CONFOCAL MICROSCOPY CORE UNIT
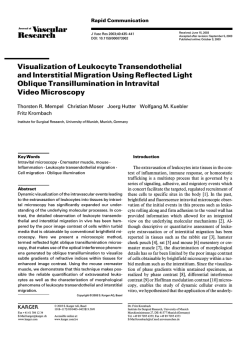
Direction of Innovation CONFOCAL MICROSCOPY CORE UNIT Biotechnology Programme | Confocal Microscopy Core Unit Diego Megías Core Unit Head RESEARCH HIGHLIGHTS Technicians Manuel Pérez, Joaquim Soriano The Confocal Microscopy Core Unit is equipped with 3 laser scanning confocal systems ( Leica SP2 and SP5 ) that incorporate UV and multiphoton excitation, as well as a white light laser and a Hybrid Detector. We also have 2 wide field systems, namely, a Deltavision 4D deconvolution station and a Leica DMRI6000 system, equipped with microinjection. All the microscopes are automated and equipped with incubators for live-cell imaging. In addition, the Unit has applied high throughput ( HT ) technologies to confocal microscopy using 2 different systems : ɗɗ An Opera ( Perkin Elmer ) High-Content Screening ( HCS ) system, which allows running HCS experiments on fixed and live cells in multi-well plates, and enables the monitoring of cell dynamics ( translocation, cell division, etc.) by means of fluorescence markers. ɗɗ A Matrix Screening Application integrated into the SP5 confocal systems, allowing HT feeding of the instrument, not only in multi-well plates but also in tissue sections. Figure Microfluidic experiment under different excitation wavelengths dedicated to a FRET assay ( right ). The Figure shows Fret activity before and after induction of glucose stimuli. have incorporated new flow control devices for more accurate dynamic live cell-based assays in perfusion chambers. These technological advances increase the level of information obtained from a sample and allow for the automated HT screening of cell behaviour in response to different treatments. We are very proud to have co-organised 2 international conferences : ɗɗ The 2nd Spanish National Advanced Microscopy Network Meeting, which took place at the CNIO, brought together a large number of international speakers and attendees. This event explored new advances and techniques in microscopy. ɗɗ The III Core Management Workshop, focused on the general management, funding and stability of core units for all disciplines. s During 2014, the Confocal Microscopy Unit significantly refurbished its computational capacity for image analysis by upgrading its HTS Opera microscope and the Unit’s servers ; we have developed new programmed routines that have now made it possible for us to manage, stitch and analyse a huge amount of images ; something that was previously impossible. Additionally, we have upgraded our laser scanning confocals with a new time gating application that has now made it possible for us to run acquisitions, not only based on intensity, but also taking fluorophore life time into account, thus allowing us to control contamination from light reflection and autofluorescence. We OVERVIEW ∞∞ The Confocal Microscopy Unit provides the CNIO Research Groups with all the standard methodologies as well as the latest advances in microscopy. The Unit offers access to state-of-the-art equipment and software packages related to confocal microscopy, including technical and scientific advice and support to the CNIO scientists. The Unit is also actively involved in developing, testing and implementing new microscopy technologies, tools and imaging applications that could be of interest to Research Groups at the CNIO. Training activities are also an essential component of our mission. SCIENTIFIC REPORT 2014 “ The Confocal Microscopy Unit is fully committed to the implementation of advanced microscopy methodologies in cancer research, with the aim of creating a benefit for society by increasing our understanding of the biology and disorders of cells that cause cancer.“ 152 ∞∞ ∞∞ PUBLICATIONS Miranda-Lorenzo I, Dorado J, Lonardo E, Alcala S, Serrano AG, Clausell-Tormos J, Cioffi M, Megias D, Zagorac S, Balic A, Hidalgo M, Erkan M, Kleeff J, Scarpa A, Sainz B Jr, Heeschen C ( 2014 ). Intracellular autofluorescence : a biomarker for epithelial cancer stem cells. Nat Methods 11, 1161-1169. Alonso-Curbelo D, Riveiro-Falkenbach E, Perez-Guijarro E, Cifdaloz M, karras P, Osterloh L, Megías D, Cañón E, Calvo TG, Olmeda D, Gómez-Lopez G, Graña O, Sánchez-Arévalo VJ, Pisano DG, Wang ∞∞ H-W, Ortiz-Romero P, Tormo D, Hoeck K, Rodríguez-Peralto JL, Joyce JA, Soengas MS ( 2014 ). RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell 26, 61-76. Piazzolla D, Palla AR, Pantoja C, Cañamero M, de Castro IP, Ortega S, Gómez-López G, Domínguez O, Megías D, Roncador G, Luque-Garcia JL, Fernandez-Tresguerres B, Fernandez AF, Fraga MF, Rodriguez-Justo M, Manzanares M, Sánchez-Carbayo M, García-Pedrero JM, Rodrigo JP, Malumbres M, Serrano M ( 2014 ). Lineage-restricted function of the SPANISH NATIONAL CANCER RESEARCH CENTRE, CNIO ∞∞ ∞∞ ∞∞ pluripotency factor NANOG in stratified epithelia. Nat Commun 5, 4226. Epifano C, Megias D, Perez-Moreno M ( 2014 ). p120-catenin differentially regulates cell migration by Rho-dependent intracellular and secreted signals. EMBO Rep 15, 592-600. Morgado-Palacin L, Llanos S, Urbano M, Blanco-Aparicio C, Megias D, Pastor J, Serrano M ( 2014 ). Non-genotoxic activation of p53 through the RPL11-dependent ribosomal stress pathway. Carcinogenesis 35, 2822-2830. Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra ∞∞ C, Muñoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, Plaza C, de Vicente E, Prados S, Tabernero S, Barbacid M, Lopez-Rios F, Hidalgo M ( 2014 ). Reply : ‘ Comments on Stromal disrupting effects of nab-paclitaxel in pancreatic cancer ’. Br J Cancer 111, 1677-1678. Hergueta-Redondo M, Sarrió D, Molina-Crespo Á, Megias D, Mota A, Rojo-Sebastián A, García-Sanz P, Morales S, Abril S, Cano A, Peinado H, Moreno-Bueno G ( 2014 ). Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS One 9, e90099. 153
© Copyright 2026