Red Blood Cell Glycophorins
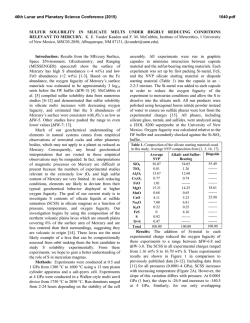
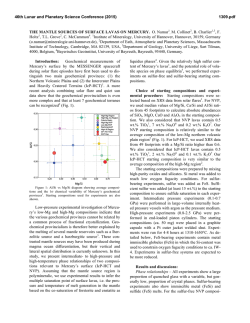
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. REMEW ARTICLE Red Blood Cell Glycophorins By Joel Anne Chasis and Narla Mohandas G r e ~ e a t s . 2 ~Although 3~~ the genomic structure of GPE is LYCOPHORIN-A (GPA), GPB, GPC, and GPD well-characterized, search for the protein encoded by this constitute a group of red blood cell (RBC) transmemgene has yielded negative results. All the expressed glycophbrane proteins that, although perhaps not widely appreciorins are 0-glycosylated proteins with their amino terminal ated in clinical hematology, have been important players in domains exterior to the lipid bilayer and a single membranethe fields of membrane biochemistry and cellular biology spanning domain. GPA and GPB share extensive sequence for several decades. GPA was the first membrane protein to homology with one another, while GPC and GPD are be sequenced1,2and has subsequently served as a model for closely related proteins-with no structural homology to topology of receptors and other transmembrane glycoproGPA and GPB. teins in both erythroid and nonerythroid cells. Initially, In this review we will discuss the genomic organization hematologic interest in the glycophorins was limited to and primary structure of cDNA and protein for each of the blood bank serologists and the characterization of blood glycophorins, with particular emphasis on the relationship group antigens located on these sialoglycoproteins. Howof these biochemical characteristics to the functional role of ever, with emerging data from functional studies, it is the proteins in the cell membrane. Recent molecular becoming apparent that certain glycophorins play imporbiologic analyses have also provided a detailed characterizatant, but differing, roles in regulating RBC membrane tion of the primary structure of naturally occurring mutant mechanical properties and in maintaining RBC shape. forms of these molecules. This information, in association Because several of these glycophorins are also expressed in with biophysical studies of normal and mutant RBCs, has various nonerythroid tissues, the functional importance of enabled us to begin to understand the contributions of their interactions with the membrane skeleton may have a glycophorins to the regulation of membrane material behavwidespread biologic significance. ior. In the sections that follow we will describe these recent The presence of glycophorins in the RBC membrane was studies and the new insights that they have stimulated. initially detected by Fairbanks et aL3 The four varieties of glycophorin comprise approximately 2% of the total RBC GPA AND GPB membrane protein, with GPA as the major component Structural characterization of GPA. Because of extensive present at 5 to 9 x lo5 copies per cell, while the less similarities in genomic organization, it appears that the abundant GPB, GPC, and GPD are present at 0.8 to 3 x genes for GPA and GPB arose from a common ancestral lo5, 0.5 to 1 X 105, and 0.2 x lo5 copies per cell, gene through homologous recombinant events involving re~pectively.4-~ Because of their high sialic acid content, A h sequences. The GPA gene, located on chromosome these molecules account for approximately 60% of the 4q28-q31,25,26 contains 7 The first exon, as well as RBC’s negative surface charge. As such, they play a pivotal part of the second, encode a cleavable leader peptide. The role in modulating RBC-RBC interactions, as well as RBC exoplasmic domain of GPA, composed of 70 amino acid interactions with the vascular endothelium and other circuresidues (Fig l), is encoded by the second through fourth lating blood cells. Over the past 2 decades, an unfortunate exons with the codons for M- and N-blood group antigens confusion was created in the glycophorin field by the contained in the NHz-terminal26 residues encoded by exon appearance of four different nomenclatures (Table l).3,6,s-10 2. M- and N-phenotypes differ from one another at amino Fortunately for us, a consensus has recently been reached acid residues one and five, with the N-phenotype containing among the investigators in the field, who have agreed to leucine at residue 1 and glutamic acid at residue 5, while the designate the various glycophorins as GPA, GPB, GPC, and M-phenotype is characterized by serine at residue 1 and GPD. A membrane-spanning domain of glycine at residue 5.9,z9,30 Protein, cDNA and genomic sequence analysis have 22 amino acids and a cytoplasmic domain of 39 residues are provided a detailed characterization of the primary strucencoded by exons 5 and 6, respectively. With its cleavable ture of GPA, GPB, and GPC.1,2J1-1s The primary structure signal peptide and single membrane-spanning domain, of GPD is currently under study, but immunochemical and biochemical data imply that this is a protein closely related to GPC. Although these four sialoglycoproteins share the From the Division of Cell and Molecular Biology, Lawrence “glycophorin” name, suggesting a common genetic origin, Berkeley Laboratory, University of Califomia, Berkeley; and the Department of Medicine, University of California, San Francisco. this is partially a misnomer, because recent molecular Submitted June 2, 1992; accepted June 15, 1992. biologic studies have firmly established that three of these glycophorins constitute different gene p r o d u ~ t s . *GPA, ~ , ~ ~ , ~ ~ Supported by National Institutes of Health Grants No. DK26263 and DK32094 and by the Director, office of Health and EnvironmenGPB, and GPC are encoded by three different genes on two tal Research Division of the US Department of Energy, under contract different chromosomes. GPC and GPD do, however, apno. DE-ACO3-76SF00098. pear to be closely related, arising from the same gene Address reprint requests to Joel Anne Chasis, MD, Lawrence through use of alternative translation initiation sites.21,Z2 Berkeley Laboratoiy, MS 74-157, I Cyclotron Rd, Berkeley, CA 94720. Recently, a novel GPE gene was isolated that might have This is a USgovemment work. There are no restrictions on its use. evolved from GPA by homologous recombination at Alu 0006-497119218008-0008$0.00/0 Blood, Vol80, N o 8 (October 15). 1992: pp 1869-1879 1869 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1870 CHASIS AND MOHANDAS Table 1. RBC Membrane Glycophorins Alternative nomenclature Chromosomal location Copies per cell ( x 10-1) Apparent molecular mass (Kd) Amino acid residues Oligosaccharide side chains 0-linked N-linked Blood group antigens RBC specific GPA GPB GPu PAS-1 GP6 PAS-3 GPC GPD GPB PAS-2 4 4 2 500-900 80-300 50-100 36 131 20 72 32 128 15 1 11 0 MN Yes Yes 12 1 Ge:3 No ss 2 20 - 23 107 -6 0 Ge:2,3 Unknown GPA is characterized as a class I transmembrane protein. Analysis of the secondary structural organization of GPA based on circular dichroism spectra and conformational prediction from primary structure suggests that the molecule is about 20% p sheet.3I." One short stretch of p sheet, composed of residues 90 through 93, is of particular interest, as it may play a role in the formation of GPA dimers, which are the predominant species in the native membrane. This segment of p sheet lies closely adjacent to the helical region within the bilayer; formation of intermolecular parallel p sheets between the two monomers could then control alignment and packing of the helical regions of the two molecules in the bilayer.32 Structural characterization of GPB. Five exons of the GPB gene27.2slocalized to chromosome 4 encode a 70 amino acid class I transmembrane protein that possesses significant structural similarity to GPA (Figs 1 and 2). The nucleotide sequences of exons 1 through 5 of the GPB gene are very similar to exons 1 through 5 of the GPA gene; however, the 3' proximal sequences differ. The NH2terminal 26 amino acids of GPB, encoded by exon 2, are homologous to those of GPA molecules of the blood group antigen N-phenotype. Although GPB genomic DNA conI I I31 GPA NH2 COW I I I I GPB I GPD 361 Iy) 107 COW N% I I Fig 1. Schematic representation of extracellular, intramembranous and cytoplasmic domains of GPA, GPB, GPC, and GPD. GPA and GPBamino acids 1-26, homologous (solidareas); GPA71-101 and GPB 34-72, strikingly similar (very heavily shaded areas); GPA 58-71 and GPB 26-34, some homology (heavily shaded areas); GPA 26-58 and 101-131. no homology (open areas). GPC amino acids 1-128 and GPD 1-107. homologous (lightly shaded areas). Extracellular domains are on the left side of the bilayer and cytoplasmic domains on the right side of the bilayer. tains nucleotide sequences quite similar to GPA exon 3 and its flanking introns, these sequences are not expressed in GPB messenger RNA (mRNA). The cDNA of GPB thus lacks nucleotides that encode residues 27 through 55 of GPA, which includes the site at which GPA is N-glycoslated. The extraccllular domain of GPB, encoded by exon 4, expresses the Ss-blood group polymorphism at amino acid residue 29, with mcthionine and threonine imparting S-and s-phenotypes, respectively.I2 Thc transmembrane domain of GPB encoded by exon 5,like that of GPA, contains about 20 hydrophobic amino acids. In both sialoglycoproteins, the cytoplasmic-transmembranejunction contains 3 to 4 basic amino acids, which function as stop transfer signaP and, in addition, may interact with the phospholipids to anchor the proteins in the bilayer.34 Of potential functional significance is the fact that the cytoplasmic domain of GPB is shorter than that of GPA, containing only 3 residues in addition to the 3 membrane-anchoring amino acids. Structural characterization of GPE gene. A new member of the GPA and GPB gene family has very recently been isolated and charactcrized.23.24.3.c Initially called invariant (inv) but renamed GPE, this gene may have evolved from the GPA gene in a fashion analogous to that postulated for the GPB gene. As both the GPE and GPB genes contain similar 3' sequences and 3' Alu repeats, they may have arisen from GPA by homologous recombination at A h repeats.24Although this newly discovered gene is effectively transcribed, there is to date no evidence of protein expression. cDNA sequencing studies show that the gene would encode a 78 amino acid protein containing a 19 residue leader ~ e p t i d e . 2The ~ ~ 29 ~ ~ N-terminal amino acids are identical to those of blood group M-type GPA, but residues 27 through 59 differ significantly from GPA and GPB. Comparison of genomic and cDNA sequence shows that the gene consists of 4 exons, with the nucleotide sequence of exons 1 and 2 homologous to GPA and GPB. In contrast, exon 3 differs from the GPB gene by several point mutations, a 24-bp insertion, and a stop codon that shortens the reading frame. However, in the region 3' of exon 3, the sequence of GPE is virtually identical to that of GPB. Along with the genes for GPA and GPB, the gene for GPE has been localized to chromosome 4 (Fig 2).24Based on genomic analysis of glycophorin variants, an interesting model has been proposed for the tandem organization of the three genes along chromosome 4 with the order GPA, GPB, and GPE.3s Deletion of structural genes within this region could position the promoter of one glycophorin gene upstream from the body of another glycophorin gene, thereby generating hybrid gene structures.3sIn support of this model are the observations that, except for a few point mutations, the sequences of both the promoter region and exon 1 of the GPA, GPB, and GPE genes are highly homologou~.~~ Moreover, the rare point mutations do not affect the potential cis-acting elements (CACC, NF-El, and NF-E2) that are present in the promoter region. Glycophorin variants. RBC membranes containing hybrid glycophorin variants and glycophorin deficiencies were initially identified by serologic and immunochemical assays, but have recently been characterized at the molecular level. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. RED BLOOD CELL GLYCOPHORINS -G P A - 1871 -WE- -0PB- Normal Mi V 5*4-j>,sY4-zzqf - - - - - - - - - - pbe> Y4-13 16 Fig 2. Alignmentof GPA, GPB, and OPE genes on chromosome 4 in normal and deletion variants. The first exon of each gene is separatedfrom the other exons by at least 15 kb. (- 4 Deletions. (Modified and reprinted with permission.=) - Among these variant phenotypes are several that have been of particular interest because of the insight they provide into the functional role of glycophorin in the membrane. This group of mutants includes Miltenberger V, in which the RBC membrane contains biochemically altered GPA m o l e c ~ l e s , ~ .as ' ~ well . ~ ~ as the En(a-) and MkMkphenotypes, in which the membrane is totally deficient in GPA.3X-40 The Miltenberger V gene, as a consequence of unequal crossing over between GPA and GPB genes, is composed of exons 1 through 3 of the GPA gene and exons 3 through 5 of the GPB gene (Fig 2).2R.3s.37 As a result, the glycoprotein encoded by the Miltenberger V gene is a hybrid molecule composed of the cxoplasmic domain of GPA (residues 1 through 58) fused to the transmembrane and cytoplasmic domains of GPB (residues 27 through 72). The extremely rare En(a-) phenotype characterized by membranes totally lacking in GPA results from several different mutations. In what is categorized as the Finnish En(a-) phenotype, En(Fin), the individual is homozygous for a complete deletion of the GPA gene with normal gcnes encoding GPB and GPE (Fig 2):' However, the English variant of En(a-), En(UK), is a more complex gcnctic story, in part, because the individual is assumed to be heterozygous for En(UK) and Mk. The presence of the Mk gene supresses the expression of GPA and GPB, due to deletion of both the GPA and GPB genes (Fig 2).".42 Although molecular biologic studies are still incomplete, conclusions from protein and DNA analysis suggest that the En(UK) gene is a fusion product formed from GPA and GPB genes, which encodes a hybrid glycophorin with the N-terminal domain of blood group M-type GPA and the C-terminal domain of GPB.4'-434sTogether, the Miltenberger V, En(Fin), and Mk mutations have been extremely Fig 3. RBC-RBC interactions. Rouleau formation (right hand panel), which occurs physiologically, resuits from weak cell-cell interactions, which temporarily overcome the repulsive negative surface charges but are readily dissociated. RBC aggregation (left hand panel) results from strong associations, such as those produced by Ig binding, and require much greater forces for cell dissociation. useful in studies defining the biologic functions of GPA, as described below. Biologic function of GPA. GPA expression is uniquely erythroid. Studies of multiple nonerythroid tissues and cell lines by both Northern analysis of RNA preparations and immunocytochemical analysis of cell surface expression have confirmed that GPA expression is restricted to the erythroid li11eage.4"~ During normal erythropoiesis, GPA can be detected on the surface of the proerythroblast, but not on the surface of the earlier burst-forming uniterythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) p r 0 g e n i t o r s . 4 ~Because ~ ~ ~ ~ the biologic function of GPA during terminal erythroid differentiation is entirely unknown, this review will focus on functional studies in the mature, circulating RBC. GPA, with its high sialic acid content, is the major contributor to the net negative surface charge of the mature RBC membrane. This underappreciated biologic role is critical for minimizing RBC-RBC interactions and preventing RBC aggregation (Fig 3). In circulation, the net aggregation energy between adjacent RBCs is determined by the balance between the aggregatingenergy from macromolecules bridging RBCs and the disaggregating energy produced by the mechanical shear stress and the electrostatic repulsive energy (as recently reviewed by Chien and Sung53).The net negative electrical charges contributed by sialic acid residues on glycophorin produce the electrostatic repulsive energy. Biochemical studies of two mutant phenotypes, En(a-) Finnish type and MkMk, suggest that a mechanism may exist in the RBC to maintain the surface content of sialic acid within a certain range. In both En(a-) and MkMkcells, which completely lack GPA,3xv39 an intriguing, associated surface change is an increased glycosylation From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1872 CHASIS AND MOHANDAS 06- 05- = d 04- t 9 03- 5 2 02- 01O 00- I 5 I 1 l I I 10 I 20 I 40 1 I I 60 1 1 1 1 80 1W I 150 Shear stress (dynes/cm') '1' 00 0 I I 50 I I I 100 I 150 I 1 200 Time (seconds) Fig 4. Membrane deformabilityand mechanicalstability of RBCs deficient in various glycophorins.(Left) RBC membranes deficient in GPC and GPD (-) required significantly higher values of applied shear stress as compared with normal membranes (shaded area) to reach equivalent deformation. The lines are parallel; thus, GPC- and GPD-deficient membranes required 2.5-fold higher shear stress than did normal membranes to reach equivalent deformation at all points along the curve, indicating that the membranes had 0.4 times normal deformability. In contrast, GPA-deficient I--) and GPB-deficient (- -) membranes required the same shear stress as normal membranes to reach equivalent deformation, implying normal membrane deformability. (Right) The deformability index of RBC membranes deficient in GPC and GPD I-) decreased more rapidly with time than normal RBC membranes (shaded area) when exposed to constant shear stress, implying decreased mechanical stability. In contrast, the rate of decline of deformability index of RBC membranes deficient in GPA (--) and GPB (- -) was normal, implying that these membranes had normal membrane mechanicalstability. (Reprinted with permission.w) - - of band 3 resulting from the addition of sialic acid moitie~.~ This ~ , posttranslational ~~,~~ modification of band 3 partially compensates for the loss of sialic acid residues on GPA. As a consequence, these mutant RBCs maintain a net negative surface charge that is only 20% less than normal, rather than a 60% decrease, which would be expected in a GPA-deficient RBC. Individuals with En(a-) and MkMk RBCs are clinically well. Thus, increased glycosylation of band 3 in En(a-) and MkMkcells functionally substitutes for the loss of GPA and accounts for the normal RBC behavior observed in these individuals. Mutations that result in GPA deficiency are extremely rare and we speculate that without concomitant increase in glycosylation of other membrane proteins these mutations may be incompatible with survival. Further support for the thesis that the erythrocyte harbors a mechanism for preserving surface glycosylation is provided by Dantu-positive erythrocytes. Although these cells lack 57% of their normal GPA, they contain a GPB-A hybrid molecule in a hybrid:GPA ratio of 2.4:1.55 Compensating for the increased glycosylation contributed by the glycophorin hybrid is a decrease in the glycosylated residues on band 3 . From this body of data, we conclude that maintaining its negative surface charge is crucial to the RBC and that the critical biologic function of GPA is to minimize cell-cell interactions in circulation. Characterization of the cellular consequences of En(a-) and MkMkmutations have also provided crucial leads for understanding the role of GPA in regulating membrane biophysical properties. Individuals with these two phenotypes have normally discocytic RBC morphology and no clinically significant anemia. When membrane material properties of En(a-) RBCs were characterized by ektacyt ~ m e t r y both , ~ ~ membrane deformability and membrane mechanical stability were found to be normal (Fig 4). These studies unequivocally show that GPA plays no role in regulating cell shape, membrane deformability, or membrane mechanical stability. Although the findings outlined above suggest that in its native state GPA does not regulate either cell shape or membrane material properties, a number of recent studies have shown that, following binding of a ligand specific for this protein, profound changes occur in membrane material behavior. Such an inducible change in membrane behavior initiated by ligand-receptor interaction is a form of signal transduction akin to ligand-induced changes in granulocyte and lymphocyte function. The study of this form of signal transduction in the RBC began with the initial observation that binding of the lectin, wheat germ agglutinin, to the RBC surface inhibited chemically induced echinocytic transformatiod7 and also markedly reduced RBC membrane def~rmability.~~ Subsequent studies showed that monoclonal antibodies (MoAbs) specific for the exoplasmic domain of GPA, as well as their monovalent Fab fragments, also decreased membrane deformability, confirming that this process was mediated by GPA and suggesting that the process involved a transmembrane communication rather than the formation of an external lattice of GPA molecules cross-linked by lectin or divalent IgG.5s The importance of the cytoplasmic domain in this transmembrane process was underscored by the finding that ligand binding decreased the lateral mobility of normal GPA molecules within the but did not change the lateral mobility of the Miltenberger V hybrid glycophorin A,6owhich has a significantly truncated cytoplasmic domain of only six amino acids (Fig 5, left and middle panel^).*^,^^,^^ Based on these findings, the following model has been proposed. In the native state, the cytoplasmic domain of GPA has little or no interaction with the skeletal network. However, ligand binding induces a conformational change in the cytoplasmic domain that results in its increased association with the From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1873 RED BLOOD CELL GLYCOPHORINS A iB Outside UPlD LIPID inside Normal BILAYER COOH Inside Mi V + R10 + 10F7 + + R10 10F7 Fig 5. Schematic diagram of normal and variant Miltenberger V GPA and the effect of R-10 and lOF7 on the relative rigidity of normal and Miltenberger V RBCs. (Left) Normal GPA with cytoplasmic domain containing 39 amino acid residues. (Middle) Hybrid Miltenberger V molecule composed of the exoplasmic domain of GPAfused t o the transmembrane and cytoplasmic domains of GPB and containing 6 amino acid residues in its cytoplasmic tail. (Right) The deformability of nonliganded normal cells (lane 1) and Miltenberger V cells (lane 4) were normal with a relative rigidity of 1. The relative rigidity of normal cells after R10 (lane 2) and lOF7 (lane 3) binding was 13.1 and 13.9, respectively. The rigidity of Miltenberger RBCs after R-10 (lane 5) and 10F7 (lane 6) binding was only minimally increased. These results imply that marked increases in antibody-induced rigidity require the presence of the cytoplasmic domain of GPA. (Reprinted with permission.62) membrane skeleton and a decrease in lateral mobility. As a consequence of this increased association, membrane deformability decreases (Fig 5, right panel). In the RBC, this form of ligand-induced signal transduction appears to involve only GPA molecules because binding of ligands specific for other RBC surface components, including band 3 and blood group antigens A, B, Rh, and Kell, does not alter membrane pr~perties.~*-~l An important feature of this ligand-induced membrane rigidity is that the extent of rigidity can be modulated by varying the site on the exoplasmic domain to which the ligand binds (Fig 6).62For example, binding of an MoAb to an epitope close to the N-terminus of GPA increased membrane rigidity 5.8-fold, binding to an epitope in the midregion of the exoplasmic domain resulted in a 10.8-fold increase in membrane rigidity, while binding to an epitope 9/43 20 -1 T 15- 10 t .-.. 0, c a, m $ 5 Control 9A3 10F7 R-10 B14 Fig 6. Schematic diagram of antibody binding sites on GPA and the maximum relative rigidity induced by antibody binding. (Left) The peptide and 1 N-linked (A)tetrasaccharide is shown traversing the lipid bilayer. The blood group antigens M and N are backbone with its 15 0-linked (0) determined by variations within the first five amino acids in the amino terminal end of the molecule. The MoAb 9A3 has anti-M specificity and binds t o the amino terminus. Antibodies R-10 and 1 0 R bind in the midregion of the extracellular portion of GPA distal t o the trypsin cleavage site. 814 detects an epitope closely adjacent t o the lipid bilayer, between residues 56 and 67. (Right) Antibody binding t o different regions of GPA induces different degrees of increase in membrane rigidity. Control M M RBCs with no bound antibody (lane 1) have normal deformability and relative rigidity of 1. Binding of 9A3 (lane 2) produces cells with a relative rigidity of 5.8 f 1.5; 10F7 (lane 3) cells with a relative rigidity of 10.8 f 1.4; R-10 (lane 4) cells with a relative rigidity of 10.8 f 2.1; and 814 (lane 5) cells with a relative rigidity of 18.2 f 2.7. (Reprinted with permission.6Z) From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1874 between residues 56 and 67 close to the bilayer increased rigidity 18-fold (Fig 6). While the mechanism for this modulatable process has not yet been characterized, we speculate that the site of ligand binding determines the extent of conformational change in the cytoplasmic domain. This modulatable process represents an intriguing mechanism whereby a single receptor could communicate across the membrane in multiple ways. Although these studies clearly establish a ligand-induced increased association of the cytoplasmic domain of GPA with the membrane skeleton, the specific nature of this association has not yet been characterized. What remains to be elucidated is whether the changes in membrane material properties result from a specific protein-protein interaction or whether the ligand-induced conformational change in the GPA cytoplasmic domain causes this region to become physically entangled with the dense underlying skeletal lattice. Two membrane proteins that GPA might specifically interact with after ligand binding are protein 4.1 and band 3. Anderson and Marchesi have reported that an association occurs between protein 4.1 and GPA that is modulated by phosphoin~sitides.~~ However, it is doubtful that this proposed interaction plays any role in the observed membrane changes because RBCs totally deficient in protein 4.1 become rigid after ligand binding.58Parenthetically, it is difficult to assign a physiologically relevant role for a GPA and protein 4.1 interaction in light of the observed normal membrane behavior of En(a-) cells completely deficient in GPA. A second candidate protein for interaction with GPA is the other major RBC transmembrane protein, band 3. Accumulated data suggest that at least some subpopulations of band 3 and GPA molecules are in close proximity within the bilayer. For example, antibodyinduced cross-linking of GPA molecules has been shown to affect the rotational mobility of band 3.64Moreover, studies on Wrb blood antigen expression showed that MoAbs specific for Wrb immunoprecipitated both GPA and band 3 and reacted by radioimmunoassay only with cells in which both of these proteins were e ~ p r e s s e d A . ~ novel ~ concept that emerges from these observations is that interaction between these two major integral proteins may contribute significantly to the biologic function of the RBC membrane. A number of bacterial antigens bind to carbohydrate residues on GPA.66,67An intriguing, but as yet untested hypothesis, is that such binding induces membrane rigidity that then stimulates increased entrapment and phagocytosis. In this scenario, GPA binding would provide a mechanism for enhanced antigen clearance. While much remains to be learned regarding ligand-induced signal transduction and GPA-band 3 associations in the membrane, it can be stated with certainty that a major physiologic function of GPA is to bestow negative surface charge to the membrane, thus minimizing cell-cell interactions. We have limited our discussion in this section to GPA because, to date, no biologic function has been ascribed to GPB other than that of carrying blood group antigens Ss and U. CHASE AND MOHANDAS GPC AND GPD Structural characterization of GPC and GPD. Unlike GPA and GPB, which are encoded by two distinct genes, GPC and GPD are encoded by a single gene located on chromosome 2q14-q21.1y,21 Although GPD contains a truncated amino terminal domain, the remaining polypeptide (residues approximately 21 to 128) is identical to that of GPC (Fig l).18,68,69 While the mechanism of production of these two proteins from the same gene is currently under active investigation, evidence gathered to date suggests that these polypeptides arise from the use of two different translation initiation sites (AUGs) within the same reading frame through a leaky scanning mechanism. Translation initiated at the first AUG generates GPC, while initiation at the second AUG gives rise to GPD.4y Structurally, the GPC gene is organized (over 13.5 kb) into four exons with exons 1 through 3 encoding the extracellular domain and exon 4 encoding both the membrane spanning and carboxy-terminal domains.70Interestingly, exons 2 and 3 are within a 3.4-kb DNA fragment containing two repeated domains with less than 5% nucleotide sequence d i ~ e r g e n c e These . ~ ~ two exons vary from one another only in that exon 3 contains 27 additional nucleotides, which encode residues 42 through 50 of the exoplasmic domain. Therefore, it appears likely that these tandem repeated domains result from duplication of a region of an ancestral gene. In contrast to GPA, GPC does not express a cleavable signal sequence and thus belongs to the type 111 class of membrane protein^.^^,^^ cDNA analysis suggests a protein 128 amino acids in length. The extracellular amino terminal domain, composed of 57 hydrophilic residues, contains 12 0-glycosylation sites and one N-glycosylation site,13 as well as the Gerbich (Ge:3) blood group antigens (residues 41 through 50).71The membrane spanning domain contains 24 nonpolar residues (58 through 81), while the cytoplasmic domain is composed of 47 residues (82 through 128). It may be functionally significant that the cytoplasmic domains of GPC and GPD are the longest of the four glycophorins. Several variant forms of GPC initially identified by immunochemical and serologic have recently been characterized on a molecular leve1.21,22,75 These variant phenotypes include Gerbich, Yus, and Webb, in which the RBC membrane contains normal amounts of GPC, but with altered biochemical composition, as well as the Leach phenotype, in which the membrane is totally deficient in GPC. Polymerase chain reaction (PCR) amplification of Yus mRNA and sequencing of the mutant fragment showed a 57-bp deletion corresponding to exon 2 that resulted in a deletion of N-terminal amino acids 17 to 35 (Fig 7).75 Similar analysis of the Gerbich phenotype showed a deletion of the 84-bp exon 3, which produced a deletion of N-terminal amino acids 36 to 63 (Fig 7).75It is postulated that the Yus and Gerbich deletions within the GPC gene were produced by an unequal crossover between the homologous 3.4-kb repeat sequences. If a crossover occurs 5’ to misaligned exons 2 and 3 in the two chromosomes, an altered gene exhibiting an exon 2 deletion would be produced (Yus-type). Alternatively, crossover 3’ to mis- From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1875 RED BLOOD CELL GLYCOPHORINS A Fig 7. PCR analysis of GPC mRNA from indhriduals with normal and abnormal GPC proteins. (A) A model of GPC mRNA with the coding region shaded and exons 1 through 4 indicated. Arrows show location of 5’ (sense strand) and 3’ (antisense strand) oligonucleotidesusedto amplify a 395-bp band including the entire coding regionand few flanking bases of untranslated sequence. (B) Products obtained after amplification of cDNA from individuals with the phenotypes indicated above each lane. Molecular weight standards are in the left lane (0 x 174 DNA cut with Hae 111). Deduced structure of each GPC mRNA is shown at the right. (Reprintedwith permission.”) B PCR products aligned exons 2 and 3 would yield a gene containing an exon 3 deletion (Gerbich phenotype). In contrast to the exon deletions seen in the Yus and Gerbich phenotypes, the Webb phenotype is the result of a substitution of serine for asparagine at position 8.’5 To date, two different mutations have been characterized that result in a total deficienty of GPC in the membrane, known as the Leach phenotype. Southern blot analysis of genomic DNA suggests that the most common genetic basis for this deficienty involves a deletion of exons 3 and 4 of the GPC gene.22.76.n The second mutation to be identified is a single nucleotide deletion in codon 45 within exon 3, which causes a frameshift mutation in the mRNA, resulting in a premature stop codon.77 Proteins translated from this mRNA would presumably be truncated and not inserted into the lipid bilayer. Characterization of the Leach, Yus, and Gerbich mutations has provided important insights into the biologic function of GPC, as we will discuss in the following section. Biologic function of GPC. It is clearly apparent that GPC, unlike GPA and GPB, has a pattern of expression that is not limited to the erythroid lineage. Immunocytochemical analysis of cell surface expression as well as Northern analysis of RNA preparations confirm the presence of GPC in multiple nonerythroid tissues, including breast, liver, and kidne~.*~.~l Interestingly, the level of mRNA expression is lower in nonerythroid tissues. This difference may be the consequence of the use of different transcription start sites in different tissues; for example, in a lymphoid cell line transcription begins 11 nucleotides upstream from the site used in erythroid cells, while in a megakaryocytic cell line the transcription start site is 57 nucleotides upstream.” Similar differences in the level of expression of other genes such as c-myc and band 3 have been attributed to variable transcription sites.m*w Two murine MoAbs, one glycosylation dependent (MR4130) and the other sialic acid independent (AP03), have been useful probes in examining the developmentallyregulated expression of GPC during terminal erythroid differentiati~n.~.~*.*l While CFU-E-derived erythroblasts react with both antibodies, erythroblasts derived from BFU-E are APOfpositive but MR4-130-negative, indicating that a desialated form of GPC is inserted into the membranes of earlier progenitors. With this pattern of expression, GPC, deduced mRNA structures like carbonic anhydrase I and blood group antigen A, can be used as a marker for identifying very early erythroid precursors during normal and leukemic erythroid differentiation?‘ In the mature RBC, GPC, in contrast to GPA, appears to play a critical role in regulating cell shape, membrane deformability, and membrane mechanical stability. A number of mutations involving the GPC gene (which have been described above), result in RBCs that are either completely deficient in GPC or else contain a variant form of the gly~oprotein.4.~~’.~~ Characterization of the cellular consequences of these mutations56.n2 have provided crucial leads for understanding the function of GPC. Although a complete deficiency of GPC, as seen in the Leach phenotype, does not cause clinically significant anemia, it does result in elliptocytosis,4as well as a decrease in membrane deformability and a marked reduction in membrane mechanical stability (Fig 4).56These abnormalities in cell shape and membrane properties are in dramatic contrast to the normal discocytic morphology and normal membrane properties observed in the En(a-) GPA-deficient RBCs. The data obtained with Leach RBCs thus imply that GPC plays a critical role in regulating both RBC shape and membrane material properties. Yus, Gerbich, and Webb mutations, as described above, result in qualitative changes in the GPC rather than quantitative changes of the Leach mutation^.^^.^^-^^ Interestingly, these three mutations are all limited to the region of the gene encoding the exoplasmic domain, leaving the primary structure of the cytoplasmic domain By characterizing the cellular properties of these mutant phenotypes, we have been able to define the role of various domains in regulating membrane function. In contrast to RBCs of the Leach phenotype, Gerbich, Yus, and Webb RBCs have normal discocytic morphology and exhibit normal membrane deformability and mechanical stability (Fig 8).R2The normal membrane properties of these phenotypes, in the context of a structurally normal cytoplasmic domain, implies that the cytoplasmic domain of GPC is the critical region of the molecule for maintaining normal shape and for regulating membrane properties. An abundance of information is currently available regarding the role of band 3 in anchoring the spectrin-based membrane skeleton to the lipid bilayer through interaction From www.bloodjournal.org by guest on February 6, 2015. For personal use only. CHASIS AND MOHANDAS 1876 vant interaction does indeed occur between the cytoplasmic domain of GPC and protein 4.1. Although the molecular nature of the interaction between the two RBC membrane constituents, GPC and protein 4.1, remains to be elucidated, it seems clear that GPC plays a functionally important role in regulating RBC shape and membrane properties, and that protein 4.1 serves as a membrane anchor for this sialoglycoprotein. I CONCLUSIONS /Leach O0.1 '*I GPA and GPB, having arisen from a common ancestral gene through homologous recombinant events involving A h sequences, share extensive sequence homology with one another. GPC and GPD, which have no structural homology to GPA and GPB, are encoded by a single gene, probably through the use of different translation initiation sites via a leaky scanning mechanism. Translation initiated at the first AUG generates GPC, while initiation at the second AUG gives rise to GPD, which contains 21 fewer amino acid residues in its amino terminal domain than does GPC. The structural data accumulated on the GPA gene family serves as a springboard for detailed analysis of multiple GPA variants. It has already been shown that certain of these variants are encoded by genes produced by a variety of recombinations and deletions within and between the glycophorin genes. With their extensive diversity, the glycophorins could, therefore, serve as an impor- I 0.0 0 50 100 150 200 Time (seconds) Fig 8. Membrane mechanical stability of RBCs with various mutant GPC polypeptides. The deformability index of resealed membranes prepared from RBCs of the Leach phenotype decreased more rapidly with time when compared with normal membranes (shaded area), implying decreased mechanical stability. In contrast, the rate of decline of deformability index of membranes of the Gerbich and Yus phenotypes was normal, implying these membranes had normal mechanical stability. (Reprinted with permission.=) of its cytoplasmic domain with ankyrin. Less well-appreciated, but strongly substantiated by the observations outlined above, is a similarly important anchoring function for the cytoplasmic domain of GPC. Evidence to date implies that protein 4.1 is the membrane skeletal component with which GPC interacts. Mueller and Morrison originally suggested this specific interaction based on the observation that nonionic detergents do not extract GPC from normal membranes, but do extract this sialoglycoprotein from membranes deficient in protein 4.1.x3 Subsequent studies substantiating this hypothesis include the observation that GPC and protein 4.1 copurify after extraction from the membranew and that GPC content of the membrane is related to protein 4.1 content.""' For example, flow cytometric analysis showed that the GPC content of membranes with 50% deficiency of protein 4.1 was 44% of normal, while it was only 9% of normal in membranes totally deficient in protein 4.1. In these individuals, molecular analysis of both GPC and protein 4.1 genes documented only an abnormality in the protein 4.1 gene, implying that GPC deficiency in these membranes is secondary to protein 4.1 deficiency rather than the result of a primary defect in the glycoprotein itself.% Convincing evidence that protein 4.1 anchors GPC in the bilayer has recently been provided by a series of experiments in which detergent extractability of GPC was examined in protein 4.1-deficient RBCs in their native state and after reconstitution with exogenously purified protein 4.1.M While GPC was readily extractable from protein 4.1-deficient membranes, the glycoprotein was retained with the skeletal proteins after reconstitution of the 4.1-deficient membranes with purified protein 4.1 (Fig 9). These data, considered with that obtained with the mutant RBCs, strongly suggest that a physiologically rele- - Glycophorin C dimer - Glycophorin C monomer a a ' b b ' c c' Fig 9. Western blot analysis using anti-GPC antibody on membranes and detergent extracted membrane skeletons from protein 4.1-deficient RBCs before and after reconstitution with purified protein 4.1. The first two lanes show the presence of GPC In membranes (lane a) and detergent extracts (lane a') from normal RBCs. The third lane shows that GPC is present in membranes totally deficient in protein 4.1 (lane b), but absent from detergent extracts preparedfrom these membranes (lane b'). The last two lanes show the results of tests on protein 4.1-deficient membranes that have been reconstituted with exogenous protein 4.1. GPC is present in both the membrane (lane c) and the detergent extracted membrane skeletons (lane c'). (Reprinted with permission.-) From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1877 RED BLOOD CELL GLYCOPHORINS tant model for future study of polymorphisms in other human genes. Certain important and previously unsuspected functions for various RBC sialoglycoproteins have recently been identified. GPA, with its abundant sialic acid content, is the major contributor to the net negative surface charge of the mature RBC membrane. The electrostatic repulsive energy produced by these negative charges plays a critical role in minimizing cell-cell interactions in the circulation. An intriguing hypothesis is that the erythrocyte harbors a mechanism for preserving negative surface charge. This thesis is suggested by the apparent compensatory changes in glycosylation of band 3 observed in mutant phenotypes [En(a-), MkMk,Dantu-positive] in which GPA molecules are either deficient or present in an overly glycosylated variant form. While GPA, in its native state, does not appear to regulate either cell shape or membrane material properties, ligand binding induces both a profound increase in membrane rigidity and a decrease in the lateral mobility of GPA molecules. This ligand-induced change in membrane properties results from increased association of the cytoplasmic domain of GPA with the skeletal network and can be modulated by varying the site on the exoplasmic domain to which the ligand binds. This inducible change in membrane behavior, mediated by the cytoplasmic domain, can be considered a form of signal transduction akin to ligandinduced changes in cellular functions described in granulocytes and platelets. Moreover, recent studies show that different VLA integrin a subunit cytoplasmic domains mediate distinct cellular functions.R8Molecular character- ization of the mechanism involved in GPA signal transduction may, therefore, provide insights into novel cell communication pathways present also in nonerythroid cells. GPC in the native state, in contrast to GPA, appears to play a pivotal role in regulating cell shape, membrane deformability, and membrane mechanical stability. The GPC-related regulation of these membrane functions appears to be through the interaction of the cytoplasmic domain of the sialoglycoprotein with protein 4.1. A deficiency in the anchoring protein 4.1 results in GPC-deficient RBC membranes. The coexistence of GPC and protein 4.1 in a wide variety of tissues raises the possibility that the interaction between these two proteins may have important but as yet undefined functions in these nonerythroid cells. Because the level of GPC mRNA transcription is higher in erythroid than in nonerythroid tissues, this sialoglycoprotein will be a relevant molecule for future study of differential tissue specificity and factors regulating gene expression. It is now abundantly clear that the glycophorins function as more than just blood group antigens. Indeed, the glycophorins can continue to serve as models for exploring mechanisms of human gene polymorphisms, differential tissue expression, signal transduction, and surface component glycosylation, thereby contributing to our understanding of the biology of both erythroid and nonerythroid cells. ACKNOWLEDGMENT We thank Dr Ross Coppel for assistance with the computerdrawn figures, Dr Marion Reid for advice, Ricky Winardi for useful discussions on secondary structure, and Cynthia Long for assistance in preparation of the manuscript. REFERENCES 1. Tomita M, Marchesi VT: Amino-acid sequence and oligosac- charide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci USA 72:2964, 1975 2. Tomita M, Furthmayr H, Marchesi VT: Primary structure of human erythrocyte glycophorin-A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry 17:4756, 1978 3. Fairbanks G, Steck TL, Wallach DFH: Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2602, 1971 4. Anstee DJ, Parsons SF, Ridgwell K, Tanner MJA, Merry AH, Thomson EE, Judson PA, Johnson P, Bates S, Fraser ID: Two individuals with elliptocytic red cells lack three minor erythrocyte membrane sialoglycoproteins. Biochem J 218:615,1984 5. Merry AH, Thomson EE, Anstee DJ, Stratton F: The quantification of erythrocyte antigen sites with monoclonal antibodies. Immunology 51:793, 1984 6. Dahr W: Immunochemistry of sialoglycoproteins in human red blood cell membranes, in Vengelen-Tyler V, Judd WJ (eds): Recent Advances in Blood Group Biochemistry. Arlington, VA, American Association of Blood Banks, 1986, p 23 7. Merry AHJ, Hodson C, Thomson E, Maallinson G, Anstee DJ: The use of monoclonal antibodies to quantify the levels of sialoglycoproteins (Y and 6 variant sialoglycoproteins in human erythrocyte membranes. Biochem J 233:93, 1986 8. Dahr W, Uhlenbruck G, Knott H: Immunochemical aspects of the MNSs blood group system. J Immunogenet 2:87,1975 9. Furthmayr H: Glycophorins A, B, C: A family of sialoglycoproteins. Isolation and preliminary characterization of trypsin-derived peptides. J Supramol Struct 9:79,1978 10. Anstee DJ: The blood group MNSs-active sialoglycoproteins. Semin Hematol18:13,1981 11. Furthmayr H: Structural comparison of glycophorins and immunochemical analysis of genetic variants. Nature 271519, 1978 12. Dahr W, Beyreuther K, Steinbach H, Gielen W, Kruger J: Structure of the Ss blood group antigens. 11. A methionine/ threonine polymorphism within the N-terminal sequence of the Ss glycoprotein. Hoppe Seyler’s Z Physiol Chem 3612395, 1980 13. Dahr W, Beyreuther K, Kordowicz M, Kriiger J: N-terminal amino acid sequence of sialoglycoprotein D (glycophorin C) from human erythrocyte membranes. Eur J Biochem 12557,1982 14. Siebert PD, Fukuda M: Isolation and characterization of human glycophorin A cDNA clones by a synthetic oligonucleotide approach: Nucleotide sequence and mRNA structure. Proc Natl Acad Sci USA 83:1665,1986 15. Siebert PD, Fukuda M: Molecular cloning of human glycophorin-B cDNA: Nucleotide sequence and relationship to glycophorin-A. Proc Natl Acad Sci USA 85:421,1987 16. Colin Y, Rahuel C, London J, Romeo PH, d’Auriol L, Galibert F, Cartron JP: Isolation of cDNA clones and complete amino acid sequence of human erythrocyte glycophorin C. J Biol Chem 261:229,1986 17. Blanchard D, Dahr W, Hummel M, Latron F, Beyreuther K, Cartron JP: Glycophorin-B and C from human erythrocyte mem- From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1878 branes: Purification and sequence analysis. J Biol Chem 262:5808, 1987 18. Blanchard D, El-Maliki B, Hermand P, Dahr W, Cartron JP: Structural homology between glycophorins C and D, two minor glycoproteins of the human erythrocyte membrane carrying blood group Gerbich antigen, in Proceedings of the IXth International Symposium on Glycoconjugates, p F54, July 6-11, 1987, Lille, France 19. Mattei MG, Colin Y, LeVan Kim C, Mattei JF, Cartron JP: Localization of the gene for human erythrocyte glycophorin-C to chromosome 2, q14-q21. Hum Genet 74:420,1986 20. Tate CG, Tanner MJA: Isolation of cDNA clones for human erythrocyte membrane sialoglycoproteins a and 6. Biochem J 254:743,1988 21. LeVan Kim C, Colin Y, Blanchard D, Dahr W, London J, Cartron JP: Gerbich group deficiency of the Ge:1,-2,-3 and Ge:1,-2,-3 types. Eur J Biochem 165:571,1987 22. Tanner MJA, High S, Martin PG, Anstee DJ, Judson PA, Jones TJ: Genetic variants of human red-cell membrane sialoglycoprotein p. Study of the alterations occurring in the sialoglycoprotein-p gene. Biochem J 250:407, 1988 23. Kudo S, Fukuda M: Identification of a novel human glycophorin, glycophorin E, by isolation of genomic clones and complementary DNA clones utilizing polymerase chain reaction. J Biol Chem 265:1102,1990 24. Vignal A, Rahuel C, London J, Cherif-Zahar B, Schaff S, Hattab C, Okubo Y, Carton J-P: A novel member of the glycophorin A and B family. Molecular cloning and expression. Eur J Biochem 191:619,1990 25. Cook PJL, Lindenbaum RH, Salonen R, De La Chapelle A, Daker MG, Buckton KE, Noades JE, Tippett P: The MNSs blood group of families with chromosome 4 rearrangements. Ann Hum Genet 45:39,1981 26. Rahuel C, London J, d’Auriol L, Mattei MG, Tournamille C, Skrzynia C, Lebouc Y, Galibert F, Cartron JP: Characterization of cDNA clones for human glycophorin A. Use for gene localization and for analysis of normal and glycophorin A deficient (Finnish type) genomic DNA. Eur J Biochem 172:147,1988 27. Kudo S, Fukuda M: Structural organization of glycophorin A and B genes: Glycophorin B gene evolved by homologous recombination at A h repeat sequences. Proc Natl Acad Sci USA 86:4619, 1989 28. Vignal A, Rahuel C, El Maliki B, LeVan Kim C, London J, Blanchard D, d’Auriol L, Galibert F, Blajchman MA, Cartron JP: Molecular analysis of glycophorin A and B gene structure and expression in homozygotes Milterberger class V (Mi”) human erythrocytes. Eur J Biochem 184:337,1989 29. Dahr W, Uhlenbruck G, Janssen E, Schmalisch R: Different N-terminal amino acids in the M,N glycoprotein from MN and NN erythrocytes. Hum Genet 35:335,1977 30. Wasniowska K, Drzeniek Z, Lisowoska E: The amino acids of M and N blood group glycopeptides are different. Biochem Biophys Res Commun 76:385,1977 31. Verpoorte JA: Purification and characterization of glycoprotein from human erythrocyte membranes. Int J Biochem 6:855, 1975 32. Welsh ET,Thom D: Molecular organization of glycophorin A Implications for molecular interactions. Biopolymers 24:2301, 1985 33. Von Heijne G: Transcending the impenetrable: How proteins come to terms with membranes. Biochim Biophys Acta 947:307, 1988 34. Segrest JP, Jackson RL, Marchesi VT, Guyer RB, Terry W: Red cell membrane glycoprotein: Amino acid sequence of an CHASIS AND MOHANDAS intramembranous region. Biochem Biophys Res Commun 49:964, 1972 35. Vignal A, London J, Rahuel C, Cartron J-P: Promoter sequence and chromosomal organization of the genes encoding glycophorins A, B and E. Gene 95:289,1990 36. Mawby WJ, Anstee DJ, Tanner MJA: Immunochemical evidence for hybrid sialoglycoproteins of human erythrocytes. Nature 291:161,1981 37. Huang CH, Blumenfeld 0: Identification of recombination events resulting in three hybrid genes encoding human MiV, MiV (J.L.) and St(a) glycophorins. Blood 77:1813,1991 38. Tanner MJA, Anstee DJ: The membrane change in En(a-) human erythrocytes. Absence of the major erythrocyte sialoglycoprotein. Biochem J 153:271,1976 39. Tokunaga E, Sasakawa S, Tamaka K, Kawamata H, Giles CM, Ikin EW, Poole J, Anstee DJ, Mawby W, Tanner MJA: Two apparently healthy Japanese individuals of type MkMkhave erythrocytes which lack both the blood group MN and Ss-active sialoglycoproteins. J Immunogenet 6:383, 1979 40. Okubo Y, Daniels GL, Parsons SF, Anstee DJ, Yamaguchi H, Tomita T, Sen0 T: A Japanese familywith two sisters apparently homozygous for Mk. Vox Sang 54:107,1988 41. Rahuel C, London J, Vignal A, Cherif-Zahar B, Coliin Y, Siebert P, Fukuda M, Cartron JP: Alteration of the genes for glycophorin A and B in glycophorin-A-deficient individuals. Eur J Biochem 177:605,1988 42. Tate CG, Tanner MJA, Judson PA, Anstee DJ: Studies on human red cell membrane glycophorin A and glycophorin B genes in glycophorin deficient individuals. Biochem J 263:993, 1989 43. Dahr W, Uhlenbruck G, Leikola J, Wagstaff W: Studies on the membrane glycoprotein defect of En(a-) erythrocytes. 111. N-terminal amino acids of sialoglycoproteins from normal and En(a-) erythrocytes. J Immunogenet 5:117,1978 44. Bigbee WL, Langlois RG, Vanderlaan M, Jensen RH: Binding specificities of eight monoclonal antibodies to human glycophorin A-Studies with MCM,and MkEN(UK) variant chimpanzee erythrocytes. J Immunol 133:3149,1984 45. Langlois RG, Bigbee WL, Jensen RH: Flow cytometric characterization of normal and variant cells with monoclonal antibodies specific for glycophorin-A. J Immunol34:4009, 1985 46. Gahmberg CG, Jokinen M, Anderson LC: Expression of major sialoglycoprotein (glycophorin) on erythroid cells in human bone marrow. Blood 52:379,1978 47. Yurchenco PD, Furthmayr H: Expression of red cell membrane proteins in erythroid precursor cells. J Supramol Struct 13:255,1980 48. Fukuda M: Tumor-promoting phorbol diester-induced specific changes in cell surface glycoprotein profile of K562 human leukemic cells. Cancer Res 41:4621, 1981 49. Cartron JP, Colin Y, Kudo S, Fukuda M: Molecular genetics of human erythrocyte sialoglycoproteins Glycophorins A, B, C and D, in Harris JR (ed): Blood Cell Biochemistry, vol 1. New York, NY, 1990, p 322 50. Robinson J, Sieff C, Delia D, Edwards PAW, Greaves M: Expression of cell-surface HLA-DR, HLA (ABC) and glycophorin during erythroid differentiation. Nature 289:68,1981 51. Sieff C, Bicknell D, Caine G, Robinson J, Lam G, Greaves M: Changes in cell surface antigen expression during hematopoietic differentiation. Blood 60703,1982 52. Fukuda M: Cell Surface glycocojugates as onco-differentiation markers in hematopoietic cells. Biochim Biophys Acta 780: 119,1985 53. Chien S, Sung LA: Physiochemical basis and clinical implications of red cell aggregation. Clin Hemorheol7:71,1987 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. RED BLOOD CELL GLYCOPHORINS 54. Gahmberg CG, Myllyla G, Leikola J, Pirkola A, Nordling S: Absence of the major sialoglycoprotein in the membrane of human En(a-) erythrocytes and increased glycosylation of band 3. J Biol Chem 251:6108,1976 55. Dahr W, Moulds J, Unger P, Kordowicz M: The Dantu erythrocyte phenotype of the NE variety. Blut 55:19,1987 56. Reid ME, Chasis JA, Mohandas N Identification of a functional role for human erythrocyte sialoglycoproteins p and y. Blood 69:1068,1987 57. Lovrien RE, Anderson RA: Stoichiometry of wheat germ agglutinin as a morphology controlling agent and as a morphology protective agent for the human erythrocyte. J Cell Biol85:534,1980 58. Chasis JA, Mohandas N, Shohet SB: Erythrocyte membrane rigidity induced by glycophorin-A-ligand interaction. Evidence for a ligand-induced association between glycophorin-A and skeletal proteins. J Clin Invest 75:1919,1985 59. Knowles D, Chasis JA, Evans E, Mohandas N: Decreased lateral mobility of glycophorin-A following ligand binding: Implications for a signal transduction process involving the cytoplasmic domain. Blood 76:10a, 1990 (abstr, suppl 1) 60. Chasis JA, Knowles D, Winardi R, George E, Mohandas N: Conformational changes in cytoplasmic domains of band 3 and glycophorin-A affect red cell membrane properties. Blood 78:252a, 1991 (abstr, suppl 1) 61. Pasvol G, Chasis JA, Mohandas N, Anstee DJ, Tanner MJA, Merry AH: Inhibition of malaria parasite invasion by monoclonal antibodies against glycophorin-A correlates with reduction in red cell membrane deformability. Blood 74:1836, 1989 62. Chasis JA, Reid ME, Jensen RH, Mohandas N: Signal transduction by glycophorin-A Role of extracellular and cytoplasmic domains in a modulatable process. J Cell Biol 1071351,1988 63. Anderson RA, Marchesi V T Regulation of the association of membrane skeletal protein 4.1 with glycophorin-A by polyphosphoinositide. Nature 318:295,1985 64. Nigg EA, Bron C, Girardet M, Cherry RJ: Band 3-glycophorin-A association in erythrocyte membrane demonstrated by combining protein diffusion measurements with antibody-induced cross-linking. Biochemistry 19:1887, 1980 65. Telen M, Chasis JA: Relationship of the human erythrocyte Wrb antigen to an interaction between glycophorin-A and band 3. Blood 76:842,1990 66. Jokinen M, Ehnholm C, Vaisanen-Rhen V, Korhonen T, Pipkorn R, Kalkkinen N, Gahmberg G: Identification of the major human sialoglycoprotein from red cells, glycophorin AM, as the receptor for Escherichia coli IH11165 and characterization of the receptor site. Eur J Biochem 147:47,1985 67. Parkkinen J, Rogers GN, Korhonen T, Dahr W, Finne J: Identification of the 0-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun 54:37,1986 68. Dahr W, Blanchard D, Kiedrowski S, Poschmann A, Cartron JP, Moulds J: High frequency antigens of human erythrocyte membrane sialoglycoproteins. VI. Monoclonal antibodies reacting with the N-terminal domain of glycophorin-C. Biol Chem Hoppe Seyler 3702349,1989 69. El-Maliki B, Blanchard D, Dahr W, Beyreuther K, Cartron JP: Structural homology between glycophorins C and D of human erythrocytes. Eur J Biochem 183:639,1989 70. Colin Y, LeVan Kim C, Tsapis A, Clerget M, d’Auriol L, London J, Cartron JP: Human erythrocyte glycophorin-C. Gene structure and rearrangements in genetic variants. J Biol Chem 264:3773,1989 71. High S, Tanner M J A Human erythrocyte membrane sialoglycoprotein-p. The cDNA sequence suggests the absence of a cleaved N-terminal signal sequence. Biochem J 243:277, 1987 1879 72. Anstee DJ, Ridgwell K, Tanner MJA, Daniels GL, Parsons SF: Individuals lacking the Gerbich blood-group antigen have alterations in the human erythrocyte membrane sialoglycoproteins p and y. Biochem J 221:97,1984 73. Dahr W, Kiedrowski S, Blanchard D, Hermand P, Moulds JJ, Carton JP: High frequency of human erythrocyte membrane sialoglycoproteins. V. Characterization of the Gerbich blood group antigens. Ge2 and Ge3. Biol Chem Hoppe Seyler 368:1375,1987 74. Reid EM, Anstee DJ, Tanner MJA, Ridgwell K, Nurse CT: Structural relationships between human erythrocyte sialoglycoproteins p and y and abnormal sialoglycoproteins found in certain rare human erythrocyte variants lacking the Gerbich blood group antigen(s). Biochem J 244:123,1987 75. Chang S, Reid ME, Conboy J, Kan YW, Mohandas N: Molecular characterization of erythrocyte glycophorin-C variants. Blood 77:644,1991 76. High S, Tanner MJA, Macdonald EB, Anstee DJ: Rearrangements of the red-cell membrane glycophorin-C (sialoglycoprotein p) gene. A further study of the alterations in the glycophorin-C gene. Biochem J 262:47,1989 77. Telen MJ, LeVan Kim C, Chung A, Cartron J-P, Colin Y: Molecular basis for elliptocytosis associated with glycophorin C and D deficiency in Leach phenotype. Blood 78:1603,1991 78. LeVan Kim C, Colin Y, Mitjavila MT, Clerget M, Dubart A, Nakazawa M, Vainchenker W, Cartron JP: Structure of the promoter region and tissue specificity of the human glycophorin C. J Biol Chem 264:20407,1989 79. Battey J, Moulding C, Taub R, Murphy W, Stewart T, Potter H, Lenoir G, Leder P: The human c-myc oncogene: Structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell 34:779, 1983 80. Kopita RR, Andersson MA, Lodish HF: Multiple tissuespecific sites of transcription initiation of the mouse antiport gene in erythroid and renal cells. Proc Natl Acad Sci USA 84:7149, 1987 81. Villeval JL, LeVan Kim C, Bettaieb A, Debili N, Colin Y, El Maliki B, Blanchard D, Vainchenker W, Cartron JP: Early expression of glycophorin-C during normal and leukemic human erythroid differentiation. Cancer Res 49:2626, 1989 82. Reid ME, Anstee DJ, Jensen RH, Mohandas N: Normal membrane function of abnormal p-related erythrocyte sialoglycoproteins. Br J Haematol67:467, 1987 83. Mueller TJ, Morrison M: Glycoconnectin (PAS 2); a membrane attachment site for the human erythrocyte cytoskeleton, in Brewer G , Eaton J (eds): Erythrocyte Membranes 11: Recent Clinical and Experimental Advances. New York, NY,Liss, 1986, p 85 84. Elliot C, Ralston GB: Solubilization of human erythrocyte band 4.1 protein in non-ionic detergent Tween-20. Biochim Biophys Acta 775:313,1984 85. Alloisio N, Morle L, Bachir D, Guetarni D, Colonna D, Delaunay J: Red cell membrane sialoglycoprotein in homozygous and heterozygous 4.1 (-) hereditary elliptocytosis. Biochim Biophys Acta 816:57,1985 86. Reid ME, Takakuwa Y, Conboy J, Tchernia G, Mohandas N: Glycophorin-C content of human erythrocyte membrane is regulated by protein 4.1. Blood 75:2229,1990 87. Sondag D, Alloisio NN, Blanchard D, Ducluzeau MT, Colonna P, Bachir D, Bloy C , Cartron JP, Delaunay J: Gerbich reactivity in 4.1( -) hereditary elliptocytosis and protein 4.1 level in blood group Gerbich deficiency. Br J Haematol65:43,1987 88. Chan BMC, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME: Distinct cellular functions mediated by different VLA integrin 01 subunit cytoplasmic domains. Cell 68:1051, 1992 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1992 80: 1869-1879 Red blood cell glycophorins JA Chasis and N Mohandas Updated information and services can be found at: http://www.bloodjournal.org/content/80/8/1869.citation.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026