LPBE kit manual
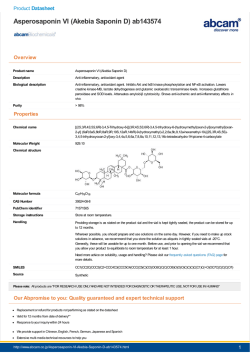
Project Directorate on Foot and Mouth Disease (FAO Regional Centre for South Asia) Mukteswar India 263138 Indian Council of Agricultural Research Instruction manual |Liquid Phase Blocking ELISA Kit (LPBE - FMD Kit)| Application: Estimation of antibodies against structural proteins of FMD virus serotypes O, A and Asia1 in all FMD susceptible species Reagents for testing minimum of 165 serum samples Store at; -20ºC Expiry: Use within 6 months after date of manufacture Page 1 PDFMD|ISO9001:2008| www.pdfmd.ernet.in Page 2 CONTENTS S. No Title 1 Page No. 02 Background 2 03 Kit Components 4 04 Other reagents/ equipments required (not provided with kit) 5 05 Before start of test 6 06 Precautions 7 07 Page 3 Liquid phase blocking ELISA Page 1 Background Quantification of protective antibody levels against FMD in animals following vaccination is essential for effective implementation of vaccination based FMD control programme in the country. Generally protective antibody level is expressed as log10 titer and titer of 1.8 is accepted as the protective level. To determine the titers of antibodies, conventionally serial two fold dilution method of test sera is performed and named as liquid phase blocking ELISA. In this assay, test serum sample is mixed with equal volume of a constant dose of viral inactivated antigen in a liquid medium and allowed to react. The antigen-antibody reaction is carried out in a suspension (or liquid medium), and the antigen is blocked by the homologous antibodies, if present, in the test serum for subsequent detection by guinea pig serum. The antigen molecules which are not completely blocked by the antibodies in the test serum are trapped to the wells of the ELISA plates with the pre-coated typespecific rabbit antibodies. Antigen and background controls are added containing antigen without serum and blocking buffer respectively as described in plate layout. Subsequently, the bound antigen is traced by sero-type specific guinea pig serum and anti-guinea pig-HRPO conjugate and substrate reaction is followed in a standard ELISA procedure. Color reaction is stopped and measured in terms of optical density (OD) at 492 nm wavelength with reference to 620nm. OD of reference antigen controls are used for normalization of data in terms of percentage inhibition and titer is calculated as the log10 of the inverse dilution at which percentage inhibition is 50%. With this kit 11 serum samples (in duplicate) can be tested in an ELISA plate for one serotype. Software for titer estimation is Page 2 included in the kit. Kit Components for 165 serum samples Reagents S No 1 96-well Nunc Maxisorp ELISA plates Quantity Number of vials/ kit 15 - Lyophilized ready-to-use Type specific anti-146S FMDV Anti-FMDV coating serum. One vial for three plates 5 vials/ serotype 3 Lyophilized ready-to-use Type specific anti-146S FMDV Anti-FMDV tracing serum. One vial for three plates 5 vials/ serotype 4 Lyophilized ready-to-use inactivated Type O, A, and Asia-1 antigens. One vial for three plates 5 vials/ serotype 5 Rabbit/ goat anti-guinea pig immunoglobulin HRPO conjugate (DAKO) One vial for three plates 15 vials 6 Substrate: OPD (Orthophenylenediamine dihydrochloride, Sigma) One vial for three plates 15 vials 7 Lyophilized ready-to-use specific Serum Controls Set of eight vials for each serotype 24 vials 8 Lyophilized Blocking Buffer One vial in a kit One vial 9 Phosphate buffer saline One sachet of one liter One sachet 10 Tween-20 10ml 10ml serotype Page 2 1 (Nunc cat. No. 442404) Other reagents/ equipments required (not provided with kit) 1. Precision pipettes: multi channel pipettes variable range from 50 to 200µl & Single channel pipettes variable range from 1 to 20µl, 20 to 100µl, 50 to 200µl and 200 to 1000µl along with disposable tips. 2. Double Distilled water 3. 1M H2SO4 4. 30% Hydrogen Peroxide 5. Wash bottle fitted with Immunowasher 6. 1 container: 1 to 2 liters 7. ELISA plate reader, 492nm filter attached with computer 8. Refrigerator: range of -20ºC to +4ºC 9. Incubator: warm wall incubator maintained at +35ºC to 37ºC. 10. pH meter: with accuracy of 0.01pH units or good quality pH strips with varying range from 2 to 10 can be used. 11. Glassware/ plastic ware: flasks (50-5000ml), graduated cylinders (10-2000ml), graduated Page 2 pipettes (1-100ml). Before start of the test Note: Equilibrate all the reagents provided in the kit at room temperature 1. Preparation of solutions/ reagents S No Reagents Reconstitute with Reconstitute Volume 1 Lyophilized ready-to-use Type specific anti-146S FMDV Anti-FMDV coating antibodies. DDW 18ml/ Vial 2 Lyophilized ready-to-use Type specific anti-146S FMDV Anti-FMDV tracing serum. PBST-0.1% 18ml/ Vial 3 Lyophilized ready-to-use inactivated Type O, A, and Asia-1 antigens. PBST-0.05% 18ml/ Vial 4 Lyophilized ready to use Rabbit/ goat anti-guinea pig immunoglobulin HRPO conjugate (DAKO) PBST-0.1% 18ml/ Vial 5 Lyophilized ready-to-use Substrate: OPD (Orthophenylenediamine dihydrochloride, Sigma) DDW 18ml/ Vial 6 Lyophilized ready-to-use specific Serum Controls DDW 100µl/ Vial 7 Lyophilized Blocking buffer PBST-0.05% 10ml serotype 2. Washing buffer: dissolve one PBS capsule in 1000ml of distilled water, check pH and if necessary adjust to pH 7.4 with 3M NaOH or 1M HCl. Add tween-20 to final concentration of 0.1% and mix it well. Page 3 3. PBS 0.05% tween: add tween-20 in PBS to final concentration of 0.05%. Precautions: 1. Use the prescribed format for ELISA only 2. Do not intermix the reagents from different batches 3. All the glass wares to be used should be clean and sterile. 4. Slight drop in pH (less than 7.2) in washing buffer can adversely affect antigen antibody reaction. Ensure correct pH every day before use of all buffers. 5. Microbial contamination/ precipitation formation of any degree in any of the reagents/ buffer will very much reduce the specificity of the test. 6. Mark the plate orientation properly with water proof marker before starting the test to avoid any confusion which may arise later. 7. Do not stack plates one over other in incubator. 8. Prepare fresh substrate solution every time. 9. Do not store prepared buffers more than 15 days. Page 4 10. Check pH of every buffer every day before starting the test. Liquid phase blocking ELISA Procedure: 1. Coating of ELISA plates: Reconstitute the ready-to-use Lyophilized serotype specific coating antibodies with 18ml of DDW and coat three plates by adding 50µl of the reconstituted solution in each wells of ELISA plate. Incubate the ELISA plates at 37ºC for 1 hour followed by incubation at 4ºC overnight. 2. Preparation of Ag-Ab mixture: Prepare two-fold dilutions (4 dilutions starting from 1:16) of test serum samples in a low binding Perspex plate with PBS containing 0.05% Tween-20. Distribute 75 µl each dilutions of the serum sample to four separate (labeled O, A, and Asia1) Perspex plates. Reconstitute the antigen vial with 18ml of PBST0.05% and add equal volume of diluted antigen (75µl) to all the dilutions of test serum samples. For antigen control, add equal volume of PBS-Tween 20 (0.05%) to the already diluted antigen. The dilution of each antigen and antibody in this step will be strictly made with PBS containing tween-20 (not the PBS containing 0.1% tween-20 as used as washing buffer for washing of the plate). Incubate the diluted antigen and antibody mixture at 4ºC overnight without disturbance. 3. Washing of ELISA plates: Wash the wells of ELISA plates by adding washing buffer (200-300µl) using immune-wash and discard the contents of wells by abrupt downward hand motion. Repeat the washing 3 times with 3 minutes of hold period between each wash. Slap the inverted microplate 3-4 times onto a dust free absorbent pad to remove all residual contents in the wells. 4. Dispensing of antigen-antibody (Ag-Ab) mixture: Dispense 50µl of the overnight incubated Ag-Ab mixture (Step 2) in duplicate to the ELISA plates as instructed in the plate lay out (Figure 1). Incubate the plates at 37°C for 1 hr. Add 50µl of the reconstituted blocking buffer to the wells of background control. 5. Wash the plate as in the step 3. 6. Tracing: Reconstitute the tracing serum vial with 18ml PBST0.1% and add the 50µl of reconstituted serotype specific tracing serum to each serotype specific plate. Incubate at Page 5 37°C for 1 hr. Wash the plate as in the step 3. 7. Conjugate: Reconstitute each vial of anti-guinea pig-HRPO conjugate with 18ml (for nine plates use three vials) PBST0.1% and add 50µl to all the wells. Incubate at 37°C for 1 hr. Wash the plate as in step 3. 8. Substrate: Reconstitute substrate vial just before use with 18ml DDW (for nine plates use three vials) and add 9.6µl of 30%H2O2 solution (or 28.8µl in 54ml for 3 vials) and add 50µl to each well of the plate and incubate at 37°C for 15 minutes. 9. Stop the color reaction by adding 50µl of stopping solution. Read the OD in the wells at 492 nm. 10. Transfer/ convert the OD sheet data into MS Excel format and analyze and interpret the results using PDFMD ELISA Analyst v2.0 as per the instructions. Figure 1: LPBE plate Format for addition of diluted test serum samples in ELISA plate (TS* Test Page 6 sample) Trouble shooting: 1. Invalid Antigen control: • Check the batch and expiry • Cross mixing of serotype specific reagents • Check the washing buffer and PBST0.05% for pH and contamination • Cross check the temperature of incubator 2. Invalid Background control • Improper washing Cross contamination of tips used for conjugate and substrate solution. • Not using the blocking buffer provided. Page • 7 3. Even OD in all the wells Notes ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. ………………………………………………………………….. Page 8 ………………………………………………………………….. PDFMD|ISO9001:2008| www.pdfmd.ernet.in Page 9 Contact: Project Director Project Directorate on Foot and Mouth Disease Mukteswar, Nainital Uttarakhand 263138 India Email: [email protected], [email protected] Phone: 05942286004; Fax: 05942-286307 Website: www.pdfmd.ernet.in Facebook: https://www.facebook.com/pages/ Project-Directorate-on-Foot-and-Mouth-Disease
© Copyright 2026