14 march, 2015 - Empower School of Health
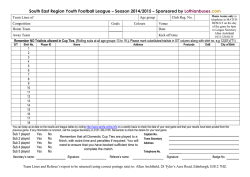
AGENDA CERTIFICATE COURSE IN ENSURING PRODUCT QUALITY IN PROCUREMENT AND SUPPLY CHAIN MANAGEMENT 09 – 14 MARCH, 2015; BANGKOK (THAILAND) TOPICS DAY WISE DAY & DATE TIMINGS 09:30-11:30 11:45-13:00 13:00-13:45 Introduction to Quality Assurance of Day 1: 09 Mar Pharmaceuticals and Monday Health Products 13:45-14:45 14:45-17:00 17:00-17:30 09:30-11:30 QA in Practice Tier 1 inspections, reviews and sampling Day 2: 10 Mar Tuesday QA in practice, Testing National QA Policy and Day 3: 11 Mar Plans Wednesday 11:45-12:45 12:45-13:45 13:45-14:30 14:45-16:00 16:00-17:00 17:00-17:30 09:30-11:00 11:15-13:00 13:00-13:45 13:45-16:00 16:00-17:00 17:00-17:30 09:30-11:30 11:45-13:15 National QA Policy, Plans and Manuals Maintaining Product Quality Throughout the Supply Chain Day 4: 12 Mar Thursday 13:15-14:00 14:00-15:30 15:30-17:00 17:00-17:30 09:30-11:00 11:15-12:45 Day 5: 13 Mar 12:45-13:30 Friday 13:30-15:00 15:00-17:00 17: 00-17:30 09:30-12:00 Human Resource Requirements Day 6: 14 Mar Saturday 12:00-12:15 12:15-12:45 12:45-13:30 13:30-14:30 SUB TOPICS SESSION WISE General course introduction; student and faculty introduction Relationship of this course to other PSM activities Initial PSM QA knowledge and experience assessment (MCQ) Introduction to Product Quality Assurance within the Procurement Supply Chain Lunch Break Product Quality Standards needed for Procurement and PSM QA ---Role of standards in PSM: What standards are available: Lack of Global Standards for health commodities : Using DTCs to select standards ---How to reference a standard in procurement, tender and contract documents: PSM QA monitoring and product standards Rational QA a Total system approach using the WHO 3 Tier system ‘Policing’ approach to QA: Applying QA concepts throughout the PSM cycle: The WHO 3 Tier system: screen a lot; test a little; assay very little Discussion and feedback Visit to delivery bay of receiving goods warehouse, Tier 1, practical visual inspection of pharmaceutical shipment Tier 1, Case Study, review of manufacturer/supplier documents Sampling of heath products–theory–random numbers–sampling rates–chain of custody Lunch Break Visit to warehouse store – practical sampling of health products Types of testing, certified laboratories, WHO basic tests, Mini-Lab Discussion and feedback Visit to testing site – demonstration of Mini-Lab in use Introduction to Product QA Plans and Manuals Lunch Break Tier 2, Visiting to QA lab, demonstration of testing techniques Managing Product Recalls – case study Discussion and feedback Elements of a procurement plan (GF sample) Practical Exercise: write a National QA Policy and Plan QA Policy: Organize the process: Major decisions: Key Approaches Lunch Break Practical Exercise – write a National QA Plan Who will – do what and pay for what?: Sampling/testing rates: Monitoring Indicators Groups present key elements of their developed plan and policy Discussion and feedback Practical exercise – DTC – selecting the right products Procuring the right products Lunch Break Good Storage and Distribution Practices Practical exercise resolving storage and distribution problems Discussion and feedback Human Resource Requirements for PSM management and QA HR needed for QA and how weak and inadequate HR in PSM can increase QA problems; advocating for adequate HR in PSM MCQ post course assessment Discussion and feedback Lunch Break Graduation Ceremony The agenda topics, date and time estimates are subject to change. Any change in the schedule will be informed in advance through mail ENSURING PRODUCT QUALITY IN PROCUREMENT AND SUPPLY CHAIN OF HEALTH COMMODITIES Bangkok (Thailand); 09-14 March 2015 Background and Details about the QA Course Key Message of the course: Health Commodity QA is about a complete system management approach with processes to be incorporated throughout the entire PSM cycle. The essence of the approach is addressing QA as an integral and essential part of the management of the PSM process. The focus of QA should not be about testing and analysis (which, following the WHO recommendations should only form a small, if somewhat expensive, component of QA activities. What the course teaches: The course is introduced and presented with the concept of understanding the management and budgeting of the QA process and connects it with the necessary resource requirements--human, financial and capital (equipment). The course follows WHO guidelines for commodity QA activities and references all the prime WHO documents. These guidelines can be applied for QA policies of the Global Fund, World Bank, UN agencies, USAID, DfiD and other donors. What the course does NOT teach: The course does not teach detailed technical aspects of QA commodity analysis and operational testing procedures. Who would benefit from this course (recommended participants) Public Health professionals; supply chain / logistics professionals working in procurement, warehousing, distribution, policy, planning, quality assurance; treatment activists at national and local level; civil society representatives; pharmacists; QA and laboratory professionals. The course has been developed for Principal Recipients and Sub Recipients of Global Fund; National Public Health programmes (HIV, TB, Malaria, and RMNCH); procurement and supply chain departments of Ministry of Health; procurement agents and NGO’s. General information: The course is intense and the participants need to be present throughout the scheduled times. Additional selfstudy may be required in the evenings for some topics. Participants are encouraged to bring examples from their own organizations/countries and these can be used for case studies and group work. Course tutors are available to discuss any specific queries prior to the course, throughout the course period and for 12 months after the course. Expected Outcome for trainees •An improved understanding of how to manage quality assurance of health commodities. •The ability to formulate plans for integrating product QA activities into overall PSM activities, with guidelines for estimating resource needs and cost estimates. Language: Participants need to be functionally literate in English Language. Background and details about the QA Course, Bangkok (Thailand); 09-14 March, 2015 DETAILED QA COURSE TOPICS INTRODUCTION TO QUALITY ASSURANCE OF PHARMACEUTICALS AND HEALTH PRODUCT SUB TOPIC COMMENTS Setting the course outline and expectations. Introducing the key concept: QA as an integral part of the PSM process. General course introduction A simple MCQ exercise to establish the overall average knowledge level of the trainees, which is used to adjust the speed and detail level of topic delivery Bringing the key message that QA issues must be incorporated throughout the Introduction to Product Quality PSM management process; and that QA really can be effectively and efficiently Assurance within the managed as part of PSM management Procurement Supply Chain This session does NOT require pharmacopeia knowledge or ISO experience The key approach is to understand the role that standards can play, their limitations, and most importantly, how to manage their use It describes how to manage the use of, and correctly apply, existing international product standards in PSM systems – especially in procurement, tender and contract documents It describes how the technical process of product standards selection can be effectively managed using Drug and Therapeutics Committee (DTC) Product Quality Standards needed Key Concept: Need for total PSM system approach in QA for Procurement and PSM QA Key Concept: Product QA is NOT about testing/analysis – it is primarily about PSM management Key Concept: Prevention is better than cure – PSM system design to prevent/limit QA problems – using a ‘policing’ approach Focus – using ABC, VEN, critical products mechanisms, to focus scarce resources on the most challenging items Explaining the WHO recommended 3 tier approach to product QA for cost control and effective management QA IN PRACTICE TIER 1 INSPECTIONS, REVIEWS AND SAMPLING SUB TOPIC COMMENTS Explaining the WHO Tier 1 classification of ‘screening’ by using visual inspections Trainees will not be required to undertake technical visual product inspections themselves – though the pharmacists will be welcome to participate if they wish. Rather, trainees should understand the management of the process, the Visit to delivery bay of receiving organization and detailed procedures required, and the resource – especially HR goods warehouse, Tier 1, practical – needed visual inspection of Trainees will be guided through the manufacturer WHO format certificates pharmaceutical shipment documentation review process using checklists sampling, types of testing Explaining the background to sampling of health commodities – the different rates of sampling depending on the source of the products – and the need to operate chain of custody mechanisms and documentations An explanation of the different categories of product testing, which can be used, when to use each type, costs of testing and management of the testing process Types of testing (certified laboratories, WHO basic tests, Mini Lab) Background and details about the QA Course, Bangkok (Thailand); 09-14 March, 2015 QA IN PRACTICE, TESTING NATIONAL QA POLICY AND PLANS SUB TOPIC COMMENTS Trainees will not be required to undertake analysis themselves – though the laboratory professionals will be welcome to participate if they wish. Rather, Visit to testing site – demonstration of Mini-Lab system trainees should understand where such tests can be undertaken – e.g. at import in use receipt points – their ease of use and rapid results, together with the resources – especially HR – needed and cost An outline of the concept and importance of national heath commodity policies, Introduction to Product QA Plans plans and manuals and Manuals An explanation of the GF QA policy requirements Trainees will not be required to undertake analysis themselves. Rather, trainees Tier 2, Visiting to QA lab, should understand the connection with the chain of custody requirements; demonstration of testing range of tests that are undertaken; the application of product standards; techniques together with the resources – especially HR – needed and costs A guided description of a product recall scenario, emphasizing the need to have established procedures in place before recalls occur; a clear delineation of Managing Product Recalls – case study exactly who will do what; checklists; tracking procedures and managing the need for urgency whilst ensuring correct stock control practice NATIONAL QA POLICY, PLANS AND MANUALS SUB TOPIC COMMENTS Trainees will not be required to write an entire National QA policy/plan – that is not practical in the time available. Rather they will be guided through managing Practical Exercise – write a National QA Policy and he process of developing and maintaining a national product QA policy and Plan QA Policy implementation plan; the key decisions that are required; the major options - Organize the process available; estimating the funding costs for QA implementation and how the whole process should be monitored - Major decisions - Key Approaches Global Fund requirements for QA policies and plans are explained during the process MAINTAINING PRODUCT QUALITY THROUGHOUT THE SUPPLY CHAIN SUB TOPIC COMMENTS Explain how to guide DTC product selection to consider QA factors – especially Practical exercise – DTC – selecting stability and deteriorating during storage and transport – so as to minimize the right products potential QA problems during the supply chain process Incorporating QA issues into procurement practice Procuring the right products Cost benefit analysis of secure sourcing versus QA costs Good Storage and Distribution Explanation of the WHO guidelines for GSP and GDP and how they affect QA Practice issues HUMAN RESOURCE REQUIREMENTS SUB TOPIC COMMENTS Human Resources required for QA activities and effects of weak and underHuman Resources resourced PSM systems on QA issues Final MCQ to measure average improvement in product QA knowledge following completion of the course MCQ post course assessment Graduation Ceremony Background and details about the QA Course, Bangkok (Thailand); 09-14 March, 2015
© Copyright 2026