formation of chloride hydrates via vapor-solid reaction at low-t
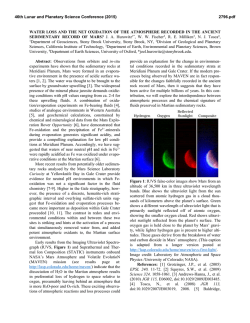
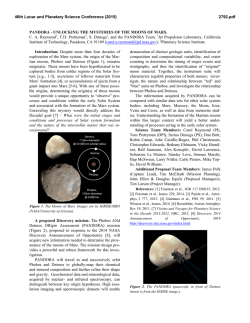
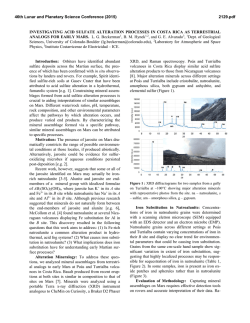
46th Lunar and Planetary Science Conference (2015) 2483.pdf FORMATION OF CHLORIDE HYDRATES VIA VAPOR-SOLID REACTION AT LOW-T -IMPLICATION FOR A H2O-RICH CRYOSPHERE IN MARS SUBSURFACE AND ON OTHER ICY PLANETARY BODIES. Alian Wang, Jie Wei, Lily Lu, and Kathryn Connor, Dept. Earth and Planetary Sciences and McDonnell Center for Space Sciences, Washington University in St. Louis ([email protected], Rudolph Hall, One Brookings Drive, St. Louis, MO, 63130, USA). A H2O-Rich Cryosphere: On Mars, ground ice was designed and conducted two sets of experiments. The identified in Polar Region by Phoenix lander [1], and results from first set of experiment was reported early was implied at high latitude region by the color change [13], which demonstrated: (1) the deliquescence of of “white” impact ejecta through orbital imaging [2]. Mg-, Ca-, Fe-, Al-chloride hydrates are heavily deIn equatorial region, a H2O-rich cryogenic environment pendents of temperature; (2) under the T and partial in subsurface sulfate-rich layer was suggested through water pressure (PH2O) relevant to Mars subsurface, the the observed color change of subsurface ferric sulfates rates of deliquescence of these chloride hydrates allow (Tyrone site at Gusev Crater), which indicates dehydrathe process to begin and to accomplish (to exhaust the tion happened after excavation and exposure to Mars tested quantity of chloride hydrates) within hours and current surface condition [3, 4]. days. These results support that the deliquescence of Cl was found existing in every sample of every surchloride hydrates can generate RSL phenomena within face exploration mission to Mars [5]. Putative chlothe time duration of RSL observed on Mars [14]. rides were suggested to exist in broad region of southThis abstract reports the second set of experiments, ern hemisphere on Mars [6]. Similar to sulfates, a thick that was designed to test if the rehydration of chlorides layer of chlorides at subsurface could maintain a lowcan happen through vapor-solid reaction at low-T reltemperature environment because their high thermal evant to Mars subsurface, i.e., if the recharging of RSL inertia, on the basis of a thermal profile model [7]. Fursource materials can happen during a local winter perithermore, chloride hydrates are the stable forms of od on Mars. chlorides at low temperature, such as CaCl2.6H2O Vapor-solid reaction at -78 °C: The experimental (antarcticite) [8]. setup is shown in Figure 1. MgCl2.6H2O was used as We would hypothesize a H2O-rich cryosphere withthe starting material. It was baked in a 200 °C oven for in the subsurface of Mars, made of ground ice layer at 48 hours. Gravimetric measurements before and after polar and high latitude regions, and of salt- or saltbaking confirmed the loss of 6.5 H2O per molecule enriched-regolith-layers at low latitude regions. One (including adsorbed H2O). Raman spectroscopic measpiece of puzzle in this hypothesis is the source materiurements before and after baking confirmed the total als of Reoccurring Slope Lineae (RSL) that has been transformation from MgCl2.6H2O to MgCl2, shown as observed during local summer season with large quanthe loss of Raman spectral features in 3000-4000 cm-1 tity and broad spreading at low-latitude region and for hydrates. Visually, original transparent equatorial area on Mars [9, 10]. MgCl2.6H2O grains (Fig. 2a) all transformed to We report here the results from a set of experiments opaque-white grains of MgCl2 (Fig. 2b) after baking. that has proved a critical concept in above RSL related For the low-T experiment, a reaction vial containstudy, i.e., the recharging of RSL source material at ing about 100 mg of MgCl2 was buried in dry ice (- 78 low-temperature (T) during local winter on Mars. The°C) in a foam box. This box-vial set was put into a se results have significant implication for Pressure Meter the process that might happen on all lowCO w/controlled P T planetary bodies, i.e., Mars, Moon, icy satellites, asteroids, and comets. Vacuum Chamber RSL & 1st set of Experiments: Based on the observations made by HiRISE and RH logger HO Flow CRISM, i.e., the tight correlation of RSL Dry Ice water bath at 35°C Control (-78°C) occurrence with temperature (T), the time duration of its occurrence and annually reExtra-dry CO Valves CO w/ wellcontrolled P occurrences, the regional correlation with Flow To Vacuum Pump putative chloride deposits, and the lack of Control VIS-NIR spectral features at RSL sites [9, Extra10, 11], we hypothesized the source matedry CO mix rials of RSL to be the subsurface chloride hydrates [12]. To test this hypothesis, we Fig. 1. Vapor-Solid Reaction at -78 C Experiment al Setup H2O Sample 2 2 2 2 H2O 2 46th Lunar and Planetary Science Conference (2015) 2483.pdf Thus, the recharging of RSL source materials annually during local Opaquewinter period is alwhite grains TransH2O ice lowed by thermodycrystals namics and kinetics. It means that the a. MgCl2.6H2O b. MgCl2 subsurface salt- or MgCl2.xH2O salt-rich-regolith c. Reaction at -78 °C d. Raman at -78 °C layers could function like a “cold-trap”, to op-wh MgCl2.yH2O react with atmospheric H2O vapor trans Reaction vial that is moving from MgCl2.zH2O dry ice Reaction vial polar region to midlow latitude regions 4000 3800 3600 3400 3200 3000 dry ice beneath a foam Raman Shift (cm-1) during local winter e. Image of reaction products period on Mars. This vapor-solid reaction would rehy(after 2 hours at Room T) drate the chlorides, which were formed during the local op-wh summer period on Mars by dehydration. The chloride trans hydrates formed in such way will be the source materials for RSL to reoccurring in next local summer. Future works: These two sets of experiments are op-wh trans proof-of-concept studies, which demonstrated the corvacuum chamber where a low vacuum near PMars was rectness of our hypothesis, and the feasibility of our maintained. H2O vapor generated from a H2O bath experimental design. A detailed systematic experi(maintained at 35 °C, thus a controlled PH2O) was mental investigation is designed and will be proposed. mixed with a flow of dry CO2 and was input into the It will concentrate on the thermodynamics and kinetics chamber. A delegate balance between the chamber properties of specific chloride hydrates that are stable pressure P and PH2O was maintained. A logger for temat lower T thus having even higher hydration degrees. perature and relative humidity (RH) was kept in the The study on reaction rates (for deliquescence and rechamber during the whole experimental duration. hydration) will be emphasized. Furthermore, experiThe vapor (H2O)-solid (MgCl2) reaction was conments to reveal the PH2O values (i.e., H2O availability) ducted for about 6 hours, and repeated three times in at low-T within an environment filled with chloride three days. Similar results were obtained each day, hydrates and sulfate hydrates will be conducted, for the which include: (1) the visual observation during the purpose to evaluate the habitable potentials of icy-saltexperiment at -78 °C through the transparent wall of environments, within Mars subsurface and on other icy vacuum chamber that indicated the formation of some planetary bodies as well. transparent grains at the surface of MgCl2 grains in the Acknowledgements: Thanks for NASA funds, reaction vial (Fig. 2c); (2) Raman spectroscopic meas#1295053 & NNX13AM22G, and for the discussion urements at -78 °C (Fig. 2d) of the reaction products with A. McEwen, J. Head, J. Dubessy, I-Ming Chou. that show various Raman peaks of chloride hydrates References: [1] Smith et al., (2009) Science, 325, p58. [2] (MgCl2.xH2O, x=TBD, Fig. 3); (3) gravimetric measByrne et al., (2008) Science, 325, p1674. [3] Wang et al., urements of the reaction products that indicate the (2008) JGR, 113, E12S40.[4] Wang and Ling (2011) JGR, gaining of 0.7 to 1.3 H2O molecule per MgCl2 mole116, E00F17. [5] Clark et al., (1978) JGR, 87, p10059. [6] cule from three repeating experiments; (4) optical miOsterloo et al., (2008) Science, 319, p1651. [7] Mellon et al., croscopic images of reaction products (after keeping at (2004) ICARUS, 169, p324. [8] Dubessy et al.,(1982) Chem. room T for two hours) that show mixtures of transparGeology, 37, p137. [9] McEwen et al., (2011) Science, 333, ent grains with opaque-white grains (Fig. 2 e). p740. [10] McEwen et al., (2013) Nature GeoScience, These results demonstrated the formations of chloDOI:10.1038; [11] Ojha et al., (2013) GRL, 40, p5621. [12] ride hydrates via a vapor-solid reaction at a temperaWang et al., (2013) 44th LPSC, abs #2606. [13] Wang (2014) ture within the T range that can be maintained by a 8th Mars, abs #1058. [14] Wang et al., (2014) GSA, abs # thick layer of salt at nowadays Mars subsurface. 248005. Figure. 2 48 h @ 200 °C Fig. 3 Raman spectra of reaction products at -78 C (MgCl2.xH2O, where x can be 2, 4, 6, 8, 12 that needs a systematic experimental study)
© Copyright 2026