . . . . . . . . . . . 65 COLOCORT . . . . . . . . . . 29 COLYTE . . . . . . . . . . . . 30 COMBIVENT RESPIMAT . . . 55 COMBIVIR . . . . . . . . . . . 37 COMETRIQ . . . . . . . . . . . 4 COMMIT LOZENGES . . . . . 67 COMMITT . . . . . . . . . . . 49 COMPAZINE . . . . . . . . . . 28 COMPLERA . . . . . . . . . . 37 COMPLETE NATALCARE PAK DHA . . . . . . . . . . 59 COMPOUND W STRIPS . . . 67 COMTAN . . . . . . . . . . . . 16 CONCERTA . . . . . . . . . . 46 condoms . . . . . . . . . . . 65 condoms - female . . . . . . . 65 CONDOMS OTC . . . . . . . . 65 CONDYLOX SOL . . . . . . . 21 conjugated estrogen/ bazedoxifene . . . . . . . 43 contraceptive foam . . . . . . 65 contraceptive gel . . . . . . . 65 COPAXONE . . . . . . . . . . 15 CORDARONE . . . . . . . . . .9 CORDRAN . . . . . . . . . . . 19 COREG . . . . . . . . . . . . . .9 CORGARD . . . . . . . . . . . .9 CORICIDIN TAB CGH/CLD . 51 Index of Covered Drugs CORN/CALLUS REMOVER PAD, CLEAR AWAY, ONE STEP OTC . . . . . . . . . 67 CORTAID . . . . . . . . . . . . 65 CORTAID SOLUTION OTC . . 19 CORTEF . . . . . . . . . . . . 25 CORTIFOAM AEROSOL . . . 21 cortisone acetate . . . . . . . 25 CORTISPORIN . . . . . . 18, 43 CORTISPORIN OTIC . . . . . 23 CORTIZONE . . . . . . . . . . 19 CORTIZONE-10 INTENSIVE HEALING . . . . . . . . . . 19 COSOPT . . . . . . . . . . . . 44 COSOPT PF . . . . . . . . . . 44 COTELLIC . . . . . . . . . . . . 4 COUMADIN . . . . . . . . . . . 7 COZAAR . . . . . . . . . . . . .8 CPM/PSE . . . . . . . . . . . 51 CREON . . . . . . . . . . . . . 30 CREON DR . . . . . . . . . . . 30 CRIXIVAN . . . . . . . . . . . . 37 crizotinib . . . . . . . . . . . . 4 CROLOM . . . . . . . . . . . . 43 cromolyn . . . . . . . . . 55, 56 cromolyn sodium . . . . . 24, 43 crotamiton . . . . . . . . . . . 21 CUPRIMINE . . . . . . . . . . 39 CURITY ALCOHOL PADS . . 65 CUTIVATE . . . . . . . . . . . 19 CUTIVATE LOTION . . . . . . 19 cyanocobalamin . . . . . . . 57 CYCLESSA . . . . . . . . . . . 41 cyclobenzaprine . . . . . . . 40 CYCLOGYL . . . . . . . . . . . 44 cyclopentolate . . . . . . . . . 44 cyclophosphamide . . . . . . . 4 cycloserine . . . . . . . . . . 33 cyclosporine . . . . . . . . 6, 45 cyclosporine, modified . . . . . 6 CYMBALTA . . . . . . . . . . . 47 cyproheptadine . . . . . . . . 23 CYPROHEPTADINE . . . . . . 23 CYSTADANE . . . . . . . . . . 28 CYSTAGON . . . . . . . . . . . 6 CYSTARAN . . . . . . . . . . . 45 cysteamine 0.44% ophthalmic solution . . . . 45 cysteamine bitartrate . . . . . . 6 CYSTOSPAZ . . . . . . . . . . 29 CYTOMEL . . . . . . . . . . . 27 CYTOTEC . . . . . . . . . . . 31 CYTOVENE . . . . . . . . . . . 35 CYTOXAN . . . . . . . . . . . . 4 D D3-50 CAP . . . . . . . . . . . 57 D5W/NACL INJ . . . . . . . . 33 dabigatran etexilate mesylate . 7 dabrafenib . . . . . . . . . . . 4 daclatasvir . . . . . . . . . . . 36 DAKLINZA . . . . . . . . . . . 36 DALLERGY DM . . . . . . . . 50 DALMANE . . . . . . . . . . . 48 danazol . . . . . . . . . . . . 41 DANOCRINE . . . . . . . . . . 41 DANTRIUM . . . . . . . . . . . 40 dantrolene . . . . . . . . . . . 40 dapsone . . . . . . . . . . . . 38 DAPSONE . . . . . . . . . . . 38 DARAPRIM . . . . . . . . . . . 38 darbepoetin alfa . . . . . . . . 7 darunavir . . . . . . . . . . . 37 darunavir/cobicistat . . . . . 38 dasatinib . . . . . . . . . . . . 4 DAYPRO . . . . . . . . . . 14, 39 DDAVP . . . . . . . . . . . . . 28 DEBROX . . . . . . . . . 22, 65 DECADRON . . . . . . . . . . 25 DE-CHLOR DM . . . . . . . . 53 DECLOMYCIN . . . . . . . . . 35 DECONEX . . . . . . . . . . . 54 DECONEX IR . . . . . . . . . . 54 decongestants . . . . . . . . 66 deferasirox . . . . . . . . . . . 61 deferiprone . . . . . . . . . . 61 DELATESTRYL . . . . . . . . . 25 delavirdine . . . . . . . . . . . 36 ALL CAPS = Brand-name drug lower case = Generic drug 73 DELFEN . . . . . . . . . . . . 65 DELOS . . . . . . . . . . . 17, 64 DELSYM . . . . . . . . . . 52, 65 DELTASONE . . . . . . . . . . 25 DELZICOL . . . . . . . . . . . 30 DEMADEX . . . . . . . . . . . 11 demeclocycline . . . . . . . . 35 DEMEROL . . . . . . . . . . . 14 DENAVIR . . . . . . . . . . . . 36 DEPAKENE . . . . . . . . . . . 17 DEPAKOTE . . . . . . 15, 16, 47 DEPAKOTE SPRINKLE . . . . 15, 16, 47 DEPEN TITRATABLE . . . . . 39 DEPO-PROVERA . . . . . . . 40 DEPO-TESTOSTERONE . . . 25 DERMAPHOR OINTMENT . . 64 DERMA-SMOOTHE OIL/FS . 19 dermatological baths . . . . . 65 DERMATOP . . . . . . . . . . 19 DESENEX . . . . . . . . . . . 20 desipramine . . . . . . . . . . 47 desmopressin . . . . . . . . . 28 desogestrel/EE . . . . . . 40, 41 DESYREL . . . . . . . . . . . . 48 DETROL . . . . . . . . . . . . 57 DETROL LA . . . . . . . . . . 57 dexamethasone . . . . . 25, 44 dexamethasone sodium phosphate . . . . . . . . . 44 DEXASOL . . . . . . . . . . . 44 dexchlorpheniramine maleate23 DEXEDRINE . . . . . . . . . . 46 DEXEDRINE SPANSULE . . . 46 dextroamphetamine . . . . . 46 dextroamphetamine extended-release . . . . . 46 dextromethorphan/ brompheniramine/ pseudoephedrine . . . . . 51 dextromethorphan/ carbinoxamine/ pseudoephedrine . . . . . 51 Index of Covered Drugs dextromethorphanguaifenesin . . . . . . 51, 52 dextromethorphan/ guaifenesin . . . . . . . . 52 dextromethorphan HBR . . . 52 dextromethorphan polistirex extended-release . . . . . 52 dextromethorphan/ promethazine . . . . . . . 52 dextromethorphan/quinidine 49 dextrose 5% w/ sodium chloride 0.45% . . . . . . 33 dextrose inj . . . . . . . . . . 33 dextrose inj . . . . . . . . . . 33 DEXTROSE INJ . . . . . . . . 33 DEXTROSTAT . . . . . . . . . 46 D.H.E. 45 . . . . . . . . . . . . 15 DHS SAL SHAMPOO OTC . . 22 DIABINESE . . . . . . . . . . . 26 DIAMOX SEQUELS . . . . . . 44 DIAQUA-2 INJ . . . . . . . . . 33 DIASTAT ACUDIAL . . . . . . . 16 DIASTAT PEDIATRIC . . . . . 16 diazepam . . . . .16, 33, 40, 46 DIAZEPAM INTENSOL . . . . 46 diclofenac 1% gel . . . . . . . 39 diclofenac potassium . . . . . 39 diclofenac sodium . . . . . . 43 diclofenac sodium delayed-release . . . . 13, 39 diclofenac sodium extended-release . . . . . 39 diclofenac sodium SR . . . . 13 dicloxacillin . . . . . . . . . . 34 DICLOXACILLIN . . . . . . . . 34 dicyclomine . . . . . . . . . . 29 didanosine . . . . . . . . . . 37 didanosine delayed-release . 37 DIDRONEL . . . . . . . . . . . 27 diflorasone diacetate . . . . . 19 DIFLUCAN . . . . . . . . 35, 42 diflunisal . . . . . . . . . . . . 39 digoxin . . . . . . . . . . . . . 9 DIHISTINE DH . . . . . . . . . 51 dihydroergotamine . . . . . . 15 DIHYDRO-PE SYP . . . . . . . 53 DILACOR XR . . . . . . . . . 10 DILANTIN . . . . . . . . . . . . 17 DILANTIN INFATABS . . . . . 17 DILATRATE-SR . . . . . . . . . 11 DILAUDID . . . . . . . . . . . . 14 diltiazem . . . . . . . . . . . . 10 diltiazem extended-release . . 10 diltiazem sustained-release . 10 DIMEATAPP DRO DECONGES . . . . . . . . 24 dimenhydrinate . . . . . . . . 66 DIMETAPP . . . . . . . . . . . 65 DIMETAPP CLD ELX/ ALLERGY . . . . . . . . . 49 DIMETAPP DM . . . . . . . . . 53 DIMETAPP DRO DCON/CGH53 DIMETAPP DRO DECONGES 65 DIMETAPP ELX CLD/ALLE . . 49 DIMETAPP SYP CGH/CLD . . 51 dimethyl fumarate . . . . . . . 15 DIOVAN . . . . . . . . . . . . . .8 DIOVAN HCT . . . . . . . . . . .9 DIPENTUM . . . . . . . . . . . 30 diphenhydramine . . . . 23, 48 diphenhydramine hcl inj . . . 32 diphenoxylate/atropine . . . . 28 diphtheria-tetanus tox adsorbed (dt) im . . . . . 62 DIPROLENE . . . . . . . 19, 20 DIPROLENE AF . . . . . . . . 19 DIPROSONE . . . . . . . . . . 19 DIP/TET PED INJ . . . . . . . 62 dipyridamole . . . . . . . . . . 7 disopyramide . . . . . . . . . . 9 disopyramide extended-release9 disulfiram . . . . . . . . . . . 45 DITROPAN . . . . . . . . . . . 57 DITROPAN XL . . . . . . . . . 57 DIURIL . . . . . . . . . . . . . 10 DIURIL ORAL SUSPENSION . 10 divalproex sodium cap sprinkle . . . 15, 16, 47 ALL CAPS = Brand-name drug lower case = Generic drug 74 divalproex sodium delayed-release . . 15, 16, 47 DIVIGEL . . . . . . . . . . . . . 42 docosanol . . . . . . . . . . . 36 docusate calcium plus . . . . 30 docusate potasssium . . . . . 30 docusate sodium . . . . . . . 30 dofetilide . . . . . . . . . . . . 9 dolasetron . . . . . . . . . . . 28 DOLOBID . . . . . . . . . . . . 39 DOLOPHINE . . . . . . . . . . 14 dolutegravir . . . . . . . . . . 38 DOMEBORO . . . . . . . . . . 65 DOMEBORO OTIC . . . . . . 22 donepezil . . . . . . . . . . . 13 dornase alfa . . . . . . . . . . 61 dorzolamide . . . . . . . . . . 44 dorzolamide HCL-timolol maleate . . . . . . . . . . 44 dorzolamide/timolol maleate 44 DOSTINEX . . . . . . . . . . . 28 DOVONEX . . . . . . . . . . . 20 doxazosin . . . . . . . . . . 8, 56 doxazosin SR . . . . . . . . . 56 doxepin . . . . . . . . . . . . 47 doxycycline delayed-release . 35 doxycycline hyclate . . . . . . 35 doxycycline monohydrate . . 35 DOXYCYCLINE MONOHYDRATE . . . . . 35 DRAMAMINE . . . . . . . . . 66 DRISDOL . . . . . . . . . . . . 57 dronabinol . . . . . . . . . . . 28 dronedarone . . . . . . . . . . 9 DROXIA . . . . . . . . . . . . . .6 DRYSOL OTC . . . . . . . . . 21 DUAVEE . . . . . . . . . . . . 43 DUETACT . . . . . . . . . . . . 26 DULCOLAX . . . . . . . . . . 66 DULERA . . . . . . . . . . . . 55 duloxetine . . . . . . . . . . . 47 DUOFILM . . . . . . . . . 21, 67 DUONEB . . . . . . . . . . . . 56 DUOPLANT GEL, COMPOUND W, SAL-PLANT OTC . . . . 67 Index of Covered Drugs DURADRYL . . . . . . . . . . 50 DURAGESIC . . . . . . . . . . 14 DURAMORPH INJ . . . . . . . 31 DURATUSS DM ELX . . . . . 51 DURICEF . . . . . . . . . . . . 34 DYAZIDE . . . . . . . . . . . . 11 DYNACIRC . . . . . . . . . . . 10 E ecothiophate . . . . . . . . . 44 ECOTRIN . . . . . . . . 7, 39, 66 ED A-HIST TABLETS AND LIQUID . . . . . . . . . . . 51 EDGE OB CHW . . . . . . . . 59 edoxaban . . . . . . . . . . . . 7 EDURANT . . . . . . . . . . . 36 E.E.S. . . . . . . . . . . . . . . 34 efavirenz . . . . . . . . . . . . 36 efavirenz/emtricitabine/ tenofovir . . . . . . . . . . 37 EFFEXOR . . . . . . . . . . . . 47 EFFEXOR XR . . . . . . . . . . 47 EFUDEX . . . . . . . . . . . . 21 ELAPRASE . . . . . . . . . . . 27 ELAVIL . . . . . . . . . . . 15, 47 elbasvir/grazoprevir . . . . . . 36 ELDEPRYL . . . . . . . . . . . 16 electrolyte . . . . . . . . . 57, 66 ELELYSO . . . . . . . . . . . . 28 ELESTRIN . . . . . . . . . . . 42 ELIDEL . . . . . . . . . . . . . 22 ELIMITE . . . . . . . . . . . . . 21 ELIQUIS . . . . . . . . . . . . . .7 ELMIRON . . . . . . . . . . . . 57 ELOCON . . . . . . . . . . . . 19 eltrombopag . . . . . . . . . . 62 elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate . . . . . . 37 EMCYT . . . . . . . . . . . . . .4 EMEND . . . . . . . . . . . . . 28 EMETROL . . . . . . . . . . . 66 EMLA . . . . . . . . . . . . . . 22 emollients . . . . . . . . . . . 64 empagliflozin . . . . . . . . . 26 empagliflozin/metformin . . . 26 emtricitabine . . . . . . . . . 37 emtricitabine/rilpivirine/ tenofovir . . . . . . . . . . 37 emtricitabine/tenofovir . . . . 37 EMTRIVA . . . . . . . . . . . . 37 enalapril . . . . . . . . . . . . . 8 enalapril/hydrochlorothiazide . 8 ENBREL . . . . . . . . . . . . 39 enfuvirtide . . . . . . . . . . . 35 ENGERIX-B . . . . . . . . . . . 62 ENJUVIA . . . . . . . . . . . . 42 enoxaparin . . . . . . . . . . . 7 entacapone . . . . . . . . . . 16 entecavir . . . . . . . . . . . . 36 ENTERIC COATEDNAPROSYN . . . . . . 14, 39 ENTEX LQ LIQ . . . . . . . . . 54 ENTOCORT EC . . . . . . . . 29 ENTRESTO . . . . . . . . . . . .9 ENULOSE . . . . . . . . . . . 30 E-OINTMENT . . . . . . . . . 64 EPANED . . . . . . . . . . . . . 8 epinephrine . . . . . . . . . . 61 EPINEPHRINE AUTO-INJECT BY IMPAX LABS . . . . . . . . 61 EPIPEN . . . . . . . . . . . . . 61 EPIPEN JR. . . . . . . . . . . . 61 EPITOL . . . . . . . . . . . . . 16 EPIVIR . . . . . . . . . . . . . . 37 EPIVIR HBV . . . . . . . . . . . 36 eplerenone . . . . . . . . . . 12 epoetin alfa . . . . . . . . . . . 7 EPOGEN . . . . . . . . . . . . .7 epoprostenol . . . . . . . . . 12 EPZICOM . . . . . . . . . . . . 37 EQUATRO . . . . . . . . . . . 16 ergocalciferol . . . . . . . . . 57 ergotamine/caffeine . . . . . 15 ergotamine tartrate/caffeine . 15 ERIVEDGE . . . . . . . . . . . .6 erlotinib . . . . . . . . . . . . . 4 ERYC . . . . . . . . . . . . . . 34 ALL CAPS = Brand-name drug lower case = Generic drug 75 ERYGEL . . . . . . . . . . . . 18 ERY-TAB . . . . . . . . . . . . 34 ERYTHROCIN . . . . . . . . . 34 erythromycin . . . . . . . 18, 45 ERYTHROMYCIN . . . . . . . 45 erythromycin delayed-release 34 erythromycin ethylsuccinate . 34 erythromycin stearate . . . . . 34 erythromycin/sulfisoxazole . . 34 ESBRIET . . . . . . . . . . . . 62 escitalopram . . . . . . . . . 47 ESGIC . . . . . . . . . . . . . 13 ESKALITH CR . . . . . . . . . 47 ESKIMO-3 . . . . . . . . . . . 58 esomeprazole . . . . . . . . . 29 estazolam . . . . . . . . . . . 48 esterified estrogens . . . . . . 42 ESTRACE . . . . . . . . . 41, 42 estradiol . . . . . . . . . . 41, 42 estradiol acetate . . . . . . . 42 ESTRADIOL TDS . . . . . . . 42 estramustine phosphate sodium . . . . . 4 ESTRASORB . . . . . . . . . . 42 ESTRING . . . . . . . . . . . . 41 ESTROGEL . . . . . . . . . . . 42 estrogen patch . . . . . . . . 42 estrogens, conjugated . . . . 42 estrogens, conjugated/ medroxyprogesterone . . 42 estrogens, conjugated, synthetic A . . . . . . . . . 42 estrogens, conjugated, synthetic B . . . . . . . . 42 estropipate . . . . . . . . . . 42 ESTROSTEP FE . . . . . . . . 41 etanercept . . . . . . . . . . . 39 ethambutol . . . . . . . . . . 33 ethionamide . . . . . . . . . . 33 ethosuximide . . . . . . . . . 16 ethynodiol diacetate/EE . . . 41 etidronate . . . . . . . . . . . 27 etodolac . . . . . . . . . . 13, 39 etonogestrel/EE . . . . . . . . 40 Index of Covered Drugs etoposide . . . . . . . . . . . . 6 etravirine . . . . . . . . . . . . 38 EULEXIN . . . . . . . . . . . . .5 EURAX . . . . . . . . . . . . . 21 EVAMIST . . . . . . . . . . . . 42 everolimus . . . . . . . . . . 4, 6 EVISTA . . . . . . . . . . . . . 27 EXCEDRIN MIGRAINE . . . . 13 EXELON . . . . . . . . . . . . 13 exemestane . . . . . . . . . . . 5 exenatide . . . . . . . . . . . 27 EXJADE . . . . . . . . . . . . . 61 exogabine . . . . . . . . . . . 16 EXTAVIA . . . . . . . . . . . . 15 EXTINA . . . . . . . . . . . . . 20 EXUVIANCE . . . . . . . . . . 18 ezetimibe . . . . . . . . . . . 11 F FABRAZYME . . . . . . . . . . 27 famciclovir . . . . . . . . . . . 36 famotidine . . . . . . . . 29, 66 FAMVIR . . . . . . . . . . . . . 36 FARESTON . . . . . . . . . . . .5 FARYDAK . . . . . . . . . . . 4, 6 FAZACLO . . . . . . . . . . . . 48 febuxostat . . . . . . . . . . . 40 felbamate . . . . . . . . . . . 16 FELBATOL . . . . . . . . . . . 16 FELDENE . . . . . . . . . 14, 39 felodipine extended-release . 10 FEMARA . . . . . . . . . . . . .5 FEMRING . . . . . . . . . . . . 42 fenofibrate . . . . . . . . . . . 11 fenoprofen . . . . . . . . 13, 39 FENTANYL CIT INJ . . . . . . 31 fentanyl citrate inj . . . . . . . 31 fentanyl transdermal . . . . . 14 FEOSOL . . . . . . . . . . 58, 67 FERGON . . . . . . . . . . . . 67 FERRIPROX . . . . . . . . . . 61 ferrous bisglycinate/ polysaccarides iron . . . . 58 ferrous sulfate . . . . . . . . . 58 fexofenadine . . . . . . . . . . 23 fexofenadine/ pseudoephedrine . . . . . 24 filgrastim . . . . . . . . . . . . 7 FINACEA . . . . . . . . . . . . 17 finasteride . . . . . . . . . . . 56 fingolimod . . . . . . . . . . . 15 FIORICET . . . . . . . . . . . . 13 FIORICET W/CODEINE . . . . 14 FIORINAL . . . . . . . . . . . . 13 FIORINAL W/CODEINE . . . . 14 FIRAZYR . . . . . . . . . . . . 61 FIRST-LANSOPRAZOLE . . . 29 FLAGYL . . . . . . . . . . 38, 42 flecainide . . . . . . . . . . . . 9 FLEET ENEMA . . . . . . . . . 66 FLEET PHOSPHO-SODA . . . 66 FLEXERIL . . . . . . . . . . . . 40 FLOLAN . . . . . . . . . . . . 12 FLOMAX . . . . . . . . . . . . 56 FLONASE . . . . . . . . . . . . 24 FLORANEX . . . . . . . . . . . 31 FLORINEF . . . . . . . . . . . 25 FLOVENT HFA . . . . . . . . . 55 FLOXIN . . . . . . . . . . . . . 34 FLOXIN OTIC . . . . . . . . . . 23 FLUARIX QUAD . . . . . . . . 62 FLUBLOK . . . . . . . . . . . . 62 FLUCELVAX . . . . . . . . . . 63 fluconazole . . . . . . . . 35, 42 flucytosine . . . . . . . . . . . 35 fludrocortisone . . . . . . . . 25 FLULAVAL QUAD . . . . . . . 62 FLUMADINE . . . . . . . . . . 36 fluocinolone acetonide . . . . 19 fluocinonide . . . . . . . . . . 19 fluoride . . . . . . . . . . . . . 58 fluoride dental rinse . . . . . 67 fluorometholone . . . . . . . 44 FLUOROPLEX . . . . . . . . . 21 fluorouracil . . . . . . . . . . 21 fluoxetine . . . . . . . . . . . 47 fluoxymesterone . . . . . . . 25 fluphenazine . . . . . . . . . 49 fluphenazine decanoate . . . 49 ALL CAPS = Brand-name drug lower case = Generic drug 76 flurandrenolide . . . . . . . . 19 flurazepam . . . . . . . . . . 48 flurbiprofen . . . . . . . . 13, 43 flutamide . . . . . . . . . . . . 5 fluticasone . . . . . . . . . . . 24 fluticasone HFA . . . . . . . . 55 fluticasone propionate . . . . 19 fluticasone/salmeterol . . . . 55 FLUVIRIN . . . . . . . . . . . . 63 fluvoxamine . . . . . . . . . . 46 fluvoxamine SR . . . . . . . . 46 FLUZONE HD PF . . . . . . . 62 FLUZONE QUAD . . . . . . . 62 FLUZONE SPLT . . . . . . . . 62 FML . . . . . . . . . . . . . . 44 FML FORTE . . . . . . . . . . 44 FML LIQUIFILM . . . . . . . . 44 folic acid . . . . . . . . . . . . 58 FOLIC ACID . . . . . . . . . . 58 FOLTABS PAK PLUS DHA RE OB + DHA PAK . . . . . . 59 FOLTABS PRENATAL . . . . . 59 FORADIL AEROLIZER . . . . 55 FORFIVO XL . . . . . . . . . . 47 formoterol . . . . . . . . . . . 55 FORTEO . . . . . . . . . . . . 27 FORTICAL . . . . . . . . . . . 27 FOSAMAX . . . . . . . . . . . 27 fosamprenavir . . . . . . . . . 37 foscarnet sodium . . . . . . . 35 FOSCAVIR . . . . . . . . . . . 35 fosinopril . . . . . . . . . . . . 8 fosinopril/hydrochlorothiazide 8 FOSRENOL . . . . . . . . . . 61 FUNGOID TINCTURE KIT . . 20 FURADANTIN SUSP . . . . . 38 furosemide . . . . . . . . . . 10 furosemide inj . . . . . . . . . 33 FUZEON . . . . . . . . . . . . 35 G gabapentin . . . . . . . . . . 16 GABITRIL . . . . . . . . . . . . 17 galantamine . . . . . . . . . . 13 Index of Covered Drugs ganciclovir . . . . . . . . . . . 35 GARDASIL . . . . . . . . . . . 62 GARDASIL 9 . . . . . . . . . . 62 gatifloxin . . . . . . . . . . . . 45 GATTEX . . . . . . . . . . . . 31 gefitinib . . . . . . . . . . . . . 4 GEL-KAM . . . . . . . . . . . . 58 gemfibrozil . . . . . . . . . . . 11 GENGRAF . . . . . . . . . . . .6 GENOTROPIN . . . . . . . . . 27 GENTAK . . . . . . . . . . 18, 45 gentamicin . . . . . . . . 18, 45 gentamicin/prednisolone acetate . . . . . . . . . . . 43 GENTEX ADE . . . . . . . . . 59 GENVOYA . . . . . . . . . . . 37 GEODON . . . . . . . . . . . . 49 GG/DM CR . . . . . . . . . . . 52 GG/PSE CR . . . . . . . . . . 52 GILENYA . . . . . . . . . . . . 15 GILOTRIF . . . . . . . . . . . . .4 GILPHEX TR . . . . . . . . . . 54 glatiramer acetate . . . . . . . 15 GLATOPA . . . . . . . . . . . . 15 GLEEVEC . . . . . . . . . . . . 4 GLEOSTINE . . . . . . . . . . . 4 glimepiride . . . . . . . . . . 26 glipizide . . . . . . . . . . . . 26 glipizide extended-release . . 26 glipizide/metformin . . . . . . 26 GLUCAGON . . . . . . . . . . 25 glucagon, human recombinant . . . . . . . . 25 GLUCOPHAGE . . . . . . . . 26 GLUCOPHAGE ER . . . . . . 26 glucose oral tablets . . . . . . 65 GLUCOTROL . . . . . . . . . 26 GLUCOTROL XL . . . . . . . . 26 GLUCOVANCE . . . . . . . . 26 glyburide . . . . . . . . . . . . 26 glyburide, micronized . . . . . 26 glycerin . . . . . . . . . . . . 30 GLYCERIN SUPPOSITORY . . 30 GLYCERIN TOPICAL . . . . . 64 glycerol phenylbutyrate . . . . 27 glycopyrrolate . . . . . . . . . 29 GLYNASE . . . . . . . . . . . . 26 GNP FISH OIL . . . . . . . . . 58 granisetron . . . . . . . . . . 28 GRANIX . . . . . . . . . . . . . .7 GRIFULVIN V . . . . . . . . . . 35 griseofulvin microsize . . . . . 35 griseofulvin ultramicrosize . . 35 GRIS-PEG . . . . . . . . . . . 35 GUAIFED . . . . . . . . . . . . 52 guaifenesin . . . . . . . . . . 52 guaifenesin extended-release 52 guaifenesin/ pseudoephedrine . . . . . 52 guaifenesin/ pseudoephedrine/ dextromethorphan . . . . 52 guaifenesin/pseudoephedrine extended-release . . . . . 52 GUAIFEN PSE . . . . . . . . . 52 guanabenz . . . . . . . . . . 12 guanfacine . . . . . . . . . . . 8 guanfacine ER . . . . . . . . 46 GUIATUSS/AC . . . . . . . . . 51 GUIATUSS DAC . . . . . . . . 51 GYNE-LOTRIMIN . . . . . 42, 64 GYNOL II . . . . . . . . . . . . 65 H HALCION . . . . . . . . . . . . 48 HALDOL . . . . . . . . . . . . 49 HALDOL DECANOATE . . . . 49 HALFPRIN . . . . . . . . . . . 39 halobetasol . . . . . . . . . . 20 haloperidol . . . . . . . . . . 49 haloperidol decanoate . . . . 49 HARVONI . . . . . . . . . . . . 36 HAVRIX . . . . . . . . . . . . . 62 HECORIA . . . . . . . . . . . . 6 HEMANGEOL . . . . . . . . . .9 heparin . . . . . . . . . . . . . 7 HEPARIN . . . . . . . . . . . . .7 ALL CAPS = Brand-name drug lower case = Generic drug 77 hepatitis a vaccine susp . . . 62 hepatitis b vaccine . . . . . . 62 HEPSERA . . . . . . . . . . . 36 hexachlorophene . . . . . . . 21 HEXALEN . . . . . . . . . . . . 4 HIPREX . . . . . . . . . . . . . 57 HISTACOL DM . . . . . . . . . 50 HISTEX . . . . . . . . . . . . . 51 HISTUSSIN HC . . . . . . . . 52 HM FISH OIL . . . . . . . . . . 58 homatropine . . . . . . . . . . 44 HUMALOG . . . . . . . . . . . 26 HUMALOG KWIKPEN . . . . 26 HUMALOG MIX 50/50 . . . . 25 HUMALOG MIX 50/50 KWIKPEN . . . . . . . . . 26 HUMALOG MIX 72/25 KWIKPEN . . . . . . . . . 26 HUMALOG MIX 75/25 . . . . 25 human papillomavirus (hpv) 9-valent recomb vac . . . 62 human papillomavirus (hpv) bival (type 16, 18) recmb vac . . . . . . . . . . . . . 62 human papillomavirus (hpv) quadrivalent recombinant vac . . . . . . . . . . . . . 62 HUMATIN . . . . . . . . . . . . 38 HUMIRA . . . . . . . . . . . . 38 HUMULIN . . . . . . . . . . . 65 HUMULIN 70/30 . . . . . . . 26 HUMULIN N . . . . . . . . . . 25 HUMULIN R . . . . . . . . . . 26 HUMULIN R 500 U/ML KWIKPEN . . . . . . . . . 26 HYCAMTIN . . . . . . . . . . . .6 HYCODAN . . . . . . . . . . . 53 hydralazine . . . . . . . . . . 12 HYDREA . . . . . . . . . . . . . 6 hydrochlorothiazide . . . . . . 10 HYDROCHLOROTHIAZIDE . 10 HYDROCODO/GG SYP . . . 52 hydrocodone/ acetaminophen . . . . . . 14 Index of Covered Drugs hydrocodone/chlorpheniramine/phenylephrine . . . 52 hydrocodone ER . . . . . . . 14 hydrocodone/guaifenesin . . 52 hydrocodone/homatropine . 53 HYDROCODONE/ TAB HOMATROP . . . . . 53 hydrocortisone . .19, 21, 25, 29 hydrocortisone acetate . . . . 21 hydrocortisone acetate w/pramoxine . . . . . . . 21 hydrocortisone/aloe . . . . . 19 hydrocortisone butyrate . . . 19 hydrocortisone crm, oint . . . 65 hydrocortisone inj . . . . . . . 25 hydrocortisone valerate . . . 19 HYDROMET SYP . . . . . . . 53 hydromorphone . . . . . . . . 14 hydroxychloroquine . . . 38, 39 hydroxyurea . . . . . . . . . . 6 hydroxyzine HCL . . . . . . . 23 hydroxyzine pamoate . . . . . 23 hyoscyamine, methenamine, phenyl salicylate, sodium phosphate monobasic, methylene blue . . . . . . 56 hyoscyamine sulfate . . . . . 29 hyoscyamine sulfate extended-release . . . . . 29 HYPERCARE 20% . . . . . . . 21 HYPERTET S/D . . . . . . . . 63 HYPOTEARS . . . . . . . . . . 66 HYTONE . . . . . . . . . . . . 19 HYTRIN . . . . . . . . . . . 8, 56 HYZAAR . . . . . . . . . . . . . 9 I IBRANCE . . . . . . . . . . . . .5 ibrutinib . . . . . . . . . . . . . 4 IBUOROFEN TAB COLD/SIN 55 ibuprofen . . . . . . . 13, 39, 66 icatibant . . . . . . . . . . . . 61 ICLUSIG . . . . . . . . . . . . . 5 idelalisib . . . . . . . . . . . . . 4 idursulfase . . . . . . . . . . . 27 iloprost . . . . . . . . . . . . . 12 imatinib mesylate . . . . . . . . 4 IMBRUVICA . . . . . . . . . . . 4 IMDUR . . . . . . . . . . . . . 12 imiglucerase . . . . . . . . . . 27 imipenem-cilastatin intravenous for soln . . . . 32 IMIPENEM/CIL INJ . . . . . . 32 imipramine HCL . . . . . . . 47 imiquimod 5% cream . . . . . 21 IMITREX . . . . . . . . . . . . 15 IMODIUM A-D . . . . . . . 28, 66 IMODIUM A-D CHW . . . . . . 28 IMODIUM A-D LIQ . . . . . . . 28 IMURAN . . . . . . . . . . . 6, 39 INCIVEK . . . . . . . . . . . . 36 INCRELEX . . . . . . . . . . . 27 indacaterol . . . . . . . . . . . 55 indapamide . . . . . . . . . . 11 INDERAL . . . . . . . . . . 9, 15 INDERAL LA . . . . . . . . . . .9 INDERIDE . . . . . . . . . . . . 9 indinavir . . . . . . . . . . . . 37 INDOCIN . . . . . . . . . 13, 39 indomethacin . . . . . . . 13, 39 INFLAMASE FORTE . . . . . . 44 influenza virus vaccine recombinant hemagglutinin (ha) . 62 influenza virus vaccine split . 62 influenza virus vaccine split high-dose pf . . . . . . . . 62 influenza virus vaccine split pf62 influenza virus vaccine split quadrivalent . . . . . . . . 62 influenza virus vaccine tiss-cult subunit . . . . . . 63 influenza virus vaccine types a&b surface antigen . . . 63 INLYTA . . . . . . . . . . . . . . 4 INSPRA . . . . . . . . . . . . . 12 insulin detemir . . . . . . . . 25 insulin glargine . . . . . . . . 25 insulin isophane . . . . . . . . 25 insulin isophane/regular . . . 26 ALL CAPS = Brand-name drug lower case = Generic drug 78 insulin lisopro pro/lispro . . . 25 insulin lisopro prot/lispro . . . 25 insulin lispro . . . . . . . . . . 26 insulin lispro prot 50%/lispro 50% . . . . . . 26 insulin lispro prot 75%/lispro 25% . . . . . . 26 insulin regular . . . . . . . . . 26 insulin . . . . . . . . . . . . . 65 INTAL . . . . . . . . . . . 55, 56 INTELENCE . . . . . . . . . . 38 interferon alfa-2b . . . . . . 5, 36 interferon beta-1a . . . . . . . 15 interferon beta-1b . . . . . . . 15 interferon gamma-1b . . . . . 38 INTRON A . . . . . . . . . . 5, 36 INTUNIV . . . . . . . . . . . . 46 INVEGA SUSTENNA . . . . . 48 INVEGA TRINZA . . . . . . . . 48 INVIRASE . . . . . . . . . . . . 37 INVOKAMET . . . . . . . . . . 26 INVOKANA . . . . . . . . . . . 26 ipratropium . . . . . . . . . . 56 ipratropium/albuterol . . 55, 56 ipratropium bromide . . . . . 24 ipratropium HFA . . . . . . . 55 irbesartan . . . . . . . . . . . . 8 IRESSA . . . . . . . . . . . . . .4 iron . . . . . . . . . . . . . . . 67 iron polysaccharides . . . . . 67 ISENTRESS . . . . . . . . . . 38 ISMO . . . . . . . . . . . . . . 11 ISOCHRON . . . . . . . . . . 11 isoniazid . . . . . . . . . . . . 33 ISONIAZID . . . . . . . . . . . 33 ISOPTO ATROPINE . . . . . . 44 ISOPTO CARPINE . . . . . . . 45 ISOPTO HOMATROPINE . . . 44 ISOPTO HYOSCINE . . . . . . 44 ISORDIL . . . . . . . . . . . . 11 ISORDIL S.L. . . . . . . . . . . 12 isosorbide dinitrate . . . . 11, 12 ISOSORBIDE DINITRATE ER . . . . . . . 11 Index of Covered Drugs isosorbide dinitrate extended-release . . . . . 11 isosorbide mononitrate . . . . 11 isosorbide mononitrate extended-release . . . . . 12 isotretinoin . . . . . . . . . . . 17 isradipine . . . . . . . . . . . 10 itraconazole . . . . . . . . . . 35 ivacaftor . . . . . . . . . . . . 61 ivermectin . . . . . . . . . 21, 35 ixazomib . . . . . . . . . . . . 5 JADENU . . . . . . . . . . . . 61 JAKAFI . . . . . . . . . . . . . . 5 JANUMET . . . . . . . . . . . 26 JANUMET XR . . . . . . . . . 27 JANUVIA . . . . . . . . . . . . 26 JARDIANCE . . . . . . . . . . 26 JENTADUETO . . . . . . . . . 26 J-TAN D PD . . . . . . . . . . . 50 J-TAN PD . . . . . . . . . . . . 23 ketoconazole shampoo . . . 20 ketoprofen . . . . . . . . 13, 39 ketoprofen SR . . . . . . . . . 13 ketorolac . . . . . . . . . . . . 43 KETOROLAC INJ . . . . . . . 31 ketorolac tromethamine . . . 13 ketorolac tromethamine im inj31 ketotifen . . . . . . . . . . . . 43 KITABIS . . . . . . . . . . . . . 61 KLARON LOTION . . . . . . . 18 KLONOPIN . . . . . . . . 16, 46 KLONOPIN WAFERS . . . . . 46 K-LOR . . . . . . . . . . . . . . 61 KLOR-CON 8 . . . . . . . . . 61 KLOR-CON 10 . . . . . . . . . 61 K-LYTE . . . . . . . . . . . . . 60 KORLYM . . . . . . . . . . . . 28 K-PHOS NEUTRAL . . . . . . 60 K-PHOS ORIGINAL . . . . . . 60 KRISTALOSE . . . . . . . . . . 30 KUVAN . . . . . . . . . . . . . 28 KYTRIL . . . . . . . . . . . . . 28 K L KALETRA . . . . . . . . . . . . 37 KALYDECO . . . . . . . . . . . 61 KAOPECTATE . . . . . . . . . 66 KAYEXALATE . . . . . . . . . 12 K-DUR 10 . . . . . . . . . . . . 61 K-DUR 20 . . . . . . . . . . . . 61 KEFLEX . . . . . . . . . . . . . 34 KENALOG . . . . . . . . . . . 19 KENALOG-40 . . . . . . . . . 25 KENALOG IN ORABASE . . . 24 KEPPRA . . . . . . . . . . . . 16 KEPPRA XR . . . . . . . . . . 16 KERALYT . . . . . . . . . . . . 18 ketoconazole . . . . . 20, 21, 35 ketoconazole foam . . . . . . 20 ketoconazole gel . . . . . . . 20 ketoconazole gel 2% & hydrocortisone gel 1% kit . . . 20 ketoconazole gel 2% & pyrithione zinc shampoo 1% kit 20 labetalol . . . . . . . . . . . . . 9 LAC-HYDRIN . . . . . . . . . . 21 lacosamide . . . . . . . . . . 16 LACTINOL . . . . . . . . . . . 21 lactobacillus . . . . . . . . . . 31 lactulose . . . . . . . . . . . . 30 LAMICTAL . . . . . . . . . . . 16 LAMICTAL CD CHEW TAB . . 16 LAMICTAL ODT . . . . . . . . 16 LAMICTAL ODT KIT . . . . . . 16 LAMICTAL STARTER KIT . . . 16 LAMICTAL XR . . . . . . . . . 16 LAMICTAL XR KIT . . . . . . . 16 LAMICTAL XR ODT KIT . . . . 16 LAMISIL . . . . . . . . . . . . . 35 LAMISIL AT . . . . . . . . . . . 20 LAMISIL GEL . . . . . . . . . . 20 LAMISIL SOLUTION . . . . . . 20 lamivudine . . . . . . . . 36, 37 lamivudine/zidovudine . . . . 37 J ALL CAPS = Brand-name drug lower case = Generic drug 79 lamotrigine . . . . . . . . . . 16 lamotrigine chew dispersable tab . . . . . . 16 lamotrigine dispersible tab . . 16 lamotrigine ODT . . . . . . . 16 lamotrigine SR . . . . . . . . 16 lamotrigine SR ODT . . . . . 16 lamotrigine starter kit . . . . . 16 lamotrigine titration kit . . . . 16 LANOXIN . . . . . . . . . . . . .9 lansoprazole . . . . . . . . . . 29 lansoprazole delayed-release 29 lansoprazole susp . . . . . . 29 lanthanum carbonate . . . . . 61 LANTUS . . . . . . . . . . . . 25 LANTUS SOLOSTAR . . . . . 25 lapatinib ditosylate . . . . . . . 4 LARIAM . . . . . . . . . . . . . 38 laronidase . . . . . . . . . . . 28 LASIX . . . . . . . . . . . . . . 10 latanoprost . . . . . . . . . . 44 laxative enemas . . . . . . . . 66 laxatives . . . . . . . . . . . . 66 ledipasvir/sofosbuvir . . . . . 36 leflunomide . . . . . . . . . . 39 lenalidomide . . . . . . . . . . 5 lenvatinib . . . . . . . . . . . . 4 LENVIMA . . . . . . . . . . . . .4 LETAIRIS . . . . . . . . . . . . 12 letrozole . . . . . . . . . . . . . 5 LEUKERAN . . . . . . . . . . . .4 LEUKINE . . . . . . . . . . . . .7 leuprolide . . . . . . . . . . . . 5 levalbuterol HCl . . . . . . . . 56 LEVAQUIN . . . . . . . . 32, 34 LEVBID . . . . . . . . . . . . . 29 LEVEMIR . . . . . . . . . . . . 25 LEVEMIR FLEXPEN . . . . . . 25 levetiracetam . . . . . . . . . 16 levetiracetam SR . . . . . . . 16 levobunolol . . . . . . . . . . 44 levocetirizine . . . . . . . . . 23 levofloxacin . . . . . . . . . . 34 levofloxacin in d5w iv soln . . 32 Index of Covered Drugs levofloxacin iv soln . . . . . . 32 levonorgestrel . . . . . . . . . 40 levonorgestrel/EE . . . . 40, 41 levothyroxine . . . . . . . . . 27 LEVOXYL . . . . . . . . . . . . 27 LEVSIN . . . . . . . . . . . . . 29 LEVSINEX . . . . . . . . . . . 29 LEXAPRO . . . . . . . . . . . 47 LEXIVA . . . . . . . . . . . . . 37 LIALDA . . . . . . . . . . . . . 30 LIBRIUM . . . . . . . . . . . . 46 LIDAMANTEL . . . . . . . . . 22 LIDEX . . . . . . . . . . . . . . 19 LIDEX E . . . . . . . . . . . . . 19 lidocaine . . . . . . . . . . . . 22 lidocaine patch . . . . . . . . 22 lidocaine-prilocaine . . . . . . 22 lidocaine/prilocaine . . . . . 22 lidocaine viscous . . . . . . . 24 LIDODERM PATCH . . . . . . 22 linaclotide . . . . . . . . . . . 31 linagliptin . . . . . . . . . . . 26 linagliptin/metformin . . . . . 26 linezolid . . . . . . . . . . . . 38 LINZESS . . . . . . . . . . . . 31 liothyronine . . . . . . . . . . 27 liotrix . . . . . . . . . . . . . . 27 LIPITOR . . . . . . . . . . . . . 11 LIPOFEN . . . . . . . . . . . . 11 LIQUIBID PD . . . . . . . . . . 54 liraglutide . . . . . . . . . . . 27 lisdexamfetamine . . . . . . . 46 lisinopril . . . . . . . . . . . . . 8 lisinopril/hydrochlorothiazide . 8 lithium carbonate . . . . . . . 47 LITHIUM CARBONATE . . . . 47 lithium carbonate extended-release . . . . . 47 LITHOBID . . . . . . . . . . . . 47 LMX-4 . . . . . . . . . . . . . . 22 LOCOID . . . . . . . . . . . . . 19 LODINE . . . . . . . . . . 13, 39 LODRANE D . . . . . . . . . . 24 LOESTRIN 1.5/30 . . . . . . . 41 LOESTRIN 1/20 . . . . . . . . 40 LOESTRIN FE 1.5/30 . . . . . 41 LOESTRIN FE 1/20 . . . . . . 40 LOFIBRA . . . . . . . . . . . . 11 LOHIST-DM . . . . . . . . . . . 53 LOMOTIL . . . . . . . . . . . . 28 lomustine . . . . . . . . . . . . 4 LONITEN . . . . . . . . . . . . 12 LONSURF . . . . . . . . . . . . 4 LO/OVRAL . . . . . . . . . . . 41 loperamide . . . . . . . . . . 28 LOPERAMIDE . . . . . . . . . 28 LOPID . . . . . . . . . . . . . . 11 lopinavir/ritonavir . . . . . . . 37 LOPRESSOR . . . . . . . . . . .9 loratadine . . . . . . . . . . . 23 loratadine/pseudoephedrine 65 loratadine/pseudoephedrine extended-release . . . . . 24 loratadine & pseudoephedrine SR . . 53 lorazepam . . . . . . . . . . . 46 LORAZEPAM INTENSOL . . . 46 LORCET . . . . . . . . . . . . 14 LORTAB . . . . . . . . . . . . 14 LORTAB ELIXIR . . . . . . . . 14 losartan . . . . . . . . . . . . . 8 losartan/HCTZ . . . . . . . . . 9 LOTENSIN . . . . . . . . . . . . 8 LOTENSIN HCT . . . . . . . . . 8 LOTRIMIN AF . . . . . . . 20, 64 LOTRISONE . . . . . . . . . . 20 lovastatin . . . . . . . . . . . . 11 LOVENOX . . . . . . . . . . . . 7 loxapine . . . . . . . . . . . . 49 LOXITANE . . . . . . . . . . . 49 LOZOL . . . . . . . . . . . . . 11 LUDIOMIL . . . . . . . . . . . 48 lumacaftor/ivacaftor . . . . . 61 LUMIZYME . . . . . . . . . . . 27 LUPRON . . . . . . . . . . . . .5 LUPRON DEPOT . . . . . . . . 5 LUPRON DEPOT 6-MONTH . .5 LUPRON DEPOT-PED . . . . . .5 LURIDE . . . . . . . . . . . . . 58 ALL CAPS = Brand-name drug lower case = Generic drug 80 LURIDE LOZI-TABS . . . . . . 58 LUVOX . . . . . . . . . . . . . 46 LUVOX CR . . . . . . . . . . . 46 LYNPARZA . . . . . . . . . . . .6 LYRICA . . . . . . . . . . . . . 17 LYSODREN . . . . . . . . . . . .6 LYSTEDA . . . . . . . . . . . . 43 M MAALOX . . . . . . . . . 28, 66 macitentan . . . . . . . . . . 12 MACROBID . . . . . . . . . . 38 MACRODANTIN . . . . . . . . 38 MAGNACET . . . . . . . . . . 14 magnesium oxide . . . . 58, 67 MAG-OX . . . . . . . . . . 58, 67 MALARONE . . . . . . . . . . 38 malathion . . . . . . . . . . . 21 MAPAP COLD TAB . . . . . . 54 maprotiline . . . . . . . . . . 48 maraviroc . . . . . . . . . . . 38 MARINOL . . . . . . . . . . . . 28 MATERNA . . . . . . . . . . . 60 MATULANE . . . . . . . . . . . 6 MATXIM LA . . . . . . . . . . . 10 MAVIK . . . . . . . . . . . . . . .8 MAXALT/MAXALT MLT . . . . 15 MAXIDEX . . . . . . . . . . . . 44 MAXITROL . . . . . . . . . . . 43 MAXZIDE . . . . . . . . . . . . 11 M-CLEAR WC . . . . . . . . . 51 measles, mumps & rubella virus vaccines for inj . . . . . . 63 mecasermin . . . . . . . . . . 27 meclizine . . . . . . . . . 28, 66 MEDIFIN PE . . . . . . . . . . 54 MEDIPLAST . . . . . . . . . . 67 MEDROL . . . . . . . . . . . . 25 medroxyprogesterone acetate . . . . . . . . 40, 42 mefloquine . . . . . . . . . . 38 MEGACE . . . . . . . . . . . . .5 megestrol acetate . . . . . . . 5 MEKINIST . . . . . . . . . . . . 5 Index of Covered Drugs MELLARIL . . . . . . . . . . . 49 meloxicam . . . . . . . . 13, 39 melphalan . . . . . . . . . . . 4 memantine . . . . . . . . . . 13 memantine SR . . . . . . . . 13 MENACTRA . . . . . . . . . . 63 MENEST . . . . . . . . . . . . 42 meningococcal (a, c, y, and w-135) . . . . . . . . . . . 63 meningococcal (a, c, y, and w-135) conjugate vaccine 63 meningococcal (a, c, y, and w-135) oligo conj vac for inj . . . . . . . . . 63 MENOMUNE . . . . . . . . . . 63 MENOSTAR . . . . . . . . . . 42 MENVEO . . . . . . . . . . . . 63 meperidine . . . . . . . . . . 14 MEPHYTON . . . . . . . . . . 58 MEPRON . . . . . . . . . . . . 38 mercaptopurine . . . . . . . . 4 mesalamine . . . . . . . . . . 29 mesalamine extended-release . . . . . 30 mesalamine supp . . . . . . . 30 mesna . . . . . . . . . . . . . . 6 MESNEX . . . . . . . . . . . . .6 MESTINON . . . . . . . . . . . 15 MESTINON TIMESPAN . . . . 15 METADATE ER . . . . . . . . . 47 METAGLIP . . . . . . . . . . . 26 METAMUCIL . . . . . . . . . . 66 metaproterenol . . . . . . . . 56 METAPROTERENOL SYRUP 56 metformin . . . . . . . . . . . 26 metformin ER . . . . . . . . . 26 metformin/glyburide . . . . . 26 methadone . . . . . . . . . . 14 methazolamide . . . . . . . . 44 methenamine hippurate . . . 57 METHERGINE . . . . . . 28, 43 methimazole . . . . . . . . . . 27 methocarbamol . . . . . . . . 40 methotrexate . . . . . . . . . 39 METHOTREXATE . . . . . . . 39 methoxsalen . . . . . . . . . . 20 methsuximide . . . . . . . . . 16 methyldopa . . . . . . . . . . 12 methyldopa/HCTZ . . . . . . 12 methylergonovine . . . . 28, 43 METHYLIN SOLUTION . . . . 46 methylphenidate . . . . . . . 46 methylphenidate extended-release . . . 46, 47 methylprednisolone . . . . . . 25 metipranolol . . . . . . . . . . 44 metoclopramide . . . . . . . 28 metolazone . . . . . . . . . . 11 metoprolol . . . . . . . . . . . 9 metoprolol succinate . . . . . . 9 METROCREAM . . . . . . . . 21 METROGEL . . . . . . . . . . 21 METROGEL 1% . . . . . . . . 42 METROGEL-VAGINAL . . . . 42 METROLOTION . . . . . . . . 21 metronidazole . . . . 21, 38, 42 MEVACOR . . . . . . . . . . . 11 mexiletine . . . . . . . . . . . . 9 MEXITIL . . . . . . . . . . . . . .9 MIACALCIN . . . . . . . . . . 27 MICATIN . . . . . . . . . . 20, 64 miconazole . . . . . . . . 20, 42 MICONAZOLE . . . . . . . . . 35 miconazole buccal . . . . . . 35 miconazole crm, soln . . . . . 64 MICONAZOLE KIT . . . . . . . 42 miconazole nitrate . . . . . . 42 miconazole nitrate kit . . . . . 20 miconazole nitrate lotion . . . 20 miconazole nitrate soln . . . . 20 MICRO-K 10 . . . . . . . . . . 61 MICRONASE . . . . . . . . . . 26 MICROZIDE . . . . . . . . . . 10 MIDAMOR . . . . . . . . . . . 10 midodrine . . . . . . . . . . . 12 mifepristone . . . . . . . . . . 28 MIGERGOT SUPPOSITORIES15 MIGRANAL . . . . . . . . . . . 15 MINIPRESS . . . . . . . . . . . .8 ALL CAPS = Brand-name drug lower case = Generic drug 81 MINOCIN . . . . . . . . . . . . 35 minocycline . . . . . . . . . . 35 minocycline SR . . . . . . . . 35 minoxidil . . . . . . . . . . . . 12 MINUTUSS DR SYP . . . . . . 53 MIRALAX . . . . . . . . . . . . 30 MIRAPEX . . . . . . . . . . . . 16 MIRAPEX ER . . . . . . . . . . 16 MIRCETTE . . . . . . . . . . . 40 mirtazapine . . . . . . . . . . 48 MIRVASO . . . . . . . . . . . . 20 misoprostol . . . . . . . . . . 31 MITIGARE . . . . . . . . . . . 40 mitotane . . . . . . . . . . . . . 6 M-M-R II . . . . . . . . . . . . . 63 MOBIC . . . . . . . . . . 13, 39 MODICON . . . . . . . . . . . 41 MODURETIC . . . . . . . . . . 10 moexipril . . . . . . . . . . . . 8 moexipril/ hydrochlorothiazide 8 mometasone . . . . . . . . . 55 mometasone/formoterol . . . 55 mometasone furoate . . . . . 19 MONISTAT . . . . . . . . 42, 64 MONISTAT 3 . . . . . . . . . . 42 MONISTAT-DERM . . . . . . . 20 MONOPRIL . . . . . . . . . . . 8 MONOPRIL-HCT . . . . . . . . .8 montelukast . . . . . . . . . . 56 morphine . . . . . . . . . . . 14 morphine extended-release . 14 morphine sulfate inj . . . . . . 31 morphine sulfate iv soln . . . 31 MORPHINE SUL INJ . . . . . 31 MOSCO CALLUS/CORN LIQUID REMOVER OTC . . . 67 MOSCO CALLUS/CORN REMOVER OTC . . . . . . . . 67 MOTRIN . . . . . . . . . . 13, 39 MOTRIN IB . . . . . . . . . . . 66 MOVANTIK . . . . . . . . . . . 31 moxifloxacin HCl . . . . . . . 45 MOZOBIL . . . . . . . . . . . . .7 MS CONTIN . . . . . . . . . . 14 Index of Covered Drugs MSIR . . . . . . . . . . . . . . 14 MUCINEX . . . . . . . . . . . 52 MUCINEX COLD LIQ /KIDS . 54 MUCINEX D . . . . . . . . . . 52 MUCINEX DM . . . . . . . . . 52 MUCINEX KIDS . . . . . . . . 52 MUCOMYST . . . . . . . . . . 61 MULTAQ . . . . . . . . . . . . . 9 MULTI SYMPTOM TAB COLD RLF . . . . . . . . . 55 multivitamins/fluoride/±iron . 58 multivitamins/minerals . . . . 58 mupirocin . . . . . . . . . . . 18 MURINE . . . . . . . . . . . . 66 MURO 128 . . . . . . . . . . . 45 MYAMBUTOL . . . . . . . . . 33 MYCELEX . . . . . . . . . 20, 35 MYCITRACIN . . . . . . . . . . 65 MYCOBUTIN . . . . . . . . . . 33 mycophenolate mofetil . . . . .6 mycophenolate sodium . . . . 6 MYCOSTATIN . . . . . . . 20, 35 MYFORTIC . . . . . . . . . . . .6 MYLANTA . . . . . . . . . 28, 66 MYLERAN . . . . . . . . . . . . 4 MYLICON . . . . . . . . . . . 66 MYSOLINE . . . . . . . . . . . 17 MYTUSSIN AC . . . . . . . . . 51 N nabumetone . . . . . . . . . . 13 nadolol . . . . . . . . . . . . . 9 NALFON . . . . . . . . . . 13, 39 naloxegol . . . . . . . . . . . 31 naloxone . . . . . . . . . . . . 48 NALOXONE INJ . . . . . . . . 48 naltrexone . . . . . . . . . 45, 48 naltrexone for IM extended release susp . . . . . . . . 48 NAMENDA . . . . . . . . . . . 13 NAMENDA XR . . . . . . . . . 13 naphazoline/antazoline . . . 43 naphazoline/glycerin . . . . . 43 naphazoline HCL . . . . . . . 43 naphazoline w/pheniramine 43 naphazoline/zinc sulfate . . . 43 NAPHCON A . . . . . . . 43, 66 NAPROSYN . . . . . . . . 13, 39 naproxen . . . . . . . . . 13, 39 naproxen delayed release 14, 39 naproxen sodium . . . . 14, 39 naratriptan . . . . . . . . . . . 15 NARCAN NASAL SPRAY . . . 48 NARIZ . . . . . . . . . . . . . . 54 NASACORT ALLERGY . . . . 24 NASACORT AQ . . . . . . . . 24 NASALCROM . . . . . . . . . 24 nasal sprays . . . . . . . . . . 65 NATACYN . . . . . . . . . . . . 45 NATALCARE . . . . . . . . . . 60 natamycin . . . . . . . . . . . 45 nateglinide . . . . . . . . . . . 26 NATROBA . . . . . . . . . . . 21 NAVANE . . . . . . . . . . . . 49 nedocromil . . . . . . . . . . 55 nelfinavir . . . . . . . . . . . . 37 NEOBENZ MICR LIQ . . . . . 17 NEO DM . . . . . . . . . . . . 50 NEO-FRADIN . . . . . . . . . . 38 neomycin/polymyxin B/ bacitracin . . . . . . . . . 18 neomycin/polymyxin B/ dexamethasone . . . . . . 43 neomycin/polymyxin B/ gramicidin . . . . . . . . . 45 neomycin/polymyxin B/ hydrocortisone . . . . 23, 43 neomycin sulfate . . . . . . . 38 NEORAL . . . . . . . . . . . . . 6 NEOSPORIN . . . . . 18, 45, 65 NEO-SYNEPHRINE . . . 24, 65 NEPHROCAPS . . . . . . . . 60 NEPTAZANE . . . . . . . . . . 44 NESTABSCBF/FA/RX . . . . . 60 NEULASTA . . . . . . . . . . . .7 NEUMEGA . . . . . . . . . . . .7 NEUPOGEN . . . . . . . . . . .7 NEURONTIN . . . . . . . . . . 16 ALL CAPS = Brand-name drug lower case = Generic drug 82 NEUTROGENA OIL FREE ACNE WASH . . . . . . . . . . . 18 NEUTROGENA RAPID CLEAR GEL . . . . . . . . . . . . . 18 nevirapine . . . . . . . . . . . 36 nevirapine ER . . . . . . . . . 36 NEXAVAR . . . . . . . . . . . . .5 NEXIUM 24 HR OTC . . . . . 29 NEXIUM DELAYEDRELEASE PACKET . . . . 29 niacin . . . . . . . . . . . . . 11 niacin extended-release . . . 11 NIACOR . . . . . . . . . . . . 11 NIASPAN . . . . . . . . . . . . 11 nicardipine . . . . . . . . . . 10 NICODERM . . . . . . . . . . 49 NICODERM CQ . . . . . . . . 67 NICORETTE . . . . . . . . . . 49 nicotine . . . . . . . . . . 49, 67 NICOTINE GUM . . . . . . . . 67 nicotine inhaler system . . . . 49 nicotine polacrilex gum . . . . 49 nicotine polacrilex lozenge . . 49 NICOTROL . . . . . . . . 49, 67 nifedipine . . . . . . . . . . . 10 nifedipine extended-release . 10 NIFEREX . . . . . . . . . 58, 67 NIFEREX-PN FORTE . . . . . 60 nilotinib . . . . . . . . . . . . . 4 nimodipine . . . . . . . . . . 10 NIMOTOP . . . . . . . . . . . 10 NINLARO . . . . . . . . . . . . .5 nintedanib . . . . . . . . . . . 62 nisoldipine . . . . . . . . . . . 10 nitazoxanide suspension . . . 38 nitazoxanide tablet . . . . . . 38 NITREK . . . . . . . . . . . . 12 NITRO-BID . . . . . . . . . . . 12 NITRO-DUR . . . . . . . . . . 12 nitrofurantoin extended-release . . . . . 38 nitrofurantoin macrocrystals . 38 nitrofurantoin susp . . . . . . 38 nitroglycerin . . . . . . . .12, 22 Index of Covered Drugs nitroglycerin extended-release12 NITROLINGUAL . . . . . . . . 12 NITROSTAT . . . . . . . . . . . 12 NIX . . . . . . . . . . . . . . . 66 NIX CREME RINSE . . . . . . 21 NIZORAL . . . . . . . . . 20, 35 NIZORAL A-D OTC . . . . . . 20 NIZORAL SHAMPOO . . . . . 21 NOLVADEX . . . . . . . . . . . .5 NORCO . . . . . . . . . . . . . 14 NORDETTE . . . . . . . . . . 41 NORDITROPIN . . . . . . . . 27 norelgestromin/EE . . . . . . 41 norethindrone . . . . . . . . . 41 norethindrone acetate . . . . 42 norethindrone acetate/EE40, 41 norethindrone acetate/EE/iron . . . 40, 41 norethindrone/EE . . . . 40, 41 norethindrone/ME . . . . . . 41 NORFLEX . . . . . . . . . . . 40 norgestimate/EE . . . . . . . 41 norgestrel . . . . . . . . . . . 41 norgestrel/EE . . . . . . . . . 41 NORPACE . . . . . . . . . . . . 9 NORPACE CR . . . . . . . . . .9 NORPRAMIN . . . . . . . . . . 47 nortriptyline . . . . . . . . . . 47 NORVASC . . . . . . . . . . . 10 NORVIR . . . . . . . . . . . . . 37 NOXAFIL . . . . . . . . . . . . 35 NUEDEXTA . . . . . . . . . . . 49 NULYTELY . . . . . . . . . . . 30 NUTROPIN AQ NUSPIN . . . 27 NUVARING . . . . . . . . . . . 40 NYMALIZE . . . . . . . . . . . 10 nystatin . . . . . . . . . . 20, 35 NYTOL QUICK CAPS . . . . . 48 O OC8 . . . . . . . . . . . . 17, 64 OCEAN NASAL SPRAY . . . . 24 octreotide . . . . . . . . . . . . 6 OCUFEN . . . . . . . . . . . . 43 OCUFLOX . . . . . . . . . . . 45 ODOMZO . . . . . . . . . . . . .6 OFEV . . . . . . . . . . . . . . 62 ofloxacin . . . . . . . 23, 34, 45 OGEN . . . . . . . . . . . . . . 42 olanzapine . . . . . . . . . . . 48 olanzapine ODT . . . . . . . . 48 olaparib . . . . . . . . . . . . . 6 olodaterol . . . . . . . . . . . 55 olsalazine sodium . . . . . . . 30 omalizumab . . . . . . . . . . 55 ombitasvir/paritaprevir/ ritonavir . . . . . . . . . . 36 ombitasvir/paritaprevir/ ritonavir/dasabuvir . . . . 36 omega-3 fatty acids . . . . . . 58 omeprazole delayed-release 29 OMNICEF . . . . . . . . . . . . 34 ondansetron . . . . . . . . . . 28 ONE TOUCH SYSTEMS (ULTRA 2, ULTRAMINI, VERIO, VERIO FLEX, VERIO IQ, VERIO SYNC) . 26 ONE TOUCH TEST STRIPS (ULTRA, VERIO) . . . . . . 26 ONFI . . . . . . . . . . . . . . 16 ONFI SUSPENSION . . . . . . 16 ONMEL . . . . . . . . . . . . . 35 OPCON A . . . . . . . . . . . 43 oprelvekin . . . . . . . . . . . . 7 OPSUMIT . . . . . . . . . . . . 12 OPTIPRANOLOL . . . . . . . 44 OPTIVAR . . . . . . . . . . . . 43 ORAP . . . . . . . . . . . . . . 49 ORAPRED . . . . . . . . . . . 25 ORKAMBI . . . . . . . . . . . 61 orphenadrine extended-release . . . . . 40 ORTHO-CEPT . . . . . . . . . 41 ORTHO COIL . . . . . . . . . 40 ORTHO-CYCLEN . . . . . . . 41 ortho diaphragm . . . . . . . 40 ORTHO EVRA . . . . . . . . . 41 ORTHO FLAT . . . . . . . . . 40 ALL CAPS = Brand-name drug lower case = Generic drug 83 ORTHO FLEX . . . . . . . . . 40 ORTHO MICRONOR . . . . . 41 ORTHO-NOVUM 1/35 . . . . 41 ORTHO-NOVUM 1/50 . . . . 41 ORTHO-NOVUM 7/7/7 . . . . 41 ORTHO-NOVUM 10/11 . . . 40 ORTHO TRI-CYCLEN . . . . . 41 ORUDIS . . . . . . . . . . 13, 39 OS-CAL . . . . . . . . . . 57, 67 oseltamivir . . . . . . . . . . . 36 OVCON 50 . . . . . . . . . . . 41 OVIDE . . . . . . . . . . . . . . 21 OVRAL . . . . . . . . . . . . . 41 OVRETTE . . . . . . . . . . . . 41 oxaprozin . . . . . . . . . 14, 39 oxazepam . . . . . . . . . . . 46 oxcarbazepine . . . . . . . . 17 OXSORALEN-ULTRA . . . . . 20 oxybutynin chloride . . . . . . 57 oxybutynin IR . . . . . . . . . 57 oxycodone . . . . . . . . . . 14 oxycodone/acetaminophen . 14 oxycodone/ acetaminophen CR . . . . 14 oxycodone/aspirin . . . . . . 14 oxycodone ER . . . . . . . . 14 OXYCONTIN . . . . . . . . . . 14 OXYFAST . . . . . . . . . . . . 14 oxymetazoline . . . . . . . . . 24 oxymorphone ER . . . . . . . 14 OXYMORPHONE ER . . . . . 14 P PACERONE . . . . . . . . . . . 9 palbociclib . . . . . . . . . . . 5 paliperidone . . . . . . . . . . 48 palivizumab . . . . . . . . . . 38 PAMELOR . . . . . . . . . . . 47 pancrealipase DR . . . . . . . 30 PANCREAZE . . . . . . . . . . 30 pancrelipase . . . . . . . . . 30 panobinostat . . . . . . . . .4, 6 PANOXYL BAR . . . . . . . . 64 PANOXYL BAR OTC . . . . . 17 Index of Covered Drugs PANOXYL CREAM . . . . . . 64 PANOXYL CREAM OTC . . . 17 PANRETIN . . . . . . . . . . . .6 pantoprazole . . . . . . . . . 29 pantoprazole delayed-release 29 PARAFON FORTE DSC . . . . 40 PARLODEL . . . . . . . . 15, 28 PARNATE . . . . . . . . . . . . 47 paromomycin . . . . . . . . . 38 paroxetine . . . . . . . . . . . 47 paroxetine SR . . . . . . . . . 47 PASER . . . . . . . . . . . . . 33 pasireotide . . . . . . . . . . . 6 PAXIL . . . . . . . . . . . . . . 47 PAXIL CR . . . . . . . . . . . . 47 PAXIL ORAL SUSP . . . . . . 47 pazopanib . . . . . . . . . . . 5 PEDIACARE LIQ MULTI-SY . . 54 PEDIALYTE . . . . . . . . 57, 66 PEDIAPRED . . . . . . . . . . 25 PEDIAZOLE . . . . . . . . . . 34 peg 3350/electrolytes . . . . 30 peg 3350/sodium bicarbonate/ sodium chloride . . . . . 30 peg 3350/sodium bicarbonate/sodium chloride/potassium chloride . 30 PEGASYS . . . . . . . . . . . . 36 PEGASYS PROCLICK . . . . 36 pegfilgrastim . . . . . . . . . . 7 peginterferon alfa-2a . . . . . 36 peginterferon alfa-2b . . . 5, 36 PEGINTRON . . . . . . . . . . 36 pegvisomant . . . . . . . . . 28 penciclovir . . . . . . . . . . . 36 penicillamine . . . . . . . . . 39 penicillin VK . . . . . . . . . . 34 PENLAC SOLUTION 8% . . . 20 PENTASA . . . . . . . . . . . . 30 pentosan . . . . . . . . . . . . 57 pentoxifylline extended-release . . . . . . 7 PEPCID . . . . . . . . . . . . . 29 PEPCID AC . . . . . . . . 29, 66 PEPCID AZ CHW . . . . . . . 29 PERCOCET . . . . . . . . . . 14 PERCODAN . . . . . . . . . . 14 PERIDEX . . . . . . . . . . . . 24 perindopril . . . . . . . . . . . 8 PERIOGARD . . . . . . . . . . 24 permethrin . . . . . . . . 21, 66 perphenazine . . . . . . . . . 49 PERSANTINE . . . . . . . . . . 7 phenazopyridine . . . . . . . 57 PHENERGAN . . . . . . . . . 28 PHENERGAN DM . . . . . . . 52 phenobarbital . . . . . . . . . 17 PHENOBARBITAL . . . . . . . 17 phenyleph/bromphen/dextromethorphan/guaifenesin 53 phenylephrine . . . . . . . . . 24 phenylephrine/ brompheniramine/ dextromethorphan . . . . 53 phenylephrine/ brompheniramine-DM . . 53 phenylephrine/ chlorpheniramine . . . . . 53 phenylephrine/ chlorpheniramine/ dextromethorphan . . . . 53 phenylephrine/chlorpheniramine/dihydrocodeine . . 53 phenylephrine/ dextromethorphan . . . . 53 phenylephrine/dextromethorphan/guaifenesin . . . . 54 phenylephrine/ephed/CPM w/carbetapentane . . . . 54 phenylephrine/guaifenesin . . 54 phenylephrine/hydrocodone/ guaifenesin . . . . . . . . 54 phenylephrine/pyrilamine/ dextromethorphan . . . . 54 phenylephrine/pyrilamine w/hydrocodone . . . . . . 54 phenylephrine tan/pyrilamine tan/carbeta tan . . . . . . 54 ALL CAPS = Brand-name drug lower case = Generic drug 84 PHENYLHIST LIQ DH . . . . . 51 PHENYTEK . . . . . . . . . . . 17 phenytoin . . . . . . . . . . . 17 phenytoin sodium extended . 17 PHISOHEX . . . . . . . . . . . 21 PHOS-FLUR . . . . . . . . 58, 67 PHOSLYRA . . . . . . . . . . . 61 PHOSPHOLINE IODINE . . . 44 phosphorus . . . . . . . . . . 60 PHRENILIN . . . . . . . . . . . 13 phytonadione . . . . . . . 33, 58 pilocarpine . . . . . . . . 24, 45 PILOPINE HS GEL . . . . . . . 45 PIMA . . . . . . . . . . . . . . 61 pimecrolimus . . . . . . . . . 22 pimozide . . . . . . . . . . . . 49 pindolol . . . . . . . . . . . . . 9 PINDOLOL . . . . . . . . . . . .9 pioglitazone . . . . . . . . . . 26 pioglitazone/glimepiride . . . 26 pioglitazone/metformin . . . 26 pioglitazone/metformin ER . 26 piperacillin sodiumtazobactam sodium for inj 32 piperacillin sod-tazobactam sod in dex iv sol . . . . . . . . 32 PIPERONYL BUTOXIDE . . . 66 piperonyl butoxide gel, liquid shampoo . . . . . . . . . 66 PIPER/TAZOBA INJ . . . . . . 32 pirfenidone capsule . . . . . 62 piroxicam . . . . . . . . . 14, 39 PLAN B . . . . . . . . . . . . . 40 PLAQUENIL . . . . . . . . 38, 39 PLAVIX . . . . . . . . . . . . . . 7 PLENDIL . . . . . . . . . . . . 10 plerixafor . . . . . . . . . . . . 7 PLETAL . . . . . . . . . . . . . .7 PLEXION . . . . . . . . . . . . 18 pneumococcal 13-valent conjugate . . . . . . . . . 63 pneumococcal vaccine polyvalent . . . . . . . . . 63 PNEUMOVAX . . . . . . . . . 63 Index of Covered Drugs PNEUMOVAX 23 . . . . . . . 63 podofilox . . . . . . . . . . . . 21 POLARAMINE . . . . . . . . . 23 polyethylene glycol 3350 . . . 30 polymyxin B/bacitracin . . . . 45 polymyxin B/trimethoprim . . 45 polysaccharide iron complex 58 POLYSPORIN . . . . . . . . . 45 POLYTRIM . . . . . . . . . . . 45 POLY-VI-FLOR . . . . . . . . . 58 POLY-VI-SOL . . . . . . . . . . 67 pomalidomide . . . . . . . . . 5 POMALYST . . . . . . . . . . . .5 ponatinib . . . . . . . . . . . . 5 posaconazole susp . . . . . . 35 potassium acid phosphate . . 60 potassium bicarbonate/potassium citrate effervescent 60 potassium chloride . . . . . . 61 POTASSIUM CHLORIDE . . . 61 potassium chloride extended-release . . . . . 61 potassium citrate . . . . . . . 57 potassium iodide . . . . . . . 61 POTIGA . . . . . . . . . . . . . 16 povidone-iodine . . . . . . . . 38 PRADAXA . . . . . . . . . . . . 7 PRALUENT . . . . . . . . . . . 11 pramipexole . . . . . . . . . . 16 pramipexole dihydrochloride SR . . . . 16 pramlintide . . . . . . . . . . 27 PRANDIN . . . . . . . . . . . . 26 PRAVACHOL . . . . . . . . . . 11 pravastatin . . . . . . . . . . . 11 praziquantel . . . . . . . . . . 35 prazosin . . . . . . . . . . . . . 8 PRECOSE . . . . . . . . . . . 26 PRED FORTE . . . . . . . . . 44 PRED-G . . . . . . . . . . . . . 43 PRED MILD . . . . . . . . . . 44 prednicarbate . . . . . . . . . 19 prednisolone . . . . . . . . . 25 prednisolone acetate . . . . . 44 prednisolone acetate . . . . . 44 prednisolone phosphate . . . 44 prednisolone sodium phosphate . . . . . . . . . 25 prednisone . . . . . . . . . . 25 PREDNISONE . . . . . . . . . 25 pregabalin . . . . . . . . . . . 17 PRELONE . . . . . . . . . . . 25 PREMARIN . . . . . . . . . . . 42 PREMPHASE . . . . . . . . . 42 PREMPRO . . . . . . . . . . . 42 prenatal vitamins w/folic acid 60 PRENATAL VITAMINS W/ FOLIC ACID . . . . . . . . 60 prenatal vit w/FE bisglyc-FE prot succ-FA . . . . . . . . . . 59 prenatal vit w/FE bisglycinate chelate-FA . . . . . . . . . 59 prenatal vit w/FE polysac cmplx-FA . . . . . 59 prenatal vit w/iron carbonyl-FA . . . . . . . . 59 prenatal vit w/o vit a w/fe bisglycinate-fa tab . . . . 59 prenatal w/o A w/ FE carbonylFE gluc-DSS-FA . . . . . . 59 prenat-FE Bis-FE prot succ-FA CA & omega 3 . . . . . . 59 prenat w/o A w/fecbn-fegl-DSSFA & DHA . . . . . . . . . 59 PREPARATION H . . . . . . . 66 PRESGEN B . . . . . . . . . . 53 PREVACID . . . . . . . . . . . 29 PREVIDENT . . . . . . . . . . 58 PREVNAR 13 . . . . . . . . . 63 PREZCOBIX . . . . . . . . . . 38 PREZISTA . . . . . . . . . . . . 37 PRIFTIN . . . . . . . . . . . . . 33 PRILOSEC . . . . . . . . . . . 29 primaquine . . . . . . . . . . 38 primidone . . . . . . . . . . . 17 PRINCIPEN . . . . . . . . . . . 34 PROAMATINE . . . . . . . . . 12 probenecid . . . . . . . . . . 40 ALL CAPS = Brand-name drug lower case = Generic drug 85 PROBENECID . . . . . . . . . 40 PROBIOTIC FORMULA . . . . 31 probiotic product . . . . . . . 31 procarbazine . . . . . . . . . . 6 PROCARDIA . . . . . . . . . . 10 PROCARDIA XL . . . . . . . . 10 prochlorperazine . . . . . . . 28 PROCRIT . . . . . . . . . . . . .7 PROCTOFOAM HC FOAM . . 21 PROCTO-PAK CREAM . . . . 21 PROCTOSOL HC CREAM 2.5% . . . . . . . 21 PROCTOZONE CREAM-HC 2.5% . . . . . 21 progesterone micronized . . . 42 PROGRAF . . . . . . . . . . . . 6 PROLASTIN . . . . . . . . . . 56 PROLIXIN . . . . . . . . . . . . 49 PROLIXIN DECANOATE . . . 49 PROMACTA . . . . . . . . . . 62 promethazine . . . . . . . . . 28 promethazine hcl inj . . . . . 33 promethazine & phenylephrine . . . . . . . 54 PROMETHAZINE SYP DM . . 52 PROMETHAZINE VC W/CODEINE . . . . . . . . 51 PROMETHAZINE W/CODEINE . . . . . . . . 51 PROMETH VC SYP . . . . . . 54 PROMETRIUM . . . . . . . . . 42 PRO NUTRIENT . . . . . . . . 58 propafenone . . . . . . . . . . 9 propantheline . . . . . . . . . 57 propranolol . . . . . . . . . 9, 15 propranolol extended-release . 9 propranolol/HCTZ . . . . . . . 9 propylthiouracil . . . . . . . . 27 PROPYLTHIOURACIL . . . . . 27 PROSCAR . . . . . . . . . . . 56 PROSOM . . . . . . . . . . . . 48 PROTONIX . . . . . . . . . . . 29 PROTONIX PAK . . . . . . . . 29 PROTOPIC 0.1% . . . . . . . . 22 Index of Covered Drugs PROTOPIC 0.03% . . . . . . . 22 protriptyline . . . . . . . . . . 47 PROVENTIL . . . . . . . . . . 55 PROVERA . . . . . . . . . . . 42 PROZAC . . . . . . . . . . . . 47 PRUET DHAEC PAK . . . . . 60 PRUET DHA PAK SETONET PAK . . . . . . 59 PSE/GG . . . . . . . . . . . . 52 pseudoephedrine . . . . . . . 54 pseudoephedrine/acetaminophen/dextromethorphan 54 pseudoephedrine/chlorpheniramine/dextromethorphan 54 pseudoephedrine/dextromethorphan/guaifenesin . . . . 55 pseudoephedrine/iburprofen 55 pseudoephedrine tan/ dexchlorphen tan/DM tan . . . . . 55 PSORCON CREAM . . . . . . 19 PSORCON OINTMENT . . . . 19 P & S SHAMPOO . . . . . . . 22 psyllium . . . . . . . . . . . . 66 PULMICORT RESPULES . . . 55 PULMOZYME . . . . . . . . . 61 PURINETHOL . . . . . . . . . . 4 PURIXAN . . . . . . . . . . . . .4 pyrazinamide . . . . . . . . . 33 PYRAZINAMIDE . . . . . . . . 33 pyrethrins/piperonyl but. . . . 21 PYRIDIUM . . . . . . . . . . . 57 pyridostigmine . . . . . . . . 15 pyridostigmine extended-release . . . . . 15 pyrilamine tan/phenyleph tan 55 pyrimethamine . . . . . . . . 38 Q QUALAQUIN . . . . . . . . . . 38 QUAL-TUSSIN SYP DC . 53, 54 QUESTRAN . . . . . . . . . . 11 QUESTRAN-LIGHT . . . . . . 11 quetiapine . . . . . . . . . . . 48 quetiapine XR . . . . . . . . . 48 quinapril . . . . . . . . . . . . . 8 quinapril/hydrochlorothiazide . 8 quinidine gluconate extended-release . . . . . . 9 QUINIDINE GLUCONATE EXT-REL . . . . . . . . . . . 9 quinidine sulfate . . . . . . . . 9 QUINIDINE SULFATE . . . . . .9 quinidine sulfate extended-release . . . . . . 9 QUINIDINE SULFATE EXT-REL 9 quinine sulfate . . . . . . . . . 38 QV-ALLERGY . . . . . . . . . 50 QVAR . . . . . . . . . . . . . . 55 R raloxifene . . . . . . . . . . . 27 raltegravir . . . . . . . . . . . 38 ramelteon . . . . . . . . . . . 48 ramipril . . . . . . . . . . . . . 8 RANEXA . . . . . . . . . . . . 12 ranitidine . . . . . . . . . . . . 29 ranitidine effer . . . . . . . . . 29 ranitidine syrup . . . . . . . . 29 ranolazine . . . . . . . . . . . 12 RAPAMUNE . . . . . . . . . . . 6 RAVICTI . . . . . . . . . . . . . 27 RAYOS . . . . . . . . . . . . . 25 RAZADYNE . . . . . . . . . . 13 REBETOL/COPEGUS . . . . 36 REBIF . . . . . . . . . . . . . . 15 RECOMBIVAX HB . . . . . . . 62 rectal crm, suppositories . . . 66 RECTIV . . . . . . . . . . . . . 22 REGLAN . . . . . . . . . . . . 28 regorafenib . . . . . . . . . . . 5 REGRANEX . . . . . . . . . . 21 RELAFEN . . . . . . . . . . . . 13 RELENZA . . . . . . . . . . . . 36 REMERON . . . . . . . . . . . 48 REMERON ODT . . . . . . . . 48 REMODULIN . . . . . . . . . . 12 RENAGEL . . . . . . . . . . . 61 ALL CAPS = Brand-name drug lower case = Generic drug 86 renat-FE bis-FE prot succ-FACA & omega DR . . . . . 60 RENVELA . . . . . . . . . . . . 61 repaglinide . . . . . . . . . . 26 REQUIP . . . . . . . . . . . . . 16 REQUIP XL . . . . . . . . . . . 16 RESCRIPTOR . . . . . . . . . 36 RESPA-BR . . . . . . . . . . . 23 RESPERAL-DM . . . . . . . . . 49 RESTASIS . . . . . . . . . . . . 45 RESTORIL . . . . . . . . . . . 48 RETIN-A . . . . . . . . . . . . 18 RETROVIR . . . . . . . . . . . 37 REVATIO . . . . . . . . . . . . 12 REVIA . . . . . . . . . . . 45, 48 REVLIMID . . . . . . . . . . . . 5 REYATAZ . . . . . . . . . . . . 37 ribavirin . . . . . . . . . . . . 36 RIDAURA . . . . . . . . . . . . 39 RID SHAMPOO/ BUTOXIDE SHAMPOO . . 21 rifabutin . . . . . . . . . . . . 33 RIFADIN . . . . . . . . . . . . . 33 rifampin . . . . . . . . . . . . 33 rifapentine . . . . . . . . . . . 33 rilpivirine . . . . . . . . . . . . 36 rimantadine . . . . . . . . . . 36 rimexolone . . . . . . . . . . . 44 riociguat . . . . . . . . . . . . 12 RISPERDAL . . . . . . . . . . 48 RISPERDAL CONSTA . . . . . 49 RISPERDAL M-TAB . . . . . . 49 RISPERDAL SOLUTION . . . 49 risperidone . . . . . . . . 48, 49 risperidone m-tab . . . . . . . 49 RITALIN . . . . . . . . . . . . . 46 RITALIN LA . . . . . . . . . . . 47 RITALIN-SR . . . . . . . . . . . 47 ritonavir . . . . . . . . . . . . 37 rivaroxaban . . . . . . . . . . . 7 rivastigmine . . . . . . . . . . 13 rizatriptan . . . . . . . . . . . 15 RMS . . . . . . . . . . . . . . . 14 ROBAXIN . . . . . . . . . . . . 40 Index of Covered Drugs ROBINUL . . . . . . . . . . . . 29 ROBINUL FORTE . . . . . . . 29 ROBITUSSIN . . . . . . . 52, 65 ROBITUSSIN CF . . . . . 52, 65 ROBITUSSIN DM . . . . . 52, 65 ROBITUSSIN LIQ CGH/ALRG . . . . . . . . 53 ROBITUSSIN LIQ CGH/CLD . 51 ROBITUSSIN LIQ CGH/CONG . . . . . . . . 52 ROBITUSSIN LIQ HD/CHST . 54 ROBITUSSIN LIQ PED NGHT 54 ROBITUSSIN PE . . . 52, 54, 65 ROBITUSSIN PED LIQ CGH/COLD . . . . . . . . 51 ROBITUSSIN PED SYP . . . . 52 ROBITUSSIN SYP CHST CNG . . . . . . . . . 52 ROBITUSSIN SYP MAX-ST . . 52 ROCALTROL . . . . . . . . . . 57 RONDEC DM . . . . . . . . . 53 RONDEC DROPS . . . . 50, 51 RONDEC SYRUP . . . . . 50, 51 ropinirole . . . . . . . . . . . 16 ropinirole hydrochloride SR . 16 ROWASA . . . . . . . . . . . . 29 ROXICODONE . . . . . . . . . 14 ROZEREM . . . . . . . . . . . 48 R-TANNAMINE . . . . . . . . . 50 rufinamide . . . . . . . . . . . 17 rufinamide suspension . . . . 17 ruxolitinib . . . . . . . . . . . . 5 RYNA-12 S . . . . . . . . . . . 55 RYNATAN PEDIATRIC SUSP . 51 RYNATUSS . . . . . . . . . . . 50 RYNATUSS PEDIATRIC SUSP54 RYTHMOL . . . . . . . . . . . .9 S SABRIL SOLUTION . . . . . . 17 sacrosidase . . . . . . . . . . 30 sacubitril/valsartan . . . . . . . 9 SALAC OTC . . . . . . . . . . 22 SALACYN . . . . . . . . . . . 22 SALAGEN . . . . . . . . . . . 24 SALEX . . . . . . . . . . . 18, 22 salicylic acid . . . . . 18, 20, 22 salicylic acid 17%/ collodion . . . . . . . 21, 67 salicylic acid film forming liquid 27.5% OTC . . . . . . . . 67 salicylic acid gel 17% OTC . . 67 salicylic acid pad 40% OTC . 67 salicylic acid plaster 40% . . 67 salicylic acid soln OTC . . . . 67 salicylic acid strip 40% . . . . 67 salicylic acid topical patch 15% OTC . . . . . . . . . 67 saline nasal spray 0.65% . . . 24 salmeterol xinafoate . . . . . 55 SAL-TROPINE . . . . . . . . . 31 SALVAX . . . . . . . . . . . . . 22 SANCTURA . . . . . . . . . . 57 SANDIMMUNE . . . . . . . . . .6 SANDOSTATIN . . . . . . . . . .6 SANTYL . . . . . . . . . . . . 21 sapropterin . . . . . . . . . . 28 sapropterin powder . . . . . . 28 saquinavir mesylate . . . . . . 37 sargramostim . . . . . . . . . . 7 SAVAYSA . . . . . . . . . . . . .7 SCALPICIN . . . . . . . . . . . 20 scopolamine . . . . . . . . . 44 SEA-OMEGA . . . . . . . . . . 58 SEBIZON . . . . . . . . . . . . 18 SECTRAL . . . . . . . . . . . . .9 SEDAPAP . . . . . . . . . . . . 13 selegiline . . . . . . . . . . . 16 selenium sulfide . . . . . . . . 22 SELSUN . . . . . . . . . . . . 22 SELSUN BLUE . . . . . . . . . 22 SELZENTRY . . . . . . . . . . 38 sennosides . . . . . . . . . . 30 SENOKOT . . . . . . . . . . . 30 SENSIPAR . . . . . . . . . . . 61 SERAX . . . . . . . . . . . . . 46 SEREVENT DISKUS . . . . . . 55 SEROMYCIN . . . . . . . . . . 33 SEROQUEL . . . . . . . . . . 48 ALL CAPS = Brand-name drug lower case = Generic drug 87 SEROQUEL XR . . . . . . . . 48 sertraline . . . . . . . . . . . . 47 sevelamer . . . . . . . . . . . 61 sevelamer hcl . . . . . . . . . 61 SIGNIFOR . . . . . . . . . . . . 6 sildenafil . . . . . . . . . . . . 12 SILVADENE . . . . . . . . . . . 18 silver sulfadiazine . . . . . . . 18 simethicone . . . . . . . . . . 66 simvastatin . . . . . . . . . . . 11 SINEMET . . . . . . . . . . . . 15 SINEMET CR . . . . . . . . . . 15 SINEQUAN . . . . . . . . . . . 47 SINGULAIR . . . . . . . . . . . 56 sirolimus . . . . . . . . . . . . 6 SIRTURO . . . . . . . . . . . . 38 sitagliptan . . . . . . . . . . . 26 sitagliptan/metformin . . . . . 26 sitagliptan/metformin ER . . . 27 SITAVIG . . . . . . . . . . . . . 36 SKLICE . . . . . . . . . . . . . 21 SOD CHLORIDE INJ . . . . . 33 sodium chloride hypertonic . 45 sodium chloride inj . . . . . . 33 sodium chloride irrigation soln 0.9% . . . . . . . . . 33 sodium chloride iv soln . . . . 33 sodium citrate/citric acid . . . 57 sodium phenylbutyrate oral powder . . . . . . . . . . 27 sodium phenylbutyrate tab . . 27 sodium polysterene sulfonate 12 sofosbuvir . . . . . . . . . . . 36 SOLU-CORTEF . . . . . . . . 25 SOLU-MEDROL . . . . . . . . 25 somatropin . . . . . . . . . . 27 SOMAVERT . . . . . . . . . . 28 SONATA . . . . . . . . . . . . 48 sonidegib . . . . . . . . . . . . 6 sorafenib . . . . . . . . . . . . 5 SORIATANE . . . . . . . . . . 20 sotalol . . . . . . . . . . . . . . 9 SOVALDI . . . . . . . . . . . . 36 Spacers . . . . . . . . . . . . 62 Index of Covered Drugs spinosad . . . . . . . . . . . . 21 SPIRIVA . . . . . . . . . . . . . 55 SPIRIVA RESPIMAT . . . . . . 55 spironolactone . . . . . . . . 11 SPIRONOLACTONE . . . . . 11 spironolactone/ hydrochlorothiazide . . . 11 SPORANOX . . . . . . . . . . 35 SPRYCEL . . . . . . . . . . . . .4 STADOL . . . . . . . . . . . . 14 STARLIX . . . . . . . . . . . . 26 STATUSS DM SYP . . . . . . . 53 stavudine . . . . . . . . . . . 37 STELAZINE . . . . . . . . . . . 49 STIVARGA . . . . . . . . . . . . 5 stool softeners . . . . . . . . 66 STRATTERA . . . . . . . . . . 46 STRENSIQ . . . . . . . . . . . 27 STRIBILD . . . . . . . . . . . . 37 STRI-DEX . . . . . . . . . . . . 18 STRIVERDI RESPIMAT . . . . 55 STROMECTOL . . . . . . . . . 35 STUART PRENATAL . . . . . . 67 SUBOXONE . . . . . . . . . . 48 SUCRAID . . . . . . . . . . . . 30 sucralfate . . . . . . . . . . . 29 SUDAFED . . . . . . . . . . . 54 sugar+orthophosphoric acid 66 SULAR . . . . . . . . . . . . . 10 sulcralfate . . . . . . . . . . . 29 sulfacetamide . . . . . . . . . 45 sulfacetamide/ phenylephrine . . . . . . . 45 sulfacetamide/pred. phos. . . 43 sulfacetamide sodium . . . . 18 sulfacetamide sodiumprednisolone . . . . . . . 43 sulfacetamide/sulfur . . . . . 18 SULFACET-R . . . . . . . . . . 18 sulfadiazine . . . . . . . . . . 34 sulfamethoxazole/ trimethoprim, DS . . . . . 34 sulfanilamide . . . . . . . . . 43 sulfasalazine . . . . . . . 30, 39 sulfasalazine delayed-release . . . . 30, 39 sulindac . . . . . . . . . . 14, 40 sumatriptan . . . . . . . . . . 15 SUMYCIN . . . . . . . . . . . . 35 sunitinib . . . . . . . . . . . . . 5 SUPER OMEGA . . . . . . . . 58 SUPRAX . . . . . . . . . . . . 34 SUPRESS-PE PEDIATRIC DROPS . . . . . . . . . . . 54 SUSTIVA . . . . . . . . . . . . 36 SUTENT . . . . . . . . . . . . . 5 SYLATRON . . . . . . . . . . . .5 SYMBICORT . . . . . . . . . . 55 SYMLIN . . . . . . . . . . . . . 27 SYMMETREL . . . . . . . 15, 36 SYNAGIS . . . . . . . . . . . . 38 SYNALAR . . . . . . . . . . . 19 SYNJARDY . . . . . . . . . . . 26 SYNTHROID . . . . . . . . . . 27 T TABLOID . . . . . . . . . . . . . 4 tacrolimus . . . . . . . . . . 6, 22 tadalafil . . . . . . . . . . . . 12 TAFINLAR . . . . . . . . . . . . 4 tafluprost . . . . . . . . . . . . 44 TAGAMET . . . . . . . . . . . 29 taliglucerase . . . . . . . . . . 28 TAMBOCOR . . . . . . . . . . .9 TAMIFLU . . . . . . . . . . . . 36 tamoxifen . . . . . . . . . . . . 5 tamsulosin . . . . . . . . . . . 56 TANAFED . . . . . . . . . . . . 50 TANAFED DMX SUSPENSION . . . . . . . 55 TANZEUM . . . . . . . . . . . 27 TAPAZOLE . . . . . . . . . . . 27 TARCEVA . . . . . . . . . . . . .4 TARGRETIN . . . . . . . . . . . 6 TASIGNA . . . . . . . . . . . . .4 TASMAR . . . . . . . . . . . . 16 TBO-filgrastim . . . . . . . . . 7 TECFIDERA . . . . . . . . . . 15 ALL CAPS = Brand-name drug lower case = Generic drug 88 TECHNIVIE . . . . . . . . . . . 36 teduglutide . . . . . . . . . . 31 TEGRETOL . . . . . . . . . . . 16 TEGRETOL-XR . . . . . . . . . 16 telaprevir . . . . . . . . . . . . 36 telbivudine . . . . . . . . . . . 36 temazepam . . . . . . . . . . 48 TEMODAR . . . . . . . . . . . .4 TEMOVATE . . . . . . . . . . . 20 temozolomide . . . . . . . . . 4 TENEX . . . . . . . . . . . . . . 8 TENIVAC . . . . . . . . . . . . 63 tenofovir . . . . . . . . . . . . 37 TENORETIC . . . . . . . . . . .9 TENORMIN . . . . . . . . . . . .9 TERAZOL 3/7 . . . . . . . . . 43 terazosin . . . . . . . . . . 8, 56 terbinafine . . . . . . . . 20, 35 terbinafine gel . . . . . . . . . 20 terbinafine HCL . . . . . . . . 20 terbutaline . . . . . . . . . . . 56 terconazole . . . . . . . . . . 43 teriflunomide . . . . . . . . . 15 teriparatide inj . . . . . . . . . 27 TESSALON . . . . . . . . . . . 49 TESTIM . . . . . . . . . . . . . 25 testosterone . . . . . . . . . . 25 TESTOSTERONE 1% TOPICAL GEL . . . . . . . 25 testosterone cypionate . . . . 25 testosterone enanthate . . . . 25 testosterone gel . . . . . . . . 25 testosterone gel topical tube, packet, and pump bottle 25 testotesterone . . . . . . . . . 25 tetanus-diphtheria toxoids . . 63 tetanus immune globulin . . . 63 TET/DIP TOX INJ . . . . . . . 63 tetrabenazine . . . . . . . . . 17 tetracycline . . . . . . . . . . 35 tetrahydrozoline/zinc sulfate . 43 tet tox-diph-acell pertuss ad . 63 thalidomide . . . . . . . . . . . 5 THALOMID . . . . . . . . . . . .5 Index of Covered Drugs THEO-24 . . . . . . . . . . . . 56 THEOCHRON . . . . . . . . . 56 theophylline . . . . . . . . . . 56 THEOPHYLLINE . . . . . . . . 56 theophylline extended-release56 THEOPHYLLINE EXT-REL . . 56 thioguanine . . . . . . . . . . . 4 thioridazine . . . . . . . . . . 49 thiothixene . . . . . . . . . . . 49 THORAZINE . . . . . . . . . . 49 thyroid tab . . . . . . . . . . . 27 THYROLAR . . . . . . . . . . 27 tiagabine . . . . . . . . . . . . 17 TIAZAC . . . . . . . . . . . . . 10 ticagrelor . . . . . . . . . . . . 7 TIGAN . . . . . . . . . . . . . . 28 TIKOSYN . . . . . . . . . . . . .9 TILADE . . . . . . . . . . . . . 55 timolol . . . . . . . . . . . . . 44 timolol maleate . . . . . . . 9, 44 TIMOPTIC . . . . . . . . . . . 44 TIMOPTIC XE . . . . . . . . . 44 TINACTIN . . . . . . . . . 20, 64 tiotropium . . . . . . . . . . . 55 tiotropium respimat . . . . . . 55 tipranavir . . . . . . . . . . . . 37 TIVICAY . . . . . . . . . . . . . 38 tizanidine . . . . . . . . . . . 40 TOBRADEX . . . . . . . . . . 43 TOBRADEX ST . . . . . . . . . 43 tobramycin . . . . . . . . . . 45 tobramycin-dexamethasone . 43 tobramycin/dexamethasone 43 tobramycin neb soln . . . . . 61 TOBREX . . . . . . . . . . . . 45 TOFRANIL . . . . . . . . . . . 47 tolazamide . . . . . . . . . . . 27 tolbutamide . . . . . . . . . . 27 TOLBUTAMIDE . . . . . . . . 27 tolcapone . . . . . . . . . . . 16 TOLINASE . . . . . . . . . . . 27 tolnaftate . . . . . . . . . 20, 64 tolterodine . . . . . . . . . . . 57 tolterodine SR . . . . . . . . . 57 TOPAMAX . . . . . . . . . . . 17 TOPAMAX SPRINKLE . . . . . 17 topical antibacterials . . . . . 65 topiramate . . . . . . . . . . . 17 topiramate sprinkle caps . . . 17 topotecan . . . . . . . . . . . . 6 TOPROL XL . . . . . . . . . . . 9 TORADOL . . . . . . . . . . . 13 toremifene . . . . . . . . . . . 5 torsemide . . . . . . . . . . . 11 TRACLEER . . . . . . . . . . . 12 TRADJENTA . . . . . . . . . . 26 tramadol . . . . . . . . . . . . 13 trametinib . . . . . . . . . . . . 5 TRANDATE . . . . . . . . . . . .9 trandolapril . . . . . . . . . . . 8 tranexamic acid . . . . . . . . 43 TRANS-VER-SAL PATCH . . . 67 TRANXENE . . . . . . . . . . . 46 tranylcypromine . . . . . . . . 47 TRAVATAN Z . . . . . . . . . . 44 TRAVATAN Z PF . . . . . . . . 45 travoprost . . . . . . . . . 44, 45 trazodone . . . . . . . . . . . 48 TRECATOR . . . . . . . . . . . 33 TRENTAL . . . . . . . . . . . . .7 treprostinil . . . . . . . . . . . 12 tretinoin . . . . . . . . . . . 6, 18 TRIACTIN CHEST CONGESTION . . . . . . . 52 triamcinolone . . . . . . . . . 24 triamcinolone acetonide . 19, 24 triamcinolone inj . . . . . . . 25 triamcinolone nasal spray . . 24 TRIAMINIC . . . . . . . . . . . 23 TRIAMINIC LIQ CHST/NAS . 54 TRIAMINIC SYR CHST/NAS . 54 triamterene/ hydrochlorothiazide . . . 11 TRIANEX . . . . . . . . . . . . 19 TRIAVIL . . . . . . . . . . . . . 47 TRIAZ CLOTHS . . . . . . . . 17 triazolam . . . . . . . . . . . . 48 TRICOR . . . . . . . . . . . . . 11 ALL CAPS = Brand-name drug lower case = Generic drug 89 TRI-FED X . . . . . . . . . . . . 55 trifluoperazine . . . . . . . . . 49 trifluridine . . . . . . . . . . . 45 trifluridine/tipiracil . . . . . . . 4 TRIGLIDE . . . . . . . . . . . . 11 trihexyphenidyl . . . . . . . . 16 TRILAFON . . . . . . . . . . . 49 TRILEPTAL . . . . . . . . . . . 17 TRILYTE . . . . . . . . . . . . 30 trimethobenzamide . . . . . . 28 trimethoprim . . . . . . . . . . 38 TRIMETHOPRIM . . . . . . . . 38 TRI-NORINYL . . . . . . . . . 41 TRIOTANN . . . . . . . . . . . 24 TRIOTANN PEDIATRIC SUSP 50 tripolidine/pseudoephedrine 55 TRIPROL/PSE SYP . . . . . . 55 TRI RX . . . . . . . . . . . . . 59 TRIUMEQ . . . . . . . . . . . . 37 TRI-VI-FLOR . . . . . . . . . . 60 TRI-VI-SOL . . . . . . . . 60, 67 TRIVORA . . . . . . . . . . . . 41 TRIZIVIR . . . . . . . . . . . . 37 TROJAN . . . . . . . . . . . . 65 trospium . . . . . . . . . . . . 57 TRUSOPT . . . . . . . . . . . 44 TRUST NATALCARE PAK DHA . . . . . . . . . . 59 TRUVADA . . . . . . . . . . . . 37 T-STAT . . . . . . . . . . . . . 18 TUCKS OINTMENT OTC . . . 21 TUMS . . . . . . . . . . . 66, 67 TUSSI 12D S . . . . . . . . . . 54 TUSSI-12 S . . . . . . . . . . . 50 TUSSIN DM . . . . . . . . . . 52 TYBOST . . . . . . . . . . . . 38 TYKERB . . . . . . . . . . . . . 4 TYLENOL . . . . . . . 13, 39, 66 TYLENOL W/CODEINE . . . 14 typhoid vaccine . . . . . . . . 63 TYVASO . . . . . . . . . . . . 12 TYZEKA . . . . . . . . . . . . 36 Index of Covered Drugs U ULORIC . . . . . . . . . . . . . 40 ULTRAM . . . . . . . . . . . . 13 ULTRAVATE . . . . . . . . . . 20 UNI-HIST DRO . . . . . . . . . 49 UNIPHYL . . . . . . . . . . . . 56 UNIRETIC . . . . . . . . . . . . 8 UNIVASC . . . . . . . . . . . . .8 urea 40% . . . . . . . . . . . 22 UREA 40% LOTION . . . . . . 22 URECHOLINE . . . . . . . . . 56 UREX . . . . . . . . . . . . . . 57 uridine . . . . . . . . . . . . . 27 UROCIT-K . . . . . . . . . . . 57 UROXATRAL . . . . . . . . . . 56 URSO . . . . . . . . . . . . . 31 ursodiol . . . . . . . . . . . . 31 URSO FORTE . . . . . . . . . 31 UTIRA C . . . . . . . . . . . . 56 V VAGIFEM . . . . . . . . . . . . 41 vaginal products . . . . . . . 64 valacyclovir . . . . . . . . . . 36 VALCYTE . . . . . . . . . . . . 35 valganciclovir . . . . . . . . . 35 VALIUM . . . . . . . . 33, 40, 46 valproic acid . . . . . . . . . . 17 valsartan . . . . . . . . . . . . 8 valsartan/hydrochlorothiazide 9 VALTREX . . . . . . . . . . . . 36 VANCOCIN/DEX INJ . . . . . 32 VANCOCIN HCL . . . . . . . . 35 VANCOCIN HCL INJ . . . . . 32 vancomycin HCl . . . . . . . 35 vancomycin hcl for inj . . . . 32 vancomycin hcl in dextrose inj . . . . . . . . 32 vandetanib . . . . . . . . . . . 5 VANTIN . . . . . . . . . . . . . 34 VAQTA . . . . . . . . . . . . . 62 varenicline . . . . . . . . . . . 49 varicella virus vac live for subcutaneous . . . . . . . 63 VARIVAX . . . . . . . . . . . . 63 VASERETIC . . . . . . . . . . . 8 VASOCIDIN . . . . . . . . . . . 43 VASOCLEAR . . . . . . . . . . 43 VASOCLEAR A . . . . . . . . 43 VASOSULF . . . . . . . . . . . 45 VASOTEC . . . . . . . . . . . . .8 VECTICAL . . . . . . . . . . . 20 VEETIDS . . . . . . . . . . . . 34 velaglucerase alfa . . . . . . . 28 VELIVET . . . . . . . . . . . . 41 vemurafenib . . . . . . . . . . 5 venlafaxine . . . . . . . . . . 47 venlafaxine XR . . . . . . . . 47 VENTAVIS . . . . . . . . . . . . 12 VENTOLIN HFA . . . . . . . . 55 VEPESID . . . . . . . . . . . . . 6 VERALAN PM . . . . . . . . . 10 verapamil . . . . . . . . . 10, 15 verapamil extended-release . 10 VESANOID . . . . . . . . . . . .6 VEXOL . . . . . . . . . . . . . 44 VFEND . . . . . . . . . . . . . 35 VIBRAMYCIN . . . . . . . . . . 35 VICODIN . . . . . . . . . . . . 14 VICODIN ES . . . . . . . . . . 14 VICOPROFEN . . . . . . . . . 14 VICTOZA . . . . . . . . . . . . 27 VICTRELIS . . . . . . . . . . . 36 VI-DAYLIN . . . . . . . . . . . . 67 VIDEX . . . . . . . . . . . . . . 37 VIDEX EC . . . . . . . . . . . . 37 VIEKIRA PAK . . . . . . . . . . 36 vigabatrin oral solution . . . . 17 VIGAMOX . . . . . . . . . . . . 45 VIMPAT . . . . . . . . . . . . . 16 VINATE AZ EX . . . . . . . . . 59 VINATE III . . . . . . . . . . . . 59 VIRACEPT . . . . . . . . . . . 37 ALL CAPS = Brand-name drug lower case = Generic drug 90 VIRAMUNE . . . . . . . . . . . 36 VIRAMUNE XR . . . . . . . . . 36 VIREAD . . . . . . . . . . . . . 37 VIROPTIC . . . . . . . . . . . . 45 VISINE . . . . . . . . . . . . . 66 VISINE-AC . . . . . . . . . . . 43 vismodegib . . . . . . . . . . . 6 VISTARIL . . . . . . . . . . . . 23 VISTIDE . . . . . . . . . . . . . 35 VISTOGARD . . . . . . . . . . 27 vitamin A . . . . . . . . . . . . 60 vitamin ADC/fluoride/±iron drops . . . . . . . . . . . 60 vitamin B-1 . . . . . . . . . . 60 vitamin B-6 . . . . . . . . . . 60 VITAMIN B-12 . . . . . . . . . 57 vitamin B complex/ vitamin C/folic acid . . . . 60 vitamin C . . . . . . . . . . . 60 vitamin D . . . . . . . . . . . . 67 VITAMIN D . . . . . . . . . 57, 67 vitamins pediatric . . . . 60, 67 vitamins prenatal . . . . . . . 67 VITAPHIL . . . . . . . . . . . . 59 VITUSSIN . . . . . . . . . . . . 52 VIVACTIL . . . . . . . . . . . . 47 VIVELLE-DOT . . . . . . . . . 42 VIVITROL . . . . . . . . . . . . 48 VIVOTIF BERNA . . . . . . . . 63 VOLTAREN . . . . . . 13, 39, 43 VOLTAREN 1% TOPICAL GEL39 VOLTAREN XR . . . . . . . . . 13 VOLTAREN XR . . . . . . . . . 39 voriconazole . . . . . . . . . . 35 vorinostat . . . . . . . . . . . . 4 VOSOL HC OTIC . . . . . . . 22 VOSOL OTIC . . . . . . . . . . 22 VOSPIRE ER . . . . . . . . . . 56 VOTRIENT . . . . . . . . . . . . 5 VPRIV . . . . . . . . . . . . . . 28 VYVANSE . . . . . . . . . . . . 46 Index of Covered Drugs W warfarin . . . . . . . . . . . . . 7 WELLBUTRIN . . . . . . . . . 47 WELLBUTRIN SR . . . . . . . 48 WELLBUTRIN XL . . . . . . . 48 WESTCORT . . . . . . . . . . 19 WYTENSIN . . . . . . . . . . . 12 X XALATAN . . . . . . . . . . . . 44 XALKORI . . . . . . . . . . . . .4 XANAX . . . . . . . . . . . . . 46 XANAX XR . . . . . . . . . . . 46 XARELTO . . . . . . . . . . . . .7 XARTEMIS XR . . . . . . . . . 14 XELODA . . . . . . . . . . . . . 4 XENAZINE . . . . . . . . . . . 17 XOLAIR . . . . . . . . . . . . . 55 XOLEGEL . . . . . . . . . . . . 20 XOLEGEL DUO KIT . . . . . . 20 XOLEGEL KIT . . . . . . . . . 20 XOPENEX RESPULES . . . . 56 XYLOCAINE . . . . . . . . 22, 24 XYZAL . . . . . . . . . . . . . 23 Z zafirlukast . . . . . . . . . . . 56 zaleplon . . . . . . . . . . . . 48 ZANAFLEX . . . . . . . . . . . 40 zanamivir . . . . . . . . . . . 36 ZANTAC . . . . . . . . . . . . 29 ZARONTIN . . . . . . . . . . . 16 ZAROXOLYN . . . . . . . . . . 11 ZARXIO . . . . . . . . . . . . . .7 ZEASORB-AF . . . . . . . . . 20 ZEBETA . . . . . . . . . . . . . .9 ZEBUTAL . . . . . . . . . . . . 13 ZELBORAF . . . . . . . . . . . .5 ZENPEP . . . . . . . . . . . . 30 ZENPEP DR . . . . . . . . . . 30 ZEPATIER . . . . . . . . . . . . 36 ZERIT . . . . . . . . . . . . . . 37 ZESTORETIC . . . . . . . . . . .8 ZESTRIL . . . . . . . . . . . . . 8 ZETIA . . . . . . . . . . . . . . 11 ZIAC . . . . . . . . . . . . . . . .9 ZIAGEN . . . . . . . . . . . . . 37 zidovudine . . . . . . . . . . . 37 zinc . . . . . . . . . . . . . . . 60 ZIOPTAN . . . . . . . . . . . . 44 ziprasidone . . . . . . . . . . 49 ZITHROMAX . . . . . . . . . . 34 ZOCOR . . . . . . . . . . . . . 11 ZOFRAN . . . . . . . . . . . . 28 ZOFRAN ODT . . . . . . . . . 28 ZOHYDRO ER . . . . . . . . . 14 ZOLINZA . . . . . . . . . . . . .4 ZOLOFT . . . . . . . . . . . . 47 zolpidem . . . . . . . . . . . . 48 ZONEGRAN . . . . . . . . . . 17 zonisamide . . . . . . . . . . 17 ZORTRESS . . . . . . . . . . . .6 ZOSTAVAX . . . . . . . . . . . 63 zoster vaccine live . . . . . . . 63 ZOSYN SOL . . . . . . . . . . 32 ZOVIA 1/35 . . . . . . . . . . 41 ZOVIA 1/50 . . . . . . . . . . 41 ZOVIRAX . . . . . . . . . . . . 36 ZYBAN . . . . . . . . . . . . . 49 ZYDELIG . . . . . . . . . . . . .4 ZYKADIA . . . . . . . . . . . . .4 ZYLOPRIM . . . . . . . . . . . 40 ZYMAR . . . . . . . . . . . . . 45 ZYPREXA . . . . . . . . . . . . 48 ZYPREXA ZYDIS . . . . . . . 48 ZYRTEC . . . . . . . . . . 23, 64 ZYRTEC D . . . . . . . . 24, 65 ZYTIGA . . . . . . . . . . . . . .5 ZYVOX . . . . . . . . . . . . . 38 ALL CAPS = Brand-name drug lower case = Generic drug 91 Notes ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ 92 “My Medications” worksheet Take this worksheet with you each time you visit a doctor. Each of your doctors should be aware of every drug you take and you should have a list as well. Name of Medicine and Strength Drug Tier I Take This Medicine For Directions Doctor Example: Lisinopril, 20 mg Tier 1 High blood pressure One tablet daily Dr. Johnson 93 © 2016 United HealthCare Services, Inc. All rights reserved. 10/16
© Copyright 2026