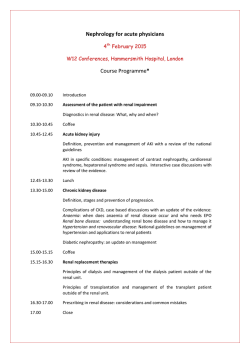
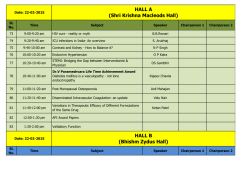
Pentosan polysulfate sodium prevents kidney morphological
Included in ISI-WOK, MEDLINE, EMBASE, IME, IBECS, SCIELO V o l u m e 3 0 - N u m b e r 6 - 2 0 1 0 TREATMENT OF DIABETIC PATIENTS WITH PERITONEAL DIALISYS A COMPETENCY-BASED MODEL FOR MEDICAL SPECIALISTS EDUCATION BK POLYOMAVIRUS-ASSOCIATED NEPHROPATHY RENAL IMMUNOEXPRESSION OF GHRELIN IN HUMAN PROLIFERATIVE GLOMERULOPATHIES URAEMIC ANOREXIA TREATMENT WITH MEGESTROL ACETATE POST-TRANSPLANT LYMPHOPROLIFERATIVE DISORDERS IN RENAL TRANSPLANTATION Sociedad Española de Nefrología Órgano Oficial de la Sociedad Española de Nefrología Versión íntegra inglés y español en www.revistanefrologia.com Nefrología Journal Editor-in-Chief: Carlos Quereda Rodríguez-Navarro Executive editor: Roberto Alcázar Arroyo Deputy editors: Andrés Purroy Unanua, Ángel Luis Martín de Francisco, Fernando García López Honorary editors: Luis Hernando Avendaño, David Kerr, Rafael Matesanz Acedos SUBJECT EDITORS (editors of thematic areas) Experimental Nephrology A. Ortiz* J. Egido de los Ríos S. Lamas J.M. López Novoa D. Rodríguez Puyol J.M. Cruzado Clinical Nephrology M. Praga* J. Ara J. Ballarín G. Fernández Juárez F. Rivera A. Segarra Diabetic Nephropathy F. de Álvaro* J.L. Górriz A. Martínez Castelao J.F. Navarro J.A. Sánchez Tornero R. Romero Hereditary Nephropathies R. Torra* X. Lens J.C. Rodríguez Pérez M. Navarro E. Coto V. García Nieto Chronic Kidney Disease A.L. Martín de Francisco* A. Otero E. González Parra I. Martínez J. Portolés Pérez CRF-Ca/P Metabolism E. Fernández* J. Cannata Andía R. Pérez García M. Rodríguez J.V. Torregrosa Arterial Hypertension R. Marín* J.M. Alcázar L. Orte R. Santamaría A. Rodríguez Jornet Nephropathy and Cardiovascular Risk J. Díez* A. Cases J. Luño Quality in Nephrology F. Álvarez-Ude* M.D. Arenas E. Parra Moncasi P. Rebollo F. Ortega Acute Renal Failure F. Liaño* F.J. Gainza J. Lavilla E. Poch Peritoneal Dialysis R. Selgas* M. Pérez Fontán C. Remón M.E. Rivera Gorrin G. del Peso Haemodialysis A. Martín Malo* P. Aljama F. Maduell J.A. Herrero J.M. López Gómez J.L. Teruel Renal Transplantation J. Pascual* M. Arias J.M. Campistol J.M. Grinyó M.A. Gentil A. Torres Paediatric Nephrology I. Zamora* N. Gallego A.M. Sánchez Moreno F. Vilalta Nephropathology J. Blanco* I.M. García E. Vázquez Martul A. Barat Cascante Evidence-Based Nephrology Vicente Barrio* (Director of Supplements), Fernando García López (Methodology assessment), Editors: María Auxiliadora Bajo, José Conde, Joan M. Díaz, Mar Espino, Domingo Hernández, Ana Fernández, Milagros Fernández, Fabián Ortiz, Ana Tato. Continued Training (journal NefroPlus) Andrés Purroy*, R. Marín, J.M. Tabernero, F. Rivera, A. Martín Malo. * Coordinators of thematic area EDITORIAL BOARD A. Alonso J. Arrieta F.J. Borrego D. del Castillo P. Gallar M.A. Frutos D. Jarillo V. Lorenzo A. Mazuecos A. Oliet L. Pallardo J.J. Plaza D. Sánchez Guisande J. Teixidó J. Alsina P. Barceló J. Bustamente A. Darnell P. García Cosmes M.T. González L. Jiménez del Cerro J. Lloveras B. Miranda J. Olivares V. Pérez Bañasco L. Revert A. Serra F.A. Valdés J. Aranzábal G. Barril F. Caravaca C. de Felipe S. García de Vinuesa A. Gonzalo R. Lauzurica J.F. Macías E. Martín Escobar J.M. Morales R. Peces J.M. Tabernero A. Vallo G. de Arriba F. Anaya A. Barrientos A. Caralps P. Errasti F. García Martín M. González Molina I. Lampreabe B. Maceira J. Mora J. Ortuño S. Pérez García J.L. Rodicio L. Sánchez Sicilia A. Vigil C. Bernis E. Fernández Giráldez F.J. Gómez Campderá P. Gómez Fernández E. Huarte E. López de Novales R. Marcén J. Montenegro A. Palma L. Piera J. Rodríguez Soriano A. Tejedor INTERNATIONAL COMMITEE BOARD E. Burdmann (Brazil) B. Canaud (France) J. Chapman (Australia) R. Coppo (Italy) R. Correa-Rotter (Mexico) F. Cosío (USA) G. Eknoyan (USA) A. Felsenfeld (USA) J.M. Fernández Cean (Uruguay) J. Frazao (Portugal) M. Ketteler (Germany) Levin, Adeera (Canada) Li, Philip K.T. (Hong Kong, China) L. Macdougall (United Kingdon) P. Massari (Argentina) S. Mezzano (Chile) B. Rodríguez Iturbe (Venezuela) C. Ronco (Italy) J. Silver (Israel) P. Stevinkel (Sweden) A. Wiecek (Poland) C. Zoccali (Italy) COUNCIL OF THE SPANISH SOCIETY OF NEPHROLOGY SUBSCRIPTIONS, ADVERTISING AND PUBLISHING Information and subscriptions: S.E.N. Secretary: [email protected] Tel: 902 929 210 Queries regarding of manuscripts: [email protected] Avda. dels Vents 9-13, Esc. B, 2.º 1.ª Edificio Blurbis 08917 Badalona Tel. 902 02 09 07 - Fax. 93 395 09 95 Rambla del Celler 117-119, 08190 Sant Cugat del Vallès. Barcelona Tel. 93 589 62 64 - Fax. 93 589 50 77 Distribuido por: E.U.R.O.M.E.D.I.C.E., Ediciones Médicas, S.L. © Copyright 2010. Grupo Editorial Nefrología. All rights reserved. • ISSN: 2013-2514 © Sociedad Española de Nefrología 2010. All international rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical, electronic, photocopying, recording or otherwise, without the prior written permission of the publisher. Nefrología is distributed exclusively among medical professionals. President: Dr. Alberto Martínez Castelao Vice-president: Dr. Isabel Martínez Secretary: Dr. José Luis Górriz Director of Nefrología Publishing Group: Dr. Carlos Quereda Rodríguez Chairperson of the Dialysis and Transplantation Registry: Dr. Ramón Saracho Treasurer: Dr. María Dolores del Pino Chairpersons of Education and Research: Ordinary members: Dr. Gema Fernández Fresnedo Dr. Juan Francisco Navarro Dr. Elvira Fernández Giráldez Dr. Julio Pascual Dr. José María Portolés Web Page of Nefrología: E-mail Editor-in-Chief: Dr. Josep Maria Cruzado Chairperson for selection of the SEN Congress presentations: Dr. Rosa Sánchez Hernández Links: www.revistanefrologia.com [email protected] [email protected] contents Included in ISI-WOK, MEDLINE, EMBASE, IME, IBECS, SCIELO Included in ISI-WOK, MEDLINE, EMBASE, IME, IBECS, SCIELO Volume 30 - Number 6 - 2010 V o l u m e 3 0 - N u m b e r 6 - 2 0 1 0 EDITORIAL COMMENT TREATMENT OF DIABETIC PATIENTS WITH PERITONEAL DIALISYS A COMPETENCY-BASED MODEL FOR MEDICAL SPECIALISTS EDUCATION 599 • The treatment of diabetic patients on peritoneal dialysis remains a challenge BK POLYOMAVIRUS-ASSOCIATED NEPHROPATHY RENAL IMMUNOEXPRESSION OF GHRELIN IN HUMAN PROLIFERATIVE GLOMERULOPATHIES 25 years later J. Portolés Pérez UREMIC ANOREXIA TREATMENT WITH MEGESTROL ACETATE. POST-TRANSPLANT LYMPHOPROLIFERATIVE DISORDERS IN RENAL TRANSPLANTATION Sociedad Española de Nefrología Órgano Oficial de la Sociedad Española de Nefrología Versión íntegra inglés y español en www.revistanefrologia.com SPECIAL ARTICLE 604 • Reinventing specialty training of physicians? Principles and challenges J. Morán-Barrios, P. Ruiz de Gauna-Bahillo Cover images. See on page 381. R. Peces et al. MALT B cell lymphoma with kidney damage and monoclonal gammopathy: A case study and literature review. SHORT REVIEWS 613 • BK virus-associated nephropathy D. Burgos, C. Jironda, M. Martín, M. González-Molina, D. Hernández 618 • Sodium-glucose cotransporter type 2 inhibitors (SGLT2): from familial renal glycosuria to the treatment of type 2 diabetes mellitus G. Pérez López, O. González Albarrán, M. Cano Megías ORIGINALS 626 • Morbidity and mortality in diabetic patients on peritoneal dialysis. Twenty-five years of experience at a single centre 633 • Renal immunoexpression of ghrelin is attenuated in human proliferative glomerulopathies F. Coronel, S. Cigarrán, J.A. Herrero M. Danilewicz, M. Wagrowska-Danilewicz 639 • Pentosan polysulfate sodium prevents kidney morphological changes and albuminuria in rats with type 1 diabetes Y. Mathison Natera, H.J. Finol, Z. Quero, R. González, J. González 646 • Treatment of uraemic anorexia with megestrol acetate M. Fernández Lucas, J.L. Teruel, V. Burguera, H. Sosa, M. Rivera, J.R. Rodríguez Palomares, R. Marcén, C. Quereda 653 • Decreased glomerular filtration rate using the Cockgroft-Gaultand MDRD formulas does not always predict cardiovascular morbidity and mortality in hypertensive primary care patients 661 • Insulin resistance in chronic kidney disease: its clinical characteristics and prognostic significance F.J. Tovillas-Morán, M. Vilaplana-Cosculluela, A. Dalfó-Pibernat, E. Zabaleta-del-Olmo, J.M. Galcerán, A. Coca, A. Dalfó-Baqué F. Caravaca, I. Cerezo, R. Macías, E. García de Vinuesa, C. Martínez del Viejo, J. Villa, R. Martínez Gallardo, F. Ferreira, R. Hernández-Gallego 669 • Post-transplant lymphoproliferative disorders in renal transplantation: two decades of experience A. Franco, l. Jiménez, C. Sillero, M. Trigueros, D. González, E. Alcaraz, J. Olivares BRIEF REPORT 676 • Scientific presentations at the meetings of the Spanish Paediatric Nephrology Association (AENP), 1988-2007 L.M. Rodríguez-Fernández, V. Recio-Pascual, M. Fernández-Fernández, M. Rosón-Varas, C. Rodríguez-Fernández, R. Morales-Sánchez, D. Mata-Zubillaga contents Included in ISI-WOK, MEDLINE, EMBASE, IME, IBECS, SCIELO Volume 30 - Number 6 - 2010 CASE REPORT 681 • MALT B cell lymphoma with kidney damage and monoclonal gammopathy: A case study and literature review R. Peces, C. Vega-Cabrera, C. Peces, A. Pobes, M.F. Fresno RESEARCH PROTOCOLS 687 • Clinical and genetic bases of hypertensive nephrosclerosis. NEFROSEN Study B. Diez Ojea, R. Marín, E. Coto, F. Fernández Vega, R. Álvarez Navascués, G. Fernández Fresnedo, A. Pobes Martínez de Salinas, A. Suárez Laurés, C. García Monteavaro, M. Gorostidi, E. Sánchez, M. Arias, F. Ortega LETTERS TO THE EDITOR A) Brief papers on research and clinical experiments 698 • Cadaveric donor procurement units faced with living donation A. Ríos, A.I. López-Navas, P. Ramírez, P. Parrilla, Proyecto colaborativo internacional donante 699 • Internal jugular vein access in a semi-seated position for catheterisation to enable haemodialysis in orthopnoeic patients R. Karatanasopuloz, V. Balbuena, M. Paiz, G. Levy, C. Martín B) Brief Case Reports 701 • Granulomatous interstitial nephritis free from extrarrenal sarcoids M. Cuxart, M. Picazo, R. Sans Lorman, M.J. Muntané 702 • Acute phosphate nephropathy after bowel cleansing: still a menace P. Santos, A. Branco, S. Silva, A. Paiva, J. Baldaia, J. Maximino, A. Loureiro, R. Henrique 704 • Paravirus B19 infection: diagnosing and treating a kidney transplant patient L.R. León, D. Curcio, D. Casadei 704 • Disseminated tuberculosis with splenic abscesses during haemodialysis 706 • Cocaine use, high blood pressure and chronic kidney disease B. Moragrega, R. Dolz, I. López Alejandre, A. Núñez Sánchez M. Picazo Sánchez, M. Cuxart Pérez, F. Martín Romero, R. Sans Lorman 707 • Delayed spontaneous rupture of the kidney graft J. Kanter Berga, C. Cáceres Borrero, T. Ripollés González, A. Ávila Bernabeu, E. Gavela Martínez, L. Pallardó Mateu 709 • Coexistence of anti-GBM antibodies and MPO-ANCA in a patient with systemic vasculitis and crescentic glomerulonephritis 710 • Treatment with rituximab for a patient with p-ANCA glomerulonephritis, alveolar bleeding and multiple relapses during haemodialysis P. Fernandes, J.A. Lopes, L. Correia, S. Gonçalves, S. Jorge M.A. Azancot, I. Agraz Pamplona, J. Fort Ros, A. Marín Valencia, I. Gil Carballeira, J. Camps Domenech 712 • Kidney failure and diabetes. Diagnostic inertia? R. Blanco García, J.J. Bravo López, A. Pérez, M. Moreiras Plaza 714 • IN MEMORY OF PROFESSOR SAULO KLAHR http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society editorial comment See original article on page 626 The treatment of diabetic patients on peritoneal dialysis remains a challenge 25 years later J. Portolés Nephrology Department. University Hospital Foundation of Alcorcón (REDinREN Carlos III. Red 06/0016). Alcorcón, Madrid, Spain Nefrologia 2010;30(6):599-603 doi:10.3265/Nefrologia.pre2010.Oct.10682 RELEVANCE OF DIABETES MELLITUS IN NEPHROLOGY Diabetes mellitus (DM) is the most important disease related to renal replacement therapy (RRT), due to its prevalence and clinical, economic and social impact. It is estimated that 0.3% of the general population suffer from type 1 DM and 7% from DM type 2.1 The prevalence of DM is dependent on the diagnostic criteria used and varies throughout the world, but the increase in the incidence of type 2 DM is estimated between 3 and 5% annually.1 This is due largely to poor health habits; therefore, its growth is even higher in developing countries. Progression to chronic kidney disease (CKD) in stage 5D increases due to a more prolonged exposure to hyperglycaemia, its association with high blood pressure (HTN), obesity, sedentary lifestyle and other risk factors, and its lower mortality, which leads to patients undergoing RRT. Therefore, the term “epidemic of the 21st century” is no exaggeration. It is estimated that the overall cost of treating patients with type 2 diabetes with target organ damage is at least €2,136 per year and may exceed €54,000 per year for patients on haemodialysis (HD). Finally, DM is a cardiovascular (CV) risk factor and a source of clinical complications, hospital admissions, poor quality of life and loss of years in full health and at work. This disease has a significant impact. Data from monitoring more than 5,000 patients in the UKPDS study allowed us to establish the clinical course of Correspondence: José Portolés Pérez Servicio de Nefrología. Hospital Universitario Fundación Alcorcón. (REDinREN Carlos III. Red 06/0016). Budapest, 1. 28922 Alcorcón. Madrid. Spain. [email protected] nephropathy in type 2 diabetes mellitus.2 Statistically, it takes 19 years to develop the disease, 11 years to go from microalbuminuria to macroalbuminuria and a decline in renal function starts 10 years later. However, patients who were included in the UKPDS with a Cr greater than 2mg/dl were undergoing RRT in just 2 years, which is the patient profile faced regularly. The objective of intervention in DM is clearly in the initial stages, focusing on renoprotection and cardioprotection, reducing CV events and the need for RRT. In fact, there is now evidence that intervention and close monitoring of patients with type 1 diabetes reduces the need for RRT in these patients. A Finnish study of 20,000 patients followed between 1965 and 1999, had dialyisis incidence rates of only 2.2% aftet 20 years, and a trend to decrease in the more recent years.3 Nevertheless, the challenge of treating DM patients on dialysis is an ongoing one. Articles like the one presented in this issue by the group from the Hospital Universitario San Carlos, Madrid, gives a historical perspective on the treatment of diabetic patients on peritoneal dialysis (PD 4). There are not many PD programmes with a experience of 25 years, as in this study. The most relevant result is the description of a worse outcome for patients with DM and the quantification of this risk in our area. 4 Patients with DM in this study have higher rates of mortality, transfer to HD, hospital admissions, non-peritoneal infections and peritonitis, in line with previous published studies. 5 For example, in the study of the Grupo Centro de Diálisis Peritoneal, GCDP (Peritoneal Dialysis Group Centre), the probability of survival at 2 years was 86.7% in patients without DM and 75.2% in patients with type 2 DM.6 In the study published in this issue, however, two different historical PD periods were compared. The most recent 599 editorial comment J. Portolés Pérez.The treatment of diabetic patients on peritoneal dialysis (post-1992) had double-bag systems, the first glucose-free solutions and the widespread use of automated systems, as well as erythropoietin. In this second phase, the rate of peritonitis was reduced accordingly and global outcome indicators improved, although the risk of death attributable to DM was not significantly reduced. The first stage of the article referred back to the 1980s (pre1992), when some groups raised concerns about the appropriateness of including patients with DM in dialysis programmes due to its high morbidity and mortality. This period (pre-1992) has some striking data reflecting a negative selection of patients for PD, which was not specifically outlined in the article. For example, the prevalence of diabetic patients on PD was 55% compared to the average reported by the register of 18% on HD, or 20% recorded by the GCDP between 2003 and 2009.5 In addition, they reported a high percentage of patients with blindness and other comorbidities, which limited the number of patients stopping the therapy to undergo transplant surgery to only 5.8% in the total follow-up of patients with type 2 DM. The low HD transfer rate could be due to the previous technique being maintained well or because patients were not able to change from one technique to another. In other words, it would be patients indicated for PD rather than those choosing PD, which is a risk factor in itself.7 Against this backdrop, the “new” integrated RRT 3.0 model offers an integrated approach for dialysis techniques and transplantation with a fluid exchange between them, as each reaches a plateau in a particular patient.8 This model is becoming a reality in many Spanish hospitals. The study published by Coronel et al. also serves as a reference for comparison for other groups starting PD. In general, these PD programmes are not large. For example, the Community of Madrid has an average size of around 25 patients, with a high turnover, fluctuations in the number and difficulties with growth. Therefore, retrospective studies of this type have been used so far as a reference to reflect the reality of PD in our area and time, highlighting differences with studies in other health systems and other countries. The collaboration between institutions is necessary to begin to have benchmarks for comparison with recent larger multicentre data.9 DM is the most important risk factor for PD patients and this poor prognosis is related to the CV pathology of patients entering PD, as indicated by other studies.6 The study by Coronel et al. shows an overall risk of death from DM of 1.96 compared to non-DM patients on PD. Although they did not have their own data on the evolution of DM patients on HD, as it was not the objective of the study, the comparison between techniques is inevitable. External references show a similar picture on the evolution of DM patients on HD. 600 According to the 2009 USRDS report, only 30% of DM patients survive 5 years after starting HD, and these data would be even worse if the early mortality of patients who did not reach 3 months in HD were included (excluded from that register10). The paper reports that half of the deaths were associated with CV events. The morbidity of DM is associated with predialysis CV damage, the concomitance of other risk factors (dyslipidaemia, HTN, etc.) and tissue deposition of advanced glycation end products (AGE). AGEs that accumulate in CKD have a direct effect on the vascular wall, promoting accelerated atherosclerosis and protein-calorie malnutrition. In fact, in some series, the risk attributable to DM greatly diminishes if corrected for the presence of previous cardiovascular events and albumin levels.11 For example, the data presented by the GCDP indicate that the risk of death in type 2 diabetes patients is 2.5 times that of non-DM after correction for age. The association between type 2 diabetes and previous cardiovascular events excludes the variable type 2 DM due to trying to put it in the same model DM and CV event prior to PD.6,7 The comparison of survival between HD and PD remains controversial, especially because the information comes from records and observational studies or from post-hoc analyses. Such questions cannot be resolved with a clinical trial design, so the information must come from observational studies with a prospective design and sufficient sample size and control of covariates and confounding factors. A recent comprehensive review in our journal concluded that both techniques were similar, with a slight advantage for PD in the first 2-3 years of evolution and HD later. In the specific case of patients with DM, younger people seem to have better outcomes with PD and the elderly with HD.12 A recent retrospective study goes beyond the multivariate analysis using the propensity score to reduce the selection bias of either technique.13 This study gives an advantage to patients on PD, particularly in the initial period, with a probability of survival of 85.5% compared with 80.7% in HD, and 71.1% versus 68% in HD after 2 years (P<.01), the trend continues without reaching significance in the third year. Overall, the risk of death favours PD by 8% in the ITT analysis. However, in the stratified analysis for diabetic patients, this benefit was only seen in the first year. The authors conclude that PD may be a good initial RRT technique. This advantage of PD in the early stages may be related to the better preservation of residual renal function and worse outcomes after a while, with the failure to control the volume or metabolic factors. In short, PD as a technique appears to be at least as good as HD for RRT patients, therefore patient choice must be considered in the decision-making process in most cases. Nefrologia 2010;30(6):599-603 J. Portolés Pérez.The treatment of diabetic patients on peritoneal dialysis WHAT ARE THE THEORETICAL ADVANTAGES AND DISADVANTAGES OF PERITONEAL DIALYSIS FOR DIABETES MELLITUS PATIENTS? PD has a number of theoretical advantages for patients with DM, such as better haemodynamic tolerance, maintenance of residual renal function, vascular capital preservation and use of peritoneal insulin for improved glycaemic control (currently not used). HD currently has a less stable electrolyte profile associated with a greater incidence of arrhythmias, the high-flow prosthetic fistulas lead to a haemodynamic overload which, along with hypertension, promote the development of left ventricular hypertrophy (LVH). These factors are behind the episodes of sudden death in HD. On the other hand, patients with DM have specific risks with this technique, primarily metabolic. Diabetic gastroparesis worsens in PD and promotes anorexia and secondary malnutrition. Glucose overload increases insulin resistance and makes it difficult to control the lipid profile. Diabetic patients have a thicker, poorly vascularised peritoneal membrane even before starting PD, as demonstrated in peritoneal biopsies obtained after inserting the catheter.14 This may influence the poorer outcome in peritoneal permeability in the medium term. INTEGRATION VIA THE RRT 3.0 MODEL FOR DIABETES MELLITUS PATIENTS We can summarise that PD seems to be a good starting technique for RRT with DM patients and has a certain advantage in the first 2 years. The current concept considers RRT as an integrated service of PD, HD and transplant (TX15). There is no evidence to guide our DM patients towards one particular technique or another, and key factors such as comorbidity, social situation and, above all, patient preference should be a starting point for RRT. In fact, the model proposed by some groups suggests the use of PD initially, and early TX, while keeping HD for those patients where PD fails.16 Early TX is the best alternative for patients with DM whose comorbidity does not prevent it. The US renal registry has a survival rate for DM undergoing TX of 67%-77% at 5 years.10 Although still lower than that of non-DM, it is a significant improvement on the 30% survival at 5 years for DM patients treated with HD or PD. It may be that recovering renal function promotes the elimination of AGEs from DM and other uraemic mediators that favour accelerated atherosclerosis and are agents of direct vascular injury. In addition, TX is associated with a better quality of life and rehabilitation, personally and at work. Therefore, TX should be offered to all diabetic patients in RRT without absolute contraindication and as early as possible. Nefrologia 2010;30(6):599-603 editorial comment FUTURE MANAGEMENT OF DIABETES MELLITUS IN RENAL REPLACEMENT THERAPY Another worth noting part of the study presented in this issue is that the prognosis of patients with type 2 diabetes does not improve in the second stage (post-1992). Despite technical advances in the treatment: double bag, cyclers, use of erythropoietic agents and the new drugs at our disposal for controlling blood pressure, dyslipidaemia, glycaemia and mineral vascular disease, the mortality is unchanged. It is true that hospital admission rate and annual stay in the most recent period are reduced, but we do not know if it is as a result of a better prognosis or overall improvement in hospital ambulatory processes and reduced stays. Although the authors have not given a detailed analysis of comorbidity between both stages, non-DM and DM type 1 patients who began PD after 1992 have improved their prognosis. Many misleading factors may interfere with an analysis like this, because other studies have reported an overall improvement in results over the years. For example, in the US registry, the mortality of diabetic patients on HD and PD was reduced from 27.4% in 1980 to 18.6% in 2007 and DM survival after PD improved by 21.8% in the last half of the 1990s.10 Other studies in the same country showed a lower peritoneal technique failure rate when comparing the 20022003 period with the 1996-1997 period.17 Although we have no data published by the Spanish registry for patients with DM, global annual mortality improved from 12% in PD in 2002 to 7.8% per annum 5 years later.18 The treatment of DM patients on PD requires dedication and integrated monitoring to reduce cardiovascular risk on all fronts. Diet, exercise and weight control are crucial, as well as control of fluid intake, which reduces the use of hypertonic solutions. A new indication for glucose-free solutions was discovered with icodextrin or amino acids as agents to reduce glucose intake for patients. In addition, the importance of preserving RRF makes all the kidney protecting measures in the pre-dialysis stage continue to have an effect. We have no evidence that glycaemic control, the use of RAAS blockers or other measures reduce the mortality of our patients. We sincerely think it is difficult to implement a randomised trial with mortality targets to test these intervention measures at present, but there is a whole pathophysiological substrate and partial evidence indicating that the hope of maintaining FRR and improving survival of DM on PD is on the right path.19 Until recently, it was accepted that diabetic patients should start dialysis early, even earlier than non-DM patients. However, the IDEAL study released this year, which is a randomised clinical trial of 828 patients followed over three and a half years, shows no benefit in starting scheduled RRT at a clearance between 10 and 14ml/min compared to doing so at 7ml/min.20 It must be made clear 601 editorial comment J. Portolés Pérez.The treatment of diabetic patients on peritoneal dialysis that the study allowed patients with symptoms or without complications to start RRT. In fact, 76% of patients assigned to a late start did so before reaching 7ml/min renal function. Finally, there was only 6 months difference between the start of RRT in the two groups. The survey is not specifically dedicated to patients with DM or PD, but provides evidence for inclusion in RRT after a patient’s complete clinical assessment and against early initiation strategy based solely on figures. In any case, PD has the added advantage of allowing a gradual start relying on and caring for RRF. Recently, it was seen that patients with preserved RRF have less vascular calcification and that this factor could be involved in the protection of that residual diuresis.21 Table 1. Measures to improve long-term outcomes in diabetic patients on peritoneal dialysisl Preserve peritoneal membrane: • Prevent peritonitis (periodic training and, after peritonitis, attention to carer fatigue, prophylaxis of orifice infection) • Reduce use of hyperosmolar and bioincompatible solutions • ˙Progressive dialysis to keep a dry day if possible Improve glycaemic control: • Reduce glucose load in solutions (icodextrin, amino acids) • Appropriate use of insulin profiles The future of PD for patients with DM is via peritoneal membrane protection, minimising glucose load, using new, more biocompatible solutions, preventing peritoneal infections and developing specific treatments to prevent peritoneal fibrosis. Control of cardiovascular risk factors specific to CKD-PD: • Avoid cardiac overload due to excess hydration (diet, diuretics) • Regulated monitoring of cardiac function • Reduce inflammation (if CRP high study possible causes) • Correct 25-OH-vitamin D deficit While we await the results of early intervention on cardiac and renal damage in our patients, we must strive to improve the prognosis of diabetic patients reaching RRT. PD appears to be a better starting technique than HD for those patients who choose it, due to its lower mortality in the first 2-3 years, greater independence and improved efficiency, because of its lower cost. At that time, we should be able to provide the patient with TX. If this is not possible, integrated control must be maintained according to Table 1 with RRF protected. Once PD is insufficient to maintain the patient’s situation, we must offer the transfer to HD within an integrated model. • Correct hyperphosphataemia Control cardiovascular risk factors: • Hypertension • Obesity • Dyslipidaemia • Smoking Elective transfer to HD when needed. Integrated RRT Modified from reference 22. KEY CONCEPTS 1. DM determines a poor prognosis in any dialysis technique mainly at the expense of added CV damage. 2. The future is early detection and intervention with kidney protection measures. 3. PD results have improved in recent years. 4. PD is a good starting technique for RRT in DM patients. Transplant is the technique of choice and should be performed as early as possible. 5. The RRT integrated model is the only technique recommended that maintains free choice and is economically sustainable. REFERENCES 1. Martínez-Castelao A. Repercusiones clínicas y sociales de la epidemia de diabetes mellitus. Nefrologia 2008;28:245-8. 2. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225-32. 602 3. Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782-7. 4. Coronel F, Cigarrán F, Herrero JA. Morbimortalidad en pacientes diabéticos en diálisisis peritoneal. Experiencia de 25 años en un solo centro. Nefrologia 2010;30(6):626-32. Nefrologia 2010;30(6):599-603 J. Portolés Pérez.The treatment of diabetic patients on peritoneal dialysis 5. Han SH, Lee SC, Ahn SV , Lee JE, Kim DK, Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients Nephrol Dial Transplant 2007;22:2653–8 doi:10.1093/ndt/gfm242. 6. Portolés J, Corchete E, López-Sánchez P, Coronel F, Ocaña J, Ortiz A. Los pacientes diabéticos tipo 2 presentan peor evolución que los no diabéticos en diálisis peritoneal a expensas de su comorbilidad cardiovascular. Nefrologia 2009;29:336-42. 7. Portolés J, Del Peso G, Fernández-Reyes MJ, Bajo MA, López-Sanchez P. Previous comorbidity and patient free choice of technique predict early mortality in peritoneal dialysis. PDI 2009;29:150-7. 8. Chung SH, Noh H, Ha H, Lee HB. Optimal use of peritoneal dialysis in patients with diabetes. Perit Dial Int 2009;29(Suppl 2):S132-4. 9. Portolés J, Ocaña J, López-Sánchez P, Gómez M, Rivera MT, Del Peso G, et al. Approach to quality objectives in incidents of patients in Peritoneal Dialysis. Nefrologia 2010;30:544-51. Doi. 10.3265/Nefrologia.pre2010.Jun.10458. 10. www.usrds.org, accessed Sept 2010. no abstract available. 11. Lowrie EG, Lew NL, Huang WH. Race and diabetes as death risk predictors in hemodialysis patients. Kidney Int Suppl 1992;38:S22-31. 12. Remón C, Quirós P, Portolés J, Marrón B. Critical analysis of survival studies on dialysis. Nefrologia 2010;30(Supl Ext 1):8-14. 13. Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 2010;21(3):499-506. 14. Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, et al. Impact of uremia, diabetes, and peritoneal dialysis it- 15. 16. 17. 18. 19. 20. 21. 22. editorial comment self on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 2008;3(3):720-8. doi: 10.2215/CJN.03630807. Gokal R. Peritoneal Dialysis in the 21st Century: An analysis of Current Problems and Future Developments. J Am Soc Nephrol 2002;13:S104-S116. Yu AWY, Chau KF, Ho YW, Li PKT. Development of the “peritoneal dialysis first” model in Hong Kong. Perit Dial Int 2007;27(Suppl 2):S53-5. Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, et al. Chronic Peritoneal Dialysis in the United States: Declining Utilization Despite Improving Outcomes. J Am Soc Nephrol 2007;18:2781-8. Doi: 10.1681/ASN.2006101130 Remón C, Quirós P, Gil Cunqueiro JM, et al. Diez años de diálisis peritoneal en Andalucía (1999-2008): datos epidemiológicos, tipos de tratamiento, peritonitis, comorbilidad y supervivencia de pacientes y técnica. Nefrologia 2010;30:46-53. Huang CC. Treatment targets for diabetic patients on peritoneal dialysis: any evidence? Perit Dial Int 2007;27(S2):S176-S179. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010;363:609-19. Yee-Moon A, Wai-Kei C, Wang M, Hiu-Shuen I, Lui S, Sanderson JE. Is Valvular Calcification a Part of the Missing Link Between Residual Kidney Function and Cardiac Hypertrophy in Peritoneal Dialysis Patients? Clin J Am Soc Nephrol 2009;4:1629-36. doi: 10.2215/CJN.03100509. Piraino B, Minev E, Bernardini J, Bender FH. Does experience with PD matter? Perit Dial Int 2009;29:256-61. Sent for review: 22 Oct. 2010 | Accepted:: 25 Oct. 2010 Nefrologia 2010;30(6):599-603 603 special article http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society Reinventing specialty training of physicians? Principles and challenges J. Morán-Barrios1, P. Ruiz de Gauna-Bahillo2, Miembros de la Junta Directiva de la Sociedad de Educación Médica de Euskadi. www.ehu.es/semde Medical Teaching Unit. Cruces Hospital. Osakidetza-Basque Health Service. Baracaldo, Biscay, Spain Department of Theory and History of Education. University of the Basque Country -Euskal Herriko Unibertsitatea. San SebastianDonostia, Guipuzkoa, Spain 1 2 Nefrologia 2010;30(6):604-12 doi:10.3265/Nefrologia.pre2010.Jul.10559 ABSTRACT In a world undergoing constant change, in the era of globalisation, the training of medical professionals should be under constant review so that it can be tailored to meet the needs of this society in transition. This is all the more true at times of economic uncertainty, such as the current conditions, which have a direct impact on health services. Professionals need new Competencies for new times. Over the last decade initiatives have emerged in various AngloSaxon countries which have defined a framework of basic Competencies that all medical specialists should demonstrate in their professional practice. In addition to this, we must respond to the creation of the European Higher Education Area which has implications for specialised training. In Spain, training for medical specialists was in need of an overhaul and the recently passed law (Real Decreto 183/2008) will allow us to move forward and implement, in medical education, initiatives and innovations required in our medical centres, to respond to the new society and bring us in line with international professional education and practice. The way forward is a Competencybased model for medical education with assessment of these Competencies using simple instruments, validated and accepted by all the stakeholders. The institutions involved (hospitals, medical centres and other health care services) should trial different approaches within the general framework established by the current legislation and be conscious of the duty they have to society as accredited training organisations. Accordingly, they should consolidate their teaching and learning structures and the various different educational roles (Director of Studies, Tutors, and Correspondence: Jesús Morán-Barrios Unidad de Docencia Médica. Hospital de Cruces. Osakidetza-Servicio Vasco de Salud. Plaza de Cruces, s/n. 48903 Baracaldo. Vizcaya. Spain. [email protected] [email protected] 604 other teaching positions), showing the leadership necessary to allow proper implementation of their training programmes. For this, the Spanish Autonomous Regions must develop their own legislation regulating Medical Specialty Training. So, medical professionals should receive training, based on ethical values, behaviours and attitudes that considers humanistic, scientific and technical factors, developing an understanding of the scientific method; ability to put it into practice; skills to manage complexity and uncertainty; a command of scientific, technical and IT terminology to facilitate independent learning; and a capacity for initiative and teamwork, as well as skills for dealing with people and for making an effective, democratic contribution both within health organisations and in the wider society. Key words: Postgraduate Medical Education. Competencybased Medical Education. ¿Reinventar la formación de médicos especialistas? Principios y retos RESUMEN En un mundo globalizado y en permanente cambio, la formación de profesionales de la medicina exige una reflexión continua para dar respuesta a esa sociedad en continua transición, máxime cuando se viven momentos económicos tan delicados como el actual que influyen directamente en el mundo sanitario. Los profesionales precisan nuevas competencias para nuevos tiempos. En la última década han surgido iniciativas en distintos países del mundo anglosajón que han definido el marco de competencias básicas necesarias que todo médico y especialista debe demostrar en su práctica profesional. Junto a ello nos encontramos ante el Espacio Europeo de Educación Superior que también influye en nuestra formación especializada. La formación sanitaria especializada en España precisaba un nuevo impulso y el reciente J. Morán-Barrios et al. Should we reinvent the training of physicians? marco regulatorio (Real Decreto 183/2008) nos permitirá avanzar y desarrollar aquellas iniciativas e innovaciones que en educación médica son imprescindibles implantar en los centros sanitarios, para responder a una nueva sociedad y adecuarnos al contexto educativo y de práctica profesional internacional. La formación médica basada en competencias y su evaluación con instrumentos, sencillos, validados y aceptados por todos los agentes implicados en la formación, es el camino a seguir. Las instituciones (centros y servicios asistenciales) deberán desarrollar sus propias experiencias dentro del marco general que proporciona la legislación vigente, y estas instituciones deben ser conscientes del compromiso adquirido con la sociedad a través de la acreditación docente, debiendo, por tanto, consolidar su organización docente y las distintas figuras de los agentes formadores (jefes de estudio, tutores y otras figuras docentes), ejerciendo el necesario liderazgo para el completo desarrollo de los programas formativos. Para ello, es preciso que las Comunidades Autónomas desarrollen sus propias normativas en formación sanitaria especializada. Finalmente, los profesionales de la medicina deben tener una formación basada en: valores éticos, hábitos y actitudes, que abarque aspectos humanísticos, científicos y tecnológicos; un conocimiento y una práctica del método científico, unidos a la gestión de la complejidad y de la incertidumbre; un manejo correcto del lenguaje científico, tecnológico e informático que facilite el aprendizaje autónomo; una capacidad de iniciativa y trabajo en equipo, así como el desarrollo de habilidades para los asuntos personales y para una eficaz participación democrática en la sociedad y en las instituciones sanitarias. Palabras clave: Formación sanitaria especializada. Formación basada en competencias. TRAINING OF PHYSICIANS IN AND FOR A HUMANISED AND FAIRER GLOBALISED WORLD Can we and should we continue training our residents with the same professional profile of as little as three years ago? What new skills must we introduce and work with in training to make future professionals more responsible and capable of managing universal and growing healthcare demands, with limited resources; professionals who have to continue acquiring knowledge (both scientific and technical) in a globalised world, full of uncertainty that should be moving towards greater equality? We live in a delicate, historic time due to the major changes that society has experienced in recent years. Our lives revolve around problems occurring elsewhere on the planet, distant each from each other and thousands of kilometres from us. Just three years ago we were far less conscious of the impact that the decisions of others could have on our Nefrologia 2010;30(6):604-12 special article lives, with consequences (the current economic crisis) that are significant for our society and that will permanently affect the political, sociocultural, and moral environment of all societies. We are seeing the other side of the coin of globalisation; a phenomenon of economic interdependence that has been intensely experienced since the 1990s and that has resulted in the neoliberal politics we see today. Globalisation is taking place in the financial sector, but also effects politics, science, culture, education and healthcare.1 In terms of the training of healthcare professionals of any kind we should ask ourselves: For what (world) and for whom (people) are we doing this training? The direction that training institutions (universities, educational centres, and health centres/hospitals) should take is based on the need to humanise society.2 In this respect, a goal of the process of training professionals must be to encourage a consciousness of universal citizenship, which will facilitate the process of change towards a new understanding of citizenship. Some time ago, organisations such as the World Health Organisation (WHO) recommended that, within the teaching environment and the practice of medicine, measures must be taken to provide education aiming to achieve equitable, efficient and comprehensive care for patients, families and communities, according to the needs and values of their society. Medical training must undergo certain changes if it is to contribute to the amelioration of some of the deficits resulting from globalisation. The commitment of training institutions must be to enable the training of professionals by and for the community, teaching them about community values of solidarity and empathy, and to be able to put themselves in the shoes of others. This commitment must not be on paper alone, but should manifest itself through training programmes and the actions of teachers. This commitment should focus on training that strengthens, among other aspects, the bonds between different cultures, life-long learning, autonomy and personal and professional responsibility, a universal vision, and, lastly, caring, creative and critical thinking.1 Training based around competencies and their evaluation allows us to tackle these challenges and commitments. THE EUROPEAN AREA AND MEDICAL TRAINING IN A CHANGING SOCIETY Medical training in Spain is facing new challenges resulting from an important change in the educational scene in Europe: the European Higher Education Area (Bologna Declaration of 19993). A process of convergence has begun that has as its aim to facilitate the mobility of graduates and adapt the content of university studies to social demands. It is an attempt to create a Europe of knowledge (a knowledge society, an expression coined by Peter Drucker in 1969,4 not 605 special article linked to the quantity of knowledge but rather to its productivity, that is, its economic impact). The Bologna Declaration states that «A Europe of Knowledge is now widely recognised as an irreplaceable factor for social and human growth and as an indispensable component to consolidate and enrich the European citizenship, capable of giving its citizens the necessary competencies to face the challenges of the new millennium, together with an awareness of shared values and belonging to a common social and cultural space».3 This does not just involve the mobility students, but also of professionals with the consequent recognition of qualifications, which implies a profound change in medical training models both at the university level as well as in specialty training. In addition, continuously changing social needs require dynamic health systems that must offer safe, effective, efficient, and high quality responses to the needs and expectations of citizens.5 The competency profile for health professionals should take into account the need for this responsiveness. Specifically, the profile must continuously be adapted and developed with new competencies, which in turn lead to modifications in learning and evaluation systems. This is important to guarantee, in the case of physicians, a good doctor-patient relationship in which there is a direct influence of demographic, epidemiological, financial, legal, and scientific-technical changes, cultural aspects, ethics and values, and new models of organisation and healthcare management (clinical management), as well as of the media and the culture of consumption.6 Regarding these changes, A. Jovell and M. Navarro7 highlight three social phenomena: changes in the labour structure of health professions, the appearance of a new patient/citizen model, and the transformation and increase in the complexity of knowledge management. To that, we must add the collectivisation of the provision of health services as a strategy to guarantee equitable access. The challenge for today’s physicians is to know how to respond effectively and efficiently to the needs of the 21st century and to the confidence placed in them by patients.5 Therefore, “the simple idea that a competent professional is one who possesses the knowledge and abilities that can lead to success in a specific profession is out of date. This idea has been replaced by the understanding that professional competency is a complex phenomenon which expresses the potential of individuals to direct their actions in the exercise of their profession with initiative, flexibility and autonomy, in diverse scenarios, based on the integration of knowledge, skills, motives and values, and is demonstrated by efficient, ethical, and socially committed professional work. It is necessary to eliminate fear of the unknown and join in the adventure of change from within; innovate and comprehend the new reality; and face up to the future and understand our role in this reality» (Pilar Martínez Clarés). Today’s training of tomorrow’s specialists means providing the competencies 606 J. Morán-Barrios et al. Should we reinvent the training of physicians? necessary to confront the uncertainties of future clinical practice, and appropriately manage future changes in society and within the medical profession itself (the areas of specialisation and collaboration between them, for example), as well as identifying and understanding the role of the physician among the various stakeholders that influence the profession of medicine (the state/governments, health organisations/corporations, the health technology and pharmaceutical industry, citizens and other health professionals8,9). It is within this context of ever greater and more complex transformations that competency-based training (CBT) emerges to enable the better adaptation and development of individuals,10 in this case, physicians. CBT focuses on learning and not on teaching, and on reaching specific objectives, i.e., on the results of learning, integrating knowledge and knowing how to do, be and act.11 THE RESIDENCY SYSTEM (SPECIALISED HEALTH TRAINING), NEW CHALLENGES AND OPPORTUNITIES Training medical specialists involves the gradual integration of recent graduates in medicine into the care activities of a health centre or hospital with growing responsibility and decreasing supervision over time. Is it possible to carry out this professional training without having the tools and resources necessary to guarantee that this integration is appropriately planned and supervised, and that the final result (a competent medical specialist) is the consequence of completing a programme designed to meet current healthcare demands?12 The Spanish system for medical graduate training, known as the MIR, was born in the 1960s as a result of the “Seminario de Hospitales” (a meeting bringing together representatives of major hospitals from across Spain: Hospital de la Santa Creu i Sant Pau, Clínica Puerta de Hierro, Hospital Marqués de Valdecilla, Fundación Jiménez Díaz, Hospital de Basurto, and Hospital General de Asturias ).13 It is based on learning on-the-job and has been one of the most important drivers of the modernisation of medical practice in Spain. The system, as regulated in 1984, has a strong state structure controlled from the Spanish Ministry of Health and Social Policy.14 The following characteristics of the system should be highlighted: the accreditation of healthcare centres and teaching units (clinical departments), whose guarantee of quality training is monitored through regular audits; a universal entrance exam; and the definition and classification of specialties and associated programmes, as regulated by the corresponding National Specialty Commissions and a National Council. However, there are considerable weaknesses in the way in which the system is put into practice within healthcare institutions. The Order on Nefrologia 2010;30(6):604-12 J. Morán-Barrios et al. Should we reinvent the training of physicians? Teaching Commissions of 1995,15 which regulates their powers and operation, as well as those of the directors of studies and tutors (educational supervisors), has not been thoroughly implemented and the evaluation system it proposed, the one currently operating, is more a system of certification of completion of a series of rotations or placements in certain care units, than a true training and assessment of competencies. A study by the National Commission on Nephrology revealed some of these weaknesses in the training of specialists, from irregular allocation of human and material resources within teaching units, to failures to fulfil teaching objectives and the role of mentoring (tutor), run on a voluntary basis, being little recognised.16 With the new law, Real Decreto 183/2008,17 the entire National Health System has had a great opportunity to develop and improve the system for specialised medical training, providing the framework for competency training and assessment, addressing, among other points, the challenges of multi-professional units, and a controversial but fundamental issue, the core curriculum. You must first be a doctor and only then a specialist. However, as noted by J. Cobo-Reinoso, the mere passing of legislation does not guarantee its successful implementation;18 this author highlights the elements that should comprise a residency programme for it to be both effective: 1) the definition of a training programme consistent with training objectives; 2) establishment of monitoring protocols; 3) adequate communication with tutors; 4) a comprehensive assessment system, essentially educational, and more demanding; and 5) quality control by the Teaching Commission. This is not possible without funding and a definitive consolidation of healthcare organisations, educational structures and training agents (directors of studies, tutors, trainers and other teaching staff), and, in Spain, this is the responsibility of the regional governments. PROFESSIONAL COMPETENCIES M.O. Bunk defines competence as behaviour resulting from a set of attitudes, skills, abilities, knowledge and values that people use to deal with specific situations related to their life and profession. 19 It is, in short, the effective ability to successfully carry out a clearly specified work activity. Professional competence is not a measure of the probability of success in the execution of a profession; it is a real and demonstrated ability that can be evaluated based on results. The start of the competency movement can be traced back to a paper published in 1973 by David McClelland, who asserted that not only aspects such as knowledge and skills, but also feelings, beliefs, attitudes and behaviours can predict high work performance.20 We are referring to Nefrologia 2010;30(6):604-12 special article empathy, intuition, integrity, perception of reality, the spirit of community, self-confidence, flexibility, and the domain of the individual. These concepts are fully applicable to the world of healthcare and indeed are today recognised as being fundamental aspects of the competencies of a medical practitioner. A Gual et al9 agree with this, stating that for the future we require: physicians who adopt a critical approach, who are communicative and empathetic, individually and socially responsible, make decisions which are good for the patient and the health care system, leaders of the health team, competent, effective and safe, honest and reliable, committed to patients and the organisation; physicians who treat patients, not diseases. In summary, the competencies of a professional combine knowledge (Know), skills and abilities (know-how), attitudes and behaviours (how to act), and values and beliefs (how to be). Faced with the aforementioned changes and challenges of the 21 st century, academic and health organisations in various countries began to define basic competencies for physicians in the 1990s and early 2000s including: Tomorrow’s Doctor 21 and Scottish Doctor 22 (UK), CanMEDS Roles23,24 (Canada), the Outcome Project of the Accreditation Council for Graduate Medical Education25,26 (USA) and the Institute for International Medical Education (IIME) in New York 27 (Table 1). The competency domains defined in these models are perfectly applicable to any specialty. Specifically, CanMEDS and the Outcome Project define what all residents must demonstrate upon completing their training. Along these lines, in 2008 the Medical Teaching Unit at Cruces Hospital (in its Competency-based Postgraduate Medical Education [CBPME] project, which started in 2004) 28 defined its model of «Being a Physician/Medical Professional» (Teaching Vision 29) for all specialties of the centre, based on the model of the IIME, and incorporating concepts from the Outcome Project, CanMEDS and the American Board of Medical Specialties. 30 Table 2 summarises the model «Being a Physician/Medical Professional at Cruces Hospital», which is to be the foundation of our competency evaluation system. These approaches do not mean that doctors, to date, have not been trained in such competencies; the difference is that in the CBPME project they are made explicit, and those competencies necessary to address changing social and healthcare needs are emphasised.31 Working with such a model facilitates the development and adaptation of the learning process (learning objectives derived from the competencies, activities, specific tasks, training plans, schedules, methodologies and teaching resources) and the implementation of a final competency assessment (outcomebased assessment). To bring about the changes required, it is very important that all professionals adopt the same approach and language, a task that will take time. 607 J. Morán-Barrios et al. Should we reinvent the training of physicians? special article Table 1. Model of competency domains CanMEDs Outcome Project (ACGME) IIEM New York Being a Physician/Professional at Hospital de Cruces 1. Expert Physician 1. Professionalism 1. Professional values, attitudes, 2. Communicator 2. Interpersonal and behaviour and ethics 1. Professionalism: professional values, attitudes, behaviour and ethics 3. Collaborator Communication Skills 2. Communication skills 4. Manager 3. Medical Knowledge 5. Health advisor 4. Patient Care 3. Scientific fundamentals of medicine 6. Scholar 7. Professional 5. Practice based on the Health System context 6. Clinical practice based on learning and improvement a 4. Clinical skills 5. Public health, health systems 6. Information management 7. Critical analysis, self-training and research 2. Clinical skills (clinical patient care expert) 3. Communication 4. Scientific fundamentals of medicine (knowledge) 5. Public health, health systems (health promoter, manager of resources) 6. Critical analysis and research. Self-training 7. Information management The competency domain of the ACGME model: “Professional practice based on learning and improvement” is broken down into two parts, in the IIEM model: “Critical analysis, self-training and research” and “Information management”. a THE EDUCATIONAL PROCESS IN THE CONTEXT OF THE WORKPLACE AND THE ROLE OF INSTITUTIONS IN PROFESSIONALISATION Training in the workplace professionalises the resident by developing their understanding of the knowledge, skills, abilities, attitudes and values that are present, these days, in the medical profession. However, it can also de-professionalise, as it is difficult to implement educational practices in each and every care setting in which residents train. The workload and other factors associated with healthcare organisations and their Table 2. Physicians/Professionals at Cruces Hospital (2008) Summary of the basic concepts of each competency domain. All residents will have and be able to demonstrate: 1. PROFESSIONAL ATTITUDES/VALUES (PROFESSIONALISM): Show integrity, accept responsibility, and complete tasks. Work within the limits of their abilities; ask for help when needed. Show respect and interest in the patients and their families. Be punctual and comply with work schedule. 2. PATIENT CARE AND CLINICAL SKILLS: Obtain a medical history and complete physical examination; request diagnostic tests as necessary and integrate the information to carry out a correct differential diagnosis. Create an appropriate treatment plan. Show skills in the performance of technical procedures for their level. 3. COMMUNICATION: Communicate effectively with patients and families, with other members of the work team and with the rest of the healthcare staff. 4. MEDICAL KNOWLEDGE: Keep their clinical knowledge up to date. Ask pertinent questions. Use their knowledge and analytical thinking to solve clinical problems. Demonstrate appropriate clinical judgment. 5. PRACTICE BASED IN THE HEALTH SYSTEM CONTEXT (PUBLIC HEALTH AND HEALTH SYSTEM): Make rational use of health resources. Work to guarantee patient safety and identify the reasons for errors; follow clinical practice guidelines (protocols). 6. PRACTICE BASED ON CONTINUOUS LEARNING AND IMPROVEMENT (CRITICAL ANALYSIS, SELF-TRAINING): Critically assess scientific literature and use available scientific evidence for patient care. Self-evaluate their clinical practice and change behaviour. Facilitate and collaborate in the learning of colleagues. 7. INFORMATION MANAGEMENT: Find, interpret, and appropriately apply clinical and scientific information. 608 Nefrologia 2010;30(6):604-12 J. Morán-Barrios et al. Should we reinvent the training of physicians? management do not facilitate interaction between tutors and residents. Though the residents should not forget that they are primarily responsible for their own training and must be proactive, this climate occasionally leads tutors and staff to forget their teaching role and that the residents are professionals in training. Institutions must guarantee: 1) the exercise of leadership from senior and middle management (directors of studies, heads of department, tutors); 2) the planning and development of a teaching strategy, with the involvement of all relevant parties (tutors and staff), facilitating decision-making and accountability, including on the part of residents; 3) the resources (structural, material, financial and organisational); 4) the development of programmes integrated into the care system in accordance with a defined profile of medical specialist; and 5) the qualitative and quantitative measurement of results. It is within this scheme that the tutor is essential as a manager of a programme of specialisation within the teaching strategy of the centre. Duties and training tasks should be specified in the training programme contracts of the centres and in the portfolio of services of teaching units. This is a reflection of the duty they have to society as accredited teaching institutions.12 Therefore, the objective is to provide a professional with: 1) broad and essential training, based on ethical values, behaviours and attitudes and that encompasses humanistic, scientific and technical aspects; 2) an understanding of the scientific method and ability to put it into practice, and to manage complexity and uncertainty; 3) a command of scientific language and skills enabling the proper use of computer technology, to facilitate independent learning; and 4) sufficient experience in the field of interpersonal relations to encourage initiative and teamwork, and the development of skills for personal relations and for effective democratic participation in society. 32 To achieve this goal, healthcare centres and teaching units (clinical departments) should be aware that there must be consistency in the training process, taking into account the three stages of the teaching/learning cycle: before, during and after. Before provides for the social environment of the specialty, the skills to be developed by level, the role of the tutor, the resident, the other trainers and the institution. During (interaction) includes training contexts, how learning will be enhanced in each of the contexts; specific, joint and individual tasks; methodological strategies; and ongoing or formative evaluation. After, involves evaluation of the learning of the resident, tutor performance, the development of the training process, the programme, the trainers and other agents of the support structure. 32 The tutor, as the pivotal figure in this complex process, with a high level of social responsibility, must acquire and develop certain teaching competencies.33 Nefrologia 2010;30(6):604-12 special article EVALUATION WITHIN THE TRAINING PROCESS Concepts and general principles Rigorous and transferable evaluation is the great unmet challenge in the specialised training system, and it will never be possible if all training agents (not just the tutors) are not sure what to evaluate, i.e., if there is not a clearly defined programme with competencies to be achieved34 (a professional profile, of the nephrologist in our case) and with instruments accepted throughout the institution. The evaluation (the after) is a moral obligation to society, the institution, and the resident (it is their right, for the purpose of guiding and supporting them in their learning, and in the acquisition and improvement of their competencies). Evaluation forms part of a complex training process and should be well defined a priori. Indeed, it is also a process in itself that generates data through assessment applying reference criteria, and this information is used to make judgments and decisions. These decisions can take many forms, but can be categorised under two broad types: a qualifying decision, a sanction, pass/fail, with no possibility of rectification, except by undergoing a new evaluation process (this type is called a summative assessment) and another type which can use the same instruments but is based on results. This latter type of evaluation allows the candidate to understand their strengths and weaknesses and create plans for improvement (it is known as formative assessment). Both demand an equal degree of rigor in their procedure and documentation. In the current training system, we must move away from the ingrained cultural concept of “pass/fail”, and thoroughly develop formative assessment. It should be realised that an evaluation process tends to fail if the following questions have not been clearly answered beforehand:34 Which competencies are to be evaluated?. How? (with what instruments?). Why evaluate? (what is the objective?). When? By whom? With what resources?, and is there collaboration across the entire team?. Therefore, every evaluation should conform to the following principles:35 it should be; 1) appropriate to the purpose (why?); 2) based on the program content (what?); 3) use methods selected in terms of validity, reliability and feasibility (based on the best available evidence); 4) have standardised, transparent and public documentation and methods; 5) provide important and positive feedback (educational evaluation); 6) be overseen by evaluators with demonstrated ability and willingness to collaborate (who?); 7) allow for lay people to assess or examine areas in which they are competent (e.g., communication, professionalism); 8) receive sufficient resources, and 9) have a comprehensive system of quality assurance. The evaluation should not be limited to the resident but rather must embrace the programme, process, structure and training agents, as the key to improvement. 609 special article J. Morán-Barrios et al. Should we reinvent the training of physicians? What to evaluate and how to assess the competencies of a professional 5. Public health systems (health promoter and manager of resources): MSF, questionnaires, and patient opinions. Evaluation of residents has, as its objective, to improve and facilitate the development of knowledge, skills, abilities, attitudes and values. To achieve this objective will require ongoing assessment of the resident in their acquisition of knowledge, skills and attitudes, and monitoring of the successes or failures of the design and operation of the training programmes. The assessment of professional competence involves various dimensions that are reflected in actions:22 1) what the doctor is able to do: technical intelligences (clinical competencies and skills), 2) how the doctor approaches their practice: intellectual (basic knowledge), emotional (attitude), the creative and analytical (reasoning) intelligences, and 3) the doctor as a professional: personal intelligences (values, ethics, professionalism). There are many validated tools and methods to assess each of these areas, which when combined, allow us to judge the competence of a professional. A description of these and their application is beyond the scope of this article, and there are excellent reviews on the subject.36,37 A particularly useful document is the Toolbox (Outcome Project, ACGME38), which describes each instrument well, its underlying concept, psychometric qualities, utility, validity and reliability. Based on that document and grouping competencies into seven domains, as described in Table 2, the potential assessment tools for each domain would be: 6. Information management: computer simulations, and cases. 1. Professional values, attitudes, behaviour and ethics: Multiple source feedback (MSF),39 commonly used in the private sector, patient point of view, and objective structured clinical examination (OSCE).40 2. Clinical skills (clinical healthcare expert): standardised patients, simulations, OSCE, clinical evaluation by direct observation of clinical practice in the workplace (MiniCEX - Mini-Clinical Evaluation Exercise, DOPs Directly Observed Procedural Skills41,42), questionnaires, case studies, clinical records, audits, peer reviews, quality indicators, reports, and portfolios.43 In relation to this, Cruces Hospital is developing its own model of reflective logbook/report to form the basis of future portfolios.44,45 Note, however, in terms of the clinical evaluation tests by direct observation, they have been developed in the Anglo-Saxon world to deal with a concern that Norcini highlights in his article,41 that the trainees are seldom observed, so the circumstances in our environment may be different. 3. Scientific foundations of medicine (medical knowledge): testing all types of knowledge, multiple-choice questions (MCQs), extended-matching items (EMIs),46 structured cases, simulations and models. 4. Communication: patient opinions, MSF, standardised patients, and OSCE. 610 7. Critical analysis and research, and self-directed learning: portfolios, reflective reports, and standardised cases. To evaluate the competencies of a professional many dimensions must be considered, it being necessary to extrapolate from partial data to arrive at a complete picture. Nevertheless, we should start with very simple, inexpensive instruments, accepted and understood by all clinical staff and the resident, and that provide added value to our Postgraduate Medical Education system. CONCLUSION Specialised training based on competencies is the answer to a globalised world in permanent change. To take this forward, medical centres and services should develop their own projects, as allowed for within the existing regulatory framework. Health institutions should be aware of their duty to society given their accreditation as teaching bodies. However, if they are to exert effective leadership in the development of training programmes it is essential that structural, organisational and human resources are made available. In Spain, this is already provided for in current legislation, and the regional governments are responsible for implementing and developing this infrastructure without further delay. Acknowledgements Acknowledgements to Pilar Martínez Clarés, Professor in the Department of Diagnostic and Research Methods applied to Education, Faculty of Education (University of Murcia) for her personal contribution to the content of this text, to the Tutors of the Competency Assessment Group (Andima Basterretxea, Elena Bereziartua, Milagros Iriberri and Agustín Martínez-Berriochoa) and to Mª Jesús González-García, secretary of the Medical Teaching Unit at Cruces Hospital. REFERENCES 1. Ruiz de Gauna P. La formación médica en una sociedad globalizada. In: Fonseca M, Ruiz de Gauna P (eds.). Avances en Educación Médica: Retos presentes para futuros profesionales de las ciencias de la salud. Oral presentation at the I SEMDE Congress, 22-25 June 2004. Bilbao: Basque Society for Medical Education (SEMDE), 2005;2:21.http://www.ehu.es/SEMDE/archivos_pdf/avances_ed_me dica.pdf 2. Mardones JM. Educar para una sociedad más humana: la educación Nefrologia 2010;30(6):604-12 J. Morán-Barrios et al. Should we reinvent the training of physicians? 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. ante la economía y la cultura. Retos educativos para la próxima década en la Unión Europea y sus implicaciones organizativas: VII Interuniversity Congress on the Organisation of Educational Institutions (VII CIOIE). San Sebastián, 4-6 July 2002;81-96. The Bologna Declaration of 19 June 1999. http:// www.bolognabergen2005.no/Docs/00-Main_doc/990719BOLOGNA_DECLARATION.PDF Stein G. Peter Drucker (I). Hacia una biografía intelectual. In: Instituto Empresa y Humanismo (Cuadernos Empresa y Humanismo n.º 73). http://www.unav.es/empresayhumanismo/publicaciones/cuadernos/catalogo07.htm White Paper on the Health Care Professions in Catalonia (Libro Blanco de las profesiones sanitarias de Cataluña). Department of Health and Social Security of the Catalan Regional Government B-18.6882003; p. 69. Jovell A. El futuro de la profesión médica. Josep Laporte Library Foundation, 2001. http://www.fbjoseplaporte.org/docs/repositori/070517121543.pdf Jovell A, Navarro M. La profesión médica en la encrucijada: hacia un nuevo modelo de gobierno corporativo y contrato social (2006). http://www.fbjoseplaporte.org/docs/repositori/070727130419.pdf Ser Médico, hoy. Retos del nuevo profesionalismo médico en España. Report of the Medical Education Foundation (FEM) for the General Council of the Colleges of Physicians (CGCOM) . Madrid 2006 http://www.educacionmedica.net/sec/serMedico 2006.pdf Gual A, Oriol-Bosch A, Pardell H. El Médico del futuro (Physician for the future). Med Clin (Barc) 2010;134:363-8. Martínez-Clares P, Martínez-Juarez M, Muñoz-Cantero JM. Formación basada en competencias en educación sanitaria: aproximaciones a enfoques y modelos de competencia. RELIEVE 2008;14(2):123. http://www.uv.es/RELIEVE Echeverría B. Gestión de la competencia de Acción Profesional. Revista de Investigación Educativa 2002;20(1):7-43. Morán Barrios J. ¿Es necesaria y compatible la existencia del tutor de médicos residentes dentro de nuestras estructuras asistenciales? Educ Med 2003;6(3):10-1. Sistema Español de Formación Especializada. Evolución, Situación actual y Perspectivas de futuro (ref 713/000155). In: Meeting of the Committee for Health and Social Security on 30 June 1992, Official Record of the Spanish Senate: commission no. 190,1992. Real Decreto 127/1984, of 11 January, regulating specialised medical training and the recognition of medical specialist. (in effect until 22 February 2008). BOE no. 26 of 31/1/1984;2524-8. Orden de 22 June 1995 regulating the Teaching Committees (Comisiones de Docencia) and systems of evaluation used in the training of medical and pharmaceutical specialists. http://www.boe.es/boe/dias/1995/06/30/pdfs/A19793-19799.pdf Quereda C, on behalf of the Spanish National Commission for the Specialty of Nephrology (Ortega F, Martín de Francisco A. L., Matesanz R, Alcázar R, Sanz Boix A, Bernis C, Abaigar P, Sánchez Casajús A, Mérida E, García Pérez MA). Algunos aspectos de la situación de la formación de especialistas de Nefrología en España. Nefrologia 2008;28(3):263-271. Real Decreto 183/2008, of 8 February, identifying and classifying specialties in the Health Sciences and specifying certain characteristics of the system for specialist health training. Nefrologia 2010;30(6):604-12 special article http://www.msc.es/profesionales/formacion/docs/realDecreto183_2008.pdf 18. Cobo-Reinoso J. La difícil transición de licenciado a especialista. Educ Med 2009;12(Supl 3):S45-S50. 19. Bunk GP. Teaching Competence in Initial and Continuing Vocational Training in the Federal Republic of Germany. Vocat Train Eur J 1994;1:8-14. 20. McClelland D. Testing for Competence rather than for Intelligence. Am Psychol 1973;28(1):1-14. 21. Tomorrow´s Doctor. http://www.gmc-uk.org/education/undergraduate/tomorrows_doctors.asp 22. The Scottish Doctor Project 2000. http://www.scottishdoctor.org/resources/scotdoc1.pdf 23. CanMEDS 2000 Project: Extract from the Societal Needs Working Group Report. Medical Teacher 2000;22(6):549-54, and the CanMEDS 2005 Framework. http://rcpsc.medical.org/canmeds/bestpractices/framework_e.pdf 24. Frank JR, Danoff D. The CanMEDS initiative: implementing an outcomes-based framework of physician competencies. Medical Teacher 2007;29(7):642-7. 25. An Introduction to Competency-based Residency Education. ©2006 ACGME. A product of the ACGME Outcome Project, 2006; http://www.acgme.org/outcome/comp/compCPRL.asp 26. Swing SR. The ACGME outcome Project: retrospective and prospective. Medical Teacher 2007;29:648-54. 27. Institute for International Medical Education, Core Committee. Global Minimum Essential Requirements in Medical Education. Medical Teacher 2002;24:130-5. 28. Morán Barrios J. Competencia profesional de médicos especialistas y modelo de gestión de la formación médica especializada basado en la experiencia del Hospital de Cruces. In: Fonseca M, Ruiz de Gauna P (eds.). Avances en Educación Médica: Retos presentes para futuros profesionales de las ciencias de la salud. Oral presentation at the I SEMDE Congress, 22-25 June 2004. Bilbao: Basque Society for Medical Education (SEMDE), 2005;9:83. http://www.ehu.es/SEMDE/archivos_pdf/avances_ed_medica.pdf 29. Morán Barrios J. Ser Médico. http://www.hospitalcruces.com/documentos/actividad Docente/VISION_DOCENTE-SER_MEDICO.pdf 30. American Board Medical Specialties. MOC Competencies and Criteria:http://www.abms.org/Maintenance_of_Certification/ABMS_MO C.aspx 31. Morán Barrios J. La Formación basada en competencias debe de introducirse también en la Formación Especializada (interview). DPM 2010;3(1):37-40. http://www.idepro.es/dpm/v3_n1/DPM3_1.pdf 32. Fonseca M, Ruiz de Gauna P. Formación médica especializada: formar en competencias para incidir en el perfil del professional que necesita la sociedad y la sanidad del siglo XXI. In: Perspectivas para el Cambio en la Formación y la Práctica Médica, pp. 67-71. Bilbao, 26-27 October 2006: II Congress of the Basque Society for Medical Education (SEMDE). http://www.ehu.es/SEMDE/publi.htm 33. Saura Llamas J. Cómo puede convertirse un tutor en un docente efectivo. Aten Primaria 2007;39(3):151-5. 34. Morán Barrios J. Estrategias e instrumentos de evaluación del residente. Valladolid, 8 and 9 October 2008: Oral presentation at a Meeting of Tutors in Specialised Health Training in de Castilla y León. http://www.ehu.es/SEMDE/publi.htm 611 special article 35. A working paper from the Postgraduate Medical Education Training Board. Principles for an assessment system for postgraduate medical training, 14 September 2004. http://www.ecompendium.nhs.uk/PMETBprinciples-forassessment-systems.asp 36. Epstein RM. Assessment in Medical Education. N Engl J Med 2007;356:387-96. 37. Wass V, Van der Vleuten C, Shatzer J, Jones R. Assessment of clinical competence. Lancet 2001;357:945-9. 38. ACGME Outcome project. Toolbox of assessment methods: http://www.acgme.org/Outcome/assess/Toolbox.pdf 39. Whitehouse A, Hassell A, Bullock A, Wood L, Wall D. 360 degree assessment (multisource feedback) of UK trainee doctors: Field testing of team assessment of behaviours (TAB). Medical Teacher 2007;29(2- 3):171-6. 40. Martínez Carretero JM. Los métodos de evaluación de la competencia profesional: la evaluación clínica objetiva estructurada (ECOE). J. Morán-Barrios et al. Should we reinvent the training of physicians? Educ Med 2005;8(Supl 2):S18-S22. 41. Norcini J, Burch V. Workplace-based assessment as an educational tool: AMEE Guide No. 31. Medical Teacher 2007;29(9-10):855-71. 42. Norcini J. The Mini Clinical Evaluation Exercise (mini-CEX). Clinical Teacher 2005;2(1):25-30. 43. Snadden D, Thomas M. The use of portfolio learning in medical education. Medical Teacher 1998;20(3):192-9. 44. Morán J, Fernández F, Lorenzo M, Sharluyan A. Memoria de Formación del Médico Residente (MFMIR). Aproximación al portafolio docente. Educ Med 2005;8(3):159. (I-8). 45. Morán J, Carballo G, Ruiz de Gauna P. Contenido reflexivo de la memoria de formación MIR del Hospital de Cruces. Bases del portafolio formativo. Educ Med 2007;10(3):169. D-6. 46. Case SM, Swanson DB. Extended-matching items: A practical alternative to free-response questions. Teaching and Learning in Medicine 1993;5(2):107-15. Sent for Review: 22 Jun 2010 | Accepted: 22 Jul. 2010 612 Nefrologia 2010;30(6):604-12 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society short reviews BK virus-associated Nephropathy D. Burgos, C. Jironda, M. Martín, M. González-Molina, D. Hernández Nephrology Department. Carlos Haya Hospital. Málaga, Spain Nefrologia 2010;30(6):613-7 doi:10.3265/Nefrologia.pre2010.Oct.10587 ABSTRACT Nefropatía asociada a infección por poliomavirus BK The infection by the BK Polyomavirus (BKV) is an emerging problem in kidney transplants that contributes to a chronic loss of kidney grafts, and in which immunosuppression plays a decisive role. Understanding its risk factors and strictly monitoring urine and serological markers of the infection could mitigate the undesirable effects of this disease. In this review, we investigate the clinical and epidemiological aspects of the BKV infection, as well as go over the available prophylactic and treatment methods currently available for controlling the infection in kidney transplant patients that receive modern immunosuppression. RESUMEN Key words: Kidney transplantation. Polyomavirus BK. Immunosuppression. Decoy cells. Palabras clave: Trasplante renal. Inmunosupresión. Células decoy INTRODUCTION BIOLOGICAL ASPECTS OF THE VIRUS Viruses are pathogens that are especially problematic in transplant recipients, since the issue of viral infections in transplantations reflects a complex equilibrium between the various viral infections that a patient may have throughout his/her lifetime, the antiviral immune response of the recipient, and the level of immunosuppression required for ensuring a functioning graft. The polyomavirus, along with the papillomavirus, belongs to the papovavirus family of pathogens. The BK virus (BKV) belongs to the polyomavirus family along with other polyomaviruses that have been detected in humans, such as the JC virus (JCV), the KI virus, the WU virus, the Merkel cell carcinoma virus, and the Simian virus 40 (SV40). Infection by polyomavirus BK (BKV) is an emergent problem in kidney transplants, and is considered to be the price paid for modern and powerful immunosuppression (IS). These are small, non-enveloped viruses, with a diameter of 42 nm. The capsid has icosahedral symmetry and houses a double circular chain genome of DNA with over 5000 base pairs, composed of an “early” region that is highly conserved and codes for the “T/t antigen” (TAg), which is implicated in transformation, viral replication, and gene regulation and expression; and a “late” region that codes for the three capsid proteins, known as VP1, VP2, and VP3, and for a protein called “agnoprotein,” a non-coding regulatory region situated between the other two, where the determinants for replication, the TAg union, and transcriptional regulation elements are located. Correspondence: Domingo Hernández Marrero Servicio de Nefrología. Hospital Carlos Haya. Avenida Carlos Haya, s/n. 29010 Málaga. Spain. [email protected] La infección por el poliomavirus BK (PBK) es un problema emergente en el trasplante renal que contribuye a la pérdida crónica de los injertos renales, y en el que la inmunosupresión desempeña un papel decisivo en su aparición. El conocimiento de los factores de riesgo y la monitorización estrecha de marcadores urinarios y serológicos de la infección pueden mitigar los efectos indeseables de esta infección. En esta revisión se profundiza en los aspectos clínicos y epidemiológicos de la infección por PBK, así como en las medias profilácticas y terapéuticas disponibles para su control en pacientes con trasplante renal que reciben moderna inmunosupresión. Poliomavirus BK. 613 short reviews The polyomavirus possesses adaptation specificity to its host; therefore, its evolution is probably associated with the host species’ evolution, and so the natural infection occurs only in a limited number of closely related species, constituting a marker for establishing the racial differences between humans. Using gene-sequencing analysis, different genotypes have been established: European, Asian, and African. The rest of the genotypes correspond to recombinations of these three, and although its origin is difficult to establish, the study of this virus could provide a tool for aiding in understanding the evolution of human migrations. BKV is associated with two complications observed in transplant recipients: BK virus-associated nephropathy (BKVN) in kidney transplants, and haemorrhagic cystitis in bone marrow transplants. In contrast to the BKV, although the JCV resides in the uroepithelium and normally reactivates, it rarely produces nephropathy, but is associated with multifocal leukoencephalopathy and encephalitis. SV40, which comes from simians, was introduced into the human population through vaccines contaminated with polio and adenovirus, and although its presence has been detected in transplanted kidney biopsies, its importance in kidney transplantation is not yet well defined. EPIDEMIOLOGY AND RISK FACTORS The primary infection occurs subclinically during the first decade of life, with a seroprevalence of over 80% in the adult population. The source of infection is exclusively human, no animals have been shown to act as reservoirs, and the transmission route can be faecal-oral, respiratory, transplacental, and through donated tissues. During the viremic phase, the virus infects the tissues, urothelium, lymph tissue, and brain, producing a latent lytic infection. After the natural viral transmission during infancy, the BKV remains in the urinary tract with intermittent reactivations and low levels of viruria (Vr), 5%–10% in immunocompetent adults.1,2 In immunocompromised individuals, the frequency of BK Vr increases to 20%–60%, and even greater levels of viruria and the appearance of decoy cells in urine are also frequent.3 In kidney transplantation, the prevalence of nephropathies associated with BK virus (BKVN) oscillates between 1% and 10%,4 based more on the immunosuppression treatment and diagnostic methods than due to real epidemiological differences. In 2004, the treatment of the BKV infection after kidney transplantation was included in the American database as a variable for post-transplant evolution (TBKV); the data were 614 D. Burgos et al. BK Virus Nephropathy later analysed, resulting in a total of >48 000 transplants, 1474 of which were treated within 24 months. The cumulative incidence of TBKV increased with time, going from 3.45% at 24 months to 6.6% at 60 months after the transplantation. Graft failure secondary to BKVN occurs at a rate of 50%–100% at 24 months in centres with no screening programs, which highlights the importance of an early diagnosis of the disease.5 Different IS protocols have been identified as risk factors for the development of BKVN, especially the use of triple therapies with anticalcineurinic drugs, mycophenolate mofetil (MMF), and steroids,5,6 but BKVN cases have also been described when using other IS regimens, which indicates that the intensity of IS treatment, and not the specific drug itself, is the risk factor in this case. Other types risk factors also exist, such as patient factors (males >50 years of age, BKV seronegative recipient), graft factors (BKV seropositive donor, HLA incompatibilities, immunological or ischaemic injury), and viral factors (latent viral load, capsid serotype, and capacity for replication).7 BKVN HISTOLOGICAL DIAGNOSIS AND PROGRESSION Decoy cells, viruria, and viremia only indicate viral replication, not nephropathy, but they are key tools for preventing and monitoring the disease. The only clinical sign of BKVN is the deterioration of kidney function, and when this occurs, it is already too late to intervene, since the renal damage has already been produced. The diagnosis of the disease can only be performed with a graft biopsy in which the typical basophilic nuclear viral inclusions are found in the epithelial cells (tubular, Bowman’s capsule, and/or urothelium), and signs of inflammation with tubulitis (Figure 1A), similar findings to those that appear in acute transplant rejection by T-cells. Only by using the immunohistochemical technique for SV-40 LTAg can we observe a positive nuclear staining and identify the polyomavirus (BK, JC) as that responsible for the inflammation, thus discarding the diagnosis of acute T-cell rejection (Figure 1B) and confirming the diagnosis of BKVN. BKVN histological lesions are focal and heterogeneous, and so a negative biopsy cannot exclude the diagnosis. As such, this test must be repeated if the viral load in the patient’s blood remains persistently high. The histological patterns of BKVN2,8,9 are based on the identification and extension of the inflammatory infiltrate Nefrologia 2010;30(6):613-7 D. Burgos et al. BK Virus Nephropathy short reviews consequence of viral reactivation and replication in the urinary tract, with the appearance of typical decoy cells, (Figure 4) which are easy to identify using routine urine cytology tests. However quantification of Vr using PCR techniques is more sensitive than using cytology, and allows for distinguishing between BKV and JCV infections. A When viruria is >10 5 copies/ml and persists, it is followed weeks or months later by the development of viremia (Vm) at >10 7 copies/ml and, finally, BKVN. BK Vr is not diagnostic of renal parenchymal damage, but the simultaneous appearance of Vm and Vr is pathognomonic of renal parenchymal damage (BKVN). Maintained, or more typical, increasing Vm is a predictive factor for deteriorating kidney function, and is correlated with the presence and severity of histological lesions. In patients with normal or moderately low kidney function, the probability of finding histological indicators of BKVN is directly proportional to the duration and severity of viremia. Elevated and sustained viremia identifies those patients with uncontrolled viral replication that leads to kidney damage. B Figure 1. Basophilic nuclear viral inclusions in epithelial cells and tubulitis in the BK-virus nephropathy (A) and immunohistochemistry for the antigen SV-40 LTAg (B). In conclusion, early diagnosis and intervention minimises the damage to the transplant. Figure 5 demonstrates a diagnostic algorithm based on previous publications. 4,9 BKVN TREATMENT and viral infection-associated fibrosis, which allows for three histological patterns to be established (Figure 2). CLINICAL EVOLUTION AND OPPORTUNITIES FOR EARLY PREVENTION AND DIAGNOSIS The common clinical evolution of BKVN9 is represented in Figure 3, which shows how the development of the disease is predicted by the appearance of BK viruria (BK Vr), a Pattern A - Viral cytopathic changes in normal renal parenchyma - Insignificant or absent FIAT and inflammation The best treatment for BKVN is an early diagnosis of the disease in order to act before renal damage is caused. For this reason, KDIGO Guides10 suggest using a screening process for all kidney transplant patients by testing monthly Vm levels during the first 3 months (2D) and every three moths until the end of the first year (2D), whenever renal dysfunction is produced with no visible alternative cause (2D), and after treatment for en episode of acute rejection (2D). Pattern B - Combination of viral cytopathic changes and areas of FIAT and focal/multifocal inflammation Pattern C - Few cytopathic changes - Extensive FIAT and inflammation B1<2.5% FIAT B2 26%-50% FIAT B3>50% FIAT Figure 2. Histological patterns of the BK virus-associated nephropathy. Nefrologia 2010;30(6):613-7 615 D. Burgos et al. BK Virus Nephropathy short reviews Stereotypical evolution of polyoma virus allograft nephropathy (PVAN) Viruria + Viremia Increasing viruria, viremia, serum creatinine & PVAN Viruria Viruria + Viremia + PVN Endstage PVN Vr Vm Cc Bx+ E F G Post-trasplant follow up (months) Figure 3. Phases of evolution of the BK virus-associated nephropathy. Figure 4. Disperse decoy cells and cellular cylinders containing compacted decoy cells. When they appear, these cylinders are pathognomic of kidney damage. A reduction in IS is also suggested when Vm is persistently greater than 107 copies/ml (2D). determine because they have not been administered in combination with a reduction in IS and because of the lack of controlled and randomised prospective studies. Regarding the reduction in IS, the first step consists of implementing the standard protocol (not giving CAN or antiproliferative treatments above the levels indicated for the therapeutic range), followed by measuring viremia every 4 weeks, reducing NAb by 15%-20%, reducing MMF and/or MMF suppression by 50%, and/or substituting TAC by CsA or an ISP (Figure 6).11 With regard to antiviral treatments, i.v. immunoglobulins, ciclofovir, leflunomide, and quinolones have been used empirically, and their efficacy is currently difficult to BK Management Test results NBK Indication Intervention 1.st Step Screening Urine cytology decoy cells BKV DNA urine Possible No 2.nd Step Confirmation Probable Yes BKV DNA Urine > 107 cop ml BKV DNA Plasma > 104 cop ml Probable Yes 3.rd Step Biopsy BKVN A BKVN B BKVN C Definitive Yes Monitoring Plasma BKV DNA Negative Resolved Finally, we would like to comment on kidney retransplantation in patients that have lost a graft due to BKVN. The recurrence of the disease in short studies is 12%. The recommendations that must be taken into account in these situations are: 1) inform the patient as to the increased potential risk of recurrence of BKVN; 2) confirm the absence of viral replication (blood and urine PCR when the patient is included on the transplant list and every 6 months thereafter), the patient must receive the transplant with negative PCR results from blood samples, and 3) adapt the IS to the pathology.12-14 Ginevri, et al. AJT 2007;7:2727. - Implement standard protocol - Viremia >4 weeks ACN 15-20% - 4.th Step Figure 5. BKVN diagnostic algorithm. 616 MMF 50% Stop MMF - Substitute TAC by CsA or ISP Figure 6. BKVN treatment algorithm. Nefrologia 2010;30(6):613-7 D. Burgos et al. BK Virus Nephropathy short reviews KEY CONCEPTS 1. The powerful and modern forms of immunosuppression could be responsible for the increasing prevalence of this infection 2. BK virus infection in immunocompromised patients could affect the function and survival of kidney transplants 3. Early diagnosis by strictly monitoring urine decoy cell count and/or viruria and viremia is crucial for avoiding the negative impacts of this complication 4. No evidence exists of a specific effective treatment for this infection. Only a reduction in immunosuppression treatment can minimise virulence. REFERENCES 1. Polo C, Pérez JL, Mielnichuk A, et al. Prevalence and patterns of polyomarvirus urinary excretion in immunocompetent adults and children. Clin Microbiol Infect 2004;10:640. 2. Drachemberg CB, Hirsh HH, Ramos E, et al. Polyomavirus disease in renal transplantation: Review of Pathological findings and diagnostic methods. Hum Pathol 2005;36:1245. 3. Drachemberg CB, Hirsh HH, Papadimitriou JC, et al. Cost efficiency in the prospective diagnosis and follow-up of polyomavirus allograft nephropathy. Transplant Proc 2004;36:3028. 4. Hirsh HH, Brennan DC, Drachemberg CB, et al. Polyomavirus associated nephropathy in renal transplantation: Interdisciplinary analysis and recommendations. Transplantation 2005;79:1277. 5. Ramos E, Drachemberg CB, Portocarrero M, et al. BK virus nephropathy diagnosis and treatment: Experience at the University of Maryland Renal Transplant Program. Clin Transpl 2002;43. 6. Hirsh HH, Friman S, Wiecek A, et al. Prospective study of Polyomavirus BK viruria and viremia in the novo renal transplantation. Am J Transplant 2007;7:150. 7. Binggeli S, Engli A, Schaub S, et al. Polyomavirus BK specific-cellular immnune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant 2007;7:1131. 8. Drachemberg CB, Papadimitriou JC, Hirsh HH, et al. Histological patterns of polyomavirus nephropathy: Correlation with graft outcome and viral load. Am J Transplant 2004;4:2082. 9. Ramos E, Drachemberg CB, Wali R, Hirsh HH. The decade of polyomavirus BK-Associated Nephropathy: State of Affairs. Transplantation 2009;87:621. 10. Kidney Diseases Improving Global Outcomes (KDIGO). Transplant Work Group. Am J Transplant 2009;9(Suppl 3):S1-S157. 11. Ginevri F, Azzi A, Hirsch HH, Bassoo S, Fontana I, Cioni M, et al. Prospective Monitoring of Poliomavirus BK Replication and Impact of Pre-Emptive Intervention in Pediatric Kidney Recipients. Am J Transplant 2007;7:2727. 12. Nickeleit V. Animal Models of Polyomavirus Nephropathy: Hope and Reality. Am J Transplant 2006;6:7. 13. Ramos E, Vincenti F, Lu WX, Shapiro R, Trofe J, Stratta RJ, et al. Retransplantation in patients with graft loss caused by polyoma virus nephropathy. Transplantation 2004;77:131. 14. Womer KL, Meier-Kriesche HU, Patton PR, Dibadj K, Bucci CM, Foley D, et al. Preemptive Retransplantation for BK Virus Nephropathy: Successful Outcome Despite Active Viremia. Am J Transplant 2006;6:209. Sent for Review: 28 July 2010 | Accepted: 5 Oct. 2010 Nefrologia 2010;30(6):613-7 617 short reviews http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society Sodium-glucose cotransporter 2 (SGLT2) inhibitors: from renal glycosuria to the treatment of type 2 diabetes mellitus G. Pérez López, O. González Albarrán, M. Cano Megías Endocrinology Department. Ramón y Cajal Hospital. Madrid, Spain Nefrologia 2010;30(6):618-25 doi:10.3265/Nefrologia.pre2010.Sep.10494 ABSTRACT For centuries, the kidney has been considered primarily an organ of elimination and a regulator of salt and ion balance. Although once thought that the kidney was the structural cause of diabetes, which in recent years has been ignored as a regulator of glucose homeostasis, is now recognized as a major player in the field of metabolic regulation carbohydrate. During fasting, 55% of the glucose comes from gluconeogenesis. Only 2 organs have this capability: the liver and kidney. The latter is responsible for 20% of total glucose production and 40% of that produced by gluconeogenesis. Today we have a better understanding of the physiology of renal glucose transport via specific transporters, such as type 2 sodiumglucose cotransporter (SGLT2). A natural compound, phlorizin, was isolated in early 1800 and for decades played an important role in diabetes and renal physiology research. Finally, at the nexus of these findings mentioned above, recognized the effect of phlorizin-like compounds in the renal glucose transporter, which has offered a new mechanism to treat hyperglycemia. This has led to the development of several potentially effective treatment modalities for the treatment of diabetes. Key words: Type 2 diabetes mellitus. Familial renal glucosuria. SGLT2 inhibitors. Inhibidores del cotransportador sodio-glucosa tipo 2 (SGLT2): de la glucosuria renal familiar al tratamiento de la diabetes mellitus tipo 2 RESUMEN Durante siglos, el riñón se ha considerado principalmente un órgano de eliminación y un regulador de la sal y del equilibrio iónico. A pesar de que una vez se pensó que era la causa estructural de la diabetes, y que en los últimos años ha sido ignorado como regulador de la homeostasis de la glucosa, actualmente es reconocido como un actor importante en el ámbito de la regulación del metabolismo glucídico. Durante el ayuno, el 55% de la glucosa proviene de la gluconeogénesis. Sólo 2 órganos tienen esta capacidad: el hígado y el riñón. Este último es responsable del 20% de la producción total de glucosa y del 40% de la producida por la gluconeogénesis. Hoy en día tenemos una mejor comprensión de la fisiología del transporte de glucosa renal a través de transportadores específicos, como el cotransportador sodio-glucosa tipo 2 (SGLT2 por sus siglas en inglés: Sodium Glucose Cotransporter). Un compuesto natural, floricina, se aisló a principios de 1800 y durante décadas desempeñó un papel importante en la diabetes y la investigación de la fisiología renal. Finalmente, en el nexo de estos descubrimientos antes mencionados, se reconoció el efecto de compuestos floricina-like en los transportadores de glucosa renal, lo que ha ofrecido un nuevo mecanismo para el tratamiento de la hiperglucemia. Esto ha llevado al desarrollo de varias modalidades terapéuticas potencialmente eficaces para el tratamiento de la diabetes. Palabras clave: Diabetes mellitus tipo 2. Glucosuria renal familiar. Inhibidores de SGLT2. INTRODUCTION In 2009, De Fronzo1 described the rapid advances in the knowledge of the various pathophysiological pathways related to the development of diabetes. Correspondence: Gilberto Pérez López Servicio de Endocrinología. Hospital Universitario Ramón y Cajal. Carretera de Colmenar Viejo, km 9,100. 28034 Madrid. Spain. [email protected], [email protected] 618 This was explained by the change from the triumvirate to the ominous octet, referring to the important role seemed to be played in carbohydrate metabolism by the kidney2-4 (increasing the reabsorption of glucose), the small intestine and alpha cells (decreasing the incretin effect and increasing the production of glucagon), and the dysfunction of neurotransmitters in the central nervous system, together with the classic insulin resistance G. Pérez López et al. SGLT2 inhibitors components (decreased insulin production, increased hepatic glucose production, decreased glucose uptake by skeletal muscle and increased lipolysis). short reviews Clinically, the most common cause of glycosuria is diabetes. Patients do not excrete glucose in the urine until the concentration of blood glucose is over 180mg/dl, which does not normally occur in people without diabetes. THE KIDNEY AND GLUCOSE HOMEOSTASIS The kidney was traditionally considered as one of the main organs responsible for glucose homeostasis. However, we now understand that it plays an important role in glucose homeostasis in two ways: 1) gluconeogenesis, and 2) glomerular filtration and reabsorption of glucose in the proximal convoluted tubules. With a better understanding of the renal mechanisms responsible for glucose homeostasis and the ability to manipulate that system, the kidney has become a key component in the treatment of hyperglycaemia. Filtration and the reabsorption of glucose For a healthy adult, approximately 180g of glucose is filtered by the glomerulus every day. 5 Under normal circumstances, almost all of this glucose is reabsorbed with less than 1% being excreted in the urine. 6 Glucose reabsorption in the tubules is a multi-step process involving several transport mechanisms. Glucose is filtered through the tubule and then transported via the tubular epithelial cells through the basolateral membrane into the peritubular capillary. Under optimal conditions, when tubular glucose load is approximately 120mg/min or less, there is no glucose loss in urine. However, when the glucose load exceeds approximately 220mg/min (glucose threshold), glucose starts to appear in the urine. The blood glucose level required to provide such a tubular load covers a range of values in humans. A study of this process reported that the blood glucose concentration required to exceed the tubular glucose threshold ranged between 130 and 300mg/dl.7 In addition, the study found a relationship between age and increased threshold levels. 90% of filtered glucose is reabsorbed by the high absorption capacity of SGLT2 transporter in the convoluted segment of the proximal tubule, and the remaining 10% of filtered glucose is reabsorbed by the SGLT1 transporter in the straight segment of the descending proximal tubule.2 As a result, no glucose appears in the urine. The maximum renal capacity for tubular reabsorption (Tm) of glucose is greater in animal models with type 1 and type 2 diabetes. 8 In people with type 1 diabetes, Mogensen et al.9 showed that the glucose Tm is increased. Conflicting results have been reported in patients with type 2 diabetes. Nefrologia 2010;30(6):618-25 Role of the SGLT2 transporter The first step in the reabsorption of urine glucose involves the transport of glucose from the tubules to peritubular capillaries via tubular epithelial cells.10 This is accomplished with the family of sodium-glucose cotransporters (SGLT), see Figure 1. The SGLTs include a variety of membrane proteins that act on the transport of glucose, amino acids, vitamins, ions and osmolytes across the brush border membrane of the renal proximal tubules and the intestinal epithelium. 11 SGLT1 is a low capacity and high affinity carrier. It is found mainly in the gastrointestinal tract, but can also be found in the S3 segment of the renal proximal tubule. Although SGLT1 is the key transporter for glucose absorption in the gastrointestinal tract, its impact on the kidney is less important; representing about 10% of glucose reabsorption. This has been of some pharmacological interest because blocking this transporter theoretically reduces the gastrointestinal absorption of glucose and may provide a method for inducing weight loss or reducing postprandial hyperglycaemia. By contrast, SGLT2 transporter has a high capacity and low affinity, and is found mainly in the kidney. Table 1 compares the SGLT1 and SGLT2 transporters. A third member of this family, SGLT3, is widely found in skeletal muscle and the nervous system. SGLT3 is not believed to be a glucose transporter, but acts as a sensor. 12 Although other members of this family have been identified (SGLT4, SGLT5 and SGLT6), their role in humans is not known at this time (Table 2). The most prevalent and functionally most important transporter in the kidney is SGLT2. It is responsible for 90% of glucose reabsorption in the kidney, and has become the subject of much interest in the diabetes field. This transporter is found in a relatively high proportion in the initial segment of the proximal tubule (S1). SGLT2 transports glucose by using the energy gradient of sodium reabsorption in the tubular filtration. This process is called secondary active transport and is driven by the electrochemical gradient of sodium in the tubular filtration. 619 G. Pérez López et al. SGLT2 inhibitors short reviews Capillary Santer et al.13 conducted a genetic study on 23 families diagnosed with renal glycosuria and found 21 different mutations of the SLC5A2 gene. Fourteen out of the 21 families were homozygous and had glycosuria between 15 and 200g/day. Heterozygotes typically had glycosuria of under 4.4g/day, although some did not. ATPase S1 Proximal tubule protein. Autosomal dominant and recessive inheritance patterns have been reported. As a result of this mutation, patients with renal glycosuria excrete in their urine more than 100g of glucose in 24 hours. Glucose Glucose Figure 1. Mechanism of action of SGLT2 On the luminal side of the S1 segment of the proximal tubule, the absorption of sodium creates an energy gradient which allows glucose uptake via SGLT2 (sodium-glucose cotransporter type 2). On the other side of the cell, sodium is transported through the blood capillary basement membrane by the sodium-potassium ATPase pump. This phenomenon in turn creates another energy gradient and glucose is transported to capillary flow by glucose transporter 2 (GLUT210). RENAL GLYCOSURIA: SGLT2 TRANSPORTER INHIBITION MODEL Renal glycosuria is a genetic condition where the effects of the inhibition of SGLT2 transporter can be observed. Patients with this condition are asymptomatic, even though in most cases they have a SLC5A2 gene mutation (solute carrier family 5A), responsible for encoding SGLT2 transporter Two families diagnosed with renal glycosuria did not have the SLC5A gene mutation, but may have had mutations of the genes encoding GLUT2 (type 2 glucose transporter), HNF-1· (hepatic nuclear factor 1 alpha) which regulates the transcription of SGLT2 or genes related with SGLT1 or SGLT3. Except for glycosuria, there were no other associated diseases. Plasma glucose was high or low, and blood volume remained essentially normal due to sodium reabsorption via other transporter channels. Renal and bladder function was normal, and this group of patients had no increased incidence of diabetes, kidney disease or urinary tract infections, compared with the general population.14 Figure 2 schematically shows the reabsorption of glucose in normal individuals and patients with renal glycosuria. As mentioned previously, the maximum renal capacity of tubular reabsorption (Tm) for glucose is variable, although for physiological studies (theoretical, continuous black line) it is about 198mg/dl (11mmol/l). The glucose Tm usually observed is below this figure (broken black line), and is saturated with glucose concentrations near 180mg/dl (10mmol/l15). Renal glycosuria can be classified into two types.13 Type A has a glucose Tm lower than in normal subjects (blue line). Table 1. Comparison of SGLT1 and SGLT2 transporters SGLT1 SGLT2 Location Small intestine and kidney Kidney Substrates Glucose or galactose Glucose Glucose affinity High Low Glucose transport capacity Low High Function - Intestinal absorption of glucose and galactose - Renal reabsorption of glucose Renal reabsorption of glucose In the kidney, SGLT2 transporter is responsible for 90% of tubular reabsorption of glucose, whereas SGLT1 is responsible for the remaining 10%. The low affinity for glucose and its high carrying capacity, make inhibition of SGLT2 a pharmacological mechanism for the treatment of type 2 diabetes mellitus.14 620 Nefrologia 2010;30(6):618-25 G. Pérez López et al. SGLT2 inhibitors short reviews Table 2. Sodium-glucose cotransporter family Transporter Substrate Tissue distribution SGLT1 Glucose and galactose Kidney, small intestine, heart and trachea SGLT2 Glucose Kidney SGLT3 Glucose sensor Small intestine, thyroid, testes, uterus and lung SGLT4 Mannose, glucose, fructose, galactose and AG Kidney, small intestine, liver, stomach and lung SGLT5 Glucose and galactose Kidney SGLT6 Myo-inositol, glucose, xylose and chiro-inositol Kidney, small intestine, spinal cord and brain 1,5 AG: 1,5-anhydro-D-glucitol12 These patients have decreased SGLT2 transporter activity as well as more significant glycosuria. from healthy individuals and diabetic patients, and were cultured in a hyperglycaemic medium. In type B renal glycosuria, the SGLT2 transporter has no affinity for glucose, resulting in a decrease in the reabsorption rate of glucose, but a normal glucose Tm (green line). As shown in Figure 3, the HEPTC of diabetic patients showed a statistically significant higher expression of SGLT2 and GLUT2 compared with non-diabetic individuals. They also determined the renal glucose uptake using methyl-α-D[U14C]-glucopyranoside (AMG), which is an analogue of glucose. More glucose uptake was also observed in diabetes patients? HEPTC than in individuals without diabetes. TYPE 2 DIABETES MELLITUS AND THE SGLT2 TRANSPORTER Type 2 diabetes mellitus is associated with increased expression and activity of SGLT2. In a study16 of the SGLT2 transporter, human exfoliated proximal tubular epithelial cells (HEPTC) were used, which were obtained from urine samples. HEPTC were isolated These findings prove that the renal system noticeably contributes to the body’s energy balance by regulating glucose uptake, and that diabetic patients appear to be poorly adapted to this mechanism. In diabetes, glucose reabsorption may be increased in absolute terms by an increase of glucose Tm. SGLT2 TRANSPORTER INHIBITORS FOR THE TREATMENT OF TYPE 2 DIABETES MELLITUS Theoretical Observed Normal Type B Glucose reabsorption Type A 5 10 15 Plasma Glucose Concentration (mmol/l) Figure 2. Comparison of the maximum tubular glucose reabsorption capacity (Tm) The solid black line shows the theoretical glucose Tm, while the broken line shows the glucose Tm in healthy subjects. The glucose Tm in patients with type A renal glycosuria is shown by the blue line and type B in green.13 Nefrologia 2010;30(6):618-25 In 1835, French chemists isolated a substance called phlorizin from the roots of apple trees. Although it was believed that phlorizin was a compound for treating fever, infectious diseases and malaria, it was not until 50 years after its discovery that it was found that high doses of phlorizin caused glycosuria.17 For several decades, phlorizin was used in the assessment of renal physiology. Then in 1970, it was discovered that glycosuria could be caused by phlorizin inhibiting an active transport system for tubular reabsorption of glucose. Between 1980 and 1990, the SGLT2 transporter was identified, and the inhibition of this transporter began to be profiled as a treatment for type 2 diabetes mellitus. Phlorizin was therefore the first known SGLT2 inhibitor. However, phlorizin could not be used as a treatment for type 2 diabetes mellitus for several reasons. Firstly, because intestinal absorption is very poor and, secondly, because it 621 G. Pérez López et al. SGLT2 inhibitors short reviews Glucose transporter expression AMG Uptake SGLT2 inhibition essentially resets the maladaptive diabetic kidney by reducing the affinity of the transporter and increasing glycosuria, which decreases blood glucose and, therefore, glucotoxicity.18 Recently, phlorizin analogues selective for SGLT2 with better intestinal absorption have been developed. Table 3 shows some drugs in this group, including dapagliflozin and canagliflozin, which are currently in phase III clinical trials. Figure 3. Comparison of the glucose tubular transporters in diabetics and non-diabetics. Comparison of the expression of SGLT2 and GLUT2, and the methyl-α-D-[U14C]-glucopyranoside (AMG) uptake in human exfoliated proximal tubular epithelial cells (HEPTCs) in healthy individuals and diabetic patients.16 CPM: counts per minute. In addition, one laboratory is currently in phase I clinical trials19 with a molecule called ISIS-388626 to reduce expression of SGLT2. This compound is an oligonucleotide that decreases transcription of the gene encoding the SGLT2 transporter. In murine and canine models, treatment with ISIS-388626 is highly selective, as it reduces the mRNA (messenger ribonucleic acid) of SGLT2 by 80% without modifying SGLT1. There was a significant reduction in fasting blood glucose, postprandial blood glucose and HbA1c (glycated haemoglobin) in animal models, while no changes were observed in plasma and urine electrolyte concentrations.20 Of the SGLT2 inhibitors, the most developed is dapagliflozin. does not just inhibit SGLT2, it is also capable of inhibiting SGLT1, causing osmotic diarrhoea in most cases. SGLT2 inhibition can reduce plasma glucose levels by reducing the glucose Tm, resulting in increased urinary excretion of glucose. In animals without diabetes, inhibition of SGLT2 has no effect on plasma glucose, because hepatic glucose production is increased to compensate for glycosuria. However, in diabetic animals, administration of SGLT2 inhibitors produces dose-dependent glycosuria and a significant reduction in plasma glucose. Dapagliflozin is rapidly absorbed after oral administration in an average time of 1 hour (0.5hr-4.0hr) in patients with type 2 diabetes mellitus. A phase I study (in healthy volunteers) suggested that absorption was slower when given with meals, although this difference was minimal.21 The half-life of dapagliflozin is approximately 16 hours. Glycosuria is dosedependent. Dapagliflozin renal clearance is minimal (3-6ml/min) and renal excretion is low (less than 2.5% in urine over 24h). In vitro studies have suggested that dapagliflozin is metabolised by metabolic inactivation of the enzyme glucuroniltransferase.22 Table 3. SGLT2 transporter inhibitor drugs and development phase Active ingredient Company Clinical trials phase Dapagliflozin BMS/Astra Zeneca III Canagliflozin Johnson & Johnson II Sergliflozin GSK Failed in phase I Remogliflozin (KGT-1611) Kiseei Pharmaceuticals Failed in phase I BI-10773 Boehringer Ingelheim II BI-44847 Boehringer Ingelheim II YM-543 Astellas II AVE-2268 Sanofi-Aventis II Information source: www.clinicaltrials.gov. BMS: Bristol Myers Squibb; GSK: GlaxoSmithKline. 622 Nefrologia 2010;30(6):618-25 G. Pérez López et al. SGLT2 inhibitors Dapagliflozin has showed a hypoglycaemic effect at daily doses of 2.5mg, 5mg, 10mg, 20mg and 50mg in phase II clinical trials. Most of the ongoing phase III trials are evaluating the effects of daily doses of 2.5mg, 5mg and 10mg. The randomised, double-blind, placebo-controlled phase II study on dapagliflozin assessed dosedependent effects in patients with type 2 diabetes mellitus. A total of 389 type 2 diabetic patients without treatment and with HbA 1c higher than 7% were randomly assigned to a placebo group or a group treated with increasing doses of dapagliflozin for 12 weeks. 23 Metformin XR was the active comparator, although no statistical comparisons were made. Fasting blood glucose, postprandial blood glucose using prolonged oral glucose overload (3h) and HbA 1c were assessed. Baseline HbA 1c ranged between 7.7% and 8.0% in all groups. In the dapagliflozin group, the decrease in HbA 1c was around 0.8%, while in the placebo group it was 0.2% (P<.01). Dapagliflozin patients had glycosuria between 52-85g/day, with a reduction in fasting blood glucose between 16-30mg/dl. A weight loss of 2.2kg-3.2kg was observed in the group treated with dapagliflozin, equivalent to an average weight loss of 2.5%-3.4%. An increase in urine volume from 107 to 470ml/day was also observed. Regarding adverse effects, there was a slight increase in the incidence of urinary tract infections, although this was not statistically significant. There were no differences in the frequency of episodes of hypoglycaemia and hypotension between the groups. Dapagliflozin is currently in advanced development for use alone or in combination with other hypoglycaemic agents. The drug was well tolerated in early stages, with the following as the most common side effects: urinary tract infections, dizziness, headache, fatigue, backache, and nasopharyngitis. 24 Phase III studies include monotherapy in patients with type 2 diabetes mellitus not controlled with diet and exercise, and in combination therapy with metformin, sulphonylureas, thiazolidinediones and insulin. 25-29 SGLT2 inhibitors are a novel group of drugs that appear to provide several advantages in the treatment of type 2 diabetes mellitus: 1. Weight: SGLT2 inhibitors promote weight loss by increasing glycosuria (1g of glucose is equivalent to 4kcal), which lowers plasma glucose levels and stimulates lipolysis. Nefrologia 2010;30(6):618-25 short reviews 2. It corrects a defective mechanism in type 2 diabetes mellitus: Increased tubular reabsorption of glucose has been shown in diabetic patients. 3. Adverse effects: Hypoglycaemia is usually a barrier when considering strategies for optimal glycaemic control. As inhibition of SGLT2 is completely independent of insulin secretion, there is no increased risk of hypoglycaemia. 4. Treatment of hyperglycaemia: The unique mechanism of SGLT2 inhibitors means they can probably be used alongside other hypoglycaemic treatments. The main concerns regarding inhibition of SGLT2 are the risk of urinary tract infections, reduced intravascular volume secondary to osmotic diuresis, electrolyte imbalance, nephrotoxicity due to the accumulation of advanced glycation end products, nocturia and drug interactions. Long-term studies are required to address these concerns, although the evidence obtained so far is sufficient to consider SGLT2 inhibitors as safe drugs. SGLT2 inhibitors may not be effective in patients with renal failure due to a reduced glomerular filtration rate, although this is currently under investigation. Studies are underway to identify the glomerular filtration rate cutoff point to contraindicate SGLT2 inhibitors. CONCLUSION Inhibition of the SGLT2 glucose transporter is a new therapeutic approach to type 2 diabetes mellitus. Studies in experimental models for diabetes have shown that induction of glycosuria reverts glucotoxicity, restores normoglycaemia and improves beta cell function and insulin sensitivity. The fact that there are genetic mutations of the SGLT2 glucose transporter, as occurs in renal glycosuria, supports the long-term inhibition of this transporter in humans. Preliminary results with dapagliflozin provide evidence of the efficacy of SGLT2 inhibitors in reducing fasting and postprandial blood glucose and decreasing HbA 1c in diabetic patients. Understanding the pathophysiology of type 2 diabetes is a dynamic process: When new pathophysiological concepts arise, new potential therapeutic tools are found. The optimal treatment of type 2 diabetes mellitus requires a multiple approach to different defects in glucose homeostasis. 623 short reviews G. Pérez López et al. SGLT2 inhibitors KEY CONCEPTS 1. 55% of glucose production comes from gluconeogenesis. The kidney is responsible for 40% of the glucose produced in gluconeogenesis, which is equivalent to about 20% of total glucose production. 2. In the kidney, the main carrier for tubular reabsorption of glucose is SGLT2. 3. In renal glycosuria there is a mutation of the SCL5A2 gene, which encodes SGLT2. In these patients, there is no increase in the inciden- ce of diabetes or kidney disease. The only finding in most cases is asymptomatic glycosuria. 4. An increase in SGLT2 transporter activity has been observed in diabetic patients, resulting in increased tubular reabsorption of glucose. 5. SGLT2 inhibitors are emerging as a treatment for type 2 diabetes mellitus, due to inducing glycosuria and thus lowering plasma glucose and glucotoxicity. REFERENCES 1. De Fronzo RA. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes Care 2009;58:773-95. 2. DeFronzo RA, Abdul-Ghani M. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocrine Practice. Endocr Pract 2008 Sep;14(6):782-90. 3. Noonan WT, Shaprio VM, Banks RO. Renal glucose reabsorption during hypertonic glucose infusion in female streptozotocininduced diabetic rats. Life Sci 2001;68:2967-77. 4. Dominguez JH, Camp K, Maianu L, Feister H, Garvey WT. Molecular adaptations of GLUT1 and GLUT2 in renal proximal tubules of diabetic rats. Am J Physiol 1994;266:F283–F290. 5. Guyton AC, Hall JE. Urine formation and the kidneys. In Textbook of Medical Physiology. 9th ed. Philadelphia, Pa.: W.B. Saunders, 1996;332-5. 6. Wright EM. Renal Na-glucose transporters. Am J Physiol Renal Physiol 2001;280:F10-F18. 7. Butterfield WJH, Keen H, Whichelow MJ. Renal glucose threshold variations with age. BMJ 1967;4:505-7. 8. Kamran M, Peterson RG, Dominguez JH. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephol 1997;8:943-8. 9. Mogensen CE. Maximum tubular reabsorpiton capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scan J Clin Lab Invest 1971;28:101-9. 10. Hediger MA, Rhoads DB. SGLT2 Mediates Glucose Reabsorption in the Kidney. Physiol Rev 1994;74:993-1026. 11. Bakris GL, Fonseca V, Sharma K, Wright E. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009;75:1272-7. 12. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 2007;261:32-43. 624 13. Santer R, Kinner M, Lassen CL, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 2003;14:2873-82. 14. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 2007;261:32-43. 15. Ganong WF. Review of Medical Physiology. 19th ed. Stamford, CT: Appleton & Lange, 1999;667-95. 16. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005;54:3427-34. 17. White JR. Apple Trees to Sodium Glucose Co-Transporter Inhibitors. Clinical Diabetes 2010;28(1):5-10. 18. Abdul-Ghani MA. Inhibition of renal glucose absorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 2008;14:782-90. 19. Isis Pharmaceuticals. Pipeline [article online]. Available from http://www.isispharm.com/Pipeline/index.htm. 20. Wancewicz EV, Siwkowski A, Meibohm B, et al. Long term safety and efficacy of ISIS-388626, an optimized SGLT2 antisense inhibitor, in multiple diabetic and euglycemic species. Diabetes 2008;57(Suppl 2):Abstract 334-OR. 21. Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Pfister M. Dapgliflozin, a novel, selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009;85:520-6. 22. Brooks AM, Thacker SM. Dapagliflozin for the treatment of type 2 diabetes. Ann Pharmacotherapy. Published electronically on 7 July 2009; DOI 10.134/aph.1M212. 23. List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes mellitus. Diabetes Care 2009;32:650-7. 24. List JF, Woo V, Morales E, Tang Q, Fiedorek FT. Efficacy and safety of Nefrologia 2010;30(6):618-25 G. Pérez López et al. SGLT2 inhibitors dapagliflozin in a dose-ranging monotherapy study of treatmentnaïve patients with type 2 diabetes [abstract]. Diabetologia 2008;51(Suppl. 1):S22-S23. 25. Bristol-Myers Squibb, AstraZeneca. A Phase III study of BMS-512148 (dapagliflozin) in patients with type 2 diabetes who are not well controlled with diet and exercise [online article]. http://clinicaltrials.gov/show/NCT00528372 (NLM Identifier: NCT00528372). 26. Bristol-Myers Squibb, AstraZeneca. Efficacy and safety of dapagliflozin in combination with metformin in type 2 diabetes patients [articulo online]. http://clinicaltrials.gov/show/NCT00660907 (NLM Identifier: NCT00660907). short reviews 27. AstraZeneca, Bristol-Myers Squibb. Efficacy and safety of dapagliflozin in combination with glimepiride (a sulphonylurea) in type 2 diabetes patients [online article]. http://clinicaltrials.gov/show/NCT00680745 (NLM Identifier: NCT00680745). 28. Bristol-Myers Squibb, AstraZeneca. Add-on to thiazolidinedione (TZD) failures [online article]. http://clinicaltrials.gov/show/NCT00683878 (NLM Identifier: NCT00683878). 29. AstraZeneca, Bristol-Myers Squibb. Efficacy and safety of dapagliflozin, added to therapy of patients with type 2 diabetes with inadequate glycemic control on insulin [online article]. http://clinicaltrials.gov/show/NCT00673231 (NLM Identifier: NCT00673231). Sent for review: 22 Jun. 2010 | Accepted: 27 Sep. 2010 Nefrologia 2010;30(6):618-25 625 originals http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society See editorial comment on page 599 Morbidity and mortality in diabetic patients on peritoneal dialysis. Twenty-five years of experience at a single centre F. Coronel1, S. Cigarrán2, J.A. Herrero1 1 Nephrology Department. San Carlos Clinical Hospital. Madrid (Spain). 2 Nephrology Department. Hospital da Costa. Burela, Lugo Spain. Nefrologia 2010;30(6):626-32 doi:10.3265/Nefrologia.pre2010.Jul.10553 ABSTRACT Aims: To describe PD outcomes over 25 years in a single centre, comparing hospitalisation rate, technique withdrawal, and survival between diabetic (DM) and non-diabetic (NonDM) patients. Differences between type 1 (DM1) and type 2 (DM2) diabetics were also analysed. Patients and methods: One hundred and eighteen DM patients (52 year old average, 74 men, 44 female) and 117 Non-DM (53 year old average, 64 men, 53 female), with at least 2 months on PD, 25±20 (2-109) and 29.4±27 (2-159) months respectively, were included. Diabetics were divided in 66 DM1 and 52 DM2. The survival and hospitalisation study was also analysed in two different time periods: before 1992 (1981-1992) and after 1992 (1993-2005). Results: 93% Non-DM and 75% DM were self-sufficient to manage the PD technique (P<.001) as well as 65% of 44 blind patients. 28% of Non-DM and 15% of DM received a renal allograft (P<.001). There was no difference in transfer to haemodialysis. 18.6% of DM and 4.3% of Non-DM patients presented >4 comorbid factors on starting PD (P<.001). Hospitalisation (admissions/year) was higher in DM than in Non-DM patients (3.4 vs 1.8, P<.01) and also hospitalisation length (46 vs 22 days/year, P=.01), without differences between DM1 and DM2. Admissions due to cardiovascular events, infections, technical problems and peritonitis were more frequent in DM2 than in Non-DM and DM1 patients (P<.05). However, DM2 patients admitted to hospital for peritonitis did not spend more days in hospital than Non-DM or DM1 patients. Mortality was 48% in DM and 22% in Non-DM (P<.001). Survival adjusted for comorbidity was higher in Non-DM (P<.001). Cerebrovascular disease was the highest risk factor for mortality in DM. Mortality was higher in DM2 than in DM1 and Non-DM(P<.001). Age Correspondence: Francisco Coronel Servicio de Nefrología. Hospital Clínico San Carlos. Prof. Martín Lagos, s/n. 28040 Madrid. Spain. [email protected] 626 (HR 1.052, P=.001), DM2 (HR 1.96, P<.01) and cerebrovascular disease (HR 4.01, P<.001) were the most important risk factors. In the post-1992 period, the hospitalisation rate and survival improved in DM1 and Non-DM patients. Conclusions: DM patients more often require outside assistance to perform PD and have more comorbidity, lower survival, and higher admissions than Non-DM, but there is no difference in HD discontinuation. Age and cardiovascular comorbidity are the factors involved in mortality. Technological advances and cumulative center experience may achieve dialysis outcome improvements in diabetic patients. Key words: End-stage renal disease. Peritoneal dialysis. Diabetes mellitus. Comorbidity. Hospitalization. Survival. Morbimortalidad en pacientes diabéticos en diálisis peritoneal. Experiencia de 25 años en un solo centro RESUMEN Objetivos: Describir la experiencia de 25 años de tratamiento con diálisis peritoneal (DP) en un solo centro, comparando la hospitalización, abandono de la técnica y supervivencia entre pacientes diabéticos (DM) y no diabéticos (NoDM) y analizando las diferencias entre diabéticos tipo 1 (DM 1) y tipo 2 (DM 2). Material y métodos: Se incluyen 118 DM (52 años, 74 hombres y 44 mujeres) con, al menos, 2 meses de permanencia en DP y media de 25 ± 20 meses (2-109), divididos en 66 con DM 1 (45 años) y 52 con DM 2 (65 años) y 117 NoDM (53 años, 64 hombres y 53 mujeres), con un tiempo en DP de 29,4 ± 27 meses (2-159). Por el largo período estudiado, en el análisis de hospitalización y de supervivencia se evalúa, además, el seguimiento en dos períodos: 1981 a 1992 (pre-92) y 1993 a 2005 (post-92). Resultados: El 93% de los NoDM y el 75% de los DM fueron autosuficientes para realizar DP (p F. Coronel et al. Morbidity and mortality of Diabetics on PD <0,001) y también el 65% de 44 pacientes ciegos. Han sido sometidos a trasplante el 28% NoDM frente al 15% DM (p <0,001) y no hay diferencia en la transferencia a HD. El 18,6% de los DM frente al 4,3% de los NoDM (p <0,001) presentan cuatro o más factores comórbidos al iniciar DP. La hospitalización (ingresos/año) fue mayor en DM (3,4 frente a 1,8) que en NoDM (p <0,01) y también los días/año (46 frente a 22; p <0,01), sin que exista diferencia entre DM 1 y DM 2. Los ingresos por causas cardiovasculares, infecciones, problemas técnicos e infección peritoneal fueron más frecuentes en DM 2 (p <0,05) que en NoDM y DM 1, pero no los días de ingreso por peritonitis. El 48% de los DM y el 22% de los NoDM fallecen (p <0,001). La supervivencia ajustada a factores de comorbilidad es mayor en NoDM (p <0,001), con la enfermedad cerebrovascular como factor mayor de impacto en la mortalidad de DM. La mortalidad es mayor en DM 2 que en DM 1 y NoDM (p <0,001). La edad (HR 1,052; p <0,001), la condición de DM 2 (HR 1,96; p <0,01) y la enfermedad cerebrovascular (HR 4,01; p <0,001) son los más importantes factores de riesgo. En el período post-92 mejora de manera importante la tasa de hospitalización y la supervivencia de pacientes NoDM y, sobre todo, de DM 1. Conclusión: Los pacientes con DM precisan más frecuentemente ayuda para realizar la DP y presentan más comorbilidad, menor supervivencia y mayor hospitalización que los pacientes NoDM, mientras que es comparable la tasa de abandono de la técnica. La edad y las complicaciones cardiovasculares (sobre todo cerebrales) son los factores implicados en la mayor mortalidad. Los avances tecnológicos y la mayor experiencia de los centros pueden mejorar las expectativas de los DM en diálisis. Palabras clave: Enfermedad renal crónica. Diálisis peritoneal. Diabetes mellitus. Comorbilidad. Hospitalización. Supervivencia. INTRODUCTION At present, diabetic nephropathy is the leading cause of inclusion of patients with chronic kidney disease in dialysis programmes.1,2 Because of the difficulty of creating vascular access for haemodialysis (HD) and the haemodynamic instability in diabetic patients, during the eighties there were abundant descriptions of promising experiences with patients in continuous ambulatory peritoneal dialysis (CAPD).3-6 Good haemodynamic tolerance and good glycaemic control with intraperitoneal insulin were the keys to optimism in using CAPD as preferential treatment in diabetics. The frequency of complications in diabetic patients treated with peritoneal dialysis (PD) in all its forms was higher than in non-diabetic patients, in relation to the severity of multiorgan systemic diseases such as diabetes mellitus (DM). After a period of frequent publications on the development of diabetic patients on dialysis, in recent years literature searches only reveal reviews of the topic and there are few Nefrologia 2010;30(6):626-32 originals original articles. However, the characteristics of diabetic patients starting haemodialysis (HD) or PD in recent years have changed, and unlike the eighties and nineties, the inclusion of diabetic patients on dialysis today occurs if they are suffering from Type 2 diabetes. DM has become a true pandemic, with a higher prevalence of adult DM.7 This difference can change the outcome of patients on renal replacement therapy. The aim of this study is to provide the experience of a single centre over 25 years of treating diabetic patients with PD, analysing survival and hospitalisation in relation to nondiabetic patients, and studying the difference between Type 1 and Type 2 diabetics. PATIENTS AND METHOD After years of intermittent use of PD in a small number of patients and the description of CAPD in 1977, the Hospital Clínico San Carlos (Madrid) started the CAPD programme in 1981 and in 1982 they included the first diabetic patient. It is a retrospective, observational study on patients who started the PD programme at this centre, since the programme’s inception until 2005. Data has been collected from 235 patients with PD stays over 2 months and with sufficient documentation to follow-up (in 12 patients the clinical history data were insufficient to evaluate), 118 diabetics (50.2%) who met the criteria for diabetic nephropathy and 117 non-diabetics (Non-DM) (49.8%). Demographic data on DM and Non-DM are shown in Table 1. Age, gender distribution, PD time and accumulated follow-up time were not different between DM and Non-DM patients. The DM patient group consisted of 66 Type 1 DM, 44.9±10.4 years (41 men and 25 women) and accumulated follow-up time of 2.08 years, and 52 Type 2 DM, 62.6 years (33 men and 19 women) and 2.1 years accumulated followup time. The aetiologies of the renal disease most common in NonDM patients are divided into interstitial 14.4%, 12.6% vascular-ischaemic, 6.5% glomerular, 4.8% polycystic disease, and the rest belong to other causes and unknown origin. Due to changes that occur over such a long period of followup in terms of material, techniques and experience, the study of hospitalisation and survival is also performed by dividing the 25 years of experience into two periods: the first period, from 1981 to 1992 (pre-92) and the second period, from 1993 to 2005 (post-92). Major developments that occurred from the nineties and that justify this division include erythropoietin, which began to be used in 1990, CAPD double bag systems that were introduced in 1992 and automated PD (APD) with cyclers, which began to be implemented in Spain in the early nineties. 627 F. Coronel et al. Morbidity and mortality of Diabetics on PD originals Table 1. Characteristics of Diabetic and Non-Diabetic Patients on PD and Risk Factors Upon Initiation of PD Age (years) Sex (M/F) PD time (months) Accumulated experience (years) Four or more risk factors (% of patients) Diabetics Non-diabetics P 51.9 52.7 NS 74/44 64/53 NS 25±20 (2-109) 29±27 (2-159) NS 2.5 2.5 NS 18.6% 4.3% <0.001 Data in averages ± SD; PD: peritoneal dialysis; NS: not significant: Risk factors: hypertension, obesity, heart failure, heart disease, peripheral vasculopathy. In the results sections the following aspects are evaluated: 1) self-sufficiency in carrying out PD, 2) number and frequency of comorbid conditions at the start of PD, such as obesity, hypertension, heart failure, heart disease, cerebral vascular disease and peripheral vascular disease, 3) discontinuation of the technique, 4) hospitalisation and causes of admission, 5) patient survival, and 6) causes of death. In all cases, we compared data from patients with DM against those without DM, and between Type 1 and Type 2 DM. 16.5 and 25.9%, respectively, in the post-92 period. 93% of Non-DM and 75% of DM were self-sufficient for PD (P<.001) and also 65% of 44 blind patients or with severe impairment of visual acuity (legally blind). At the beginning of PD, DM patients had high comorbidity, higher than NonDM patients (Table 1). 18.6% of DM compared to 4.3% of Non-DM (P<.001) had four or more risk factors. Admissions Statistical Analysis The continuous variables are expressed as average and standard deviation (SD). Comparisons were carried out using the Student’s t or Chi-squared tests according to the nature of the variables. Survival was analysed using the Kaplan-Meier log rank test and confidence intervals (CI), considering other events as appropriate and the forward conditional Cox regression model to identify the influence of risk factors. In terms of patient mortality, the event is death; and leaving the programme for any other reason (transplant, transfer, etc.) is considered a loss. Discontinuation of the technique includes the move to HD, transplantation and recovery of renal function; the failure of the technique only includes transfer to HD. The heterogeneity of the groups were analysed with the Chisquared test for N-1 degrees of freedom with an alpha of .05 for statistical significance. Data are expressed as mean survival probability with 95% CI. Data were processed with the SPSS 16.1 statistical software for Windows. RESULTS Prevalence DM prevalence and type changed over the two periods analysed, so that in the period from 1981 to 1992, 58% of patients were diabetic and in the period from 1993 to 2005 the percentage dropped to 40.5%. Meanwhile, the type of diabetes changed in the two periods, with a DM1 percentage of 39.5% and DM2 of 18% in pre-92, which is reversed to 628 DM patients are admitted more than Non-DM (1.38±1.1 vs 0.88±0.9 admissions/year, P<.001) and have more days of accumulated stay (20.7±25.4 versus 13.2±19.0 days/patient/year, P=.018). Peritoneal infection is the leading cause of hospital admission in all patient groups, and has a higher rate for DM than Non-DM (33 vs 28%, P<.05) (Table 2). This is due mainly to the subgroup of Type 2 diabetes, with 46.2% of admissions due to peritoneal infection compared to 22.7% of Type 1 DM (P<.001) (Table 2). However, no significant differences were found between Type 1 DM and Type 2 DM in the accumulated number of stays per year due to peritoneal infection (11.1±18.6 versus 7.8±14.0 days/patient/year, P=.150). The percentage of patients hospitalised due to infectious peritonitis, cardiovascular or dialysis technique-related problems, is shown in Table 2. When analysing separately the two periods into which we divided the study, we see a progressive tendency towards reduced admissions in all subgroups of patients. Therefore, the Non-DM move from 1.2±1.1 in the first period (pre-92) to 0.63±0.64 in the second period (post-92) (P<.01), with a reduction in inpatient days (32.8±25.8 compared with 15.1±22.6, P<.01). The same applies to DM patients, whose admission rate decreases from 1.58±1.18 in pre-92 to 1.13±1.0 in post-92 (P<.01), with a consequent reduction of days of accrued stay (51±61 versus 40.6±48.7, P<.01). Technique Change The analysis of technique changes are detailed in Table 2. There were no differences in the transition to HD between Nefrologia 2010;30(6):626-32 F. Coronel et al. Morbidity and mortality of Diabetics on PD originals Table 2. Causes of hospitalisation and PD discontinuation in diabetic and non-diabetic patients Patients Non-diabetics Diabetics DM 1 DM 2 (n = 117) (n = 118) (n = 66) (n = 52) Peritonitis 28.2% 33%a 22.7% 46.2%b Infection (not peritonitis) 10.3% 14.4% 6% 25%b Technical cause 8.5% 11.9% 9.1% 25.4%b CV Cause 16.2% 17.8% 12.1% 25%b Move to tx 28.2% 15.3%b 22.7% 5.8%b Move to HD 26% 28% 39.4% 13.5%b Recovery RF 0.9% 0.9% a a PD: Peritoneal dialysis; DM 1: Type 1 diabetic: DM 2: Type 2 diabetic; CV: cardiovascular; Tx: kidney transplant; HD: haemodialysis; RF: renal function; % percentage of patients; aP <.05; bP<.001. DM and Non-DM but surprisingly, the move to HD is more frequent in Type 1 DM (39.4%) than in Type 2 (13.5%), (P<.001). As expected, kidney transplantation was the main reason for discontinuing DP. As such, the amount of NonDM patients who discontinued DP for this reason was twice that of DM patients (Table 2). Among diabetic patients who underwent transplantation, 22.7% have Type 1 DM compared to only 5.8% of Type 2 DM. 0.70-9.92, P<.001). In DM2 patients, it is heart failure (HR 1.64, 95% CI, 0.67-3.93, P<.001) and stroke (HR 6.94, 95% CI, 2.32-20.7, P<.001) in both periods. The leading cause of death in DM patients is a cardiovascular event. Some 15.3% died due to heart problems compared with 8.5% of Non-DM (P<.001) and stroke (8.5% in DM compared to 2.6% in Non-DM, P<.01). Peritoneal infection as a cause of death is equal in both DM and Non-DM (5.1 vs 5.1%). Survival Survival by periods for each of the subgroups (DM Type 1, DM Type 2 and Non-DM) is shown in Figure 2, Figure 3 and Figure 4, where we can see a slight difference in survival between the two periods in Non-DM patients (P<.046) and a significant increase in DM1 patients (P<.008), without significant differences between the two periods in DM2 patients. In the Cox regression analysis by periods, Non-DM patients hypertension (HR 1.6, 95% CI, 1.17-1.86, P=.017) and stroke (HR 4.7, 95% CI, 1.6 to 14.4, P<.001) mark the difference between the two periods. In DM1 patients it is ischaemic heart disease (HR 2.6, 95% CI, Nefrologia 2010;30(6):626-32 DISCUSSION Our study describes the experience in treating PD at a single centre over 25 years. Overall we found the best results in non-diabetic patients in the most recent period (post-1992). Although previous studies highlight the weight of DM as a prognostic factor, it is important to have data from our area and for such a long period. 1.0 Accumulated survival (%) Some 48.3% of DM patients died in PD during the study period, compared to 27.4% of Non-DM patients (P<.001), with a HR of 1.96 (95% CI, 1.1-3.3). The Kaplan-Meier analysis (Figure 1) reflects a higher survival rate for NonDM patients, although for up to 4 years they show a similar survival as Type 1 diabetics, about 60%, and always higher than Type 2 DM. The HR of Type 2 DM patients compared with Non-DM is 2.18 (95% CI, 1.042-4.51). If we carry out a forward, stepwise multivariate Cox regression analysis, based on the age and added comorbidity, the presence of Type 2 diabetes with a HR of 1.96 (95% CI, 1.13-3.39, P<.01), along with age with a HR of 1.052 (1.019 to 1.079) (P<.001) and cerebrovascular disease (HR 4.013, 95% CI, 2.119-7.601, P<.001) are the factors with greater weight in terms of mortality. 0.8 Log Rank: 14.19 0.6 p <.001 0.4 Diabetic groups DM I DM II Non-diabetic censored DM I - censored DM II – censored 0.2 0.0 0.0 2.50 5.00 7.50 10.00 12.50 Time on PD (years) P<.001 between non-DM and DM II Figure 1. Survival of Patients with Type 1 Diabetes (DM1), Type 2 (DM2) and Non-Diabetic (ND) in Peritoneal Dialysis (PD) 629 F. Coronel et al. Morbidity and mortality of Diabetics on PD originals COX REGRESSION Group: Non-diabetic Year group at start Post-1992 Pre-1992 Accumulated survival 1.0 0.8 0.6 Log Rank: 0.746 p <.046 0.4 0.2 0.0 0.0 2.00 4.00 6.00 0.00 10.00 Time on PD (years) Survival averages 3.83 (1.65-6.02) from 1981 to 1992 (pre-92) compared to 3.17 (2.7-3.6) from 1993 to 2005 (post-92). Log rank 0.746, P<.046. Figure 2. Comparison of the Survival of Non-Diabetic Patients in the Two Study Periods Most diabetic patients had injury in various organs and systems at the time of starting PD, which determines a high comorbidity. This, in turn, can influence their adaptation to the technique, their maintenance on it and their survival. With regard to adaptation, it is relevant that we have achieved a sufficient level of self-care so that the patient is responsible for their dialysis, even with the large number of DM patients who are blind. As for continuation of the technique and survival, our data show increased mortality, especially cardiovascular, as other authors have also reported.8, 9 One of the merits of this study is that it brings together a long experience in a single centre, more than 25 years. This has allowed us to differentiate two time periods, in which progressive improvement is seen, and although DM patients have a worse prognosis in both periods, in post-92 an improvement is seen in survival in Non-DM and DM1 patients. The worst prognosis of DM patients is often reported in the medical literature until the late nineties10-13 and more sporadically in recent years.14-16 Accordingly, the long-term trends described here are influenced by changes in the prescription of dialysis over time, improvements in technology and greater experience in treating these patients. Dividing the study into two phases, with a time point in 1992, coincides with major changes in PD technology, such as the consolidation of double-bag systems and the introduction of new cyclers that have allowed an increasing number of patients in ADP. A learning curve is common in almost all complex medical activities, but here it is clear that this improvement in results is not limited to the first months or years of application of the technique, but persists in time and can be maintained for years.17 This improvement in overall performance is more evident in younger patients with Type 1 diabetes, which have reduced mortality in the comparison between the two periods. In one of the few recent studies on the evolution of DM patients on PD, Fang et al.15 indicate advanced age as the most important factor affecting mortality in diabetic patients. Our results are along the same lines, and age, along with cardiovascular comorbidity are the most significant factors with regards the mortality of our patients. Other authors report similar results, describing how heart disease primarily affects DM2 patients.14,16 In our study the CV event with the COX REGRESSION COX REGRESSION Group: Type 2 DM Group: Type 1 DM Accumulated survival 1.0 0.8 Log Rank: 3.575 0.6 P <.008 0.4 0.2 0.8 Log Rank: 0.115 NS 0.6 0.4 0.2 0.0 0.0 0.0 1.00 2.00 3.00 4.00 5.00 6.00 Time on PD (years) Survival averages: 2.08 (1.39-2.76) from 1981 to 1992 (pre-92) compared to 4.0 (2.9-5.1) from 1993 to 2005 (post-92). Log rank 3.57, P<.008. Figure 3. Comparison of the Survival of Type 1 Diabetic (DM1) Patients in the Two Study Periods 630 Year group at start post-1992 pre-1992 1.0 Accumulated survival Year group at start Post-1992 Pre-1992 0.0 2.00 4.00 6.00 8.00 10.00 Time on PD (years) Survival averages 1.83 (1.29-2.38) from 1981 to 1992 (pre-92) compared to 1.75 (0.85-2.65) from 1993 to 2005 (post-92). Log rank 0.115, P=NS. Figure 4. Comparison of the Survival of Type 2 Diabetic (DM2) Patients in the Two Study Periods Nefrologia 2010;30(6):626-32 F. Coronel et al. Morbidity and mortality of Diabetics on PD greatest weight on outcome is stroke (CVA). Type 2 DM patients are older and have a higher prevalence of stroke, and the three factors combine to worsen the prognosis of these patients. Others have suggested the prognostic value of cerebrovascular disease in DM patients, both in PD18 and in HD.19 In a previous study by our group on survival in DM patients and the renal function with which they started PD, cerebrovascular disease and heart failure also appear as the factors with greatest impact on mortality.20 The Type 1 and Type 2 DM patient profiles are completely different, and therefore we analysed both groups separately. Type 1 DM patients had similar survival rates to the NonDM in the first 4 years of treatment, as shown in Figure 1, and improved survival and hospitalisation in the post-92 period, which is not seen in the DM2 patients. We found a higher hospitalisation rate in diabetic patients, especially in Type 2 diabetics, although some studies do not report differences in hospitalisation between DM and NonDM on PD.14 We have previously discussed how technical advances can have lead to the improved results for the second period. One recent study, with a similar design which divides a long-term monitoring in two periods of time, explained how technological advances in PD may influence the results.21 A significant percentage of DM patients underwent kidney transplantation, although due to their higher comorbidity, it was a lower number than Non-DM. The transfer to HD due to failure or fatigue of the technique is, however, same for the DM and Non-DM patients. This study has several limitations. On one hand, a long evolution involves the incorporation of changes and improvements in treatment, but the prognosis for DM is maintained in both periods. On the other hand, as it is a single-centre study, we cannot ensure that results can be generalised. This is a retrospective study with asymmetry in the size of the groups, which limits the survival analysis. However, we have included all patients who have gone through the PD programme, data has been reviewed and a correct analysis was carried out. Therefore, this study provides a good description of PD treatment in the real world, away from the constraints of clinical trials. In conclusion, in the 25 year monitoring, diabetic patients had worse clinical status at the start of PD and had a poorer outcome in overall results such as hospitalisation and patient survival. The leading cause of death is a cardiovascular event, and it is possible that the vascular damage present before the start of the PD affects these results. Therefore, DM patients require special attention from the CKD early stages. The programme experience and developments in PD may be responsible for the better results with Type 1 DM patients in the second half of the period. Nefrologia 2010;30(6):626-32 originals Acknowledgements Thanks to Dr J. Portolés for his comments and advice on the statistical treatment of the study. REFERENCES 1. US Renal Data System. Annual Data Report. Bethesda, MD: The National Institutes of Health, National Institute of Diabetes and Kidney Disease, 2006. 2. Registro Español de Enfermos Renales. Informe de Diálisis y Trasplante 2006. Cádiz: XXXVII Congreso Nacional de la Sociedad Española de Nefrología, 2007. 3. Amair P, Khanna R, Leíble B, et al. Continuous ambulatory peritoneal dialysis in diabetics with end-stage renal disease. N Engl J Med 1982;306:625-30. 4. Madden MA, Zimmerman SW, Simpson DP. Continuous ambulatory peritoneal dialysis in diabetes mellitus. The risk and benefits of intraperitoneal insulin. Am J Nephrol 1982;2:133-9. 5. Grefberg N, Danielson BG, Nilsson P. Continuous ambulatory peritoneal dialysis in the treatment of end-stage diabetic nephropathy. Acta Med Scand 1984;215:427-34. 6. Coronel F, Naranjo P, Torrente J, et al. A 2 years evaluation of diabetic patients on continuous ambulatory peritoneal dialysis. J Diab Comp 1987;1:20-5. 7. Martínez-Castelao A. Repercusiones clínicas y sociales de la epidemia de diabetes mellitus. Nefrologia 2008;28:245-8. 8. Korevaar JC, Feith GW, Dekker FW, Van Manen JG, Boeschoten EW, Bossuyt PM, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003;64:2222-8. 9. Peréz-García R, Dall´Anesse C, Jofré R, et al. Tratamiento sustitutivo de la función renal (TSFR) en diabéticos: diecisiete años de experiencia. Nefrologia 1996;16(Supl. 3):52-8. 10. Coronel F, Hortal L, Naranjo P, et al. Analysis of factors in the prognosis of diabetics on continuous ambulatory peritoneal dialysis (CAPD): Long-term experience. Perit Dial Int 1989;9:1215. 11. Marcelli D, Spotti D, Conte F, et al. Prognosis of diabetic patients on dialysis: Analysis of Lombardy Registry Data. Nephrol Dial Transplant 1995;10:1895-900. 12. Zimmerman SW, Oxton LL, Bidwell D, Wakeen M. Long-term outcome of diabetic patients receiving peritoneal dialysis. Perit Dial Int 1996;16:63-8. 13. Lee HB, Song K II, Kim JH, et al. Dialysis in patients with diabetic nephropathy: CAPD versus Hemodialysis. Perit Dial Int 1996;16(Supl.1):S269-S274. 14. Miguel A, García-Ramón R, Pérez-Contreras J, et al. Comorbidity and mortality in peritoneal dialysis: a comparative study of type 1 and type 2 diabetes versus nondiabetic patients. Peritoneal dialysis and diabetes. Nephron 2002;90:290-6. 15. Fang W, Yang X, Kothari J, et al. Patient and technique survival of diabetics on peritoneal dialysis: one-center’s experience and review of the literature. Clin Nephrol 2008;69:193-200. 16. Portolés J, Corchete E, López-Sánchez P, Coronel F, Ocaña J, Ortiz 631 originals A, y GCDP. Los pacientes diabéticos tipo 2 presentan peor evolución que los no diabéticos en diálisis peritoneal a expensas de su comorbilidad cardiovascular. Nefrologia 2009;29:336-42. 17. Piraino B, Minev E, Bernardini J, Bender FH. Does experience with PD matter? Perit Dial Int 2009;29:256-61. 18. Miguel Carrasco A, García Ramón R, Gómez Roldán C, et al. Morbimortalidad en los pacientes diabéticos con IRC en diálisis peritoneal. En: Coronel F (ed.). Diálisis peritoneal y diabetes. Barcelona: Editorial Médica JIMS, S.L., 1999;13-8. F. Coronel et al. Morbidity and mortality of Diabetics on PD 19. Mattana J, Effiong C, Gooneratne R, Singhal PC. Risk of fatal cerebrovascular accident in patients on peritoneal dialysis versus hemodialysis. J Am Soc Nephrol 1997;8:1342-7. 20. Coronel F, Cigarrán S, Herrero JA. Early initiation of peritoneal dialysis in diabetic patients. Scand J Urol Nephrol 2009;43:14853. 21. Moraes TP, Pecoits-Filho R, Ribeiro SC, et al. Peritoneal dialysis in Brazil: Twenty-five years of experience in a single center. Perit Dial Int 2009;29:492-8. Sent for Review: 20 Jul. 2010 | Accepted: 22 Jul. 2010 632 Nefrologia 2010;30(6):626-32 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society originals Renal immunoexpression of ghrelin is attenuated in human proliferative glomerulopathies M. Danilewicz, M. Wagrowska-Danilewicz Department of Nephropathology. Medical University of Lodz. Lodz, Poland Nefrologia 2010;30(6):633-8 doi:10.3265/Nefrologia.pre2010.Jul.10312 ABSTRACT Background: Ghrelin is a novel 28 amino acid growth hormone-releasing peptide hormone that has been shown to inhibit cell proliferation and to decrease the production of proinflammatory cytokines by monocytes/macrophages. Moreover it decreases the release of endothelin-1 (ET-1), as well as mononuclear cell binding. Material and methods: Seventeen patients with proliferative glomerulopathies (PG) and 15 patients with non-proliferative glomerulopathies (NPG) were examined by percutaneous renal biopsy. As a control 11 biopsy specimens of the kidneys removed because of trauma were used. The immunoexpression of ghrelin and ET-1 was assessed semiquantitatively whereas the interstitial monocytes/macrophages and interstitial area were evaluated quantitatively. Results: The mean value of the immunoexpression of ghrelin was significantly diminished in PG patients as compared to both NPG group and controls while the mean values of ET-1, interstitial CD68+ cells, as well as interstitial area were in PG group increased in comparison with controls and NPG patients, most of them significantly. In all groups there were significant negative correlations between immunostaining of ghrelin and ET-1, whereas negative correlation between immunostaining of ghrelin and CD68+ cells was significant only in PG group. Conclusions: We can confirm the presence of ghrelin in tubular epithelial cells in normal and diseased human kidneys. Lack or low level of this protein in proliferative glomerulopathies may be, in part, responsible for interstitial accumulation of monocytes/macrophages in these cases. Key words: Ghrelin. Endothelin-1. Monocytes/macrophages. Glomerulopathies. Correspondence: Marian Danilewicz Department of Nephropathology. Medical University of Lodz. Poland Pomorska 251, 92-213. Lodz, Poland. Tel: +48426757633 [email protected] [email protected] La inmunoexpresión renal de la grelina se atenúa en las glomerulopatías proliferativas humanas RESUMEN Antecedentes: La grelina es un péptido de reciente descubrimiento de 28 aminoácidos que libera la hormona del crecimiento y que se ha demostrado que inhibe la proliferación celular al igual que disminuye la producción de citoquinas proinflamatorias mediante monocitos/macrófagos. Además, reduce la liberación de endotelina-1 (ET-1), así como la unión de células mononucleares. Materiales y métodos: Se practicó biopsia renal percutánea a 17 pacientes con glomerulopatías proliferativas (GP) y a 15 pacientes con glomerulopatías no proliferativas (GNP). Como grupo de control se utilizaron 11 biopsias de riñones que habían sido extirpados por traumatismo. La inmunoexpresión de la grelina y la ET-1 se determinó semicuantitativamente mientras que se realizaba un análisis cuantitativo de la zona intersticial y de los monocitos/macrófagos intersticiales. Resultados: El valor medio de la inmunoexpresión de la grelina se vio considerablemente disminuido en pacientes con GP, en comparación con el grupo de pacientes con GNP y de control, mientras que los valores medios de ET-1, células CD68+ intersticiales, así como de la zona intersticial, se vieron incrementados en el grupo de pacientes con GP en comparación con el grupo de control y los pacientes con GNP, la mayoría de ellos de forma significativa. En todos los grupos se observaron correlaciones negativas importantes entre la expresión de grelina y de ET-1, mientras que la correlación negativa entre la expresión de grelina y de células CD68+ era relevante únicamente en el grupo de pacientes con PG. Conclusiones: Puede confirmarse la presencia de grelina en las células epiteliales tubulares en riñones humanos normales y enfermos. La falta o el reducido nivel de esta proteína en las glomerolopatías proliferativas pueden ser, en parte, la causa de la acumulación intersticial de monocitos/macrófagos en estos casos. Palabras clave: Grelina. Endotelina 1. Monocitos/macrófagos. Glomerulopatías. 633 originals M. Danilewicz et al. Renal immunoexpression of ghrelin is attenuated in human proliferative glomerulopathies INTRODUCTION Ghrelin is a 28 amino acid peptide hormone, first discovered in stomach cell extracts, that promotes the release of growth hormone.1,2 It is generated by processing of a 117 amino acid peptide, preproghrelin, by specific proteases and stored in secretory vesicles of endocrine cells.3 Human ghrelin gene is located on chromosome 3 and consists of 4 exons and 3 introns. 4 A natural receptor for ghrelin (GHS-R) is a classical G-protein coupled receptor, consists of 336 amino acids and it was found to contain seven putative alphahelical membrane spanning segments and three intracellular and extracellular loops.5 Apart from the gastric mucosa ghrelin is also expressed in other tissues, such as hypothalamus, pituitary, pancreas, immune cells, lung, ovary, testes, intestine, placenta and various tumors.3,5 Interestingly, Aydin et al.6 found that the urinary ghrelin level was higher than blood level, suggesting that the kidney might produce more ghrelin than stomach. It was also proposed that this locally produced ghrelin may modulate cell pathophisiology through an autocrine mechanism.2 Ghrelin is a multifunctional molecule, involved in many biological processes.3 Apart from the regulation of a growth hormone release it takes place in appetite regulation7 and gut motility.8 Moreover, it was suggested that ghrelin inhibits cell proliferation,9 decreases the production of proinflammatory cytokines by monocytes/macrophages,10,11 endothelin-1 (ET-1) release,12,13 and mononuclear cell binding.11 In view of the above, the aim of the present study was to evaluate renal ghrelin immunoexpression in proliferative (PG) and so called non-proliferative glomerulopathies (NPG) as well as to find whether this immunoexpression could correlate with ET-1 immunoexpression, interstitial monocytes/macrophages and interstitial fibrosis. MATERIAL AND METHODS Patients Kidney tissue biopsies were obtained for diagnostic purposes percutaneously from 17 patients in PG group (11 males and 6 females, aged 22-58, mean age = 39.7) and 15 from NPG patients (9 males and 6 females, aged 25-63, mean age = 46.9). PG group included 9 cases of IgA nephropathy with diffuse mesangial proliferation, 5 cases of mesangiocapillary glomerulonephritis type I and 3 cases of proliferative mesangial glomerulopathy with IgM depositions. In this group 6 patients showed hematuria alone, 4 nephrotic syndrome, 4 proteinuria <3.5 g/day and 3 poteinuria and hematuria. Renal function impairment (serum creatinine >1.5 mg/dl) was noted in 4 cases. NPG group incorporated 10 cases of membranous glomerulopathy and 5 cases of minimal change disease. In these patients nephrotic syndrome was seen in 8 cases, proteinuria <3.5 g/day in 4, proteinuria and hematuria in 634 3 and renal function impairment (serum creatinine >1.5 mg/dl) in 1. The mean duration of PG prior to biopsy taking was 6.3 months (range 4.7-8.2 months), and the mean duration of NPG prior to biopsy was 7.7 months (range 7.5-9 months). At the biopsy none of the patients had been treated with immunosuppressive drugs. As a control 11 biopsy specimens of the kidneys removed because of trauma were used (the male to female ratio was 7:4, the mean age was 38.1 ± 7.2). None of the persons from whom renal tissue originated were known to have had previous or actual renal disease. Before the semiquantitative and quantitative examinations were carried out, all control specimens were histologically examined by a nephropathologist and found to be normal renal tissue. In all cases, diagnosis of glomerulopathies were based on characteristic findings by light microscopy (sections stained with hematoxylin and eosin, Masson-Trichrome, Jones’ silver impregnation and periodic acid-Schiff followed by Alcian Blue) as well as electron-microscopy and immunofluorescence using standard protocols. Thickness of each section was controlled according to the method described by Weibel.14 Immunohistochemistry Paraffin sections were mounted onto superfrost slides, deparaffinized, then treated in a microwave oven in a solution of citrate buffer, pH 6.0 for 20 min at 750 W and transferred to distilled water. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in distilled water for 5 min, and then sections were rinsed with Tris-buffered saline (TBS, DakoCytomation, Denmark) and incubated with: rabbit-anti-human ghrelin antibody (Phoenix Pharmaceuticals. Inc., dilution 1:400), monoclonal mouse anti-human anti-endothelin-1 antibody (clone TR.ET.48.5, Sigma, Saint Louis, USA, dilution 1:250), and monoclonal mouse anti-human CD68 antibody (DakoCytomation, Denmark, dilution 1:100). Afterwards LSAB+/HRP Universal kit (DakoCytomation, Denmark) prepared according to the instructions of the manufacturer was used. Visualisation was performed by incubating the sections in a solution of 0.5 mg 3,3’-diaminobenzidine (DakoCytomation, Denmark), per ml Tris-HCl buffer, pH 7.6, containing 0.02% hydrogen peroxide, for 10 min. After washing, the sections were counter-stained with hematoxylin and coverslipped. For each antibody and for each sample a negative control were processed. Negative controls were carried out by incubation in the absence of the primary antibody and always yielded negative results. For ghrelin positive control was performed using gastric mucosa. In each specimen staining intensity of ghrelin in tubuli and ET-1 in the endothelium of peritubular capillaries as well as arterioles and in the renal tubular epithelial cells were recorded semiquantitatively by two independent observers in 7-10 Nefrologia 2010;30(6):633-8 M. Danilewicz et al. Renal immunoexpression of ghrelin is attenuated in human proliferative glomerulopathies adjacent high power fields and graded from 0 (staining not detectable), 1 (weak immunostaining), 2 (moderate immunostaining intensity) and 3 (strong staining). The mean grade was calculated by averaging grades assigned by the two authors and approximating the arithmetical mean to the nearest unity. Morphometry Histological morphometry was performed by means of image analysis system consisting of a PC computer equipped with a Pentagram graphical tablet, Indeo Fast card (frame grabber, true-color, real-time), produced by Indeo (Taiwan), and color TV camera Panasonic (Japan) coupled to a Carl Zeiss microscope (Germany). This system was programmed (MultiScan 8.08 software, produced by Computer Scanning Systems, Poland) to calculate the number of objects (semiautomatic function) and the surface area of a structure using stereological net (with regulated number of points). The coloured microscopic images were saved serially in the memory of a computer, and then quantitative examinations had been carried out. Interstitial area was measured as a surface fraction in sections stained with Masson trichrome using point counting method which is an adaptation of the principles of Weibel.14 The point spacing was 16 µm. Total numbers of the points of a net was 169, and total area was 36,864 sq µm. Under the net described above 8-10 randomly selected adjacent fields of the renal cortex were investigated. Glomeruli and large blood vessels were neglected. The percentage interstitial area was an expression of the number of points overlying renal cortical interstitium as a percentage of the total points counted. originals RESULTS In all renal biopsy specimens in both controls and NPG groups ghrelin was detected in the renal tubular epithelial cells of thin portion of the Henle’s loops and some distal tubules. This immunoexpression was focal and confined to the cytoplasm (Figure 1 and 2). In 14 PG patients (3 cases were completely negative) only slight focal immunoexpression of ghrelin in tubular epithelial cells of thin portion of the Henle’s loops and distal tubules was seen (Figure 3). In all groups ghrelin immunoexpression was absent from glomerular and interstitial areas. The semiquantitative data of the immunoexpression of ghrelin in tubuli and ET-1 in the endothelium of peritubular Figure 1. Strong focal immunoexpression of ghrelin in tubular epithelium of thin portion of the Henle’s loops in control case. x200. Interstitial monocytes/macrophages were determined by counting CD68+ cells (semiautomatic function) in a sequence of ten consecutive computer images of 400x high power fields - 0.0047 mm 2 each. The only adjustments of field were made to avoid glomeruli and large vessels. The results were expressed as a mean number of CD68 immunopositive cells per mm2. Statistical methods All values were expressed as the mean ± SD (standard deviation). The differences between groups were tested using Student t-test for independent samples preceded by evaluation of normality and homogenity of variances with Levene’s test. Additionally the Mann-Whitney U test was used where appropriate. Correlation coefficients were calculated using Spearman’s method. Results were considered statistically significant if P <.05. Nefrologia 2010;30(6):633-8 Figure 2. Intense focal immunoexpression of ghrelin in epithelial cells of thin portion of the Henle’s loops and several distal tubuli in NPG case. x200. 635 originals M. Danilewicz et al. Renal immunoexpression of ghrelin is attenuated in human proliferative glomerulopathies DISCUSSION Figure 3. Weak, focal immunoexpression of ghrelin in tubular epithelium of thin portion of the Henle’s loops and distal tubuli in PG patient. x200. capillaries and arterioles and in the renal tubular epithelium as well as morphometric data of the interstitial CD68+ cells and interstitial area appear from Table 1. The mean value of the immunoexpression of ghrelin was significantly diminished in PG patients as compared to both NPG group and controls whereas this immunoexpression did not differ significantly between controls and NPG group. On the other hand the mean values of ET-1, interstitial CD68+ cells, as well as interstitial area were in PG group increased in comparison with controls and NPG patients, most of them significantly, meanwhile the differences between controls and NPG patients were not significant. The correlations between the tubular immunoexpression of ghrelin and ET-1, CD68+ cells as well as interstitial area are shown in Table 2. In all groups there were significant negative correlations between immunostaining of ghrelin and ET-1, whereas negative correlation between immunostaining of ghrelin and CD68+ cells was significant only in PG group. The relationships between ghrelin and interstitial area were in all groups negative, but weak and not significant. The reports on ghrelin in a kidney tissue are very scanty. In study of Gheraldoni et al. Ghrelin was not detectable in the kidney. 15 Mori et al. 16 demonstrated the ghrelin immunoreactivity in the mouse kidney which was much more abundant then that in the mouse plasma. They concluded that ghrelin is produced locally in the kidney and suggested the possible endocrine and/or paracrine role of this peptide. Although these authors found that ghrelin gene was expressed in glomerulus and renal cells, in our study the glomeruli showed no ghrelin immunoexpression in all cases investigated, whereas epithelial tubular cells were focally positive. Similarly, Dagli et al. 2 noticed ghrelin immunoexpression in tubular epithelium of human normal kidney, but glomeruli were absent from ghrelin immunoreactivity. In the kidneys of normal rats, mice, hamsters and diabetic rats positive ghrelin immunoexpression was observed in distal tubular epithelium. 17,18 There was no positive staining in the proximal tubules and glomeruli. However, to the best of our knowledge, this is the first study of ghrelin immunoexpression in human glomerulopathies. We found immunoexpression of ghrelin in normal kidneys and in both proliferative glomerulopathies usually accompanied with prominent interstitial leukocyte infiltrates and non proliferative with only a little interstitial damage. However, the major finding in the present study was the observation that tubular immunoreactivity of ghrelin in proliferative glomerulopathies was significantly diminished as compared to non proliferative patients and controls. Emerging evidence indicates that ghrelin has antiinflammatory activity19-21 as it decreases the release of proinflammatory cytokines from T cells and monocytes.22 It was documented that ghrelin administration in mice significantly decreased serum TNF-alpha, IL-1 beta and IL-6 and as well as had protective effect against endoxemiainduced kidney injury.13 This raises the interesting possibility that low level or lack of ghrelin in proliferative glomerulopathies might have a role in interstitial damage in Table 1. Renal immunoexpression of ghrelin, ET-1, and analysis of interstitial volume as well as CD68+ cells in PG, NPG and controls Number of cases Ghrelin ET-1 CD68+ (mean score) (mean score) (cells /1/mm ) (%) 1.1 ± 0.7 1.6 ± 0.7 38.78 ± 20.67 12.04 ± 2.17 Proliferative glomerulopathies (n = 17) 0.3 ± 0.2 2.5 ± 0.9 108.25 ± 46.21 19.25 ± 7.36 Non proliferative glomerulopathies (n = 15) 0.9 ± 0.6 1.9 ± 0.8 58.25 ± 36.21 14.35 ± 5.86 <0.005 <0.01 <0.001 <0.005a Controls (n = 11) P value a a = 0.44 (NS) = 0.4 (NS) = 0.12 (NS) = 0.23 (NS)b <0.03c = 0.56 (NS)c <0.003c <0.05c b a Interstitial area 2 b b Between controls and PG group. b Between controls and NPG group. c Between and PG and NPG groups. NS: not significant. 636 Nefrologia 2010;30(6):633-8 M. Danilewicz et al. Renal immunoexpression of ghrelin is attenuated in human proliferative glomerulopathies originals Table 2. The correlations between renal immunoexpression of ghrelin and selected parameters in PG, NPG and controls Correlation between PG (n = 17) NPG (n = 15) Controls (n = 11) Immunoexpression of ghrelin and ET-1 r = –0.56, p <0.02 r = –0.52, p <0.05 r = –0.65, p <0.03 Immunoexpression of ghrelin and CD68+ cells r = –0.49, p <0.05 r = –0.11, p = 0.69 (NS) r = –0.30, p = 0.24 (NS) r = –0.28, p = 0.27 (NS) r = –0.27, p = 0.27 (NS) r = –0.18, p = 0.59 (NS) Immunoexpression of ghrelin and interstitial volume these cases which in part may also depend on ET-1. We found that immunoexpression of ET-1 was significantly increased in PG as compared to controls. In comparison to NPG, however, this difference was not significant. Moreover, in the present study there were negative significant correlations between immunoexpression of ghrelin and ET-1 in all groups investigated. Endothelin is a potent biological mediator which exists in three isoforms: endothelin-1, -2 and -3, however ET-1 is considered the clinically most important endothelin in human kidney disease.13,24 The main vascular effect of ET-1 is transient vasodilatation and profound and sustained vasoconstriction.25 It is also regarded as a potent mitogen for mesangial cells and as a pro-fibrotic protein.26,27 On the other hand ghrelin is known to inhibit ET-1 release probably via TNF-alpha pathway,12,13 thus high immunoexpression of ET-1 in PG patients may suggest, among others, an involvement of this protein in interstitial renal damage in these cases. As might be expected interstitial monocytes/macrophages were in PG group significantly more numerous than in NPG and controls. Moreover, CD68+ cells in PG patients significantly, negatively correlated with the immunoexpression of ghrelin. Monocytes/macrophages seem to be the major cell targets in the inhibition of the high mobility box 1 secretion in which ghrelin blocked its cytoplasmic translocation. 21 In vitro study of Li et al.11 showed that ghrelin inhibited mononuclear cell binding, whereas findings of Chen et al.10 documented that ghrelin attenuates proinflammatory cytokine production in lung macrophages in rats. Our present finding suggests the interesting opportunity that ghrelin might have the potential to reduce monocytes/macrophages recruitment in the kidney tissue. Renal interstitial fibrosis is the final common pathway leading to end-stage disease in various nephropathies. We found the interstitial area to be significantly increased in PG as compared to NPG and controls. Although the correlations between interstitial area and ghrelin were in all groups negative, they were weak and not significant, thus no casual associations can be made between these parameters. In conclusion, we can confirm the presence of ghrelin in tubular epithelial cells in normal and diseased human kidneys. Lack or low level of this protein in proliferative Nefrologia 2010;30(6):633-8 glomerulopathies may be, in part, responsible for interstitial accumulation of monocytes/macrophages in these cases. Acknowledgement This work was supported by grant No NN402088735. REFERENCES 1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656-60. 2. Dagli F, Aydin S, Karaglou A, Akpolat N, Ozercan IH, Ozercan MR.Ghrelin expression in normal kidney tissue and renal carcinomas. Pathol Res Pract 2009;205:165-73. 3. Grönberg M, Tsolakis AV, Magnusson L, Janson ET, Saras J. Distribution of obestatin and ghrelin in human tissues: immunoreactive cells in the gastrointestinal tract, pancreas, and mammary glands. J Histochem Cytochem 2008;56:793-801. 4. Wajnranch MP, Ten IS, Gertner JM, Leibel RL. Genomic organization of human ghrelin gene. J Med Gen 2000;1:231-3. 5. Katergari SA, Milousis A, Pagonopoulou O, Asimakopoulos B, Nikolettos NK. Ghrelin in pathological conditions. Endocr J 2008;55:439-53. 6. Aydin S, Karatas F, Geckil H. Simultaneous quantification of acylated and desacylated ghrelin in biological fluids. Biomed Chromatogr 2008;22:1354-9. 7. Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001;120:337-45. 8. Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 2006;55:327-33. 9. Volante M, Allis E, Fulcheri E, Cassoni P, Ghigo E, Muccioli G, et al. Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am J Pathol 2003;162:645-54. 10. Chen J, Liu X, Shu Q, Li S, Luo F. Ghrelin attenuates lipopolysaccharide-induced acute lung injury through NO pathway. Med Sci Monit 2008;14:BR141-146. 11. Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factorkappaB activation in human endothelial cells. Circulation 2004;109:2221-6. 637 originals M. Danilewicz et al. Renal immunoexpression of ghrelin is attenuated in human proliferative glomerulopathies 12. Wu R, Dong W, Zhou M, Cui X, Hank Simms H, Wang P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res 2005;68:318-26. 13. Wang W, Bansal S, Falk S, Ljubanovic D, Schrier R. Ghrelin protects mice against endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol 2009;297:F1032-1037. 14. Weibel ER. Point Counting Methods. In: Weibel ER. Stereological Methods. vol. 1. London, New York, Toronto, Sydney, San Francisco: Academic Press, 1979;101-159. 15. Ghelardoni S, Carnicelli V, Frascarelli S, Ronca-Testoni S, Zucchi R. Ghrelin tissue distribution: comparison between gene and protein expression. J Endocrinol Invest 2006;29:115-21. 16. Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, et al. Kidney produces a novel acylated peptide, ghrelin. FEBS Lett 2000;486:213-6. 17. Kuloglu T, Dabak DO. Determination of ghrelin immunoreactivity in kidney tissues of diabetic rats. Ren Fail 2009;31:562-6. 18. Yabuki A, Taharaguchi S, Ichii O, Kojima M, Nishi Y, Mifune H, et al. Immunohistochemical localization of ghrelin in rodent kidneys. Histochem Cell Biol 200;126:231-8. 19. Demers A, Caron V, Rodrigue-Way A, Wahli W, Ong H, Tremblay A. A concerted kinase interplay identifies PPARgamma as a molecular target of ghrelin signaling in macrophages. PLoS One 2009;4:e7728. 20. García EA, Korbonits M. Gherelin ans cardiovascular health. Curr Opin Pharmacol 200;6:142-7. 21. Chorny A, Anderson P, González-Rey E, Delgado M. Gherelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol 200;180:8369-77. 22. Dixit VD, Yang H, Cooper-Jenkins A, Giri BB, Patel K, Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood 2009;113:5202-5. 23. Luscher TF, Boulanger CM, Dohi Y, Yang ZH. Endotheliumderived contracting factors. Hypertension 1992;19:117-30. 24. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411-5. 25. Kiowski W, Luscher TF, Lindner L, Buchler FR. Endothelin-1-induced vasoconstriction in humans. Reversal by calcium channel blockade, but not by nitrovasodilatators or endothelium-derived relaxing factor. Circulation 1991;83:469-75. 26. Chahdi A, Sorokin A. Endothelin-1 induces p66Shc activation through EGF receptor transactivation: Role of beta(1)Pix/Galpha(i3) interaction. Cell Signal 2010;22:325-9. 27. Leask A. Signaling in fibrosis: targeting the TGF beta, endothelin-1 and CCN2 axis in scleroderma. Front Biosci 2009;1:115-22. Sent to review: 8 Jun. 2010 | Accepted: 18 Jul. 2010 638 Nefrologia 2010;30(6):633-8 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society originals Pentosan polysulfate sodium prevents kidney morphological changes and albuminuria in rats with Type 1 diabetes Y. Mathison Natera1, H.J. Finol2, Z. Quero1, R. González2, J. González3 Chair of Pharmacology. Escuela José María Vargas. School of Medicine. Universidad Central de Venezuela. Caracas, Venezuela Dr. Mitsuo Ogura Electron Miscroscope Centre. School of Sciences. Universidad Central de Venezuela. Caracas, Venezuela 3 Centre for Medical and Biotechnology Research. Universidad de Carabobo. Valencia, Venezuela 1 2 Nefrologia 2010;30(6):639-45 10.3265/Nefrologia.pre2010.Jul.10543 ABSTRACT Decreased levels of glycosaminoglycans (GAGs) have been observed in the kidney and other organs, in human and animal models of diabetes. Long-term administration of heparins and other glycosaminoglycans has demonstrated a beneficial effect on morphological and functional kidney abnormalities in diabetic rats. We assessed the effect of pentosan polysulfate sodium (PPS), a semi-synthetic glycosaminoglycan with low anticoagulant activity, on kidney involvement in streptozotocin diabetic rats. Diabetes was induced in male Sprague-Dawley rats by i.v. administration of streptozotocin (STZ). Animals were randomly allocated to three groups: C = control, STZ and STZ + PPS = pretreated with PPS (15mg/kg, s.c.). After three months of follow-up, blood and 24 h-urine samples were obtained, the animals were sacrificed and the kidney microdissected for morphometric analysis. Urinary albumin excretion was markedly increased in untreated diabetic rats (C = 0.26 ± 0.03 vs STZ = 7.75 ± 1.8 mg/24 h) and PPS treatment partially prevented the albumin rise (3.7 ± 0.7 mg/24 h), without affecting the metabolic control HbA1c (C = 3.6 ±1.7; STZ = 8.82 ± 0.47; STZ + PPS = 8.63 ± 0.54). Electron microscope observation revealed typical renal lesions described in experimental diabetes (STZ group). PPS administration prevents the tubular basement membrane thickening and the loss of cytoarchitecture induced by experimental diabetes. Our data demonstrate that long-term administration of PPS has a favourable effect on morphological and functional abnormalities in kidneys of diabetic rats and suggests a potential therapeutic use for this compound. El pentosán polisulfato de sodio previene las alteraciones morfológicas renales y la albuminuria en ratas con diabetes tipo 1 RESUMEN Se ha reportado una disminución de los valores de glicosaminoglicanos (GAG) en el riñón y otros órganos en modelos experimentales de diabetes y en humanos. La administración a largo plazo de heparina y otros GAG previene las alteraciones morfológicas y funcionales del riñón en ratas diabéticas. Evaluamos el efecto del pentosán polisulfato de sodio (PPSNa), un mucopolisacárido semisintético similar a los GAG y de baja actividad anticoagulante, sobre la función renal y los cambios estructurales en ratas diabéticas. La diabetes fue inducida a ratas Sprague-Dawley mediante la administración i.v. de estreptozotocina (STZ). Los animales fueron distribuidos al azar en tres grupos (C = control, STZ y STZ + PPSNa = pretratados con 15 mg/kg/día de PPSNa s.c.). Después de 3 meses se tomaron muestras de sangre y de orina de 24 horas; los animales fueron sacrificados y los riñones extraídos mediante microdisección para el análisis morfométrico. Los animales del grupo STZ presentaron un incremento importante de la excreción de albúmina en orina (C = 0,26 ± 0,03 frente a STZ = 7,75 ± 1,8 mg/24 h), que fue parcialmente revertido por el pretratamiento con PPSNa (3,7 ± 0,7 mg/24 h), sin afectar al control metabólico, HbA1c (C = 3,6 ± 1,7; STZ = 8,82 ± 0,47; STZ + PPSNa = 8,63 ± 0,54). En las micrografías electrónicas se observan las lesiones renales típicas descritas en la diabetes experimental (grupo STZ). La administración de PPSNa previene el engrosamiento de la membrana basal tubular y la pérdida de la citoarquitectura inducida por la diabetes. Nuestros resultados demuestran que la administración de PPSNa previene parcialmente el daño renal en este modelo experimental y sugieren un potencial uso terapéutico de este compuesto. Key words: Microalbuminuria. Glicosaminoglicanos. Kidney damage. Diabetes Palabras clave: Microalbuminuria. Glicosaminoglicanos. Daño renal. Diabetes. Correspondence: Yaira Mathison Natera Cátedra de Farmacología. Escuela José María Vargas. Facultad de Medicina. Universidad Central de Venezuela. Calle LaTrinidad Res. Uracoa Apto, 10-B, Santa Paula, 1060, Caracas, Venezuela. [email protected] INTRODUCTION The efficacy of glycosaminoglycans (GAG) in decreasing urinary albumin excretion in diabetic nephropathy has 639 originals Y. Mathison Natera et al. PPS in the prevention of kideny damage in Type 1 DM been widely demonstrated in various experimental studies and in humans.1-3 This has stimulated clinical research using these compounds not only in this pathology but also in other microvascular complications of diabetes4,5 and in different types of glomerulonephritis.6 the Escuela José María Vargas, School of Medicine, UCV, Caracas, Venezuela. These rats were kept with alternating periods of light and dark, and they were allowed free access to water and food (standard feed for laboratory rats, containing approximately 20% protein). Studies have been conducted with modified molecules in order to obtain preparations with low anticoagulant activity to improve the safety profile. The modified molecules derived from heparin have a similar effectiveness to dermatan sulfate for lowering albumin excretion and inhibiting the expression of TGFb1. This proves that the fraction of heparin is not essential for this activity.7 Furthermore, studies have been performed with molecules with different degrees of sulfation,8 with proteoglycans that bind TGFb such as decorin,9 and GAG with a similar chain to heparan sulfate such as danaparoid sodium.10 This yielded mixed results. However, the most commonly used GAG in medical practice is sulodexide, a GAG compound of a fast-moving heparin fraction and dermatan sulfate.11-15 The rats were randomly assigned to two groups: a control group and a group that was induced with diabetes. All experiments were performed following the best practices for handling laboratory animals25 and were approved by the Bioethics Committee of the Escuela de Medicina (School of Medicine) José María Vargas. Pentosan polysulfate sodium (PPS) is a semisynthetic mucopolysaccharide that is structurally similar to GAGs. This has been widely used in the treatment of interstitial cystitis, showing efficacy in controlling symptoms and an appropriate safety profile and tolerance for the range of doses used.16-18 PPS shows some similarity with the effects of sulodexide. They both have fibrinolytic effect and activate the lipoprotein lipase and hepatic triglyceride lipase,19-22 effects that would be beneficial in diabetic patients. PPS anticoagulant activity is very low, about 15 times lower than that of heparin, has a better absorption profile through oral administration than GAG16 and in vitro studies have shown that it inhibits cell proliferation mediated by the heparin-binding growth factors.23 Pharmacokinetic studies on rats, through the administration of radiolabeled PPS, show that it concentrates mainly in the kidneys and urinary tract.24 This suggests that it may have a possible functional role in these structures. The possibility that PPS prevents or decreases albumin excretion in diabetic nephropathy has not been evaluated. Therefore, our aim is to evaluate whether PPS decreases urinary albumin excretion in rats that have been made diabetic through the administration of streptozotocin (STZ) and whether it can prevent the morphological changes in the kidney caused by diabetes. MATERIAL AND METHODS Induction of Experimental Diabetes Diabetes was induced through the administration of a single injection of STZ (Sigma Chemical Co., St. Louis, MO), at a dose of 60mg/kg of weight, in the caudal vein. The induction of diabetes was confirmed by measuring blood glucose levels using an enzymatic method (Glucose HK Reagent, Bayer) at 2 and 7 days after STZ administration. A control group was kept in which diabetes was not induced. This group was only administered a saline solution in the caudal vein. After 7 days, the control and diabetes-induced animals were randomly assigned to the following treatment groups: Control: saline solution or vehicle. STZ-Control: STZ. STZ-PPS: STZ + 15mg/kg/day of PPS administered subcutaneously for 3 months. PPS was administered subcutaneously 5 days a week (Monday to Friday). The animals did not receive insulin during the experiment. During the monitoring period, the animals’ weight, blood pressure and capillary glucose levels were measured every month. At the end of the treatment period, blood samples from the caudal vein in the tail were taken to determine glycaemia and HbA1c. The animals were placed in metabolic cages to collect urine for 24 hours in order to determine albuminuria. The animals were then put down and their kidneys were extracted by microdissection for morphometric analysis. Glycaemia was measured in the blood samples through an enzymatic assay using a commercial kit (Glucose HK Reagent, Bayer). HbA1c and urinary albumin excretion were measured using commercial kits (DCA 2000®, Bayer). Animals Measuring Blood Pressure The laboratory animals were male albino Sprague-Dawley rats, between 6 and 7 weeks of age, weighing between 220g and 250g, originating from the laboratory animal facility of 640 Blood pressure readings were conducted on conscious rats employing a non-invasive method using a digital Nefrologia 2010;30(6):639-45 Y. Mathison Natera et al. PPS in the prevention of kideny damage in Type 1 DM plethysmograph of the tail (LE 5000, LETICA Scientific Instrument, Barcelona, Spain). The rats were subjected to heat (42ºC) for 15 minutes in an oven (Memmert, 854 Schawabach, Germany) to vasodilate the peripheral vessels.26 Subsequently, they were immobilised in a restraint and the blood pressure measuring device was attached to their tail. This device was connected to a pulse transducer that recorded this parameter. The readings reported are the average of three successive readings. Ultrastructural and Morphometric Analysis Kidney cortex samples, 2mm in diameter, were extracted. The samples were fixed in 3% glutaraldehyde and 1% OsO4 in Milloning’s phosphate buffer (pH = 7.4, 320 mOsmol). They were then dehydrated in increasing concentrations of ethanol and embedded in Epon. After being embedded, the samples were sectioned in a Porter-Blum MT2-B ultramicrotome, contrasted with uranyl acetate and lead citrate and examined under a JEM-1011 transmission electron miscroscope with an accelerating voltage of 80 kV. The digital records of the images were analysed with a morphometry software (Image-Tool version 3.0), which measured the basement membrane thickness (n=16/each treatment). Statistical Analysis The data were expressed as the mean ± SEM and were plotted and analysed using the GraphPad Prism version 4.1 and STATISTICS version 7 software. The comparison between the arithmetic means of the groups was performed using analysis of variance (ANOVA). Those values with P<.05 were considered statistically significant. RESULTS Table 1 shows the values for weight, glycaemia and urine volume for the groups studied. The groups which were originals induced with diabetes showed a significant increase in glycaemia and urine volume excreted in 24 hours, as well as a lower weight compared with the control group after 3 months of treatment. Induction of diabetes with STZ produced a significant increase in urinary excretion of albumin as compared to the control group (C=0.27 ± 0.03; STZ=7.8mg/24 h ± 1.8). Treatment with PPS for 3 months partially prevented this increase (STZ+PPS=3.7mg/24h ± 0.70), decreasing albumin excretion by 52.5% compared to the untreated diabetic group (Figure 1). HbA1c values in the treatment groups were: C=3.6 ± 1.7; STZ=8.8 ± 0.47 and STZ+PPS=8.65% ± 1.23. This clearly shows that treatment with PPS does not alter the increase in the HbA1c values which are characteristic of this diabetes model. Additionally, there were no significant differences between values of mean arterial pressure between the different treatment groups (C=122.8 ± 5.1; STZ=134.6 ±12 and STZ+PPS=110mm Hg ± 5.7). The electron micrographs (Figure 2) show the normal appearance of kidney tubules in the control rats (A) with adequate interdigitation and basement membrane of normal thickness. Note that the capillary has a uniform and thin endothelium. In contrast, significant tubular damage was observed in the STZ (B) group with loss of cytoarchitecture as evidenced by the disappearance of interdigitations and thickening of the basement membrane. Treatment with PPS (C) partially prevented tubular damage since more interdigitations were observed than with STZ but they were arranged irregularly and did not correspond with the number of mitochondria. Morphometric analysis of the thickness of the tubular basement membranes for the various treatment groups (Figure 3) produced the following values: C=0.077µm ± 0.003; STZ=0.266µm ± 0.021 and STZ+PPS=0.082µm ± 0.04. The thickness of the basement membrane increased significantly in the STZ group compared to the control group, while in the group of rats with diabetes who received treatment with PPS the thickening of the basement membrane was much lower. There was no significant difference with the control group but there was a statistically significant difference with the STZ group. Table 1. Parameters evaluated at 3 months Control Body weight (g) Glycaemia (mg/dl) Urinary Vol. (ml/24 h) STZ STZ + PPS 383.1 ± 3.7 283 ± 7.1a 299.7 ± 3.2a 95 ± 7.6 632 ± 27a 17.4 ± 0.4 556.8 ± 88a 109.3 ± 12.9 a 105.2 ± 25a Data correspond to the mean ± SEM of 6-10 rats. p <.01 compared to control a Nefrologia 2010;30(6):639-45 641 Y. Mathison Natera et al. PPS in the prevention of kideny damage in Type 1 DM originals A 12 10 Albuminuria 8 6 4 2 B 0 Control STZ STZ + PPSNa Data correspond to the mean ± SEM of 6-10 rats. *P <.05 **P <.01 versus control and P <.01 comparing the STZ + PPS group versus the STZ group Figure 1. Urinary albumin excretion in 24 hrs in control rats, diabetic (STZ) rats y diabetic rats treated with pentosan polysulfate sodium (STZ + PPS). C DISCUSSION GAG, particularly heparan sulfate, are synthesised in endothelial and mesangial cells and, after a process of sulfation in the Golgi apparatus, are incorporated into the extracellular matrix in the glomerulus and the great arteries, where they help maintain the structural integrity of the basement membrane and the vascular wall.27 A general reduction of the negative charges of the extracellular matrix and the plasma membranes associated with a reduced content of heparan sulfate or with changes in its degree of sulfation has been reported in diabetes.28-31 This alteration in the charge of the basement membrane would result in a loss of charge selectivity and facilitate increased elimination of proteins in the urine.30,32 In this way, experimental models of diabetes in rats and mice have found a decrease in proteoglycan synthesis in the glomerulus and decreased content of basement membrane heparan sulfate proteoglycans.33,34 Furthermore, it has been reported that GAG content was reduced in the kidneys and in the intima of the aortas obtained from autopsies done on diabetic patients.35,36 This suggests that alterations in the 642 Figure 2. (A) Control: Regular arrangement of interdigitations and mitochondria (circle), basement membrane of normal thickness (arrow) and uniform capillary endothelium (triangle). Magnification x12 000. (B) STZ: loss of interdigitations, mitochondria arranged randomly (asterisks) and thickened basement membrane (arrow). Magnification x18 000. (C) STZ + PPS: preserved interdigitations but irregular areas devoid of mitochondria (asterisks). Magnification x21 000. metabolism of heparan sulfate are not restricted to the kidney and may be involved in the pathogenesis of other complications of diabetes. Nefrologia 2010;30(6):639-45 Y. Mathison Natera et al. PPS in the prevention of kideny damage in Type 1 DM originals It has been shown that the changes in the metabolism of GAG, produced in an experimental diabetes model in rats, may be modified if they are administered exogenously, with the consequent restoration of normal kidney function.1,2 glomerulus in patients with diabetic nephropathy.40 A significant reduction in microalbuminuria or macroalbuminuria in diabetic patients who have been treated with GAG has also been observed.11,37-39 Our results are consistent with those found in medical literature since the kidney alterations brought on by diabetes are partially prevented by administrating PPS for 3 months following administration of STZ in rats. These findings indicate that GAG may play an important role in the pathophysiology of diabetic nephropathy, and that an abnormal metabolism of GAG may be the cause of this disease.1,41 The subcutaneous administration of PPS for 3 months prevented the increase in renal excretion of albumin in this experimental model. This coincides with what has been previously reported in medical literature,1,2 and corresponds to the effect of decreased renal excretion of albumin demonstrated in patients with diabetes mellitus through the administration of other GAGs.12,14,15,37-39 The administration of sulodexide is the most studied GAG. The mechanism by which GAG exert this protective effect in diabetic nephropathy is not fully understood. Initially it was proposed that its effect was limited to restoring glomerular permselectivity by replacing negative charges, thus reducing urinary albumin excretion and restoring renal function.3,32,42 It is now known however that GAG can modulate protein synthesis in the extracellular matrix, an effect that may contribute to its therapeutic usefulness. Similarly, treatment with PPS protects the kidneys from the structural changes caused by diabetes. This prevents the basement membrane from thickening and the loss of cytoarchitecture. This coincides with the results of other authors who have shown that the administration of heparin and other GAGs prevents diabetic nephropathy in rats. It also maintains the normal thickness of the basement membrane and the anionic charge density, and simultaneously delays the onset of microalbuminuria.1-3 The administration of low molecular weight heparin and dermatan sulfate prevents the thickening of the basement membrane, the reduction of anionic charges and the onset of albuminuria in rats with STZ-induced diabetes.1 Additionally, it has been shown that the administration of heparin reduces the overexpression of collagen, possibly blocking the TGF-b1-mediated pathway that is activated in diabetic nephropathy.2,7 Recently, Lewis and Xu (2008) demonstrated that GAG inhibit the heparanase enzyme, which is stimulated by hyperglycaemia, thereby preventing the breakdown of heparan sulfate.31 Through these combined effects, GAG are able to prevent structural and functional alterations, mainly mesangial, that occur in diabetic nephropathy. A correlation has been found between albuminuria and heparan sulfate content of the basement membrane of the 0.32 In nephrectomised rats, PPS has been shown to be capable of preventing atrophy of epithelial cells and decreasing the inflammatory infiltrate in the interstitium.43 It also inhibits the extracellular matrix from proliferating, reducing type I and IV collagen in mesangial cell cultures44 and in vascular smooth muscle cells obtained from patients in whom the implant failed to obtain vascular access for haemodialysis.45 PPS also reduces cyclosporine-induced nephropathy in rats subjected to salt depletion. This is clearly seen by the decrease in the number of affected arterioles and tubulointerstitial lesions.46 These findings, together with our results, clearly show the nephroprotective role of PPS. 0.24 0.16 0.06 Mean Mean ± SE 0.00 Control STZ STZ + PPSNa Data are expressed as mean ± SEM of n=16 measures/treatment. *P <.05 versus control; +P<.05 versus STZ + PPS. Figure 3. Diameter of basement membrane. Nefrologia 2010;30(6):639-45 Our findings may be explained by the similarity between the effects of PPS and sulodexide; namely, the fibrinolytic effect and activation of the lipoprotein lipase and the hepatic triglyceride lipase, 19-22 as well as the structural similarity with GAG. The anti-inflammatory action of PPS also plays a role in its efficacy for treating interstitial cystitis, 47,48 and may be involved in its nephroprotective effect. Thus, in models of nephrectomised rats, the nephroprotective effect of PPS, as evidenced by 643 originals Y. Mathison Natera et al. PPS in the prevention of kideny damage in Type 1 DM decreased sclerosis and tubular dilation, was associated with a reduction in the infiltration of lymphocytes and macrophages, in a similar way to the effects reported for losartan.43,49 This experimental model of diabetic nephropathy shows that PPS partially prevents kidney damage. The physiological and ultrastructural findings were consistent. This suggests that there is a potential therapeutic use for this compound. Acknowledgements To the staff of the laboratory animal facility of the Escuela José María Vargas, CLINIFAR Laboratories, the Electron Microscope Laboratory of the Escuela de Medicina (School of Medicine) José María Vargas and the IVAX Laboratories for the donation of pentosan polysulfate sodium. This study was subsidised by the PG09-11-5102-2003 and PG0911-51022007 projects of the Consejo de Desarrollo Científico y Humanístico (Council for Scientific and Humanistic Development) of the Universidad Central de Venezuela. REFERENCES 1. Gambaro G, Cavazzana A, Luzi P, Piccoli A, Borsatti A, Crepaldi G, et al. Glycosaminoglycans prevents morphological renal alterations and albuminuria in diabetic rats. Kidney Int 1992;42:285-91. 2. Gambaro G, Venturini A, Noonam D, Fries W, Re G, Garbisa S, et al. Treatment with a glycosaminoglycans formulation ameliorates experimental diabetic nephropaty. Kidney Int 1994;46:797-806. 3. Gambaro G, Van der Woude F. Glycosaminoglycans: use in treatment of diabetic nephropathy. J Am Soc Nephrol 2000;11:359-68. 4. Kristová V, Kriska M, Babál P, Djibril MN, Slámová J, Kurtansky A. Evaluation of endothelium-protective effects of drugs in experimental models of endothelial damage. Physiol Res 2000;49(1):123-8. 5. Koblik T, Sieradzki J, Sendur R, Biernat J, Czarnobilski K, Gryz E, et al. The effect of insulin and sulodexide (Vessel Due F) on diabetic foot syndrome: pilot study in the elderly patients. J Diabetes Complications 2001;15(2):69-74. 6. Gluhovschi G, Schiller A, Raica M, Petrica L, Trandafirescu V, Velciov S, et al. The effects of the therapy with natural glycosaminoglycans (Sulodexide) on proteinuria in different types of glomerulonephritis. Facta Universitatis, Medicine and Biology 2001;8(1):26-30. 7. Ceol M, Gambaro G, Sauer U, Baggio B, Anglani F, Fiorino M, et al. Glycosaminoglycans therapy prevents TGF-b1 overexpression and pathologic changes in renal tissue of long-term diabetic rats. J Am Soc Nephrol 2000;11:2324-36. 8. Yard B, Chorianopoulos E, Herr D, Van der Woude F. Regulation of endothelin-1 and transforming grow factor-b1 production in culture proximal tubular cells by albumin and heparan sulphate glycosaminoglycans. Nephrol Dial Transplant 2001;16:1769-75. 9. Boarder W. Natural inhibitor of transforming growht factor b protects against scarring in experimental kidney diseases. Nature (Lond) 1992;360:361-4. 644 10. Van der Pijl J, Lemkes H, Frölich M, Van der Woude F, Van der Meer F, Van Es L. Effect of danaparoid sodium on proteinuria, von Willebrand factor, and hard exudates in patients with diabetes mellitus type 2. J Am Soc Neprhol 1999;10:1331-6. 11. Solini A, Carraro A, Barzon I, Crepaldi G. Therapy with glycosaminoglycans lower albumin excretion rate in non-insulin dependent diabetic patients with macroalbuminuria. Diab Nutr Metab 1994;7:304-7. 12. Solini A, Vergnani L, Ricci F, Crepaldi G. Glycosaminoglycans delay the progression of nephropathy in NIDDM. Diabetes Care 1997;20(5):819-23. 13. Dedov I, Shestakova M, Vorontzov A, Palazzini E. A randomixed, controlled study of sulodexide therapy for the treatment of diabetic nephropathy. Nephrol Dial Transplant 1997;12:2295-300. 14. Gambaro G, Kinalska I, Oksa A, Pont’uch P, Hertlová M, Olsovsky J, et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S randomized trial. J Am Soc Nephrol 2002;13(6):1615-25. 15. Achour A, Kacem M, Dibej K, Skhiri H, Bouraoui S, El May M. One year course of oral sulodexide in the management of diabetic nephropathy. J Nephrol 2005;18(5):568-74. 16. Fisher A, Merton R, Marsh Ngaffney P, Barrowcliffe T, Thomas D. A comparison of pentosan polysulfate and heparin II: effect of subcutaneous injection. Thromb Haemostas 1982;47:109-13. 17. Daha LK, Lazar D, Simak R, Pflûger H. The effects of intravesical pentosan polysulfate treatment on the symptoms of patients with bladder pain syndrome/intersticial cystitis: preliminary results. Int Urogynecol J Pelvic Floor Dysfunct 2008;19(7):987-90. 18. Davis EL, El Khoudary SR, Talbott EO, Davis J, Regan LJ. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for intersticial cystitis: a randomized double-blind clinical trial. J Urol 2008;179(1):177-85. 19. Bonanno G, Bonaccorso R, DellÁli C, Salanitri G. Sulodexide in the treatment of atherosclerosis: A controlled clinical trial. Acta Therapeutica 1985;11:87-98. 20. Marsh N, Peyser P, Creighton L, Mahmoud M, Gaffney P. The experimental pentosan polysulphate (SP54) on the fibrinolytic enzyme system-a human volunteer and experimental animal study. Thromb Haemost 1985;54(4):833-7. 21. Mac Gregor I, Dawes J, Pepper D, Prowse C, Stocks J. Metabolism of sodium pentosan polysulphate in man measured by a new competitive binding assay for sulphated polysaccharidescomparison with effects upon anticoagulant activity, lipolysis and platelet alpha-granule protein. Throm Haemost 1985;53(3):411-4. 22. Ceriello A, Quatraro A, Ettorre M, Marchi E, Barbanti M, Giugliano D. Glucosaminoglycans administration decreases high fibrinogen plasma levels in diabetic patients. Diab Nutr Metab 1993;6(3):1-4. 23. Czubayko F, Smith R, Chung H, Wellstein A. Tumor growth and angiogenesis induced by a secreted binding protein for fibroblast growth factors. J Biol Chem 1994;269(45):28243-8. 24. Odlind B, Dencker L, Tengblad A. Preferential localization of 3Hpentosanpolysulphate to the urinary tract in rats. Pharmacol Toxicol 1987;61(3):162-6. 25. NIH Guide for the care and use of laboratory animals. NIH Guide 1996;25(28):1-111. 26. Sobin S. Accuraty of indirect determination of blood pressure in rat: Nefrologia 2010;30(6):639-45 Y. Mathison Natera et al. PPS in the prevention of kideny damage in Type 1 DM 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. relation to temperature of plethismograph and width of cuff. Am J Physiol 1983;146:179-82. Gallagher J, Lyon M, Steward W. Structure and function of heparan sulphate proteoglycans. Biochem J 1986;236:313-25. Wu V, Wilson B, Cohen M. Disturbances in glomerular basement membrane glycosaminoglycans in experimental diabetes. Diabetes 1987;36:679-83. Deckert T, Kofoed-Enevoldsen A, Borch-Johnsen K, Rasmussen B, Jensen T. Microalbuminuria: Implications for micro-and macrovascular diseases. Diabetes Care 1992;15(9):1181-91. Jensen T. Pathogenesis of diabetic vascular disease: evidence for the role of reduced heparan sulfate proteoglycan. Diabetes 1997;46(S2):S98-S100. Lewis E, Xu X. Abnormal glomerular permeability characteristic in diabetic nephropathy. Implications for the therapeutic use of lowmolecular weight heparin. Diabetes Care 2008;31(S2):S202-S206. Deckert T, Kofoed-Enevoldsen A, Vidal P, Norgaard K, Andreasen HB, Felt-Rassmussen B. Size and charge selectivity of glomerular filtration in type 1 (insulin-dependent) diabetes with and without albuminuria. Diabetologia 1993;36:244-51. Kanwar Y, Rosenzweig L, Linker A, Jakuboswski M. Decreased the novo synthesis of glomerular proteoglycans in diabetes: Biochemical and autoradiographic evidences. Proc Natl Acad Sci USA 1983;80:2272-5. Rohrbach D, Hassel J, Kleinman H, Martín G. Alterations in the basement membrane (heparan sulfate) proteoglycan in dibetic mice. Diabetes 1982;31:185-8. Parthasarathy N, Spiro R. Effect of diabetes on the glycosaminoglycans component of the human glomerular basement membrane. Diabetes 1982;31:738-41. Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993;36(4):316-22. Myrup B, Hansen P, Jensen T, Kofoed-Enevoldsen A, Feldt-Rasmussen B, Gram J, et al. Effect of low-dose of heparin on urinary excretion in insulin-dependent diabetes mellitus. Lancet 1995;345:421-2. Tamsma J, Van der Woude Y, Lemkes H. Effect of sulphated glycosaminoglycans on albuminuria in patients with overt diabectic (type 1) nephropathy. Nephrol Dial Transplant 1996;11(1):182-5. Velussi M, Cernigoi A, Dapas F, De Monte A. Glycosaminoglycans oral therapy reduces microalbuminuria, blood fibrinogen levels and limb arteriopthy clinical sings in 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. originals patients with non-insulin dependent diabetes mellitus. Diab Nutr Metab 1996;9:53-8. Tamsma J, Van der Born J, Brujin J, Assmann K, Weening J, Berden J, et al. Expression of glomerular extracelllular matrix components in human diabetic nephropathy: decrease of heparan sulphate in the glomerular basement membrane. Diabetologia 1994;37:313-20. Gambaro G, Baggio B. Glycosaminoglycans: a new paradigm in the prevention of proteinuria and progression of glomerular disease. Nephrol Dial Transplant 1996;11:762-4. Gambaro G, Skrha J, Ceriello A. Glycosaminoglycans therapy for the long-term diabetic complications. Diabetologia 1998;41:975-9. Bobadilla NA, Tack I, Tapia E, Sánchez-Lozada LG, Santamaría J, Jiménez F, et al. Pentosan polysulfate prevents glomerular hypertension and structural injury despite persisting hypertension in 5/6 nephrectomy rats. J. Am Soc Nephrol 2001;12:2080-7. Elliot SJ, Striker LJ, Stetler-Stevenson W, Jacot TA, Striker GE. Pentosan polysulfate decreases proliferation and net extracellular matrix production in mouse mesangial cells. J Am Soc Nephrol 1999;10:62-8. Elliot SJ, Striker LJ, Connor E, Stetler-Stevenson W, McQuinn WC, Blagg CR, et al. Pentosan polysulfate decreases proliferation and extracellular matriz deposition by vascular smooth muscle cells isolated from failes hemodialysis access grafts. Clin Nephrol 2000;54(2):121-7. Schwedler SB, Bobadilla N, Striker LJ, Vaamonde CA, Herrera-Acosta J, Striker GE. Pentosan polysulfate treatment reduces cyclosporineinduced nephropathy in SALT-depleted rats. Transplantation 1999;68(10):1583-8. Sadhukhan PC, Tchetgen MB, Rackley RR, Vasavada SP, Liou L, Bandyopadhyay SK. Sodium pentosan polysulfate reduces urothelial responses to inflammatory stimuli via an indirect mechanism. J Urol 2002;168(1):289-92. Chiang G, Patra P, Letourneau R, Jeudy S, Boucher W, Green M, et al. Pentosanpolysulfate inhibits mast cell histamine secretion and intracellular calcium ion levels: an alternative explanation of its beneficial effects in interstitial cystitis. J Urol 2000;164(6):2119-25. Herrera-Acosta J, Tapia E, Sánchez-Lozada LG, Franco M, Striker LJ, Striker GE, et al. Restoration of glomerular haemodynamics and renal injury independent of arterial hypertension in rats with subtotal renal ablation. J Hypertension 2002;20(S3):S29-35. Sent for Review 20 July 2010 | Accepted: 22 Jul. 2010 Nefrologia 2010;30(6):639-45 645 originals http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society Treatment of uraemic anorexia with megestrol acetate M. Fernández Lucas, J.L. Teruel, V. Burguera, H. Sosa, M. Rivera, J.R. Rodríguez Palomares, R. Marcén, C. Quereda Nephrology Department. Ramón y Cajal Hospital. Madrid. Spain Nefrologia 2010;30(6):646-52 doi:10.3265/Nefrologia.pre2010.Aug.10546 ABSTRACT Background: Anorexia is a common disorder in patients treated with regular hemodialysis and a contributing factor of malnutrition. The aim of this study was to evaluate the effectiveness of megestrol acetate, an appetite stimulant used in cancer patients as a treatment for anorexia in dialysis patients. Material and methods: In 2009, 16 patients in our hemodialysis unit, three with diabetes mellitus were treated with megestrol (160 mg/day single dose) for anorexia defined as a Likert scale of Appetite. The pattern and dialysis dose were not changed during the study. Results: In the third month of treatment is aimed, in the total group, an increase of dry weight (60.8 vs. 58.9 kg, p <.01), concentration of albumin (4.02 vs 3.8 g/dl, p <.05), creatinine concentration (9.73 vs. 8.26 mg/dl, p <.01) and protein catabolic rate (1.24 vs. 0.97 g/kg/day, p <0001). Non significant variations in the concentration of hemoglobin, erythropoietin dose, and concentration of lipids were found. One patient with diabetes mellitus had to increase the dose of insulin and two other patients have had some mild hyperglycemia. Megestrol acetate did not suppress the secretion of pituitary sex hormones, but in 3 of 10 patients studied was found inhibition of ACTH secretion. The response was not homogeneous: a patient did not respond and reduced its dry in weight, 5 weight gain was quiet (less than 1 kg) and the remaining ten the response was good with an increase in dry weight ranged between 1.5 and 5.5 kg. Conclusions: Megestrol acetate can improve appetite and nutritional parameters in patients treated with periodic hemodialysis who report anorexia. Megestrol acetate Correspondence: Milagros Fernández Lucas Servicio de Nefrología. Hospital Ramón y Cajal. Ctra. Colmenar, km 9,100, 28034. Madrid. Spain. [email protected] 646 can induce hyperglycemia and inhibit the secretion of ACTH in some patients. These side effects should be assessed when given this treatment. Key words: Megestrol acetate. Anorexia. Malnutrition. Hemodialysis. Tratamiento de la anorexia urémica con acetato de megestrol RESUMEN Introducción: La anorexia es un trastorno frecuente en el enfermo tratado con hemodiálisis periódica, y factor contribuyente de la malnutrición. El objetivo del presente trabajo es comprobar la eficacia del acetato de megestrol, un estimulador del apetito utilizado en enfermos con cáncer, como tratamiento de la anorexia del enfermo sometido a diálisis. Material y métodos: En el año 2009, 16 enfermos de nuestra unidad de hemodiálisis, tres de ellos con diabetes mellitus, fueron tratados con acetato de megestrol (160 mg/día en dosis única), por anorexia definida según una escala Likert de apetito. La pauta y la dosis de diálisis no fueron modificadas durante el estudio. Resultados: Al tercer mes de tratamiento se objetivó, en el grupo total, un aumento del peso seco (60,8 frente a 58,9 kg; p <0,01), de la concentración de albúmina (4,02 frente a 3,8 g/dl; p <0,05), de la concentración de creatinina (9,73 frente a 8,26 mg/dl; p <0,01) y de la tasa de catabolismo proteico (1,24 frente a 0,97 g/kg/día; p <0,001). No hemos constatado variaciones significativas en la concentración de hemoglobina, dosis de eritropoyetina y concentración de lípidos. En un enfermo con diabetes mellitus hubo que aumentar la dosis de insulina y en otros 2 enfermos se detectó una hiperglucemia leve. El acetato de megestrol no suprimió la secreción de hormonas sexuales hipofisarias, pero en 3 de 10 enfermos estudiados se constató una inhibición de la secreción de corticotropina. La respuesta no fue homogénea: un enfermo no respondió y disminuyó su peso seco, en cinco el incremento de peso fue discreto (in- M. Fernández Lucas et al. Megestrol acetate in haemodialysis ferior a 1 kg) y en los 10 restantes la respuesta fue buena, con un incremento de peso seco que osciló entre 1,5 y 5,5 kg. Conclusiones: El acetato de megestrol puede mejorar el apetito y los parámetros nutricionales en enfermos tratados con hemodiálisis periódica que refieran anorexia. El acetato de megestrol puede inducir hiperglucemia e inhibir la secreción de corticotropina en algunos pacientes. Estos efectos secundarios deben ser valorados cuando se administre este tratamiento. Palabras clave: Acetato Desnutrición. Hemodiálisis de megestrol. Anorexia. originals good, with a rate of side effects no higher than those observed in placebo groups, 17 but adverse effects have been reported such as gastrointestinal intolerance, hyperglycaemia, and inhibition of secretion of pituitary hormones such as corticotropin (ACTH) and the gonadotropins. 14 The use of megestrol acetate in patients undergoing dialysis has limited experience; the doses used have varied, as have the results.18-23 In January 2009 we began a protocol of treatment of anorexia in dialysis patients with megestrol acetate. In this paper we describe our experience with the patients that began treatment during its first year of use. INTRODUCTION MATERIAL AND METHOD Anorexia, defined as a lack of desire to eat, is a frequent disorder among patients treated with periodic haemodialysis. A loss in appetite was noted by 33% of the patients in the HEMO 1 study, by 24% of those in the DOPPS2 study, and in up to 38% in other series.3 Anorexia is one of the factors that contribute to malnutrition among patients undergoing dialysis. 4,5 Insufficient food intake is the main cause of type 1 uraemic malnutrition, which is characterised by weight and muscle mass loss with a modest impact on albumin concentrations, in contrast to type 2, which is more related to concurrent inflammatory processes. 6,7 Apart from its impact on nutrition, anorexia is itself an independent risk factor for morbidity and mortality. 1,2 Its pathogenesis is unknown. Inflammatory cytokines, deregulation of hormones and neuropeptides that control appetite, retention of medium molecular weight molecules, and alterations in amino acid concentrations seem involved.4,8,9 The control of anorexia is important for the prevention and treatment of malnutrition associated with renal failure; however, few studies have focused on appetite and specific measures to stimulate it. In the HEMO study, it was found that neither the administration of a greater dose of haemodialysis than that currently considered adequate nor the use of high-flux dialysers resulted in an improvement in appetite.10 Appetite is lower on the day of haemodialysis, 11 but an increase has been reported as the number of sessions increases.12 Steroids, progestogens, and serotonin agonists have been used to stimulate appetite in various clinical situations.13 Of all substances with orexigenic effects, the best known is megestrol acetate. It is a synthetic progestin that is used to increase appetite and weight in cancer patients or those infected with the HIV virus. 14,15 Two systematic reviews concluded that treatment with megestrol acetate is effective in those cases.16,17 Its tolerance is considered to be Nefrologia 2010;30(6):646-52 For the definition of anorexia, we used the appetite questionnaire from the HEMO 11 and DOPPS 2 studies. It reflects the patient’s current appetite as they see it on a Likert scale with five possibilities: very good, good, fair, poor, or very poor. Next the patients are asked if in the last four week their appetite has improved, stayed the same, or worsened. Anorexia is diagnosed when a patient reports that their current appetite is fair, poor, or very poor, and that in the last 4 weeks it has not changed or has worsened. In 2009, 99 patients with chronic renal failure were cared for in our haemodialysis unit. During this year, 18 patients with anorexia gave their informed consent to receive treatment with megestrol acetate. One patient was excluded who stopped the treatment one month after starting, without obvious cause, and another was diagnosed with multiple myeloma and died from his illness two months later. The 16 remaining cases were treated with megestrol acetate for 3 months and make up the object of this study. Age, gender, time on haemodialysis, and the possible cause of anorexia are shown in Table 1. Three patients had insulindependent diabetes mellitus (cases 1, 2 and 16). Eight patients (cases 3, 6, 7, 8, 11, 15, and 16) had resumed haemodialysis after a failed kidney transplant; none of them received immunosupression at the time of beginning treatment with megestrol acetate. In 7 patients, an intercurrent process triggered anorexia: initiation of treatment for hepatitis C with interferon (case 6); HIV infection treated with antiretroviral drugs (case 11), and admission to hospital for various complications (cases 2, 4, 7, 13 and 16). In the 9 remaining cases, the lack of appetite could not be attributed to a specific cause. All patients started treatment as outpatients. In the cases in which anorexia was brought on by intercurrent conditions that required hospital admission, the treatment with megestrol acetate started after the patients were discharged. 647 M. Fernández Lucas et al. Megestrol acetate in haemodialysis originals Table 1. Baseline data on patients and progress at three months of treatment with megestrol acetate Age (years) Months on HD Cause of anorexia 75 5 None 78 163 Gastric ulcer with Weight lost in the Subjective improvement two months before in appetite at treatment month 3 Weight gain at month 3 Case 1 Male 3 kg Yes 2 kg pyloric oedema 4.5 kg Yes 5.5 kg 2.5 kg Yes 1.5 with post-surgical sepsis 4 kg Yes 2.5 kg 1 kg Yes 1.5 2 kg No 0.5 kg transplantectomy 0 Yes 2 kg Case 2 Female Case 3 Female 44 9 None 72 4 Heminephrectomy Case 4 Male of a single kidney Case 5 Female 82 19 None 52 72 Treatment with Case 6 Female interferon Case 7 Male 59 10 Graft intolerance Case 8 Female 72 7 None 1.5 kg No 0.5 kg 79 1 None 0 Yes 4 kg 78 43 None 2.5 kg Yes 0.5 kg 53 45 HIV infection 5 kg No –1 kg 71 62 None 1 kg Yes 0.5 41 34 cerebral haematoma 9 kg Yes 5.5 kg Case 9 Female Case 10 Male Case 11 Female Case 12 Female Case 13 Male Surgery for Case 14 Female 40 6 None 0 Yes 2 62 8 None 0.5 kg Yes 2 kg 67 4 0.5 kg Yes 0.5 Case 15 Male Case 16 Female Infection of arteriovenous fistula wound The starting dose of megestrol acetate was 160 mg daily in a single dose. No calorie or protein supplements were administered orally or intravenously during haemodialysis. After 3 months of treatment, response was assessed through a survey in which patients were asked whether their appetite had improved and the evolution of dry weight and laboratory 648 parameters were analysed. The follow-up period ended 30 June 2010. The patients had dialysis three times per week, 3.5-4 hours per session, with high-flux dialysis and ultra-pure dialysis liquid. The dialysis dose was calculated using standard urea Nefrologia 2010;30(6):646-52 M. Fernández Lucas et al. Megestrol acetate in haemodialysis clearance (Kt/V) obtained using the simplified monocompartmental Daugirdas formula. The protein catabolic rate (PCR) was obtained using the Borah formula as modified by Sargent.24 Dry weight was determined using clinical criteria. Twelve patients had residual diuresis less than 150 ml/day when treatment with megestrol acetate was indicated. Blood samples for the laboratory measurements were obtained immediately before the first haemodialysis session of the week, after the long interdialytic interval. Data are expressed as mean (SD). For the statistical analysis we used the Student’s t test for paired data. Values with P<.05 were considered statistically significant. RESULTS The loss of dry weight in the two months prior to treatment with megestrol acetate, the subjective evaluation of appetite, and the evolution of dry weight at the three-month follow-up are shown in Table 1. Thirteen patients considered that their appetite had improved. Dry weight increased in 15 patients, although in five of them the increase was less than 1 kg. The evolution of the parameters related to nutrition analysed after the third month of treatment is shown in Table 2. Overall, the group saw a significant increase in dry weight, in concentrations of albumin and creatinine, and in the rate of protein catabolism. The last two parameters were analysed only in the 12 patients without diuresis, in order to avoid the possible influence of variations in residual renal function. The dialysis dose did not vary significantly: baseline spKtV 1.59 (0.37) and at 3 months: 1.63 (0.29). Statistically significant changes were not observed in the concentration of haemoglobin (baseline: 10.6 [1.2]; 3 months: 11.5 [1.4] g/dl). They were also not seen in the intravenous dose of recombinant human erythropoieitin (baseline dose: 19.062 [17.448]; 3 months: 15.906 [14.262] U/week). originals The treatment with megestrol acetate did not have a statistically significant effect on total cholesterol (baseline: 152.3 [36]; 3 months: 145.4 [34.6] mg/dl), HDL cholesterol (baseline: 35.8 [14.6]; 3 months: 33.3 [8.2] mg/dl) and LDL cholesterol (baseline: 98.6 [37] mg/dl; 3 months: 86.2 [30.2] mg/dl). The follow-up period from the start of treatment, the time of treatment with megestrol acetate, the cause of withdrawal and weight gain are shown in Table 3. Three patients died during the follow-up period. Cases 2 and 10, with severe vascular disease, died as a consequence of this disease, and case 3, due to tuberculosis pericarditis in a patient with AIDS. The 13 remaining patients were alive when the study ended. In 5 patients the dose of megestrol acetate was increased to 320 mg/day due to a lack of response to the initial dose (cases 6, 8, and 12) or due to a later decrease of appetite (cases 9 and 14), with good appetite evolution and weight in all cases. At the time the study ended, 3 patients continued taking megestrol acetate. In two of them, attempts to withdraw the treatment were associated with loss of appetite and the need to reintroduce the treatment (cases 1 and 7). Side effects Of the three patients with insulin-dependent diabetes mellitus (cases 1, 2, and 6), two did not need their insulin dosage to be modified after administering megestrol acetate. In case 2, the dose of Lantus insulin needed to be increased from 15 to 18 U/day. An increase in glucose concentration was found in two of the 13 remaining patients. In one patient (case 13) who had dialysis in the afternoon, the postprandial blood glucose, which had been less than 125 mg/dl, increased starting the second month of treatment (maximum concentration 156 mg/dl) and returned to normal figures one month after the end of treatment. In case 6, baseline glucose increased with the administration of interferon up to a maximum of 153 mg/dl. Table 2. Changes in dry weight, albumin and creatinine concentrations, lymphocyte count and protein catabolic rate (PCR) at the third month of treatment with megestrol acetate Baseline Three months P Weight (kg) 58.9 (10.8) 60.8 (10.8) <.01 Albumin (g/dl) 3.8 (0.55) 4.02 (0.41) <.05 Lymphocytes/µl 1393 (762) 1507 (831) NS Creatinine (mg/dl)a 8.26 (1.90) 9.73 (2.75) <.01 PCR (g/kg)a 0.97 (0.26) 1.24 (0.30) <.001 a Analysed only for the 12 patients without residual renal function. Nefrologia 2010;30(6):646-52 649 M. Fernández Lucas et al. Megestrol acetate in haemodialysis originals One patient (case 9), who received low doses of steroids for necrotising vasculitis with leukocyte cytoplasmic antibodies, had left deep femoral vein thrombophlebitis after the sixth month of treatment and needed anticoagulation for 3 months, with complete repermeabilisation of the femoral vein. A study of the pituitary-adrenal axis was conducted in 10 patients during the treatment with megestrol acetate. We determined baseline ACTH, baseline cortisol, and at 30 and 60 minutes after stimulation with ACTH (0.25 mg of ACTH i.v.) (Table 4). Three patients had a basal cortisol level below the normal range, associated in 2 cases with concentrations of ACTH at the lower limits of normality. Concentrations of FSH and LH gonadotropins were measured in 8 patients, which were within the normal range in all of them (data not shown). DISCUSSION We describe here our experience with the use of megestrol acetate in 16 patients treated with haemodialysis who reported anorexia. The diagnosis of anorexia was made using the HEMO and DOPPS questionnaire on subjective appraisal of appetite. The validity of the questionnaire was demonstrated in both studies by showing that the evaluation of appetite is a faithful reflection of the consumption of food,1,11 has a good correlation with nutritional parameters and those of quality of life,1,2 and is a predictor of morbidity and mortality.1-3 The initial dose of megestrol acetate was 160 mg/day. This is an intermediate dose among the wide range of doses used in patients on dialysis: 40 mg (18), 80 mg (22), 160 mg (21), 400 mg (20), and 800 mg daily.19,23 At 3 months, 13 of the 16 patients believed their appetite had improved. Overall, the group saw an increase in dry weight, an improvement in nutritional parameters related to protein metabolism such as albumin concentration and protein catabolic rate (a reflection of the consumption of protein) and an increase in the concentration of creatinine (an indicator of muscular mass). The effect of megestrol acetate on albumin concentration has been observed in other studies with patients on dialysis.18,20-22 Treatment with megestrol acetate is usually temporary. In our series, 87% of the cases were treated for less than one year (4-11 months), stimulating appetite itself in clinically stable patients, or helping to recover from intercurrent conditions, which are frequent in patients on dialysis. At the dose used, clinical tolerance was good and no patient reported symptoms such as headache, diarrhoea, confusion, or dizziness, which have been reported at doses of 800 mg per day.19 However, we did observe other side effects attributable to the use of megestrol acetate. One diabetic patient needed an increase in her dose of insulin, and in 2 Table 3. Follow-up from the start of treatment until the end of the study (30 June 2010), time of treatment with megestrol acetate, cause of withdrawal and weight gain. Case a Months of Months of treatment Reason for Weight follow-up with megestrol ending gain (kg)a 1 17 17 Continued 1 2 7 7 Death 11.5 3 18 4 No need 2 4 18 7 No need 13 5 12 6 No need 2 6 18 11 No need 3 7 13 13 Continued 7 8 14 11 No need 10 9 11 9 No need 4.5 10 4 4 Death 0.5 11 4 4 Death ?1 12 10 8 No need 6 13 8 4 No need 6.5 14 12 7 No need 4.5 15 8 5 No need 4.5 16 6 6 Continued 0.5 Until the end of treatment or the end of follow-up. 650 Nefrologia 2010;30(6):646-52 M. Fernández Lucas et al. Megestrol acetate in haemodialysis originals Table 4. Study of the pituitary-adrenal axis using the ACTH test (0.25 mg of ACTH i.v.) Case Dose (mg/day) Duration of treatment (months) ACTH Baseline Cortisol Cortisol 30 min Cortisol 60 min 1 160 16 20.1 8.4 18.1 20.1 4 160 1 21 11 24.2 25.6 6 160 11 10.4 13.2 31.5 40.7 7 160 12 5 1 2.7 4.5 8 320 11 14.3 4.6 14.1 17.1 12 320 8 12.5 11.7 9.23 23.2 13 160 2 20.5 10.6 35.7 37.8 14 320 6 7 2.9 14.1 14.3 15 160 4 25.5 11.9 21.4 27.9 16 160 5 12.2 11.3 45.4 43.2 Normal range for ACTH: 5-46 pg/ml; normal range for baseline cortisol: 5-25 µg/dl. patients an increase in plasma glucose concentration was detected. The inhibitory effect of megestrol acetate on the pituitary-adrenal axis was a more significant revelation. In 3 of 10 patients studied, a reduction in baseline cortisol was found, with low concentrations of corticotropin in two cases. These possible effects on the endocrine system have not been analysed in other studies on dialysis patients. One patient had thrombophlebitis. Relating this with megestrol acetate is hypothetical, since the patient was receiving steroids for necrotising vasculitis. We did not observe any effect of megestrol acetate on lipid profiles or on treatment for anaemia. No inhibition of FSH or LH concentrations was found in any of the patients studied. We can conclude that megestrol acetate stimulates appetite in patients on haemodialysis who report anorexia. The increase in appetite is accompanied by an increase in weight and an improvement in other nutritional parameters related to protein metabolism. However, induced hyperglycaemia and inhibited secretion of ACTH are possible side effects that should be taken into account when administering this drug. The dose and duration of treatment should be established in future studies. 3. 4. 5. 6. 7. 8. 9. 10. REFERENCES 1. Burrowes JD, Larive B, Chertow GM, Cockram DB, Dwyer JT, Greene T, et al., for the HEMO Study Group: Self-reported appetite, hospitalization and death in haemodialysis patients: findings from the Hemodialysis (HEMO) Study. Nephrol Dial Transplant 2005;20:2765-74. 2. Lopes AA, Elder SJ, Ginsberg N, Andreucci VE, Cruz JM, Fukuhara S, et al. Lack of appetite in haemodialysis patients-associations with Nefrologia 2010;30(6):646-52 11. 12. patients characteristics, indicators of nutritional status and outcomes in the international DOPPS. Nephrol Dial Transplant 2007;22:3538-46. Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 2004;80:299-307. Bossola M, Tazza L, Giungi S, Luciani G. Anorexia in hemodialysis patients: An update. Kidney Int 2006;70:417-22. Heng AE, Cano NJM. Nutritional problems in adult patients with stage 5 chronic kidney disease on dialysis (both haemodialysis and peritoneal dialysis). NDT Plus 2010;3:109-17. Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant 2000;15:953-60. Locatelli F, Fouque D, Heimburger O, Drücke TB, Cannata-Andía JB, Hörl WH, et al. Nutritional status in dialysis patients: a European consensus. Nephrol Dial Transplant 2002;17:563-72. Aguilera A, Selgas R, Díez JJ, Bajo MA, Codoceo R, Álvarez V. Anorexia in end-stage renal disease: pathophysiology and treatment. Expert Opin Pharmacother 2001;2:1825-38. Carrero JJ, Aguilera A, Stenvinkel P, Gil F, Selgas R, Lindholm B. Appetite disorders in uremia. J Ren Nutr 2008;18:107-13. Rocco MV, Dwyer JT, Larive B, Greene T, Cockram DB, Chumlea WC, et al., for the HEMO Study Group: The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: Results of the HEMO Study. Kidney Int 2004;65:2321-34. Burrowes JD, Larive B, Cockram DB, Dwyer J, Kusek JW, McLeroy S, et al. Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: crosssectional results from the HEMO study. J Ren Nutr 2003;13:191-8. Galland R, Traeger J, Arkouche W, Cleaud C, Delawari E, Fouque D. Short daily hemodialysis rapidily improves nutritional status in hemodialysis patients. Kidney Int 2001;60:1555-60. 651 originals 13. Loprinzi CL, Hesketh PJ, Savarese DMF, Jatoi A. Pharmacologic management of cancer anorexia/cachexia. 2010 UpToDate. www Uptodate.com 14. Corcoran C, Grinspoon S. Treatments for wasting in patients with the acquired immunodeficiency syndrome. N Engl J Med 1999;340:1740-50. 15. López AP, Fíguls MR, Cuchi GU, Berenstein EG, Pasies BA, Alegre MB, et al. Systematic review of megestrol acetate in the treatment of anorexia-cachexia syndrome. Pain Symptom Manage 2004;27:360-9. 16. Ruiz-García V, Juan O, Pérez Hoyos S, Peiró R, Ramón N, Rosero MA. Acetato de megestrol: una revisión sistemática de su utilidad clínica para la ganancia de peso en los enfermos con neoplasia y caquexia. Med Clin (Barc) 2002;119:166-70. 17. Berenstein EG, Ortiz Z. Megestrol acetate for the treatment of anorexia-caquexia syndrome. Cochrane Database Syst Rev 2005:CD004310. 18. Lien YH, Ruffenach SJ. Low dose megestrol increases serum albumin in malnourished dialysis patients. Int J Artif Organs 1996;19:147-50. M. Fernández Lucas et al. Megestrol acetate in haemodialysis 19. Boccanfuso JA, Hutton M, McAllister B. The effects of megestrol acetate on nutritional parameters in a dialysis population. J Ren Nutr 2000;10:36-43. 20. Rammohan M, Kalantar-Zadeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr 2005;15:345-55. 21. Golebiewska J, Lichodziejewska-Niemierko M, Aleksandrowicz E, Majkowicz M, Lysiak-Szydlowska W, Rutkowski E. Influence of megestrol acetate on nutrition and inflammation in dialysis patients-preliminary results. Acta Biochim Pol 2009;56:733-7. 22. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Ren Nutr 2009;19:167-71. 23. Yeh SS, Marandi M, Thode HC, Levine DM, Parker T, Dixon T, et al. Report of a pilot, doubleblind, placebo-controlled study of megestrol acetate in elderly dialysis patients with caquexia. J Ren Nutr 2010;20:52-62. 24. Sargent JA. Control of dialysis by a single-pool urea model: The National Cooperative Dialysis Study. Kidney Int 1983;23(Suppl 13):S19-S25. Sent for review: 20 Aug. 2010 | Accepted: 31 Aug. 2010 652 Nefrologia 2010;30(6):646-52 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society originals Decreased glomerular filtration rate as calculated by the Cockckroft-Gault and MDRD formulas does not always predict cardiovascular morbidity and mortality in hypertensive patients treated in primary care F.J. Tovillas-Morán1,2, M. Vilaplana-Cosculluela2,3, A. Dalfó-Pibernat4, E. Zabaleta-del-Olmo2, J.M. Galcerán5, A. Coca6, A. Dalfó-Baqué2,3 Martí i Julià Primary Care Team (PCT). Cornellá, Barcelona, Spain. 2 Gòtic PCT. Barcelona. Spain. 3 Vallcarca PCT. Barcelona, Spain. Instituto de Investigación en Atención Primaria (Primary Care Research Institute) (IDIAP) Jordi Gol. Barcelona, Spain. 5 ALTHAIA Foundation. Manresa Barcelona, Spain. 6 Hypertension Unit. Institute of Medicine and Dermatology. Teaching Hospital. University of Barcelona. Barcelona, Spain. 1 4 Nefrologia 2010;30(6):653-60 doi:10.3265/Nefrologia.pre2010.Jun.10297 ABSTRACT Background: A decrease in renal function is associated with cardiovascular morbidity and mortality. The aim of this study was to analyse the association of cardiovascular morbidity and mortality with baseline glomerular filtration rate (GFR), calculated according to the CockcroftGault and MDRD formulas, with the incidence of major adverse cardiovascular events (MACEs) in a cohort of hypertensive individuals followed for 12 years. Method: We performed a prospective study of a random sample of 223 hypertensive patients free of MACEs, who were followed in an urban Primary Care Centre. GFR was estimated using both formulas. MACEs were considered as the onset of ischaemic heart disease, heart failure, heart attacks, peripheral vascular disease or cardiovascular death. Data were analysed using the life-table method and Cox regression modeling. Results: The median follow-up was 10.7 (interquartile range, 6.5-12.1) years. Follow-up was completed in 191 participants (85.7%). The cumulative survival was 64.7% (95% Confidence Interval [CI], 57.9-71.6). The incidence of MACEs during the follow-up period was 3.6 (95% CI, 2.7-4.4) per 100 subject-years. The final multivariable model showed that the most predictive variables of MACEs in the study population were the presence of diabetes mellitus and the estimation of GFR >60ml/min/1.73 m2 by the MDRD equation. Conclusions: There was a rela- Correspondence: Francisco Javier Tovillas-Morán Equipo de Atención Primaria (EAP) Martí i Julià. Avenida Baix Llobregat, 17. 08940 Cornellà. Barcelona. Spain. [email protected] [email protected] tionship between the occurrence of MACEs and an estimated GFR by MDRD above 60 ml/min/1.73 m2 at study entry, inversely to what was expected. GFR estimated by the C-G formula was not associated with cardiovascular risk. Key words: Hypertension. Cardiovascular disease. Primary Health Care. Survival Analysis. Glomerular Filtration Rate. Renal Insufficiency. El filtrado glomerular reducido según las fórmulas de Cockcroft-Gault y MDRD no siempre predice la morbimortalidad cardiovascular en los pacientes hipertensos atendidos en atención primaria RESUMEN Antecedentes: El deterioro de la función renal se ha asociado con un incremento de la morbimortalidad cardiovascular. El objetivo del estudio fue analizar la asociación del filtrado glomerular (FG) basal, según las fórmulas de Cockcroft-Gault y MDRD, con la incidencia de eventos cardiovasculares (ECV) en una cohorte de personas hipertensas seguida durante 12 años. Métodos: Estudio prospectivo de una muestra aleatoria de 223 hipertensos libres de ECV atendidos en un centro de atención primaria urbano. Se estimó el FG mediante ambas fórmulas. Se consideró ECV la aparición de cardiopatía isquémica, insuficiencia cardíaca, accidente cerebrovascular, vasculopatía periférica o muerte por ECV. Se analizaron los datos mediante el método actuarial y modelos de regresión de Cox. Resultados: La mediana de tiempo de seguimiento fue de 10,7 años (rango intercuartílico, 6,5-12,1). El seguimiento fue completo en 191 participantes (85,7%). La supervivencia acumulada fue del 64,7% (inter653 originals F.J. Tovillas-Morán et al. GFR as a predictor of CV morbidity and mortality in AHT valo de confianza [IC] del 95%: 57,9-71,6%). La tasa media de incidencia de ECV durante todo el período de seguimiento fue de 3,6 (IC del 95%, 2,7-4,4%) por 100 personas hipertensas/año. El modelo multivariable final mostró que las variables con mayor poder predictivo de ECV en la población de estudio fueron la diabetes y la estimación del FG >60 ml/min/1,73 m 2 mediante fórmula MDRD. Conclusiones: Se observó una relación entre la aparición de ECV y los valores de FG estimados por la fórmula MDRD al inicio del seguimiento superiores a 60 ml/min/1,73 m2, inversa a la esperada. La estimación del FG mediante fórmula de Cockcroft-Gault no se asoció con el riesgo cardiovascular. Palabras clave: Hipertensión. Enfermedad cardiovascular. Atención Primaria de salud. Análisis de supervivencia. Filtrado glomerular. Insuficiencia Renal. detected and controlled mainly in PC, it stands to reason that the general hypertensive population is better represented by the population cared for in PC. In 1993, a prospective study was conducted in which the prevalence of left ventricular hypertrophy was determined in a general hypertensive population that was free of cardiovascular disease and that was being treated in a primary care centre.16 The cohort of patients was monitored from that year onwards.17 The aim of this study was to analyse the association between cardiovascular morbidity/mortality and initial renal function, according to the Cockcroft-Gault and MDRD formulas, in a cohort of hypertensive individuals followed during 12 years. MATERIAL AND METHOD INTRODUCTION Arterial hypertension (AHT) is a major health problem since it is a known risk factor for developing cardiovascular disease.1 One of the organ disorders derived from AHT is chronic kidney disease (CKD), defined initially by the presence of kidney damage and in later stages, as a decrease in glomerular filtration rate (GFR). Various publications have shown that CKD is responsible for increased cardiovascular morbidity and mortality, which is proportional to kidney function deterioration.2-4 For this reason, various professional associations and organisations5-8 include reduced GFR as one of the consequences to consider in hypertensive patients. They also state that it is useful in therapeutic decision tables. Current recommendations9,10 are aimed more towards minimising the progression of kidney deterioration and treating the complications inherent in kidney failure, thus reducing the cardiovascular risk associated with CKD. CKD is a condition that can be diagnosed in primary care (PC) in its early stages by estimating GFR through various formulas (hidden CKD) since serum creatinine is not usually altered until more advanced stages. The most widely used and validated are the Cockcroft-Gault 11 and MDRD 12 formulas, which have various advantages and limitations. They are recommended depending on the stage of renal function alteration in the literature. 13-15 In addition, their association with the appearance of cardiovascular events (CVE) has been discovered. There have been few studies carried out in Spain which have analysed the evolution of long-term cardiovascular morbidity and mortality in cohorts of hypertensive patients monitored in PC, according to renal function. Since AHT is 654 The «Gòtic» prospective study of a cohort of hypertensive patients treated at a health centre in Barcelona (Spain) was the source of data for this study. Monitoring began in 1993 and lasted 12 years. The selection criteria of the population and the variables analysed have been previously published.17 For this study, we also excluded patients with extremes of body weight (body mass index [BMI] less than 19 kg/m2 or greater than 35 kg/m2), significant alterations in muscle mass, acute renal failure, pregnancy, severe liver disease, generalised oedema or ascites, and those who continued treatment with drugs that blocked secretion of creatinine. Finally, the study cohort included a total of 223 hypertensive individuals. GFR was calculated according to the Cockcroft-Gault (GFR=[140-age]*weight(kg)/[serum creatinine*72]*0.85, if female)11 and MDRD (GFR=186*serum creatinine—1.154*age— 0.203 *1.212, if black*0.742, if female)12 formulas and was classified according to the CKD stage.9 A CVE was considered to be the appearance of the following episodes during the follow-up period: heart failure; ischaemic cardiopathy (angina, acute myocardial infarction); stroke (permanent or temporary); peripheral vasculopathy (symptoms of intermittent claudication or confirmation through eco-Doppler); death due to cardiovascular event and sudden death. Sudden death was defined as death occurring within one hour after onset of symptoms or individuals dying without any witnesses who had no prior diagnosis of coronary heart disease or other presumably deadly diseases. The recording of the onset of CVE was done during AHT follow-up visits. All CVEs recorded were confirmed with the patients’ doctor and with the information and registration systems in hospitals and PC centres. Strategies were developed to minimise losses during the followup period and to recover information related to mortality.17 Nefrologia 2010;30(6):653-60 F.J. Tovillas-Morán et al. GFR as a predictor of CV morbidity and mortality in AHT originals The CVEs and the causes of death were evaluated by an external committee of doctors who were unaware of the patients’ situations regarding their kidney function. and 17 (6.4%) for other reasons, such as consumption of drugs that blocked creatinine secretion, presence of severe liver disease, and creatinine readings were not available. The study was approved by the Ethics Committee for Clinical Research of IDIAP Jordi Gol of the Catalan Institute of Health. Table 1 shows the characteristics of the participants at the start of monitoring, as well as the mean analytical values, the frequency of different cardiovascular risk factors (CRF), the distribution of GFR estimates according to the CockcroftGault and MDRD formulas, and the proportion of patients treated with antihypertensive drugs. Survival time was measured from the date when the subject was included in the study until the occurrence of a CVE, if such an event occurred. The censoring variable was the presence or absence of a CVE from the inclusion of subjects in the study (1993) to the end date of the follow-up (2005). Only the initial event was taken into account if there were various episodes of the same event in the same patient. Survival and incidence rates were estimated using the actuarial method. For the bivariate analysis, we used the Student’s t-test or its corresponding nonparametric test in the case of continuous variables, and the Chi-squared test in the case of categorical variables. We used the kappa index to evaluate the correlation between the estimates of GFR carried out with both formulas. 18 The prognostic value of GFR calculated with each of the two formulas was evaluated using Cox proportional hazards regression models, as well as the prognostic value of the adjustment factors. One model was evaluated for each of the formulas used. The covariate adjustments for the initial models were: age (years), sex (male/female), time of AHT diagnosis (months), mean systolic and diastolic blood pressure of the last two visits, obesity at the start of the study (BMI>30 kg/m2), diabetes mellitus (DM), dyslipidaemia and tobacco consumption. The variables included in the final model were selected by combining statistical and substantive criteria.19,20 We evaluated the confounding and interaction effects between the model’s variables. Furthermore, we checked the proportional hazards assumption for the various covariates and for the group of those included in the final model, as well as the presence of multicollinearity. Quantitative data were described by estimating the mean and standard deviation when they followed a normal distribution. Otherwise, the median was used along with the interquartile range (IQR). The estimates related directly with the objective of the study are accompanied by their respective 95% confidence intervals (CI). The accepted level of statistical significance was P<.05. The data analysis was performed using the SPSS 15.0 statistical software for Windows. The median follow-up time was 10.7 (IQR 6.5 to 12.1) years. Follow-up was complete in 191 participants (85.7%) and incomplete in a total of 32 (14.3%). The latter group differed from the first group in mean age (68.5 years versus 64.2 years) and mean time to diagnosis of hypertension (149 months versus 82 months). The distribution of the remaining study variables was similar in both groups. The cumulative survival, or the proportion of participants who remained free of cardiovascular events by the end of the study, was 64.7% (95% CI, 57.9-71.6). The mean incidence of CVE during the entire follow-up period was 3.6 (95% CI, 2.7-4.4) per 100 hypertensive individuals/year. The distribution of estimated GFR with both formulas at the start of the study is shown in Table 2. The agreement between the two estimates was moderate (kappa=0.501; 95% CI, 0.399-0.604). Most patients were classified in stages 2 and 3 by both formulas. Within stage 3, stage 3a was more frequent: 86.8% for calculations performed using the Cockcroft-Gault formula and 76.1% when using the MDRD formula. Table 3 shows the results obtained in the bivariate analysis, which compared participants according to whether or not they suffered a CVE during follow-up. Only the presence of DM was related with a higher probability of presenting at least one CVE during follow-up. Conversely, female sex and a GFR<60 ml/min/1.73 m2 according to the MDRD formula were associated with a lower risk of CVE. However, there was no association between the occurrence of CVE and a GFR as estimated by the Cockcroft-Gault formula. In the final multivariable model (Table 4), only a GFR >60 ml/min/1.73 m2 estimated using the MDRD formula and the presence of DM at the start of the study were associated with an increased cardiovascular risk. DISCUSSION RESULTS Of the 265 individuals in the cohort at baseline, GFR could only be measured in 223 (84.2%). The relationship and reasons for exclusion were: 25 (9.4%) had a BMI>35 kg/m2 Nefrologia 2010;30(6):653-60 In this study, a moderately reduced GFR was not associated with increased cardiovascular risk. Even patients with a GFR >60 ml/min/1.73 m2 according to the MDRD formula had a higher incidence of events. This was the opposite to what has 655 originals F.J. Tovillas-Morán et al. GFR as a predictor of CV morbidity and mortality in AHT Table 1. Characteristics of the study cohort at the start of follow-up (n=223) Variablesa Total (n = 223) Age 64.8 (10.1) 63.5-66.1 63.7 57.1-70.2 Sex, % female 95% CIb Time from diagnosis of hypertension; months 91.5 (96.7) 78.6-104.4 Mean systolic BP in the last two visits; mm Hg 159.2 (18.0) 156.9-161.6 Mean diastolic BP in the last two visits; mm Hg 89.6 (10.2) 88.3-91.0 Body mass index; kg/m2 28.3 (3.4) 27.9-28.8 Obesity; % 33.2 26.8-39.6 Diabetes mellitus; % 14.8 9.9-19.7 Dyslipidaemia; % 42.6 35.9-49.3 229.7 (44.6) 223.8-235.6 Total cholesterol; mg/dl HDL cholesterol; mg/dl 47.6 (12.4) 45.9-49.3 LDL cholesterol; mg/dl 158.1 (41.2) 152.0-164.2 14.3 9.5-19.2 - None 30.0 23.8-36.3 - One 40.0 33.3-46.6 - Two or more 30.0 23.8-36.3 Left ventricular hypertrophy (LV): % 64.1 57.6-70.6 1.1 (0.2) 1.09-1.15 60.1 (11.1) 58.6-61.6 51.6 44.8-58.4 61.1 (15.6) 59.0-63.1 Glomerular filtration rate according to Cockcroft-Gault formula <60 ml/min/1.73 m2; % 49.8 42.9-56.6 Antihypertension drug treatment; % 74.4 68.5-80.4 Smokers; % Additional cardiovascular risk factors;% Creatinine; mg/dl Glomerular filtration rate according to MDRD; ml/min/1.73m2 Glomerular filtration rate according to MDRD <60 ml/min/1.73 m2; % Glomerular filtration rate according to Cockcroft-Gault formula; ml/min a The quantitative variables are expressed as mean and standard deviation (SD). b Confidence Interval (CI). been described in the literature.2,21 These results are not confirmed by the measurement of GFR according to the Cockcroft-Gault formula. The MDRD formulas is useful to estimate GFR below 60 ml/min/1.73 m2 and has a tendency to underestimate higher values.14,15,22 Its author has recently explained12 the new equation CKD-EPI that has a greater precision than MDRD for GFR >60 ml/min/1.73 m2.12 The Cockcroft-Gault formula, which is also appropriate for these values of GFR, underestimates renal function less for values above 60 ml/min/1.73 m2. Other studies have produced conflicting results regarding the performance of the MDRD formula depending on the stages of kidney failure. In a study performed in Spain with a retrospective cohort of the general population between 35 and 75 years of age 656 and with stage 3 renal function (GFR between 30 and 60 ml/min/1.73 m 2), Buitrago et al 14 reported that the Cockcroft-Gault formula detected more hidden CKD in men, with higher cardiovascular risk and greater age. MDRD, on the other hand, did this more in women with higher obesity, higher diastolic blood pressure and higher triglyceride readings. The MDRD equation could therefore avert significantly important cases from the point of view of cardiovascular event prevention. These authors found only moderate consistency between the estimates performed with both formulas, which was similar to the results in our study. As with our study, they also did not find any evidence of a relationship between hidden CKD detected by one or the other formula and cardiovascular morbidity and mortality. 23 Similar observations have been reported in other studies with larger sample sizes, such as the NHANES-I 24 study in the general U.S. population. Nefrologia 2010;30(6):653-60 F.J. Tovillas-Morán et al. GFR as a predictor of CV morbidity and mortality in AHT originals Table 2. Distribution of the study population according to glomerular filtration rates (GFR) at the start of the study, estimated using the MDRD and Cockcroft-Gault formulas GFR (ml/min/1.73 m2) according to Cockcroft-Gault GFR (ml/min/1.73 m ) according to MDRD GFR> _90 GFR 60-89 GFR 30-59 GFR 15-29 GFR<15 GFR> _90 1 1 0 0 0 2 GFR 60-89 8 74 24 0 0 106 GFR 30-59 1 27 85 1 0 114 GFR 15-29 0 0 0 1 0 1 GFR<15 0 0 0 0 0 0 Total 10 102 109 2 0 223 2 This questions the importance of GFR as a predictive tool for cardiovascular risk by itself, or at least the usefulness of considering Stage 3 as a single group.25 Indeed, various Total studies found that the risk increased significantly with GFR below 45 ml/min/1.73 m2 (stage 3b), with the risk of patients with higher GFR (stage 3a) comparable to those with normal Table 3. Characteristics at the start of the study of patients who completed the follow-up (n=191) according to the presence or absence of cardiovascular events (CVE) during that period Variablesa (n = 191) Age Sex, % female CVE Total No Yes (n = 125) (n = 66) p 64.2 (10.3) 63.2 (11.0) 66.0 (8.8) 0.125 63.4 68.8 53.0 0.031 Time from diagnosis of hypertension; months 82.0 (85.8) 85.1 (92.9) 76.7 (68.3) 0.968 Mean systolic BP in the last two visits; mm Hg 158.7 (18.1) 159.7 (19.1) 156.8 (15.9) 0.287 Mean diastolic BP in the last two visits; mm Hg 89.7 (9.9) 90.7 (9.2) 87.7 (10.8) 0.099 Body mass index; kg/m2 28.2 (3.3) 28.4 (3.2) 27.9 (3.6) 0,327 Obesity; % 31.9 30.4 34.8 0.531 Diabetes mellitus; % 13.6 8.8 22.7 0.008 Dyslipidaemia; % 42.4 45,6 36,4 0.219 229.5 (45.7) 227.6 (45.5) 233.1 (46.3) 0.427 0.070 Total cholesterol; mg/dl HDL cholesterol; mg/dl 48.2 (12.8) 49.3 (13.4) 45.9 (9.7) LDL cholesterol; mg/dl 158.3 (42.7) 158.1 (44.7) 158.9 (38.8) 0.911 Smokers; % 14.1 12.8 16.7 0.466 Additional cardiovascular risk factors; % 69.1 68.0 71.2 0.648 0.785 - None 30.9 32.0 28.8 - 40.3 40.8 39.4 - Two or more 28.8 27.2 31.8 Left ventricular hypertrophy (LV): % 62,3 60.8 65.2 One Creatinine; mg/dl 0.555 1.1 (0.2) 1.1 (0.2) 1.1 (0.2) 0.264 60.5 (10.6) 59.1 (11.0) 63.3 (9.3) 0.009 49.7 57.6 34.8 0.003 61.6 (15.0) 61.6 (16.2) 61.6 (12.4) 0.992 Cockcroft-Gault formula <60 ml/min/1.73 m2; % 48.7 52.0 42.4 0.208 Antihypertensive drug treatment; % 72.8 72.0 74.2 0.741 Glomerular filtration rate according to MDRD; ml/min/1.73 m2 Glomerular filtration rate according to MDRD <60ml/min/1.73 m2; % Glomerular filtration rate according to Cockcroft-Gault formula; ml/min/1.73 m2 Glomerular filtration rate according to a The quantitative variables are expressed as mean and standard deviation (SD). Nefrologia 2010;30(6):653-60 657 F.J. Tovillas-Morán et al. GFR as a predictor of CV morbidity and mortality in AHT originals Table 4. Final Cox regression model to determine the predictive power of cardiovascular events on the study variables at 12 years follow-up of the cohort of hypertensive individuals (n=223) Variables Age >65 years Comparison Beta coefficient Standard error Hazard Ratio (95% CI) P versus <65 years 0.492 0.260 1.6 (0.98-2.72) 0.058 versus GFR <60 ml/min/1.73 m2 0.832 0.281 2.3 (1.33-4.00) 0.003 Versus male -0.450 0.264 0.6 (0.38-1.07) 0.637 Yes versus no 0.637 0.301 1.9 (1.05-3.41) 0.035 GFR according to MDRD >60 ml/min/1.73 m2 Female Diabetes renal function.2,26 In our study, most patients in stage 3 corresponded to stage 3a, both by the Cockcroft-Gault and the MDRD formulas. The controversy between formulas persists even in more advanced stages of kidney failure (4 and 5) for which some authors advocate the superiority of the Cockcroft-Gault formula over the MDRD.27 Furthermore, ageing reduces the relationship between estimated GFR and morbidity and mortality as shown in some studies.28 Other authors29 did not report any increase in mortality in elderly patients with GFR between 45 and 59 ml/min/1.73 m2. Other factors, such as sex, may also affect the prognostic value of GFR. A lower correlation has been reported in women25,30 and it is females that make up the majority of our sample. As with other studies2-4 that did find a relationship between reduced GFR and cardiovascular morbidity and mortality, a first group corresponded to the general population. The mean age in our study was higher than those of patients in the Go et al. and Hallan et al. studies. This may contribute to a different cardiovascular risk and may change the importance of the renal function prognosis. The Keith et al. study, with a mean sample age similar to ours, found that the prognostic differences between stage 2 and 3 were less than 5%, with statistical significance in large samples but not in smaller ones such as ours. Furthermore, the Go et al. study reported that the greatest prognostic differences occurred when GFR<45 ml/min/1.73 m2 (stages 3b to 5), especially between 60 and 80 years of age, and with an GFR<30 ml/min/1.73 m2 in those over 80 years of age, which was a lot less among stages with better renal function. In studies of hypertensive patients, Ruilope et al.31 found that GFR had a predictive value, but in patients with high cardiovascular risk. Our study has various limitations. One of them lies in the greater likelihood of losses due to the long duration of the follow-up (longer than in other studies). This could increase the possibility of a selection bias that would affect internal validity. However, a number of strategies used17 meant that in the end the percentage of losses was 14.3%. 658 Another drawback was the lack of monitoring of certain parameters such as the evolution of GFR over time. This could be of great importance since it has been recently reported that the rate of decline in renal function for individuals over 65 years old is higher in relation to cardiovascular risk than baseline creatinine.32 Also, albuminuria and proteinuria were not recorded systematically at the start of the study. In 1993, their measurement was not included in the assessment protocols for hypertensive patients. Several studies have emphasised the importance of this parameter, which has a greater prognostic value than GFR estimated by MDRD. Hemmelgarn et al.26 found an increased cardiovascular risk and progression of renal deterioration in patients in stages 12 with proteinuria compared to patients in stage 3a without proteinuria. Furthermore, the PREVEND study33 showed that individuals with GFR<60 ml/min/1.73 m2 with no proteinuria did not have a higher cardiovascular risk than those with higher GFR. Combining reduced GFR with proteinuria may improve risk prediction.25,26,34 Other markers, such as cystatin C, have recently been shown to be predictors of cardiovascular risk and renal disorders in elderly patients with GFR >60 ml/min/1.73 m2 to a greater extent than GFR according to MDRD25,35 as occurs with the aforementioned CKD-EPI.15 However, there is little access to both of these methods in PC. Lastly, the expected influences of antihypertensive treatment were not evaluated during the follow-up, nor were other treatments such as hypolipidaemic or antiplatelet therapy. In conclusion, this study does not demonstrate an increased cardiovascular risk in hypertensive patients with moderately reduced GFR. The formula estimates of GFR on its own, currently recommended by clinical practice guidelines, may be less useful for predicting CVE in hypertension as they do not reflect the real risk of some important populations. It may be advisable to consider redefining the values of risk factors of renal function (stages 3 or 3b) and assess the possible benefit of incorporating gender and the value of albuminuria to improve the predictive value of estimated GFR. Nefrologia 2010;30(6):653-60 F.J. Tovillas-Morán et al. GFR as a predictor of CV morbidity and mortality in AHT Hypertensive patients in stages 2-3a are the most prevalent in PC and are therefore the ones who need the best strategies to prevent cardiovascular events. Acknowledgements This study was made possible thanks to the collaboration of the entire “Gothic” PCT of Barcelona who contributed to the optimal monitoring of the patients. Furthermore, the study was supported by the IDIAP Jordi Gol, as well as assistance (FIS Exp. PI040356) from the Carlos III Health Institute of the Ministry for Science and Innovation. The authors of this study declare that there are no conflicts of interest. REFERENCES 1. Kaplan NM. Clinical Hypertension (9. th ed.) Philadelphia: Lippincott Williams & Wilkins, 2006. 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. 3. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004;164:659-63. 4. Hallan SI, Dahl K, Oien CM, Grootendorst DC, Aasberg A, Holmen J, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ 2006;333:1047. 5. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72. 6. Brosius FC, III, Hostetter TH, Kelepouris E, Mitsnefes MM, Moe SM, Moore MA, et al. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease: a science advisory from the American Heart Association Kidney And Cardiovascular Disease Council; the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: developed in collaboration with the National Kidney Foundation. Circulation 2006;114:1083-7. 7. Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007;25:1105-87. 8. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009;27:2121-58. 9. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and Nefrologia 2010;30(6):653-60 originals stratification. Am J Kidney Dis 2002;39:S1-266. 10. Alcázar R, Egocheaga MI, Orte L, Lobos JM, González PE, Álvarez GF, et al. Documento de consenso SEN-SEMFYC sobre la enfermedad renal crónica. Nefrologia 2008;28:273-82. 11. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41. 12. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. 13. Gracia S, Montanes R, Bover J, Cases A, Deulofeu R, Martín de Francisco AL, et al. Documento de consenso: Recomendaciones sobre la utilización de ecuaciones para la estimación del filtrado glomerular en adultos. Nefrologia 2006;26:658-65. 14. Buitrago F, Calvo JI, Gómez-Jiménez C, Canon L, Robles NR, Angulo E. Comparación y concordancia de las ecuaciones de estimación de filtrado glomerular de Cockcroft-Gault y MDRD en el diagnóstico de enfermedad renal crónica oculta. Nefrologia 2008;28:301-10. 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. 16. Dalfo A, Bayo J, Gil M, Campillo M, Botey A, Vila MA, et al. Hipertrofia ventricular izquierda en una población hipertensa general de Barcelona. Med Clin (Barc) 1995;105:361-6. 17. Tovillas-Morán FJ, Zabaleta-del-Olmo E, Fo-Baque A, VilaplanaCosculluela M, Galceran JM, Coca A. Cardiovascular morbidity and mortality and left ventricular geometric patterns in hypertensive patients treated in primary care. Rev Esp Cardiol 2009;62:246-54. 18. Abraira V. El índice Kappa. SEMERGEN 2000;27:247-9. 19. Kleinbaum DG, Klein M. Survival Analysis. A Self-Learning Text (2.th ed.). New York: Springer Science Business Media, 2005. 20. Katz MH. A multivariable Analysis. A Practical Guide for Clinicians (2.th ed.). New York: Cambridge University Press, 2006. 21. Redon J, Cea-Calvo L, Lozano JV, Fernández-Pérez C, Navarro J, Bonet A, et al. Kidney function and cardiovascular disease in the hypertensive population: the ERIC-HTA study. J Hypertens 2006;24:663-9. 22. Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 2007;18:2749-57. 23. Calvo Hueros JI, Gómez JC, Canon BL, Martín Hidalgo-Barquero MV, Robles Pérez de Monteoliva NR, Buitrago RF. Episodios cardiovasculares en pacientes con insuficiencia renal oculta detectada mediante fórmulas de filtrado glomerular. Aten Primaria 2008;40:623-30. 24. Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int 2002;61:1486-94. 25. Lou Arnal L, Campos B, Gracia O, López I, Turón A. Fórmulas de cálculo de la función renal: fortalezas y debilidades. Nefrologia 2009;29(Sup. Ext. 5):94-100. 26. Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010;303:423-9. 659 originals F.J. Tovillas-Morán et al. GFR as a predictor of CV morbidity and mortality in AHT 27. Teruel JL, Sabater J, Galeano C, Rivera M, Merino JL, Fernández LM, et al. La ecuación de Cockcroft-Gault es preferible a la ecuación MDRD para medir el filtrado glomerular en la insuficiencia renal crónica avanzada. Nefrologia 2007;27:313-9. 28. O’Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol 2006;17:846-53. 29. Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003;63:1121-9. 30. Derose S, Crooks P, Rutkowski M, Levin N. Age, GFR and the risk of ESRD or death. En: Renal Week Abstracts Archive. Washington: American Society Nephrology;2007:46A. 31. Ruilope LM, Zanchetti A, Julius S, McInnes GT, Segura J, Stolt P, et al. Prediction of cardiovascular outcome by estimated glomerular filtration rate and estimated creatinine clearance in the high-risk hypertension population of the VALUE trial. J Hypertens 2007;25:1473-9. 32. Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 2009;20:2625-30. 33. Brantsma AH, Bakker SJ, Hillege HL, De ZD, De Jong PE, Gansevoort RT. Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant 2008;23:3851-8. 34. Cirillo M, Lanti MP, Menotti A, Laurenzi M, Mancini M, Zanchetti A, et al. Definition of kidney dysfunction as a cardiovascular risk factor: use of urinary albumin excretion and estimated glomerular filtration rate. Arch Intern Med 2008;168:617-24. 35. Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, StehmanBreen C, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 2006;145:237-46. Sent for Review 7 Jun. 2010 | Accepted: 23 Jun. 2010 660 Nefrologia 2010;30(6):653-60 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society originals Insulin resistance in chronic kidney disease: its clinical characteristics and prognostic significance F. Caravaca, I. Cerezo, R. Macías, E. García de Vinuesa, C. Martínez del Viejo, J. Villa, R. Martínez Gallardo, F. Ferreira, R. Hernández-Gallego Nephrology Department. Infanta Cristina Hospital. Badajoz, Spain Nefrologia 2010;30(6):661-8 doi:10.3265/Nefrologia.pre2010.Aug.10491 ABSTRACT Introduction: Insulin resistance (IR) increases significantly the risk for cardiovascular disease (CV) in the general population. IR is a common metabolic disorder in patients with chronic kidney disease (CKD). However, the influence of IR on the evolution of CKD patients has scarcely been studied. Objective: This study aims to determine whether IR is associated with the progression of CKD, the development of new CV events, or all-cause mortality of non-diabetic patients with CKD stage 4 or 5 not yet on dialysis. Material and methods: The study group consisted of 365 non-diabetic patients (63 ± 16 year, 169 females) with GFR <30 ml/min. The degree of IR was estimated by the Homeostasis Model Assessment parameter (HOMA). The outcome measures were: progression of CKD (composite of initiation of dialysis or doubling of baseline serum creatinine level), new cardiovascular events, and all-cause mortality. Unadjusted and multivariable-adjusted relative risks were calculated for HOMA either as a continuous or qualitative variable (tertiles), using Cox proportional hazards models. Results: Mean HOMA value (± SD) was 4.28 ± 2.07. HOMA values correlated significantly with body mass index (beta = 0.37; p <.0001), plasma triglycerides (beta = 0.22; p <.0001), plasma albumin (beta = 0.19; p = .007), and serum phosphate (beta = 0.17; p = .031). Progression of CKD was observed in 234 patients (64%) with a median follow-up of 542 days. Patients with HOMA values in the lower tertile (<3.13) showed a slower progression of CKD than that of the rest of study patients (log rank 4.16, p <.05). In adjusted models for age, sex, baseline GFR, body mass index, and proteinuria, HOMA values in the lower tertile entered as an independent variable in the best predictive equation for progression of CKD (HR 0.72, p <.03). Fifty-one patients developed a new CV event and 103 patients died during the study period (median follow-up of 1,103 days). HOMA did not relate to the development of Correspondence: Francisco Caravaca Servicio de Nefrología. Hospital Infanta Cristina. Avda. Elvas, s/n. 06080 Badajoz. Spain. [email protected] new CV events or all-cause mortality in unadjusted or adjusted models for age, sex, comorbid index, plasma albumin, and C-reactive protein. Conclusions: In conclusion, progression of renal disease was slower in those non-diabetic CKD patients with low HOMA values; however, HOMA values did not relate to the development of new CV events or all-cause mortality. Key words: Chronic kidney disease. Mortality. Progression renal insufficiency. Insulin resistance. Cardiovascular risk. Resistencia a la insulina en la enfermedad renal crónica: características clínicas asociadas y significado pronóstico RESUMEN Introducción: La resistencia a la insulina (RI) es una alteración prevalente en los pacientes con enfermedad renal crónica (ERC). Su relación con la morbilidad cardiovascular (CV) y la mortalidad en la ERC ha sido poco estudiada. Objetivos: Los objetivos de este estudio fueron determinar la relación de la RI con la progresión de la ERC, el desarrollo de nuevos eventos CV y la mortalidad por cualquier causa en pacientes con ERC prediálisis. Material y métodos: Estudio de cohorte prospectivo observacional en el que se incluyeron 365 pacientes no diabéticos (63 ± 16 años, 169 mujeres) con un filtrado glomerular <30 ml/min. El grado de RI fue estimado mediante el parámetro «Homeostasis Model Assessment» (HOMA). Los sucesos evolutivos analizados fueron: progresión de ERC (entrada en diálisis o duplicar creatinina sérica inicial), desarrollo de nuevos procesos CV, o la mortalidad por cualquier causa. Resultados: Los pacientes con valores HOMA en el tercil inferior (<3,13) mostraron una progresión más lenta de la ERC en un modelo de regresión de Cox ajustado a edad, sexo, filtrado glomerular basal, índice de masa corporal y proteinuria, (razón de riesgo = 0,72; p = 0,03). Durante el período total de seguimiento 51 pacientes desarrollaron nuevos eventos CV y 103 fallecieron. Los valores HOMA no se relacionaron con el desarrollo de nuevos eventos CV ni con la mortalidad en modelos no ajustados o ajustados a 661 originals F. Caravaca et al. Insulin resistance in kidney disease edad, sexo, índice de comorbilidad, albúmina sérica y proteína C reactiva. Conclusiones: En conclusión, la progresión de la ERC fue más lenta en pacientes con los valores HOMA más bajos, aunque este parámetro no fue capaz de predecir el desarrollo de nuevos eventos cardiovasculares o la mortalidad. follows: patients over 18 years of age with no previous diagnosis of diabetes mellitus and baseline (fasting) blood glucose levels below 126 mg/dl, in a stable clinical condition with no acute intercurrent disease at the time of the baseline study and receiving no treatment with corticoids or other drugs with a significant “anti-insulin” action. Palabras clave: Enfermedad renal crónica. Mortalidad. Progresión insuficiencia renal. Resistencia insulina. Riesgo cardiovascular. The aetiology of renal insufficiency was: undetermined origin (154 patients), primary glomerulonephritis (76 patients), chronic interstitial nephritis (66 patients), polycystic disease (31 patients), ischaemic nephropathy (30 patients) and other etiologies (8 patients). INTRODUCTION Although none of the patients included in the study had diabetes mellitus, other comorbid diseases were common: 41 patients had a previous history of ischaemic cardiopathy, 55 of heart failure, 61 of cerebral or peripheral vascular disease, 23 of malignant disease and 40 of chronic obstructive pulmonary disease, and 19 patients had other significant comorbidities. Insulin resistance (IR), which is characterized by a functional deficit in this hormone despite high plasma levels, leads to a series of changes in the composition of plasma lipids, coagulation, endothelial function and vascular resistance, as well as endocrine changes and obesity. In combination, this increases the risk of developing high blood pressure and accelerated atherosclerosis.1-4 The increase in cardiovascular risk associated with IR has been shown in the general population.1-4 The biochemical data of a high proportion of patients with chronic kidney disease (CKD) is compatible with IR,5-8 even in the earliest stages of renal insufficiency. Although this metabolic disorder, which is associated with uraemia, was first described in the 1980s by DeFronzo and Alvestrand,9,10 its physiopathological mechanisms are not yet fully understood. CKD patients have a very high risk of developing cardiovascular (CV) diseases11,12 and the link between these processes and traditional CV risk factors is specific.13 The role that IR plays in the development of CV disease and mortality in the CKD population has not been subject of many studies.7,14-16 The objectives of this study were to determine the prevalence and clinical and biochemical characteristics associated with IR in a patient population with advanced CKD prior to dialysis, and to establish the prognostic value of IR in the progression of CKD, the development of new CV processes and all-cause mortality. MATERIAL AND METHODS Patients In the study, 365 patients (average age 63 ± 16 years, 169 females) with stage 4-5 chronic kidney disease, who were monitored by a specialist in advanced chronic kidney disease (ACKD) were included. The inclusion criteria were as 662 The drugs which were most frequently prescribed were antihypertensive agents (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, betablockers), diuretics, statins, antiplatelet drugs and phosphate binders. Clinical Data and Laboratory Analysis In addition to demographic data, systolic and diastolic blood pressure measurements, and body mass index were included. Comorbidity levels were quantified using the method developed by Davies et al.17 Because of their potential link to insulin resistance, variables included the regular consumption of drugs such as betablockers, diuretics and angiotensinconverting enzyme inhibitors (ACEI) and/or angiotensin receptor antagonists (ARA). Blood samples were taken from patients after a prolonged fasting period (at least 8 hours) in order to determine the following parameters: haemogram, glucose, urea, creatinine, uric acid, calcium, phosphate, total cholesterol, triglycerides, albumin (Advia Chemistry Multianalyzer, Siemens Healthcare Diagnostics) and venous bicarbonate (ABL 800 FLEX analyzer). The plasma concentration of highly sensitive C-reactive protein was determined by nephelometry (N High Sensitivity CRP, Behring, Marburg, Germany). The plasma concentrations of PTH were determined by IRMA (184 N-tact PTH IRMA Diasorin). Glomerular filtration was estimated by means of the 4variable MDRD formula. The protein catabolic rate (NPNA) was calculated by measuring urinary nitrogen excretion using the combined formulas of Cottini et al and Maroni et al, in accordance with the description given by Bergström et al.18 The NPNA was adjusted to the actual weight of the patient. Nefrologia 2010;30(6):661-8 F. Caravaca et al. Insulin resistance in kidney disease Plasma concentrations of insulin were determined by means of two-site, solid-phase chemiluminiscent immunometric assays (Immulite® 2000 Immunoassay System Siemens Healthcare Diagnostics). The intra-assay and total coefficients of variation were 6.1 and 7.1% respectively. To establish the level of insulin resistance we used the Homeostasis Model Assessment Insulin Resistance (HOMA)19 parameter, the utility and reliability of which have been validated in patients with chronic kidney failure.20 This parameter is calculated by means of the following formula: fasting insulin (µU/ml) x fasting glucose (mmol/l)/22.5. Study Design and Statistical Analysis This study is divided into two section, a first section consisting of a transversal analysis, in which the clinical and analytical characteristics associated with different levels of insulin resistance are described, and a second section, in which, by means of a prospective analysis, an attempt is made to establish the prognostic value of the HOMA parameter on three outcome events: a) CKD progression, defined as the initiation of dialysis or the doubling of baseline serum creatinine; b) the development of new severe acute cardiovascular processes (myocardial infarction, unstable angina, the need for coronary intervention, transistory or established cerebrovascular accidents or severe ischaemia of the lower limbs) and c) all-cause mortality. After an initial assessment, patients were monitored by means of regular visits every 1-3 months, while they continued to be seen by a specialist due to ACKD, or by the reporting of any change in the progression of the disease after the initiation of dialysis. Patients were ruled out for study purposes in cases of all-cause mortality, renal transplant, follow-up losses (16 patients) or completion of the study period (1 November 2008). Censoring date considered for patients who were lost was their last consultation. Median follow-up until the initiation of dialysis was 542 days (interquartile ranges: 221–922 days) and median follow-up until death or censoring date was 1,103 days (interquartile ranges: 643–1,707 days). The comparison of continuous variables between groups was performed using analysis of variance (ANOVA) or the Kruskal-Wallis test, depending on the distribution characteristics of the variables. The Scheffe test was used for post hoc comparisons. For the comparison of two independent continuous variables the Student’s t test for unpaired samples was used or the non-parametric MannWhitney test, depending on the distribution characteristics of the variables. The Chi-square test was employed to compare discrete variables. Nefrologia 2010;30(6):661-8 originals To analyze the variables that showed the best links with the HOMA parameter (continuous variable), multivariate linear regression models were used, covariables being automatically selected by the (backward) conditional elimination process. To establish whether there was an independent link between the HOMA parameter and study outcomes, multivariate Cox proportional hazard models were used and the relative risks and 95% confidence intervals were determined. The HOMA parameter was analyzed both as a continuous and a discrete entity (terciles). The models were adjusted by introducing variables or risk factors with a potential influence on the final events which were the subject of the study (age, sex, comorbidity index, plasma albumin, C-reactive protein, residual renal function, proteinuria, etc.). The selection of the variables which best fitted the models was done automatically by the progressive conditional elimination process. To confirm risk proportionality, in all the survival studies we examined the graphs which were obtained by correlating the logarithm (-survival rate logarithm) with the survival time logarithm, as well as the graphs correlating the partial residues of each covariable against survival time. The percentage of missing data was below 1% for all the variables. The quantitative variables which were lost were made up by adding the arithmetic mean value for the rest of the present data. The data for this study are presented as the mean plus standard deviation (± SD) or as the median and interquartile ranges or minimum-maximum value. A p value of <.05 was regarded as statistically significant. The SPSS software version 15.0 (SPSS, Chicago, USA) was used for the statistical analysis and graphs. RESULTS Clinical Characteristics Associated with Insulin Resistance The average insulin and HOMA parameter values were: 17.31 ± 7.54 mU/ml and 4.28 ± 2.07 mU/ml x mmol/l respectively. The clinical and biochemical characteristics of the patients grouped into terciles according to the distribution frequency of the HOMA parameter are shown in Table 1. Significant differences were not observed for age, sex, comorbidity index, percentage of patients with ischaemic cardiopathy and proteinuria. In patients in the upper tercile, glomerular filtration was significantly lower than in patients in the lower 663 F. Caravaca et al. Insulin resistance in kidney disease originals Table 1. Clinical and biochemical characteristics of patients grouped into HOMA parameter terciles Lower Tercile Middle Tercile Upper Tercile 60 ± 19 66 ± 14 62 ± 15 72/50 61/61 63/58 - Absent 74 61 74 - Mild-Moderate 37 52 40 - Severe 11 9 7 History of ischaemic cardiopathy (% of patients) 12 12 11 History of other vascular processes (% of patients) 18 17 15 Body mass index , kg/m2 26.3 ± 4.7 28.1 ± 4.9a 30.2 ± 5.6b SBP, mmHg 147 ± 25 153 ± 23 149 ± 25 Age (years) Sex (M/F) Comorbidity DBP, mmHg 86 ± 13 85 ± 11 87 ± 13 Glomerular filtration (ml/min/1,73 m2 15.58 ± 5.89 14,64 ± 4,71 13.12 ± 4.08c Proteinuria (mg/24 h) 2,000 ± 2,458 1.819 ± 2.223 1,723 ± 1,732 7.4 ± 1.9 7.7 ± 1.9 7.6 ± 2.3 Plasma albumin (g/dl) 3.84 ± 0.54 3.81 ± 0.50 3.98 ± 0.40e Total plasma cholesterol (mg/dl) 193 ± 56 203 ± 45 194 ± 49 Plasma triglycerides (mg/dl) 115 ± 51 126 ± 61 171 ± 111b Total serum calcium (mg/dl) 9.19 ± 0.81 9.25 ± 0.84 9.42 ± 0.90 Serum phosphate (mg/dl) 4.65 ± 0.98 4.62 ± 0.97 5.00 ± 1.12d Serum bicarbonate (mmol/l) 21.2 ± 3.4 21.9 ± 4.1 20.6 ± 3.7e Serum uric acid (mg/dl) Protein catabolic rate (g/kg/24 h) 1.03 ± 0.32 1.08 ± 0.28 1.07 ± 0.27 C-reactive protein (mg/l) 8.86 ± 13.99 9.58 ± 17.82 9.14 ± 14.54 PTH (pg/ml) 217 ± 185 244 ± 164 301 ± 274c Ferritin (ng/ml) 216 ± 248 115 ± 129 148 ± 175 2,26 3.92 6,67 (0.73-3.13) (3.14-4.90) (4.93-11.88) ACEI/ARA (% patients) 66 69 67 Betablocker (% patients) 19 17 16 Diuretics (% patients) 48 57 52 HOMA (mU/ml x mmol/l) (min.-max.) a p <.05 middle tercile compared to lower tercile; bp <.0001 upper tercile compared to other terciles; cp <.01 upper tercile compared to lower tercile; a p <.05 upper tercile compared to other terciles; ep <.05 upper tercile compared to middle tercile. d tercile. Patients in the upper HOMA terciles had a higher body mass index than patients in the lower tercile. Although differences were not observed in total plasma cholesterol concentrations, upper HOMA tercile patients showed significantly higher triglyceride levels than those in the other terciles. Serum phosphate levels were also higher in upper tercile patients than in the remaining terciles. In the multiple linear regression models, the variables included in the best predictive equation for HOMA values were: body mass index, triglycerides, plasma albumin and serum phosphate levels (table 2). Other significant differences between terciles were detected for plasma albumin, bicarbonate and PTH (table 1). During the follow-up period 234 patients (64%) met the criteria for CKD progression (6 patients doubled their initial serum creatinine levels and 228 started dialysis). Using Kaplan-Meier survival analysis (Figure 1), it was noted that only patients in the lower HOMA tercile showed more prolonged survival without meeting CKD progression criteria C-reactive protein levels were similar and there were no significant differences in the drugs prescribed in the three subgroups (table 1). 664 Insulin Resistance and CKD Progression Nefrologia 2010;30(6):661-8 F. Caravaca et al. Insulin resistance in kidney disease originals Table 2. Variables associated with the HOMA parameter according to multiple linear regression models Variable B Coefficient 95% CI B Coefficient Beta p Body mass index (kg/m ) 0.138 0.102 ; 0.174 0.357 <0.0001 Triglycerides (mg/dl) 0.005 0.003 ; 0.008 0.217 <0.0001 Albumin (g/dl) 0.807 0.414 ; 1.199 0.189 <0.0001 Serum phosphate (mg/dl) 0.311 0.130 ; 0.493 0.157 0.0001 Constant –4.976 –7.133 ; –2.819 2 R2 = 0.233. (lower tercile compared to the rest of the patients: log rank=4.19; p=.04). In the Cox regression analysis, adjusted for variables which are potentially related to the progression of CKD (age, sex, body mass index, systolic and diastolic blood pressure, baseline glomerular filtration rate, proteinuria, haemoglobin, albumin, phosphate, bicarbonate, diabetes and anti-angiotensin treatment, calcium antagonists and diuretics), a HOMA value in the lower tercile continued to correlate significantly with a slower progression of CKD (table 3 and figure 2). Insulin Resistance and the Development of New Cardiovascular Disease Episodes or Mortality During the follow-up period 51 patients presented a new cardiovascular episode and 104 died as a result of any cause. Using Kaplan-Meier survival curves, we failed to detect a significant correlation between HOMA terciles and the development of new CV episodes (log rank=0.117; NS) or with all-cause mortality (log rank=2.64; p=0.267). Survival without CKD progression 1.0 0.8 In Cox regression models adjusted for age, sex, body mass index, comorbidity index, plasma albumin, Creactive protein, baseline glomerular filtration rate, proteinuria, serum phosphate and antihypertensive medication, HOMA measured both as a continuous entity and in terciles showed no link either with the development of new CV processes (hazard ratio for the continuous variable=1.106; 95% CI, 0.901–1.358; p=0.337) or with all-cause mortality (HR continuous variable=1.091; 95% CI, 0.991–1.200; p=.076). The stratification of the models, based on a body mass index higher or lower than 30 kg/m2, failed to substantially modify the results, although the correlation between HOMA and mortality occurred at the limit of statistical significance (HR HOMA terciles=1.28; p=.060). DISCUSSION The results of this study show that the degree of severity of insulin resistance in ACKD is linked to obesity and plasma triglyceride, albumin and phosphate levels. Other parameters which show univariate correlation with HOMA values include glomerular filtration rate, serum bicarbonate and PTH. However, neither blood pressure values nor C-reactive protein levels or proteinuria magnitude correlate significantly with HOMA values. Although there are no standard values for normalized HOMA parameter measurement (in theory a young healthy subject should have a value equivalent to one19), the figures presented by the patients included in this study were very high (two thirds of the patients showed a value of >3), which confirms the high prevalence of this metabolic disorder in non-diabetic CKD. 0.6 0.4 HOMA lower tercile 0.2 HOMA middle tercile HOMA upper tercile 0.0 0 1000 2000 3000 Time (days) Figure 1. Kaplan-Meier survival curves without CKD progression criteria according to HOMA terciles Nefrologia 2010;30(6):661-8 4000 Obesity is very prevalent in CKD.21 IR and obesity-linked hyperinsulinaemia have been implicated in the development of kidney disease and accelerated atherosclerosis.22,23 Obesity was very prevalent in the patients included in this study and was a significant factor in determining the degree of severity of IR, suggesting a pathogenic link in the development of 665 F. Caravaca et al. Insulin resistance in kidney disease originals Table 3. Parameters included in the best predictive equation for CKD progression estimated by Cox regression models Variable Risk ratioª 95% CI Risk Ratio p Age (years) 0.98 0.97 ; 0.99 <0.0001 Sex (1 = male) 1.52 1.15 ; 2.00 <0.004 Body mass index (kg/m2) 0.97 0.94 ; 0.99 0.023 Plasma albumin (g/dl) 0.57 0.42 ; 0.79 <0.0001 Proteinuria (g/24 h) 1.09 1.03 ; 1.15 0.005 Baseline glomerular filtration (ml/min/1.73 m2) 0.87 0.84 ; 0.91 <0.0001 Serum phosphate (mg/dl) 1.32 1.14 ; 1.53 <0.0001 Lower Tercile HOMA (0.1) 0.72 0.54 ; 0.97 0.032 The following were not included in the best predictive equation: diabetes, systolic and diastolic blood pressure, haemoglobin, bicarbonate, triglycerides, anti-angiotensin drugs, calcium antagonists and diuretics. a Hazard ratio. The positive correlation between HOMA values and plasma albumin concentrations is a prominent finding in this study. Plasma albumin concentration reflects the presence and severity of different processes which have a negative impact on outcomes for CKD patients (e.g. poor nutrition, inflammation, hypervolaemia, etc.) and so this parameter is regarded as a “clinical index of disease”,25 determining mortality in most studies involving CKD patients. Survival without CKD progression 1.0 0.8 0.6 0.4 0.2 0.0 0 500 1000 1500 2000 2500 3000 Time (days) Figure 2. Survival curves in patients with HOMA in the lower tercile (discontinuous line) and the rest of study patients (continuous line). The model is estimation after adjustment for age, sex, body mass index, albumin, proteinuria and phosphate this metabolic disorder, irrespective of any association which can be attributed to uraemia. Hypertriglyceridaemia is pathogenically linked to IR.3,4 This lipid metabolism disorder is also very common in CKD.24 The results of this study show a significant link between HOMA values and plasma triglyceride levels, which suggests that IR has an important role in the development of this dislipidaemia in CKD. 666 In haemodialysis patients HOMA correlates positively with both the rate of synthesis and breakdown of muscle protein, although there is a tendency towards a negative correlation with net muscle protein balance.26 While these findings suggest that IR has a negative effect on nutritional status, other studies in pre-dialysis CKD patients show that the amount of protein intake determines hyperinsulinaemia levels and sensitivity to insulin, IR improving in patients on low protein and phosphate diets.27-29 A possible explanation for the positive correlation between HOMA and plasma albumin might be differences in protein intake and nutritional status between patients. Although in this study patients in the lower HOMA tercile showed a lower protein catabolic rate (NPNA) than upper tercile patients, the differences were not significant and therefore this hypothesis cannot be accepted. The plasma phosphate levels also show a positive correlation with HOMA values. In favour of the previous hypothesis, a diet which is inappropriate to the level of kidney failure might help to explain this finding. A reduction of phosphate in the diet has been shown to improve the degree of IR in CKD patients.28 We should also point out the potential link between insulin and the excretion of phosphate in urine. Insulin has an anti-phosphaturic effect,30,31 even antagonizing the phosphaturic action of PTH.32 In the present study estimates Nefrologia 2010;30(6):661-8 F. Caravaca et al. Insulin resistance in kidney disease of phosphate were not made and, consequently, this hypothetical link between IR and phosphate cannot be confirmed. Hyperinsulinaemia and IR predispose to the development or worsening of high blood pressure, and the development and progression of CKD, via mechanisms such as an increase in renal absorption of sodium, increased sympathetic activity, changes in endothelial and podocyte function, dislipidaemia, hyperglycaemia and increased renin-angiotensin activity.33 In some studies a link has been observed between the HOMA parameter and the rate of CKD progression in patients with glomerulonephritis,34 and between plasma insulin levels and the rate of progression of age-related renal deterioration.35 CKD progression, according to the criteria established in the present study, was slower in patients with a lower degree of IR (lower tercile). Although this finding is statistically significant, even after adjustment for other factors which determine the progression of CKD, and consistent with the potentially negative effects of hyperinsulinaemia, the clinical relevance of this finding does not appear to be very important, if we compare it with the effect of other factors (proteinuria, age, phosphate, etc.) on the progression of CKD. Although IR is regarded as a cardiovascular and mortality risk factor in the general population,1-4 the impact of this metabolic disorder in the CKD population is the subject of controversy.7,14-16 In CKD the link between IR and mortality has only been observed in Japanese patients,14,16 while in other ethnic groups it has not been possible to demonstrate this relationship.7,15 Neither do the results of this study support a link between the magnitude of the HOMA parameter and mortality or the development of new CV events in advanced CKD patients prior to dialysis, findings which once again differ from those observed in the general population. This study has limitations. The measurement of IR severity is based on a single HOMA parameter sample. The transversal design of the study to identify the determinants of the HOMA parameter limits its ability to adequately explain correlations. The absence of a link between HOMA levels and the development of new CV processes does not rule out a potential connection between IR and the severity and extent of vascular atherosclerotic damage which can be measured by more specific and sensitive procedures. The CKD progression criteria used in this study are not as reliable as the measurement and estimation of changes in glomerular filtration rate during the follow-up period. In conclusion, insulin resistance, estimated by the HOMA parameter, is prevalent in ACKD, although it does not seem to have a negative influence on the vital prognosis for these patients. Nefrologia 2010;30(6):661-8 originals REFERENCES 1. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996;97:2601-10. 2. Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities–the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996;334:374-81. 3. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453-8. 4. McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab 2001;86:713-8. 5. Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia 1995;38:565-72. 6. Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int 1998;53:1343-7. 7. Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, et al., MMKD Study Group. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol 2005;16:1091-8. 8. Lorenzo C, Nath SD, Hanley AJ, Abboud HE, Haffner SM. Relation of low glomerular filtration rate to metabolic disorders in individuals without diabetes and with normoalbuminuria. Clin J Am Soc Nephrol 2008;3:783-9. 9. DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest 1981;67:563-8. 10. Alvestrand A, Mujagic M, Wajngot A, Efendic S. Glucose intolerance in uremic patients. The relative contributions of impaired beta-cell function and insulin resistance. Clin Nephrol 1989;31:175-83. 11. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 1998;9(Supl 12):S16-S23. 12. Schiffri EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007;116:85-97. 13. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple J. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003;63:793-808. 14. Shinohara K, Shoji T, Emoto M, Tahara H, Koyama H, Ishimura E, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol 2002;13:1894-900. 15. Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, et al. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2599-606. 16. Takenaka T, Kanno Y, Ohno Y, Suzuki H. Key role of insulin resistance in vascular injury among hemodialysis patients. Metabolism 2007;56:153-9. 17. Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002;17:1085-92. 667 F. Caravaca et al. Insulin resistance in kidney disease originals 18. Bergström J, Fürst P, Alvestrand A, Lindholm B. Protein and energy intake, nitrogen balance and nitrogen losses in patients treated with continuous ambulatory peritoneal dialysis. Kidney Int 1993;44:1048-57. 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment. Diabetologia 1985;28:412-9. 20. Kanauchi M, Akai Y, Hashimoto T. Validation of simple indices to assess insulin sensitivity and pancreatic beta-cell function in patients with renal dysfunction. Nephron 2002;92:713-5. 21. Abrass CK. Overview. Obesity: what does it have to do with kidney disease? J Am Soc Nephrol 2004;15:2768-72. 22. Bagby SP. Obesity-initiated metabolic syndrome and the kidney: A recipe for chronic kidney disease? J Am Soc Nephrol 2004;15:277591. 23. Schelling JR, Sedor JR. The metabolic syndrome as a risk factor for chronic kidney disease: More than a fat chance? J Am Soc Nephrol 2004;15:2773-4. 24. Kwan BCH, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 2007;18:1246-61. 25. Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 2010;21:223-30. 26. Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int 2007;71:146-52. 27. Gin H, Aparicio M, Potaux L, Merville P, Combe C, De Precigout V, et al. Low-protein, low-phosphorus diet and tissue insulin 28. 29. 30. 31. 32. 33. 34. 35. sensitivity in insulin-dependent diabetic patients with chronic renal failure. Nephron 1991;57:411-5. Gin H, Combe C, Rigalleau V, Delafaye C, Aparicio M, Aubertin J. Effects of a low-protein, low phosphorus diet on metabolic insulin clearance in patients with chronic renal failure. Am J Clin Nutr 1994;59:663-6. Rigalleau V, Blanchetier V, Combe C, Guillot C, Deleris G, Aubertin J, et al. A low-protein diet improves insulin sensitivity of endogenous glucose production in predialytic uremic patients. Am J Clin Nutr 1997;65:1512-6. DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 1975;55:845-55. Allón M, Rodríguez M, Llach F. Insulin in the acute renal adaptation to dietary phosphate restriction in the rat. Kidney Int 1990;37:14-20. Gunputalli J, Rogers A, Bourke E. Effect of insulin on renal phosphorus handling in the rat: Interaction with PTH and nicotinamide. Am J Physiol 1985; 249:F610-F618. El-Atat FA, Stas SN, McFarlane SI, Sowers JR. The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol 2004;15:2816-27. Kaartinen K, Syrjanen J, Porsti I, Harmoinen A, Pasternack A, Huhtala H, et al. Insulin resistance and the progression of IgA glomerulonephritis. Nephrol Dial Transplant 2007;22:778-83. Oterdoom LH, De Vries AP, Gansevoort RT, De Jong PE, Gans RO, Bakker SJ. Fasting insulin modifies the relation between age and renal function. Nephrol Dial Transplant 2007;22:1587-92. Sent for Review: 7 Aug. 2010 | Accepted: 22 Aug. 2010 668 Nefrologia 2010;30(6):661-8 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society originals Post-transplant lymphoproliferative disorders in renal transplantation: two decades of experience A. Franco1, L. Jiménez1, C. Sillero1, M. Trigueros2, D. González1, E. Alcaraz2, J. Olivares1 1 2 Nephrology Department. General Hospital of Alicante. Alicante, Spain Anatomical Pathology Department. General Hospital of Alicante. Alicante, Spain Nefrologia 2010;30(6):669-75 doi:10.3265/Nefrologia.pre2010.Aug.10361 ABSTRACT Introduction: Post-transplant lymphoproliferative disease (PTLD) represents a heterogeneous group of diseases characterised by a proliferation of lymphocytes occurring after solid organ transplantation. Most cases of PTLD are B-cell and their development has been closely associated with the Epstein-Barr virus (EBV), whose proliferation is encouraged by the inhibition of the cytotoxic function of T lymphocytes due to immunosuppressive drug treatment for transplant recipients. Several risk factors have been described for the development of this disorder, such as the seronegative state of the EBV receptor, the degree of overall net immunosuppression, especially with the use of monoclonal and polyclonal antibodies, acute rejection and cytomegalovirus (CMV) disease. Material and method: We studied the incidence of PTLD and its relationship with EBV as well as its evolution and possible risk factors in 1176 adult recipients of cadaveric renal transplantation performed in our hospital between 1988 and 2009, with a follow-up of 1-255 months. The presence of EBV in the lymphoproliferative tissue was determined using in situ hybridisation. We analysed the incidence of PTLD over two time periods, 1988-1998 and 1999-2009 with 472 and 704 patients respectively. Results: A total of 28 recipients (2.38%), 22 men and 6 women with a mean age of 46.5 (15.36) years (18-70 years) with a mean post-transplant evolution of 72.9 (56.3) months (1-180 months), developed PTLD. Thirteen (46.4%) did not show any of the classic risk factors described. The presence of EBV in lymphoproliferative tissue was detected in 18 out of 26 patients studied (69.2%). In terms of histology, 25 out of 28 were type B (89.2%). Ten out of 28 patients diagnosed (35.7%) received treatment with rituximab, six died during the follow-up, five as a direct result of their illness. The incidence for the two time periods was very similar for both groups, with 0.003922 cases/year-pa- Correspondence: Antonio Franco Servicio de Nefrología. Hospital General de Alicante. Maestro Alonso, 109. 30010 Alicante. Spain. [email protected] tient in the 1988-1998 period and 0.003995 cases/yearpatient in the 1999-2009 period. Overall post-transplant survival for patients with PTLD was 73.6% at 5 years and 36.9% at 10 years, versus 87.8% and 75.9% for diseasefree recipients (P<.0001). We calculated a graft survival of 62.6% at 5 years and 27.3% at 10 years versus 72.4% and 53.9% for grafts in disease-free recipients (P<.0001). In our study, patient survival one year after presenting the disease was 30.9% and 23.2% at year two. For the graft, survival was 15.5% and 7.7%, respectively. Conclusions: We conclude that PTLD is a disorder that is generally type B; it is significantly associated with EBV. Its incidence has not changed over time and half of all PTLD cases had no identifiable risk factors, which led to a poor prognosis despite the development of new treatments. Key words: Renal transplantation. Post-transplant lymphoproliferative disorder. Epstein-Barr virus. Enfermedad linfoproliferativa postrasplante renal. Dos décadas de experiencia RESUMEN Introducción: La enfermedad linfoproliferativa postrasplante (ELP) representa un grupo heterogéneo de enfermedades que se caracterizan por una proliferación de linfocitos que se presenta después del trasplante de órganos sólidos. La mayoría de los casos de ELP son de estirpe B y su desarrollo se ha asociado estrechamente con el virus de Epstein-Barr (VEB), cuya proliferación se vería favorecida por la inhibición de la función citotóxica de los linfocitos T debido a la inmunosupresión farmacológica a la que se somete a los receptores de trasplante. Se han descrito varios factores de riesgo para el desarrollo de esta entidad, como son la seronegatividad del receptor para VEB, el grado de inmunosupresión neta global, sobre todo con el uso de anticuerpos monoclonales o policlonales, el rechazo agudo y la enfermedad por 669 A. Franco et al. PTLD in RT: Two Decades originals citomegalovirus (CMV). Material y métodos: Hemos estudiado la incidencia de ELP y su relación con el VEB, así como su evolución y los posibles factores de riesgo en su desarrollo, en 1.176 receptores adultos de trasplante renal de cadáver realizados en nuestro hospital, entre 1988 y 2009, con un seguimiento de uno a 255 meses. Se determinó la presencia de VEB en el tejido linfoproliferativo mediante hibridación. Analizamos la incidencia de ELP en dos períodos de tiempo, 1988-1998 y 1999-2009 con 472 y 704 pacientes, respectivamente. Resultados: Un total de 28 receptores (2,38%), 22 hombres y 6 mujeres, con una edad media de 46,5 ± 15,36 años (18-70 años) y con una evolución media postrasplante de 72,9 ± 56,3 meses (1-180 meses), desarrollaron ELP. Trece de ellos (46,4%) no presentaban ninguno de los factores de riesgo clásicos descritos. Se detectó la presencia de VEB en el tejido linfoproliferativo de 18 de los 26 pacientes estudiados (69,2%). Respecto a su estirpe histológica 25 de los 28 eran tipo B (89,2%). Diez de los 28 pacientes diagnosticados (35,7%) recibieron tratamiento con rituximab, seis de ellos fallecieron durante el seguimiento, cinco como consecuencia directa de su enfermedad. Calculada la densidad de incidencia en los dos períodos, ésta fue muy similar en ambos grupos, de 0,003922 casos/años-paciente en el período 1988-1998 y de 0,003995 casos/años-paciente en el período 1999-2009. La supervivencia global postrasplante del paciente que presentó ELP fue del 73,6% a los 5 años y del 36,9 % a los 10 años frente al 87,8% y al 75,9% del receptor libre de enfermedad (p <0,0001). Evidenciamos una supervivencia del injerto del 62,6% a los 5 años y del 27,3% a los 10 años frente al 72,4% y al 53,9% de los injertos de los receptores libres de enfermedad (p <0,0001). En nuestra serie, la supervivencia del paciente al año de presentar la enfermedad fue del 30,9%, y del 23,2% al segundo año, y para el injerto del 15,5% del 7,7%, respectivamente. Conclusiones: Concluimos que la ELP es una entidad en su mayoría de estirpe B, asociada de forma significativa con el VEB, cuya incidencia no ha variado en el tiempo y en la que en la mitad de los casos no se identifican factores de riesgo, condicionando muy mal pronóstico a pesar de los nuevos tratamientos desarrollados. Palabras clave: Trasplante renal. Enfermedad linfoproliferativa postrasplante. Virus de Epstein-Barr. INTRODUCTION Post-transplant lymphoproliferative disease (PTLD) represents a heterogeneous group of diseases characterised by the proliferation of lymphocytes occurring after solid organ transplantation.1 670 Most cases of PTLD cases are B-cell 2 and their development has been closely associated with the Epstein-Barr virus (EBV 1,3,4) whose proliferation is encouraged by the inhibition of the cytotoxic function of the T-lymphocytes due to the overall net immunosuppressive drug treatment that transplant recipients must undergo. 5 Several common risk factors have been described for the development of this disorder, such as the seronegative state of the EBV receptor, 6,7 the degree of overall net immunosuppression, especially with the use of monoclonal and polyclonal antibodies,6-8 acute rejection,7 and cytomegalovirus (CMV) disease. 9,10 Recently, Opelz described the mismatch at the DR locus as a risk factor for developing the disease, but could not conclude whether this factor reflects the need for further immunosuppression or an increased incidence of immunogenicity and acute rejection.11 Using two decades of experience, we have studied the incidence of PTLD and its possible variation over time, the relationship between EBV and PTLD, the prognosis of the condition, possible risk factors for its development and the influence of new strategies in its treatment. MATERIAL AND METHOD Patients This is a descriptive study of the incidence of PTLD in 1176 adult recipients who received cadaver donor kidney transplants over a period of 21 years, from July 1988 to December 2009. Post-transplant follow-up time was from one to 255 months. A total of 472 patients underwent transplantation in the 1988-1998 period and 705 in the 1999-2009 period. The initial immunosuppression regimen included cyclosporine, azathioprine and prednisone, substituting azathioprine with mycophenolate from 1998 on, when tacrolimus started to be used as anticalcineurin in many patients. High immunological risk recipients received induction therapy with OKT3 until 2000 and thymoglobulin thereafter. Acute rejection was diagnosed throught biopsy and treated initially with three IV boluses of 6-methyl-prednisolone (500mg). In case of corticoid resistance or Banff grade II or III, patients received monoclonal or polyclonal antibodies. The presence of common risk factors for developing the disease was evaluated by examining medical records. These risk factors included EBV seronegativity, use of monoclonal or polyclonal antibodies, CMV infection and treated acute rejection. Nefrologia 2010;30(6):669-75 A. Franco et al. PTLD in RT: Two Decades Method originals We calculated the incidence rate for the two periods. It is expressed as cases/years-patient. Diagnosis Histology Histological material and/or cellularity were obtained from all patients with the disease, and necropsy was requested for those recipients who died. The histological assessment was carried out according to morphological and immunohistochemical studies. The morphology study was performed, according to the classification of haematopoietic diseases by the World Health Organization,12 on sections stained with haematoxylin and eosin. Giemsa and PAS results were obtained from material fixed in neutral buffered formalin and embedded in paraffin. Immunohistochemical studies were performed on tissue fixed and embedded in paraffin using the streptavidin biotin peroxidase method, with antigen retrieval in a pressure cooker for 15 minutes. The following antibodies were used: CD 45 (pan-leukocyte), B lymphocytes markers (CD 20, CD 79, CD 45), T lymphocytes markers (CD 43, CD 3, CD 45RO=UCL1), cell proliferation marker Ki67, light chains, CD 30 (anaplastic), CD 15, and the bcl2 and p53 proteins. EBV Studies EBV serology was determined using EBV-VCA IgG and IgM before transplantation in all recipients by ELISA. The presence of EBV in the lymphoproliferative tissue was determined using in situ hybridisation with EBER PNA probes (Dako). The curves for graft and recipient survival were calculated using Kaplan-Maier and the different comparisons between them with the log-rank test. A comparison was considered significant if P<.05. The SPSS statistical software was used. RESULTS Out of the total population of 1176 patients, 28 were diagnosed with PTLD (2.38%), 22 of them were men (78.5%) and 6 women (21.5%), with a mean age of 46.5 (15.36) years (18-70 years). Post-transplant follow-up was 1255 months and the mean time between transplant and diagnosis of the disease was 72.9 (56.32) months (1-180). A total of five patients (17.8%) developed the disease during the first year after transplantation and 23 (82.2%) developed it later (Table 1). 78.6% were EBV seropositive before transplantation versus 21.4% who were seronegative (Table 1). Twenty-three of the 28 patients diagnosed with PTLD (82.1%) had received cyclosporine as anti-calcineurin and 16 out of the 28 patients received azathioprine (57.1%). Five patients (17.8%) received tacrolimus and 11 (39.2%) received mycophenolate in combination with cyclosporine or tacrolimus. Only one patient received sirolimus as part of their immunosuppression treatment. A total of four patients (14.2%) received treatment with monoclonal and polyclonal antibodies (Table 1). Thirteen out of the 28 patients (46.4%) showed no common risk factors. Five (17.8%) out of the 15 that did show some risk factor, had more than one (Table 1). Other studies Statistical analysis PTLD was diagnosed post mortem in six cases (21.4%), while the rest was diagnosed based on histological material or cellularity in vivo (Table 1). The lymphocyte strain detected was B in 25 out of the 28 patients (89.2%), (Table 2). The presence of EBV in the lymphoproliferative tissue was studied in 26 of the 28 patients (92.8%) and the virus was detected in 18 of them (69.2%) (Table 2). Descriptions of patient baseline characteristics are expressed as percentages for qualitative variables. For quantitative variables with a normal distribution, the mean was determined with standard deviation. For quantitative variables that did not follow a normal distribution, the median with its interquartile range was used. Although the majority of cases belonged to the B cell strain, only 10 of the 28 patients diagnosed (35.7%) received treatment with rituximab (anti-CD20) since its use did not start until 2003 and not all cases had the CD20 antigen. Of these, six died during the follow-up and five as a direct result of their disease. Independent qualitative variables were analysed using contingency tables with the associated chi-squared statistics. Comparisons between quantitative variables were analysed using the Student’s t-test. The incidence rate was similar in patients undergoing transplantation during the 1988-1998 period (0.003922) to that of recipients during the 1999-2009 period (0.003995). Imaging tests were performed that included thoracicabdominal CT in patients diagnosed with PTLD in life. Nefrologia 2010;30(6):669-75 671 A. Franco et al. PTLD in RT: Two Decades originals Table 1. Post-transplant time, diagnostic method, Immunosuppression and risk factors in recipients who developed the disease No. Months after transplantation Diagnosis Immunosuppression Risk factors 1 1 Necropsy Ab / CyA / Aza / Pred AR /Ab / CMV 2 24 Biopsy CyA / Aza / Pred AR 3 84 Cellularity CyA / Aza / Pred AR 4 84 Necropsy CyA / Aza / Pred 0 5 48 Biopsy CyA / Aza / Pred 0 6 60 Biopsy CyA / Aza / Pred 0 7 60 Necropsy CyA / Aza / Pred 0 8 36 Biopsy CyA / Aza / Pred Seronegative EBV 9 48 Necropsy CyA / MMF / Pred 0 10 156 Biopsy CyA / Aza / Pred Seronegative EBV 11 144 Biopsy CyA / Aza / Pred Seronegative EBV 12 30 Biopsy Ab / CyA / MMF / Pred Ab / Seronegative EBV 13 84 Biopsy CyA / Aza / Pred 0 14 108 Necropsy CyA / Aza / Pred 0 15 43 Biopsy CyA / Aza / Pred 0 16 24 Biopsy CyA / MMF / Pred AR 17 85 Biopsy CyA / MMF / Pred AR 18 81 Cellularity CyA / MMF / Pred 0 19 4 Biopsy FK / SRL / Pred AR 20 5 Cellularity FK / MMF / Pred AR / Seronegative EBV 21 168 Biopsy CyA / Aza / Pred 0 22 154 Biopsy CyA / Aza / Pred 0 23 92 Biopsy CyA / MMF / Pred 0 24 5 Biopsy FK / MMF / Pred Seronegative EBV 25 156 Necropsy CyA / MMF / Pred 0 26 4 Biopsy Ab / FK / MMF / Pred Ab / Seronegative EBV 27 180 Biopsy Ab / CyA / Aza / Pred Ab / AR 28 18 Biopsy FK / MMF / Pred AR AR: acute rejection; CMV: cytomegalovirus infection; EBV: Epstein-Barr virus; Ab, monoclonal or polyclonal antibodies; CyA: cyclosporine; MMF: mycophenolate mofetil; Aza: azathioprine; FK: tacrolimus; SRL: sirolimus; Pred: prednisone. Overall post-transplant survival for patients with PTLD was 73.6% at 5 years and 36.9% at 10 years versus 87.8% and 75.9% for disease-free recipients (P<.0001) (Figure 1). The graft had a survival rate of 62.6% at 5 years and 27.3% at 10 years versus 72.4% and 53.9% for grafts in disease-free recipients (P<.0001), (Figure 2). In our study, patient survival at one year of having the disease was 30.9%, and 23.2% at two years (Figure 3). Survival for the graft was 15.5% and 7.7%, respectively (Figure 3). DISCUSSION According to our experience, the relationship between EBV and the development of the disease is close, as evidenced by the fact that most recipients who developed PTLD in our study had EBV in the proliferating tissue (Table 2). Seven of 672 our patients were seronegative for this virus, i.e. a risk factor, and therefore, they were likely to develop a primary infection, which is the main risk factor identified in the large series.6,7,9 It is striking that in only half of the cases in our study we were able to identify a common risk factor (Table 1), a fact that could be explained by the existence of factors or combinations of factors still unknown that are involved in the disease. The influence of different immunosuppressive agents in the development of the disease has been extensively studied in the literature. In general, immunosuppression is a key factor in its appearance, as shown by the fact that patients undergoing transplantation who restart dialysis significantly lower the risk of developing the disease.6,13 Therefore, the use of monoclonal or polyclonal antibodies has been established as a first-order risk factor.6-8 In our study, only four out of the Nefrologia 2010;30(6):669-75 A. Franco et al. PTLD in RT: Two Decades originals Table 2. Histological classification according to WHO12, treatment with rituximab and presence of EBV in tissue of recipients with lymphoproliferative disease No. Strain Morphology EBV 1 B Polymorphous 1 Rituximab 2 2 B Burkitt 1 2 3 B Large cell nd 2 4 B Polymorphous 1 2 5 B Polymorphous 2 2 6 Hodgkin Hodgkin 1 2 7 B Large cell 2 2 8 B Polymorphous 1 2 9 B Polymorphous 2 2 10 B Polymorphous 2 2 11 B Large cell 2 1 12 B Large cell 2 1 13 B Large cell 1 1 14 Not B or T Anaplastic CD 30 + 1 2 15 B Polymorphous nd 2 16 B Burkitt 2 2 17 B Polymorphous 1 2 18 B Polymorphous 1 2 19 B Large cell 1 1 20 B Large cell 1 1 21 B Large cell 2 1 22 Hodgkin Hodgkin 1 1 23 B Plasmacytoma 1 2 24 B Large cell 1 1 25 B Polymorphous 1 2 26 B Polymorphous 1 1 27 B Plasmocitoma 1 2 28 B Large cell 1 1 1, Yes; 2, No; nd: not determined. EBV, Epstein-Barr virus 28 patients received this immunosuppressive agent (Table 1), indicating once again the existence of other factors involved in the development of this condition. The use of anti-CD25 antibodies in the induction does not add an increased risk of occurrence of the disease.7,8 As for other immunosuppressants, we should note the higher incidence of PTLD in kidney transplant recipients who have received FK instead of CsA.7,8 In our study, only five patients received this immunosuppressant (Table 1), which prevents us from reaching any conclusion. The antiproliferative azathioprine and mycophenolate are associated with a lower risk of developing the disease.7 It should be noted that the net overall immunosuppression used should be considered as the risk factor, although the importance of the use of monoclonal or polyclonal antibodies is significant. The identification of acute rejection as a common risk factor7,8 could be an indication of the Nefrologia 2010;30(6):669-75 importance of overall net immunosuppression in the development of the disease. Given the possible influence that the change in immunosuppression during the last decade could have on the incidence of PTLD, we analysed incidence rate for the past two decades without finding a significant difference. These results are supported by those reported by Opelz, which provide evidence that the incidence of this condition has remained stable over the three time periods studied.8 This information is not reassuring since the follow-up time in the second period is shorter and it is expected that late cases of the disease have not yet presented. There is controversy about whether there are two distinct conditions within PTLD; one with an early onset, which is closely related to EBV infection and prone to remission after reduction of immunosuppression, and one with a later onset, 673 A. Franco et al. PTLD in RT: Two Decades originals Years No PTL PTLD Figure 1. Overall Patient Survival (no PTLD Versus PTLD) which is barely related to EBV and that has a poor clinical outcome with the reduction of immunosuppression.1,2,6 The data from Opelz goes counter to this hypothesis since it states a similar prognosis for PTLD whether early or late.8 In our study, most cases were of late onset, and therefore their poor prognosis could be related to the poor outcome ascribed to late onset by authors such as Leblond.2 Furthermore, most of our patients had EBV in the proliferating tissue, a rare finding under conditions of late development.2,4 As for time of onset after transplantation, the greatest risk seems to appear during the first year.14 Smith has reported a greater incidence of PTLD in the first year after transplantation, which decreases in subsequent years, although their data are of limited value due to the censoring of the series for non-medical reasons at the three year follow-up.13 Van Leeuwen, using the ANZDATA data, reports a lower incidence during the two to five year period than during the first two years.6 Opelz also refers to a higher incidence during the first year that remains stable during the following 10 years.8 Other series, however, report similar numbers of early and late cases4 and some, like our series, flip the ratio to favour a late incidence of the disease.15 Only five of our patients (17.8%) developed the disease within the first year after transplantation but in all of these cases we were able to demonstrate the presence of EBV in the proliferating tissue, confirming the relationship between a precocious development of the condition and the presence of EBV.2 The mean post-transplant time in which the disease was diagnosed in our series was 77.8 months allowing us to label our patients as suffering from late PTLD, which was similar to the experience of Trappe et al. who reported a mean post-transplant time of 88 months.15 In a meta-analysis by Pascual, the time to onset was much later, around 117 months,16 while other series report mean times that are much earlier.7,17 674 Most PTLD is of the B2 strain, a fact confirmed in our series where 89.2% of patients developed B-cell proliferations (Table 2). Treatment for these cases, provided they are CD20 positive, is established with anti-CD20 and conversion to mTOR inhibitors, having reported good outcomes in other series.15,16 In our limited experience, six out of the 10 treated patients died, five of them as a direct result of the disease, which indicates that despite recent advances this condition continues to have a poor prognosis. It must be stressed that the strategy mentioned has only been applied to the last few patients diagnosed, which means that the prognosis for the disease could change in the future. The overall incidence of PTLD in our series is high, up to 2.38%, higher than other published series.6,7,17 The presence of risk factors in our patients does not explain this high incidence since only half of them showed some of the common risk factors described. It should be noted that 20% of our cases were diagnosed by performing autopsies (Table 1), a result of the departmental policy performing postmortem studies on all patients who lack a definite cause of death, thus increasing the number of cases diagnosed. According to our experience, the prognosis for patients with PTLD is poor, with patient survival at one year and at two years of 30.9% and 23.2%, respectively, which is significantly lower than that of patients who do not develop the disease. The development of PTLD therefore significantly determined patient and graft survival. Other authors confirm these data, although with higher survival rates 7, with 40% mortality at one year in the Opelz series.8 Comparing our current results with those published 8 years ago,18 which reported data from patients undergoing transplantation up to 2001, we do not observe significant changes in patient age at the onset of the disease, time of onset after transplantation, percentage of patients with risk Overall Graft Survival (No PTLD vs PTLD) Survival Survival Overall Patient Survival (No PTLD vs PTLD) Years No PTLD PTLD Figure 2. Overall Survival of Graft (no PTLD versus PTLD) Nefrologia 2010;30(6):669-75 A. Franco et al. PTLD in RT: Two Decades Survival Survival after diagnosis Month Patient Graft Figure 3. Patient survival after diagnosis. factors and prognosis of the disease. We must emphasise, however, that our incidence has almost doubled due to a much longer patient follow-up, with a significant incidence of late-developing disease after transplantation. This is confirmed by the data from Opelz, which show the existence of a year-after-year cumulative risk of developing the disease from the moment of transplantation.8 We conclude that PTLD, mostly of B cells, is a condition whose incidence has not changed over time. It is significantly associated with EBV, no risk factors are identified in half of all cases and it has a poor prognosis despite the new treatments developed. REFERENCES 1. Payá CV, Fung JJ, Nalesnik MA, Kieff F, Green M, Gores G, et al. Meeting on Epstein-Barr virus induce post-transplant lymphoproliferative disorder. Transplantation 1999;68:1517-25. 2. Leblond V, Davi F, Charlotte F, Dorent R, Bitker MO, Sutton L, et al. Post-transplant lymphoproliferative disorder not associated with Epstein-Barr virus: A distint entity? Clin Oncol 1998;16:2052-9. 3. Rosselet A, Vu DH, Meylan P, Baur Chauber AS, Schapira M, Pascual M, et al. Associations of Serum EBV DNA and gammopathy with Post-transplant lymphoproliferative disorder. Clin Transplant 2009;23:74-82. 4. Ghobrial, IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, et al. Differences between early and late post-transplant lymphoproliferative disorders in solid organ transplant patients: are originals they two different disesase. Transplantation 2005;79:244-7. 5. Sánchez Fructuoso A. Enfermedad linfoproliferativa postrasplante asociada a virus de Epstein-Barr. Nefrologia 2005;25:20-30. 6. Van Leeuwen MT, Grulich AE, Webster AC, Mc Credie MRE, Stewart JH, Mc Donald SP, et al. Immunosuppression and other risk factors for early and late non-Hodgkin lymphoma after kidney transplantation. Blood 2009;114:630-7. 7. Callard S, Dharnidharka V, Agodoa L, Bohend E, Abbott K. Post-transplant lymphoproliferative disorder after renal transplantation in the United States in Era of modern immunosuppression. Transplantation 2005;80:1233-43. 8. Opelz G, Döhler B. Lymphomas after solid organ transplantation: A Collaborative transplant study report. Am J Transplant 2003;4:222-30. 9. Opelz G, Daniel V, Naujokat C, Döhler B. Epidemiology of pretransplant EBV and CMV serostatus in relation to posttransplant nonHodgkin lymphoma. Transplantation 2009;88:962-7. 10. Mañez R, Breining MC, Linden P, Wilson J, Torres-Cisneros JU, Kusnei S, et al. Post-transplant lymphoproliferative disease in primary Epstein Barr Virus Infection after liver transplantation: The role of Cytomegalovirus disease. J Infect Dis 1997;176:1462-7. 11. Opelz G, Döhler B. Impact of HLA mismatching on incidence of posttransplant non-Hodkin lymphoma after kidney transplantation. Transplantation 2010;89:567-72. 12. Swerdlow SH. International agency for research 0n cancer, World Health Organization.WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer, 2008. 13. Smith JM, Rudser K, Gillend Kestenbaum, B, Seliger S, Weiss N, Mc Donald RA, et al. Risk of lymphoma after renal transplantation varies with time: An analysis of the United States Renal Data System. Transplantation 2006;81:175-80. 14. Morgans AK, Reshef R, Tsai DE. Post-transplant lymphoproliferative disorder following kidney transplant. Am Kidney Dis 2010;55:16880. 15. Trappe R, Hinrichs C, Appel U, Babel N, Reinke P, Neumayer HH, et al. Treatment of PTLD with Rituximab and CHOP reduces the risk of renal graft impairment after reduction of immunosuppression. Am J Transplant 2009;9:2331-7. 16. Pascual J. Post-transplant lymphoproliferative disorder. The potential of proliferation signal inhibitors. Nephrol Dial Transplant 2007;22:27-35. 17. Saadat A, Einollahi B, Ahmadzad-Asi MA, Moradi M, Nafar M, Pourfarziani V, et al. Post-transplant lymphoproliferative disorder in renal transplant recipients: Report of over 20 years of experience. Transplant Proc 2007;39:1071-3. 18. Franco A, Jiménez L, Aranda I, Álvarez L, González M, Rocamora N, et al. La Enfermedad linfoproliferativa difusa postrasplante renal y su relación con el virus Epstein-Barr. Experiencia de un Centro. Nefrologia 2002;22:463-9. Sent for Review: 22 July 2010 | Accepted: 22 Aug. 2010 Nefrologia 2010;30(6):669-75 675 short original http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society Scientific presentations at the meetings of the Spanish Paediatric Nephrology Association (AENP), 1988-2007 L.M. Rodríguez-Fernández, V. Recio-Pascual, M. Fernández-Fernández, M. Rosón-Varas, C. Rodríguez-Fernández, R. Morales-Sánchez, D. Mata-Zubillaga Unidad de Nefrología Pediátrica. Servicio de Pediatría. Hospital de León. León, Spain. Nefrologia 2010;30(6):676-80 doi:10.3265/Nefrologia.pre2010.Jun.10286 ABSTRACT Comunicaciones científicas en los congresos de la Asociación Española de Nefrología Pediátrica (AENP), 1988-2007 Objectives and study: To find out that characteristics of the scientific presentations given at the AENP’s meetings in the past 20 years. Material and Methods: We reviewed in the scientific programs of the AENP’s meetings of the past 20 years: number of presentations, number of participating institutions, institutions that provided the majority of the presentations, presentation format, number of studies involving experimental nephrology, topics most commonly presented. Results: There have been 1,119 presentations in the past 20 years, 45/year between 88-92 and 67/year between 03-07. Ninety-one institutions participated in the meetings, 17/year between 88-92 and 34/year between 0307. Pediatric Nephrology unit from the H. La Paz (Madrid) contributed the most presentations. Poster presentations were accepted at the ANEP meetings after 1995. Since then, 369 of the 815 presentations followed this format. Between 88-07 only 16 presentations dealt with experimental nephrology. The most common topics of presentation waere glomerular disease (203) and urinary tract infection/VUR (132). Fifty-one presentations dealt with dialysis (almost 2/3 peritoneal). Transplantation was the topic of 123 presentations. Of the 21 presentations on molecular genetics only one happened before 1998. Conclusions: The poster is a useful alternative in scientific presentations which has allowed an increase in presentations, authors and institutions participating in the ANEP meetings. The main topic of presentation was glomerular disease. The frequency of presentations dealing with transplantation has increased in the last years. The past decade has seen more presentations on molecular genetics, but presentations dealing with experimental nephrology are still infrequent. Objetivos: Revisar las comunicaciones científicas presentadas en los congresos de la Asociación Española de Nefrología Pediátrica (AENP). Material y métodos: En los programas científicos (1988-2007) de los congresos de la AENP se revisaron: número de presentaciones, centros participantes y con el mayor número de comunicaciones, forma de presentación, estudios experimentales y temas elegidos. Resultados: En los últimos 20 años, 91 centros presentaron 1.119 comunicaciones. El Hospital La Paz (Madrid) fue el que más comunicaciones presentó. Desde el año 1995 comenzaron a admitirse comunicaciones tipo póster y 369 de las 815 comunicaciones presentadas tuvieron ese formato. Dieciséis comunicaciones informaron de investigación animal. El tema más frecuente fue la enfermedad glomerular (203). Se presentaron 51 comunicaciones sobre diálisis. Trataron sobre trasplante renal 123 comunicaciones. Sólo una comunicación sobre genética fue presentada antes de 1998. Conclusiones: El formato póster es un método útil para las presentaciones científicas. El tema más habitual fue la enfermedad glomerular. En la última década han aparecido comunicaciones sobre genética, pero sobre experimentación animal son todavía excepcionales. Key words: Pediatric nephrology. Meetings. Scientific presentations. Palabras clave: Nefrología Comunicaciones científicas. Correspondence: Marta Fernández Fernández Unidad de Nefrología Pediátrica. Servicio de Pediatría. Hospital de León. Spain. [email protected] 676 RESUMEN pediátrica. Congresos. INTRODUCTION The Spanish Paediatric Nephrology Association (AENP) is the official scientific body that encompasses Spanish L.M. Rodríguez-Fernández et al. Presentations at AENP meetings short original practitioners (paediatric nephrologists) dedicated to the practice of this specialty and one of its statutory objectives is to promote the development of paediatric nephrology.1 It was founded in 1973 under the name Sección de Nefrología de la Asociación Española de Pediatría (Nephrology Department of the Spanish Paediatric Association) and in 1995 went on to receive its current name.2 8. Presentations on animal experimentation. 9. Topics chosen for presentations and their frequency. 10. Presentations on dialysis and kidney transplantation, molecular genetics and glomerular disease. A year after its founding, it had its first scientific meeting in Madrid followed by annual meetings that in 1999 went on to be called Congresos Nacionales de Nefrología Pediátrica (National Meetings of Paediatric Nephrology).2 Number of presentations delivered In its 37 years, the AENP has held 35 Meetings or National Congresses and, since 1992, four joint meetings with the Sociedad Portuguesa de Nefrología Pediátrica (Portuguese Society of Paediatric Nephrology). Each meeting includes round tables and conferences led by invited professors, but the research activity of paediatric nephrologists is demonstrated through scientific presentations, the fundamental basis of AENP meetings. Coinciding with the 20th anniversary of the Paediatric Nephrology Unit (PNU) of the Hospital of León, we reviewed the presentations delivered at the meetings during that period of time in order to get to know the characteristics of the research activities of AENP members. MATERIAL AND METHODS This is a retrospective, descriptive study of presentations delivered by Spanish paediatric nephrologists at national conferences of paediatric nephrology held between 1988 and 2007. The information was obtained by reviewing the programmes published in the books of the conferences and meeting abstracts published in Anales de Pediatría (Journal of Paediatrics). RESULTS There were 1119 presentations delivered in the 20 years reviewed with a significant increase in presentations per meeting in recent years (Figure 1). If the data are analysed in 5-year periods, the average number of presentations increased from 45 presentations per year between 1988-1992 to 67 between 2003-2007. Hospitals where authors come from During the time period analysed, 91 hospitals made scientific contributions. Figure 2 shows the annual change in the number of hospitals. The average number of participating hospitals was 17 per year in the period 1988-1992, increasing to 34 per year between 2003-2007. The percentage of presentations from hospitals that perform transplantation went from 50.8% between 1988 and 1992 to 39.4% between 2003 and 2007. The hospitals that delivered the most scientific presentations in the last 20 years were Hospital Materno-Infantil La Paz, Hospital de la Vall d´Hebron and Hospital Central de Asturias (HUCA). Only three centres delivered presentations in all the meetings held during these 20 years (La Paz, HUCA and Virgen del Rocío). Foreign centres provided approximately 8% of the presentations, with Portugal contributing the most (37). The following data was collected and assessed in the scientific programmes: 1. Number of presentations delivered each year and the change in number of presentations, in 5 year periods. 2. Hospitals from where the authors originate and the change in number of participating hospitals in each meeting, in 5-year periods. 3. Hospitals that have made the greatest number of scientific contributions. 4. Hospitals that have participated in all meetings by sending presentations. 5. Change in percentage of presentations from hospitals that perform transplantations, in 5-year periods. 6. Presentations from outside Spain. 7. Number of presentations delivered orally and in poster form. Nefrologia 2010;30(6):676-80 Number of presentations Four meetings were held (1992, 1999, 2000, 2005) with Portuguese paediatric nephrologists and one (2002) within the European Congress of Paediatric Nephrology. Year Figure 1. Number of presentations per year delivered at AENP meetings between 1988 and 2007 677 L.M. Rodríguez-Fernández et al. Presentations at AENP meetings short original Number of presentations on animal experimentation. Number of hospitals In these 20 years, only 16 of the 1119 presentations (1.5%) reported on animal research, of which 15 came from HUCAUniversity of Oviedo. Topics chosen for presentation Every year presentations were delivered on glomerular pathology, tubulo-interstitial nephritis and kidney transplantation. Year Figure 2. Number of centres per year that participated in AENP meetings between 1988 and 2007 Number of presentations made orally and in poster form In 1995, presentations began to be delivered in poster format. Since then, 369 of the 815 presentations delivered (45.3%) were in this format. The most frequently chosen topic was glomerular disease, followed by urinary tract infection/vesicoureteral reflux and tubulo-interstitial pathology (Figure 3). 51 presentations were delivered on dialysis (32 peritoneal/19 haemodialysis). Kidney transplantation was the topic of 123 presentations and almost three quarters of these (90) were delivered between 1998 and 2007. There were 21 presentations on molecular genetics and/or molecular biology (1.8%) and only one of these was delivered before 1998. Urinary Infection/Viscouretal Reflux/PNC 250 Hydronephrosis/obstructive uropathy Enureses 204 Lithiasis/metabolopathies Development diseases 200 Number of presentations Genetics/molecular biology Cystic diseases High blood pressure 150 132 Systemic diseases 120 123 Glomerular disease Tubulo-interstitial disease Acute respiratory infection 100 Chronic respiratory infections – General presentations Chronic respiratory infections – Growth Chronic respiratory infections – kidney osteodistrophy Haemodialysis 50 Peritoneal dialysis Kidney transplant Imaging studies Surgical techniques 0 Subject of the presentation Miscellaneous Figure 3. Most commonly chosen subjects for presentations delivered at AENP meetings between 1988 and 2007 678 Nefrologia 2010;30(6):676-80 L.M. Rodríguez-Fernández et al. Presentations at AENP meetings Some 204 presentations were delivered on glomerular disease (18.2% of the total). Twenty-three of these came from Portuguese centres. A total of 33 Spanish hospitals delivered presentations on this topic. Hospital Sant Joan de Déu and Hospital La Fe delivered the most presentations. Nephrotic syndrome was the most frequently presented glomerular disease (29 out of 69 were on its treatment). Systemic glomerular disease was the subject of 49 presentations (17 on Schönlein-Henoch nephropathy) and renal biopsy was the subject of 15. DISCUSSION Each year, paediatric nephrologists from selected Spanish hospitals take charge of the organisation of AENP meetings thus meeting the objectives of the association as stated in its statutes.1 These scientific meetings include keynote speeches given by Spanish and foreign specialists, round tables on current topics, debates, cases studies and expert Q&A.2 However, it is the scientific presentations from PNU of Spanish hospitals that give these meetings their meaning and allows for new specialists and hospitals to be incorporated into the activities of the AENP, helping the next generation of this paediatric subspecialty.3,4 From this point of view, our review provides information on the dynamic and scientific tone of the AENP and offers an idea of future expectations in this area of specific knowledge. We chose to study this 20-year period because we felt it was a sufficiently long period of time and to make it easier to access the books on the presentations, keeping in mind that publication of abstracts in scientific journals was irregular over time.2 During these 20 years, we witnessed a progressive and striking increase in the number of presentations made in each meeting that paralleled the increase in participating hospitals. A total of 91 hospitals participated in the AENP meetings. The number of hospitals participating in each meeting doubled from the first five years (17 centres per year) to the last five years analysed (34 centres per year). This increase is probably related to the creation of new PNUs as recommended in the National Plan of Paediatric Nephrology. 5,6 There are three types of units depending on the utilisation of health care and human resources, demographics and geopolitical criteria:5,6 1. Level I or basic units: Essentially preventive and care based. 2. Level II: Equipped to maintain a substitutive treatment for chronic kidney failure: haemofiltration, peritoneal dialysis and/or haemodialysis. Nefrologia 2010;30(6):676-80 short original 3. Level III: Basic infrastructure and equipment for maintaining a paediatric dialysis and kidney transplantation programme. In these 20 years, the number of level II and III PNUs has not changed and only one new hospital has been authorised to perform kidney transplantation in children: Sant Joan de Déu of Barcelona. However, thanks to the efforts of former resident physicians of the major PNUs, many level I PNUs have been added to the healthcare network, as could be expected from the historical review carried out almost a decade ago by Dr. García-Nieto.2 The members of these new units are probably responsible for the increase in the number of presentations and participating hospitals in AENP meetings. This explains why half of all presentations came from transplant hospitals between 1988 and 1992, while only 39.4% of them came from transplant hospitals in the period 2003-2007. Logically, the largest number of presentations delivered came from the two level III PNUs with a greater number of patients: University Hospital La Paz of Madrid and Hospital Vall d´Hebron of Barcelona. However, it is surprising that the level II PNU of HUCA ranks third among Spanish centres in the number of presentations, ahead of the other centres that perform transplants. It is one of the only three PNUs that delivered presentations at all the AENP meetings held in the 20 years reviewed, along with Hospital La Paz of Madrid and Hospital Virgen del Rocío of Seville. Presentations delivered from outside Spain represented only 1.5% of the total and, in general, came from Portuguese hospitals in the context of Reuniones Ibéricas de Nefrología Pediátrica (Iberian Meetings of Paediatric Nephrology).2 The remaining international presentations came from nine other countries and their number is practically symbolic. In 1995, presentations in poster form began to be accepted at AENP meetings.2 Since then, this type of presentation represents almost half of the total and its introduction has probably contributed to the increase in the number of presentations observed in recent years. Basic research using animal testing is not common in Spanish hospitals and it is poorly represented in paediatric meetings and in particular in paediatric nephrology. Clinical research significantly is the dominant topic while animal experimentation almost always depends on universities and thus reaches only 1.5% of the total, almost all coming from the University of Oviedo-HUCA. During childhood, glomerular disease is less frequent than that associated with infectious, hereditary, congenital and/or malformation symptoms, and causes little more than 20% of terminal kidney failure in childhood.7 Nonetheless, it was the most frequently chosen topic for presentation (almost 20% of 679 short original the total), followed by urinary tract infection/vesicoureteral reflux and tubulo-interstitial pathology. Of all primary glomerular diseases, nephrotic syndrome is the most common topic of presentation, probably because it deals with the most frequent primary glomerular symptom in childhood.8 As expected, there are more presentations on Schönlein-Henoch nephropathy than those of systemic glomerular diseases. However, only one third of PNUs delivered presentations on glomerular diseases, possibly because the small hospitals barely have any cases with these characteristics since these diseases are so infrequent. Hospital Sant Joan de Déu of Barcelona delivered the most presentations on this topic. In addition to glomerular disease, every year there were presentations on tubulo-interstitial nephropathy and kidney transplantation. Kidney transplantation is a frequent topic at the meetings we reviewed despite the fact that Spain only has seven level III PNUs and until recently only had six.2 Presentations on dialysis are less common and those referring to peritoneal dialysis are the most common. This is not surprising since in recent years peritoneal dialysis has been chosen as the first substitute treatment for kidney function for twice as many children as haemodialysis.7 Virtually all the studies performed on genetics and molecular biology occurred in the last decade and it is likely that this will be an increasingly frequent topic for presentation. Mutations responsible for various tubular disorders and cystic kidney diseases have been reported recently and several Spanish PNUs have been involved in this process, a process followed by paediatric nephrology on a global level.9-11 We conclude by recalling that during the past 20 years the number of participating hospitals has increased as well as the number of presentations delivered at the AENP meetings. Glomerular disease was the most common topic. Presentations on kidney transplantation have increased in frequency in recent years and in the last decade presentations began to be given on molecular genetics. However, presentations on animal experimentation remain rare. As noted by Dr. Rodríguez Soriano a few years ago, the phenomenon of subspecialisation in paediatrics is irreversible and is already setting new challenges in healthcare, teaching and L.M. Rodríguez-Fernández et al. Presentations at AENP meetings research.12 Scientific meetings with the delivery of presentations are proof of this research activity and a basis on which to support training of specialists, providing crucial contributions to the development of specific areas within Paediatrics. REFERENCES 1. Estatutos de la Asociación Española de Nefrología Pediátrica. Año 2007. Registro Nacional de Asociaciones del Ministerio del Interior. http://servicio.mir.es/webasocia 2. García Nieto V, Málaga S. Historia de la Asociación Española de Nefrología Pediátrica. En: Málaga Guerrero S, Pintos Morell G, Alonso Melgar A, Hernández Marco R, García Nieto VM (eds.). 25 años de la Asociación Española de Nefrología Pediátrica (1973-1998). Gijón: 1998;37-87. 3. Sánchez Moreno A. Futuro y devenir de la Nefrología Pediátrica en un centro de tercer nivel. Perspectivas en los próximos 10 años. Libro de Actas del XXXIII Congreso Español de Nefrología Pediátrica. Calatayud, 2007;82-85. 4. Rodríguez LM, Fernández M. Evolución de la nefrología pediátrica. Bol Pediatr 2007;47:362-6. 5. Sección de Nefrología de la Asociación Española de Pediatría. Plan Nacional de Nefrología Pediátrica. An Esp Pediatr 1984;20:720-39. 6. Hernández R, Fons J, Núñez F, Marín J. Propuesta de actualización del Plan Nacional de Nefrología Pediátrica. En: Málaga Guerrero S, Pintos Morell G, Alonso Melgar A, Hernández Marco R, García Nieto VM (eds.). 25 años de la Asociación Española de Nefrología Pediátrica (1973-1998). Gijón: 1998;101-137. 7. Zamora I, Vallo A. Registro español pediátrico de insuficiencia renal terminal, 1998. Nefrología 2000;20(Supl. 5):32-9. 8. Málaga S, Sánchez Jacob M, Santos F, García Fuentes M, Gómez S, Matesanz JL, et al. Síndrome nefrótico de la infancia: Características clínicas, terapéuticas y evolutivas de 100 casos. An Esp Pediatr 1991;34:220-4. 9. Coto E, Rodríguez J, Jeck N, Álvarez V, Stone R, Loris C, et al. A new mutation (intron 9 1 G > T) in the SLC12A3 gene is linked to Gitelman syndrome in Gypsies. Kidney Int 2004;65:22-6. 10. Claverie-Martín F, Flores C, Antón-Gamero M, González-Acosta H, García-Nieto V. The Alu insertion in the CLCN5 gene of a patient with Dent’s disease leads to exon 11 skipping. J Hum Genet 2005;50:370-4. 11. Ariceta G, Vila M, Arrojo L, Otero M, Pazos G, Alonso R, et al. Genetic diagnosis of Autosonal Dominant Polycystic Kidney Disease in children at risk. Pediatr Nephrol 1999;13:C33. 12. Rodríguez Soriano J. Nacimiento y desarrollo de la nefrología pediátrica. Una historia vivida. Bol Pediatr 2002;42:313-6. Sent for Review: 31 May 2010. Accepted: 4 Jun. 2010 680 Nefrologia 2010;30(6):676-80 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society case report MALT B cell lymphoma with kidney damage and monoclonal gammopathy: A case study and literature review R. Peces1, C. Vega-Cabrera1, C. Peces2, A. Pobes3, M.F. Fresno4 Servicio de Nefrología. Hospital Universitario La Paz. Madrid, Spain Área de Tecnología de la Información. SESCAM. Toledo, Spain 3 Sección de Nefrología. Hospital de Cabueñes. Gijón. Asturias, Spain 4 Servicio de Anatomía Patológica. Hospital Universitario Central de Asturias. Oviedo. Asturias, Spain 1 2 Nefrologia 2010;30(6):681-6 doi:10.3265/Nefrologia.pre2010.Jun.10428 ABSTRACT We report a case of low-grade B-cell lymphoma of mucosaassociated lymphoid tissue (MALT) involving the left kidney and simultaneous onset of a monoclonal gammopathy IgM kappa. No predisposing local inflammatory condition was identified. Following left nephrectomy, the renal specimen showed the centrocyte like cells and lymphoid cells in the lymphoepithelial lesions were positive for CD20 and CD79 alfa. The neoplastic cells expressed monotypic cytoplasmic IgM kappa. The demonstration of bone marrow cells of Blineage expressing the same monoclonal protein as the tumor suggested bone marrow involvement, even in the absence of identical morphology. Despite chemotherapy and rituximab treatment, clinical follow-up showed right kidney extension with high-grade transformation, and finally systemic dissemination. This case illustrates that the kidney is among the sites that may be involved by MALT B-cell lymphomas in a primary or secondary fashion, and the need for expanded investigation of the possible dissemination. We review the literature on this unusual extranodal lymphoma. Key words: B-cell lymphoma; IgM kappa; Kidney; Monoclonal gammopathy; Mucosa-associated lymphoid tissue (MALT). Linfoma de células B tipo MALT con afectación renal y gammapatía monoclonal: presentación de un caso y revisión de la literatura RESUMEN Se presenta un caso de linfoma de células B de bajo grado del tejido linfoide asociado a mucosas (MALT), afectando al riñón izquierdo, y comienzo simultáneo de una gammapatía monoclonal IgM kappa. En este paciente no pudo identificarse ningún proceso inflamatorio predisponente local. Tras la nefrectomía izquierda, el espécimen renal mostró células centrocito-like y células linfoides en las lesiones linfoepiteliales que fueron positivas para CD20 y CD79 alfa. Las células neoplásicas expresaron IgM kappa monotípica citoplásmica. La demostración de células de estirpe B de la médula ósea expresando la misma proteína monoclonal que el tumor sugirió la afectación de la médula ósea incluso en ausencia de idéntica morfología. A pesar del tratamiento con quimioterapia y rituximab, el seguimiento clínico demostró extensión al riñón derecho, con transformación a linfoma de alto grado y, finalmente, diseminación sistémica. Este caso ilustra que el riñón se encuentra entre las localizaciones que pueden verse afectadas por los linfomas de células B de tipo MALT, de forma primaria o secundaria, y explica la necesidad de extender la investigación para detectar su posible diseminación. Se revisó la literatura sobre este infrecuente linfoma extranodal. Palabras clave: Linfoma de células B; IgM kappa; Riñón; Gammapatía monoclonal; Tejido linfoide asociado a mucosa (MALT). INTRODUCTION Correspondence: Ramón Peces Servicio de Nefrología. Hospital Universitario La Paz. Madrid, Spain. [email protected] Isaacson and Wright1 initially defined malignant lymphoma of the mucosa-associated lymphoid tissue (MALT) in the gastrointestinal tract, and subsequently in the thyroid, lung, and salivary gland, as a neoplastic proliferation of centrocyte681 case report like cells, with or without lymphoplasmacytic cells, usually accompanied by lymphoepithelial lesions and benignappearing germinal centers2. More recently, B-cell lymphomas of MALT arising of a variety of sites have been described, including the breast, orbit, conjunctiva, skin, gallbladder, cervix, larynx, and trachea.2-4 Urogenital tract is among the various sites involved in MALT lymphoma.3-4 However, B-cell lymphomas of MALT type involving the kidney are relatively rare.4-20 The present paper describes the clinical presentation, pathological features and disease course of a patient with MALT B-cell lymphoma involving the kidney and a serum M-component of IgM kappa. Case report A 77-year-old man with a 30 years history of hypertension was referred to our hospital for evaluation of chronic renal failure. Four years before the patient had been diagnosed of Barrett’s esophagus and duodenal ulcer with pyloric stenosis by upper gastrointestinal endoscopy. Esophagus biopsies showed esophagitis with koilocytosis. The biopsy urease test was negative but he received specific treatment to H. pylori eradication. At that time laboratory data revealed hematocrit 40 %, hemoglobin 12.5 g/dl, white blood cells 5380/ml with normal differential count, and an erythrocyte sedimentation rate of 20 mm/h (normal 15-30 mm/h). Total serum proteins were 5 g/dl and albumin 3.1 g/dl. Urinalysis was normal and serum creatinine ranged from 1.2 to 1.4 mg/dl. He continued receiving treatment with omeprazole, and sixteen months later a barium study of the upper gastrointestinal tract showed normal findings. At admission physical examination was unremarkable. Laboratory data showed hematocrit 34.5 %, hemoglobin 11 g/dl, white blood cells 5950/ml with normal differential count, platelets 161000/ml, and an erythrocyte sedimentation rate of 100 mm/h. Blood coagulation was normal. Urinalysis revealed normal sediment and proteinuria 1.2 g/24 h. Serum urea was 70 mg/dl, serum creatinine 2.2 mg/dl, and creatinine clearance 45 ml/min. The total serum proteins were 9.6 g/dl, albumin 4.1 g/dl, and gammaglobulin 3.5 g/dl (with a monoclonal peak). The serum immunoglobulin levels measured by nephelometry were IgM 4640 mg/dl (normal 38-231 mg/dl), IgG 858 mg/dl (normal 650-1700 mg/dl), and IgA 168 mg/dl (normal 103-568 mg/dl), kappa 641 mg/dl (normal 170-370 mg/dl), lambda 106 mg/dl (normal 90-210 mg/dl). Monoclonal IgM kappa was detected in the serum by immunofixation. Serum cryoglobulin was negative and Bence-Jones proteinuria was positive. Other laboratory data showed C reactive protein 4.9 mg/dl, beta 2-microglobulin 4.9 mg/l, lactate dehydrogenase 286 U/l, serum calcium 9.9 mg/dl, and serum phosphate 3.2 mg/dl. EBV and HCV were negative. Abdominal ultrasound examination showed a solid mass in the left kidney. A skeletal roentgenogram and a bone scan revealed no abnormalities. A thoracoabdominal CT 682 R. Peces et al. MALT lymphoma involving the kidney revealed a 7 cm mass located in the midportion of the left kidney (Fig. 1). There was no evidence of lymphadenopathy. A bone marrow aspirate showed a normocellular patter and revealed 12.5 % atypical plasma cells. Bone marrow flow cytometry showed 3 % polyclonal T lymphocytes and 1 % polyclonal B lymphocytes. The plasma cells were CD38++, CD138-, CD19+, CD45+, CD56-, CD117-, and monoclonal kappa. On September 2000, a left nephrectomy was performed. The kidney weighed 240 g (including the adrenal gland), measured 20 x 12 x 8 cm, and contained an 8 x 8 x 2 cm wellcircumscribed mass involving the subcortical mid-portion of the kidney. Histologically, there was a lymphoid infiltrate extending diffusely through the pericapsular region and involving the renal cortex. Kidney architecture was effaced by the infiltrate within which occasional residual tubules and glomeruli were evident (Fig 2 A). In some areas, the infiltrate was diffuse between the reactive lymphoid follicles. Many reactive germinal centers were colonized by neoplastic cell with a residual mantle zone preserved (Fig 2 B). In other areas, the neoplasm was nodular, composed of reactive lymphoid follicles surrounded by pale zones of small lymphoid cells with pale or clear cytoplasm and slightly irregular nuclear contours (monocytoid and centrocyte-like cells) (Fig 2 C). In some areas, a prominent plasma cell and plasmacytoid lymphocytes component with occasional intranuclear inclusions (Dutcher bodies) and Russell bodies was observed (Fig 2 D). The neoplastic cells expressed monotypic cytoplasmic IgM kappa by immunoperoxidase. The neoplastic cells also reacted with the pan-B-cell antibody L26 (CD20, CD79a), coexpression of CD43 and stain of atypical lymphocytes with bcl-2 consistent with a low-grade B-cell lymphoma of MALT. Lymphoepithelial lesions were Figure 1. CT showing tumoral growth through the renal cortex of the left kidney. Nefrologia 2010;30(6):681-6 R. Peces et al. MALT lymphoma involving the kidney case report Discussion Figure 2. (A) Diffuse infiltration between reactive lymphoid follicles with expanded pale marginal zone. Some tubules and glomeruli appear scattered in the tumoral mass (H&E). (B) Germinal center colonized by tumoral cells. Note residual mantle zone preserved (H&E). (C) Neoplastic centrocyte-like and monocytoid cells with slightly irregular nuclear contours and pale to clear cytoplasm (H&E). (D) Plasmacytoid and lymphoplasmacytoid component (H&E). occasionally observed with cytokeratin immunostain. In the areas of the kidney not involved by lymphoma, there were histologic signs of nephrosclerosis. Two months after nephrectomy the serum creatinine was 3 mg/dl and the erythrocyte sedimentation rate remained elevated to 141 mm/h. The total serum proteins were 8.4 g/dl, albumin 4.1 g/dl, and gammaglobulin 2.4 g/dl with a monoclonal peak. The serum immunoglobulin levels were IgM 2970 mg/ml, IgG 865 mg/ml, and IgA 161 mg/ml. The patient was well and symptoms free, and he refused any invasive exploration. He received treatment with chlorambucil 5 mg/week and despite of this the monoclonal peak (IgM kappa) persisted and the serum IgM level remained elevated. The patient was followed by monthly routine blood examinations. On April 2003, an abdominal CT revealed a mass located in his solitary right kidney. A fine needle aspiration biopsy of the kidney revealed a B clonal kappa, CD19+, CD10+ and CD20+ cell infiltration. A highgrade MALT lymphoma transformation was considered and the patient was treated simultaneously with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy and a course of rituximab. On May 26, a second cycle of CHOP was initiated but he developed severe neutropenia and multiple complications, including sepsis and acute renal failure necessitating hemodialysis. On October 2003, dissemination of the lymphoma occurred and the patient died with multi-organ failure. Nefrologia 2010;30(6):681-6 Extranodal B-cell lymphomas of MALT arise at extranodal sites, are usually associated with chronic inflammation as a result of an infection or autoimmune disorder, and share histologic and immunophenotypic features. At these anatomic sites, MALT lymphomas are commonly associated with inflammatory conditions that predispose to lymphomagenesis. This is best established in the stomach where MALT lymphomas are commonly associated with H. pylori infection.1 The absence of lymphoid tissue in the normal renal parenchyma and the inability to rule out absolutely the presence of microscopic foci of tumor elsewhere, has led to controversy about the existence of primary renal lymphoma as a distinct disease. However, it has been suggested that lymphoma may arise from the renal hilum or from foci of inflammation that attract lymphocytes to the area, such as chronic pyelonephritis.8, 20 Other related factors contributing to the genesis of renal MALT lymphoma were Sjögren’s syndrome,4 IgA nephropathy,7 12 membranoproliferative glomerulonephritis, Epstein-Barr virus,10 actinomycosis,18 sarcoidosis,15 systemic lupus erythematosus,17 and gastric H. pylori infection.9,18 The presence of concomitant renal cell carcinoma,18 transitional cell carcinoma18 or colonic adenocarcinoma18 no appears to be a contributing factor. To our knowledge, only 30 unequivocal cases, non including the one reported here, of B-cell lymphomas of MALT involving the kidney have been reported in the English literature4-20 (Table I). Except a recent report by Garcia et al.18 of a series of 10 cases the majority were case reports. It is difficult in these patients to tell in which organ the lymphoma originated. Thus, whereas in 5 cases the primary tumor was localized in the salivary glands, the orbit or the gastrointestinal tract and the kidney lesion represented dissemination,6-8,18 in other 2 cases the tumor was simultaneously diagnosed in the kidney and the parotid gland, 4,16 and in other 3 cases MALT lymphoma involving the kidney was associated to enlarged lymph nodes.10,17,18 In all these cases secondary involvement of the kidney cannot be excluded. Only in 18 cases the tumor could have been originated in the kidney.5, 9, 11-15, 18-20 MALT lymphomas are usually confined to the sites of origin when diagnosed and are slow to disseminate. If disseminated, have a tendency to involve other mucosal organs. This explains the prolonged clinical course and the efficacy of surgical excision with no further therapy in some patients.9,11, 13-15, 18-20 Thus, in 8 cases the neoplasm involving the kidney did not recur or disseminate following nephrectomy.9, 11, 13-15, 18-20 In other case there was a remission following nephrectomy and irradiation.5 In one case with definitive resolution of the lymphoma following to chlorambucil therapy there was disappearance of serum M-component and improvement in symptomatology.7 In other case with renal actinomycosis 683 R. Peces et al. MALT lymphoma involving the kidney case report Table 1. Summary of clinical and pathologic features of B-cell lymphoma of MALT involving the kidney Ref. [4] Age/ Sex 62/F [5] 69/F Associated process Sjögren syndrome NS Cytoplasmic immunoglobulin Monotypic λ Serum immunoglobulin NS Other sites Treatment and course Parotid gland Monotypic κ NS None NS Orbit Monoclonal IgM λ Hypergamma IgG NS Gastric Nephrectomy + chlorambucil, partial remission Nephrectomy + irradiation, remission Nephrectomy, died of disease 13 years after surgery Chlorambucil, remission [6] 56/M NS [7] 62/M IgA nephropathy Monotypic λ [8] 68/M NS [9] 50/M H. pylori gastritis IgG (heavy chain) Monotypic IgM κ [10] 9/M Epstein-Barr virus infection [11] [12] 76/F 68/F NS MPGN [12] 72/F MPGN [13] [14] 77/M 43/M NS NS [15] [15] 83/F 53/M [15] [16] Bilateral renal sinus, salivary, prostate None None, no progression Nephrectomy, remission NS Axillary lymph node, lung NS NS None None Chemotherapy, died of disease at age 13 Nephrectomy, remission Prednisone, not available Monoclonal IgM κ NS NS None Prednisone, partial remission None None NS Sarcoidosis NS NS None None 72/M 45/M NS NS NS NS [17] 84/F NS [18] [18] 54/M 75/F [18] 66/M [18] [18] 83/M 65/F [18] 73/M [18] 47/M Systemic lupus erythematoso NS Transitional cell carcinoma, bladder Renal cell carcinoma NS H. pylori gastritis Adenocarcinoma colon NS Nephrectomy, remission Nephrectomy, remission at 28 months Chemotherapy, remission Partial nephrectomy, remission Nephrectomy, remission Nephrectomy, chemotherapy, multiple relapses during 13 years Chemotherapy [18] 18/F [18] [18] 77/F 65/M [19] 30/M [20] [20] Case 48/F 55/F 77/M H. pylori gastritis NS Renal actinomycosis NS Chronic pyelonephritis Chronic pyelonephritis Peptic ulcer, esophagitis Monotypic κ Monotypic IgM κ Monotypic κ Monotypic κ Monotypic κ Monotypic κ NS NS Orbit Parotid, orbit, skin, breast, prostate Para-aortic lymph nodes None None Polytypic NS None Monotypic κ Monotypic κ NS NS Monotypic κ NS Monotypic λ NS Monotypic κ NS Chemotherapy, remission Nephrectomy, persistently positive lymph nodes Chemotherapy, residual renal mass Chemotherapy, autologous stem cell transplantation Chemotherapy, remission Monotypic κ Monotypic λ NS NS None Retroperitoneal lymph nodes Ocular, bone marrow Soft tissue of flank and thigh Parotid and cervical lymph node None None NS None NS NS Monoclonal IgM κ None None Bone marrow Nephrectomy, remission at 28 months Nephrectomy Nephrectomy Nephrectomy, chemotherapy rituximab, incomplete remission, died of disease 3 years after Monotypic IgM κ Chemotherapy, remission Nephrectomy, chemotherapy, remission Nephrectomy, remission Not available, alive 3 years Antibiotics, remission NS = not stated; MPGN = membranoproliferative glomerulonephritis there was a remission following to antibiotics.18 In other case there was a partial remission at 15 months following 684 nephrectomy and chlorambucil administration.4 In some cases the tumor had indolent behavior and very slow clinical Nefrologia 2010;30(6):681-6 R. Peces et al. MALT lymphoma involving the kidney course. In one case the lymphoma progressed very slowly and the patient remained alive without any treatment for 5 years.8 Nine patients were alive with no evidence of disease from 9 to 53 months.18, 19 Three patients, including our case, died of disease from 3 to 13 years after initial diagnosis,6, 10 2 of who presented high-grade transformation and dissemination of the lymphoma. The lymphoma cells express monotypic surface immunoglobulins or, to a lesser extent, cytoplasmic immunoglobulins, usually IgM.15 On the other hand, monoclonal gammopathy is a common phenomenon in patients with MALT lymphoma, most probably due to paraprotein production by the clonal lymphoplasmacytic cells.21-23 In a study the monoclonal gammopathy corresponded with the light chains detected on biopsy by immunohistochemical on the majority of cases.22 In 18 of 31 cases of B-cell MALT-type lymphoma of the kidney (including the one reported here) monotypic cytoplasmic immunoglobulins kappa or lambda were present. However, only 3 cases of monoclonal gammopathy IgM, one with the light chain of the lambda type and 2 with kappa, have been reported so far.7, 12 The pathogenesis of B-cell lymphoma in our case is unknown, as there was no chronic inflammation identified in the kidney. The case reported here is singular as it showed the morphologic, immunologic, and phenotypic features of a B-cell MALT-type lymphoma of the kidney occurring in a patient with a monoclonal gammopathy IgM kappa. Thus, in our case, monoclonal gammopathy corresponded with the light chain detected on kidney by immunohistochemical. On the other hand, the demonstration of bone marrow cells of B-lineage expressing the same monoclonal protein as the tumor suggested bone marrow involvement, even in the absence of identical morphology. In the literature we identified only another case of MALT lymphoma involving the kidney and bone marrow.18 In addition, at 2 months of the nephrectomy the persistence of the serum M-component was suggestive of incomplete remission. Despite chemotherapy and rituximab treatment, monoclonal gammopathy IgM kappa persisted. Clinical follow-up showed right kidney extension with high-grade transformation, and finally systemic dissemination. Therefore, the presence of monoclonal gammopathy was associated with more advanced disease, with bone marrow involvement and high-grade transformation.23 Although MALT lymphoma seems to be a relatively benign disease in most patients, the clinical course of our patient clearly demonstrated the malignant potential and the importance of prompt and aggressive treatment. As occurred in our patient, adverse prognosis factors may include presence of monoclonal gammopathy, bone marrow involvement, high tumor burden, high-grade transformation, or dissemination of disease.24 In summary, this case illustrates that the kidney is among the sites that may be involved by MALT B-cell lymphomas in a Nefrologia 2010;30(6):681-6 case report primary or secondary fashion, and the need for expanded investigation of the possible relation with systemic involvement. These lymphomas, like other extranodal MALT-type lymphomas, have indolent behaviour and slow clinical course as demonstrated by the available literature. However, in some cases high-grade transformation and/or dissemination of disease occur. The treatment choice should be patient-tailored, taking into account the site, the stage and the clinical characteristics of the individual patient. REFERENCES 1. Isaacson PG, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue: a distinctive type of B-cell lymphoma. Cancer 1983;52:1410-6. 2. Isaacson PG, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer 1984;53:2512-24. 3. Thieblemont C, Berger F, Coiffier B. Mucosa-associated lymphoid tissue lymphomas. Curr Opin Oncol 1995;7:415-20. 4. Pelstring RJ, Essell JH, Kurtin PJ, Cohen AR, Banks PM. Diversity of organ site involvement among malignant lymphomas of mucosa-associated tissues. Am J Clin Pathol 1991;96:738-45. 5. Parveen T, Navarro-Román L, Medeiros J, Raffeld M, Jaffe ES. Lowgrade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the kidney. Arch Pathol Lab Med 1993;117:780-3. 6. Imahori SC. Low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue involving the kidney. Arch Pathol Lab Med 1994;118:111-2. 7. Mak SK, Wong PN, Lo KY, Wong AK. Successful treatment of IgA nephropathy in association with low-grade B-cell lymphoma of the mucosaassociated lymphoid tissue type. Am J Kidney Dis 1998;31:713-8. 8. Araki K, KubotaY, Lijima Y, Suziki H, Sasagawa I, Nakada T, et al. Indolent behaviour of low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue involved in salivary glands, renal sinus and prostate. Scand J Urol Nephrol 1998;32:234-6. 9. Colovic M, Hadzi-Djokic J, Cemerikic V, Colovic R, Jankovic G, Dacic M. Primary MALT lymphoma of the kidney. Hematol Cell Ther 1999;41:229-32. 10. Tao J, Kahn L. Epstein-Barr virus-associated high-grade B-cell lymphoma of mucosal-associated lymphoid tissue in a 9-year-old boy. Arch Pathol Lab Med 2000;124:1520-4. 11. Mhawech P, Ahearn J, Medeiros J. Pathologic quiz case. A unilateral renal mass in an elderly woman. Arch Pathol Lab Med 2000;124:919-20. 12. Stokes MB, Wood B, Alpers ChE. Membranoproliferative glomerulonephritis associated with low-grade B cell lymphoma presenting in the kidney. Clin Nephrol 2002;57:303-9. 13. Mita K, Ohnishi Y, Fdahiro T, Yamasaki A. Primary mucosa-associated lymphoid tissue lymphoma in the renal pelvis. Urol Int 2002;69:241-3. 14. Tuzel E, Mungan M, Yorukoglu K, Basakci A, Kirkali Z. Primary renal lymphoma of mucosa-associated lymphoid tissue. Urology 2003;61:463. 15. Qiu L, Unger PD, Dillon RW, Strauchen JA. Low-grade mucosa associated lymphoid tissue lymphoma involving the kidney. Report of 3 685 R. Peces et al. MALT lymphoma involving the kidney case report 16. 17. 18. 19. 20. cases and review of the literature. Arch Pathol Lab Med 2006;130:86-9. Jindal B, Sharma SC, Das A, Banerjee AK. Indolent behavior of lowgrade B cell lymphoma of mucosa-associated lymphoid tissue arising in the kidney. Urol Int 2001;67:91-3. Mortlock AM, Lim CSE, Morgan H, et al. Renal MALToma: an unusual lymphoma in a patient with lupus. Lupus 2006;15:613-5. Garcia M, Konoplev S, Morosan C, Abruzzo LV, Bueso-Ramos CE, Medeiros LJ. MALT lymphoma involving the kidney: a report of 10 cases and review of the literature. Am J Clin Pathol 2007;128:464-73. Kato Y, Hasegawa M, Numasato S, Monma N, Fujioka T. Primary mucosa-associated lymphoid tissue-type lymphoma arising in the kidney. Int J Urology 2008;15:90-2. Li B, Zhang W, Tian W, Qiu L. Primary renal mucosa-associated lymphoid 21. 22. 23. 24. tissue lymphoma, the result of chronic pyelonephritis? Chinese-German. J Clin Oncol 2008;7:55-8. Cavalli F, Isaacson PG, Gascoyne RD, Zucca E. MALT lymphomas. Hematology 2001;1:241-58. Wöhrer S, Streubel B, Bartsch R, Chott A, Raderer M. Monoclonal immunoglobulin production is a frequent event in patients with mucosa-associated lymphoid tissue lymphoma. Clin Cancer Res 2004;10:7179-81. Asatiani E, Cohen P, Ozdemirli M, Kessler CM, Nabromatis B, Cheson BD. Monoclonal gammopathy in extranodal marginal zone lymphoma (ENMZL) correlates with advanced disease and bone marrow involvement. Am J Hematol 2004;77:144-6. Canelhas A, Compérat E, Le Tourneau A, Molina T, Ramos M, Ribeiro P, et al. Marginal zone lymphoma of both spleen and kidney displaying transformation into large B-cell lymphoma. Int Urol Nephrol 2006;38:431-7. Enviado a Revisar: 19 May. 2010 | Aceptado el: 4 Jun. 2010 686 Nefrologia 2010;30(6):681-6 http://www.revistanefrologia.com © 2010 Revista Nefrología. Official Publication of the Spanish Nephrology Society research protocols Clinical and genetic bases of hypertensive nephrosclerosis. NEFROSEN Study B. Diez Ojea1, R. Marín2, E. Coto3, F. Fernández Vega4, R. Álvarez Navascués4, G. Fernández Fresnedo5, A. Pobes Martínez de Salinas6, A. Suárez Laurés7, C. García Monteavaro6, M. Gorostidi4, E. Sánchez4, M. Arias5, F. Ortega4 Nephrology Department. Valle del Nalón Hospital. Langreo, Asturias, Spain. 2 Nephrology and Hypertension Unit. Asturias Medical Centre. Oviedo, Asturias, Spain. 3 Molecular Genetics Laboratory. Central de Asturias University Hospital. Oviedo, Asturias, Spain. 4 Nephrology Department. Central de Asturias University Hospital. Oviedo, Asturias, Spain. 5 Nephrology Department. Marqués de Valdecilla University Hospital. Santander, Cantabria, Spain. 6 Nephrology Department. San Agustín Hospital. Avilés, Asturias, Spain. 7 Nephrology Department. Cabueñes Hospital. Gijón, Asturias, Spain 1 Nefrologia 2010;30(6):687-97 doi:10.3265/Nefrologia.pre2010.Jul.10372 ABSTRACT Background: Hypertensive nephrosclerosis is a chronic kidney disease (CKD) associated withto essential hypertension. The lack of correlation between hypertension control and progression to end-stageof CKD suggests an intrinsic and primitive disease. New evidence suggests that MYH9 gene alterations are associated with polymorphisms in African Americans. The aim of this study is to investigate whether a polymorphism of MYH9 in Caucasians is linked to essential hypertension and nephrosclerosis. The secondary objective is to identify the clinical risk factors of progression to end-stage renal disease (ESRD)end-stage CKD. This is a retrospective study that will compare patients with nephrosclerosis versus and essential hypertensives without renal disease, and also patients with nephrosclerosis and impaired renal function versus with those that are stable. Method: Between October 2009 and October 2010, 500 patients with stages 3-5 CKD attributed to nephrosclerosis according to usual clinical criteria, and 300 essential hypertensives (eGFR>60mL/min/1.73m2; microalbuminuria <300mg/g) are to be recruited. A total of 200 healthy controls from the general population are also to be included for the genetic study. There are two study sections, being the first and final visits to the clinic (for stage 5 cases, the start of replacement therapy will be the end of follow-up). Clinical and laboratory data will be recorded, and blood samples will be collected. Discussion:n Our study will aim to determine if there exists is a relationship between the diagnosis of nephrosclerosis and the MYH9 gene in Caucasiansthe Caucasia race, and to study possible Correspondence: Beatriz Diez Ojea Sección de Nefrología. Hospital Valle del Nalón. Polígono Riaño, s/n. 33920 Langreo. Asturias. Spain. [email protected] [email protected] risk factors for progression to ESRDend-stage CKD, on both clinical and genetic baseis. Key words: Hypertensive nephrosclerosis. Essential hypertension. MYH9 gene. Chronic kidney disease. Bases clínicas y genéticas de la nefroesclerosis hipertensiva. Estudio NEFROSEN RESUMEN Justificación: Se conoce como nefroesclerosis la enfermedad renal crónica (ERC) que complica la hipertensión arterial (HTA) esencial. La ausencia de correlación entre el control de la HTA y la progresión a ERC terminal sugiere la existencia de una enfermedad intrínseca y primitiva. Recientemente se ha asociado con polimorfismos del gen MYH9 en individuos afroamericanos. El objetivo del trabajo que presentamos es determinar si algún polimorfismo de dicho gen se relaciona en raza caucásica con la asociación de HTA esencial y nefroesclerosis y, además, conocer los marcadores de progresión a ERC terminal. Será un estudio retrospectivo que comparará a pacientes con nefroesclerosis frente a pacientes con HTA esencial sin enfermedad renal y, además, se incluirán pacientes con nefroesclerosis y progresión de la enfermedad renal frente a los que se mantienen estables. Métodos: Entre octubre de 2009 y octubre de 2010 se incluirán 500 pacientes con ERC (estadios 3-5) atribuida a nefroesclerosis según criterios clínicos habituales, y 300 pacientes afectados de HTA esencial (FGe >60 ml/min/1,73 m 2; microalbuminuria <300 mg/g). Para el estudio genético también se incluirán 200 controles sanos de población general. Habrá dos cortes del estudio, la primera visita en el hospital y la visita final (en esta687 research protocols dio 5 el inicio del tratamiento sustitutivo constituirá el final del seguimiento). Se registrarán datos clínicos y analíticos, y se recogerán muestras de sangre para el estudio genético. Discusión: Nuestro estudio, con la doble vertiente genética y clínica, tratará de determinar si en la raza caucásica existe relación entre el diagnóstico de nefroesclerosis y el gen MYH9, y estudiará, además, los posibles marcadores de progresión. Palabras clave: Nefroesclerosis. Hipertensión arterial esencial. Gen MYH9. Enfermedad renal crónica. INTRODUCTION The term nephrosclerosis is usually used for kidney disease which complicates arterial hypertension and that primarily affects the preglomerular microvasculature. 1,2 In practice, it is an entity with vague clinical profiles that groups hypertensive patients with chronic kidney disease (CKD) without other identifiable causes of disease. 3,4 In nephrosclerosis, also known as benign nephroangiosclerosis or hypertensive nephropathy, the most characteristic microscopic lesion is hyalinosis of the afferent arterioles. Vascular changes produce vasoconstriction, glomerular ischaemia (retraction of glomerular tuft, focal or global sclerosis) and in some areas, interstitial fibrosis and tubular atrophy. Other authors point out that the hyalinisation of afferent arterioles would initially cause vasodilatation, glomerular hypertrophy and, in the long-term, segmental glomerulosclerosis lesions that overall favour the appearance of proteinuria and progression of the disease. These alterations are more frequent and severe in black patients, unrelated to the control of blood pressure or the degree of proteinuria. In fact, nephrosclerosis is a form of intrarenal renovascular disease, and in some cases may represent a magnification of the changes of kidney aging, from both a histological and clinical standpoint.5-7 Its causal relation with essential hypertension is still a subject of debate. It is not clear that treated arterial hypertension can lead to end-stage CKD.8-11 Therefore, some authors have postulated that renal structural alterations may precede hypertension, and that it would be an intrinsic process of the renal microvasculature with loss of self-regulation that would mainly result in excessive preglomerular vasoconstriction4 or persistent vasodilation of the afferent arteriole.6,7 A chronically impaired renal plasma flow, in the long run, leads to hypertension and CKD. Nephroangiosclerosis could have the same clinical significance as atherosclerosis in coronary or cerebral vessels.2,3 In the US, Europe and Spain, vascular kidney disease is the second most common cause of terminal CKD. However, the 688 B. Diez Ojea et al. NEFROSEN study diagnosis of nephrosclerosis is usually made by exclusion, when no data for another type of kidney disease is found, and in very few cases is based on histological findings. There are no specific signs or symptoms, but there are some suggestive clinical findings (men aged 55-60 years, longstanding hypertension, left ventricular hypertrophy [LVH], mild CKD and proteinuria less than 0.5-1g/24 hours). As with diabetic kidney disease, a kidney biopsy is almost never performed. This attitude may be reasonable in many cases, but is a source of misdiagnoses.12 Some studies with small samples suggest that the degree of proteinuria may be variable, and may even reach the nephrotic range. However, there is general consensus that subnephrotic proteinuria values are typical of hypertensive nephrosclerosis. Clinical inclusion criteria for the African American Study of Kidney Disease and Hypertension (AASK) required a proteinuria level lower than 2.5 (UP/Cr).4,13,14 End-stage CKD onset age for AfricanAmericans varied between 45 and 64 years, while for Caucasian Americans it is over 65 years.4 Compared with early glomerular or diabetic kidney diseases, the progression of kidney failure is slow in most cases, especially in whites. Renal function can remain stable for years if hypertension is well controlled. However, in a few cases the disease progresses to endstage CKD. 5,12,15 Vascular kidney disease is the most common cause of hospital visits for CKD in nephrology departments in Spain. Up to 39% of cases have this aetiology, higher than diabetic or glomerular kidney disease (20%). 16 Despite the small percentage of patients with progression, its high prevalence makes it the second leading cause of end-stage CKD. The factors for progression of nephrosclerosis are not well recognised, which hampers the implementation of preventive measures. Usually, firstly the black race is cited, and later age, the degree of kidney failure at diagnosis, the level of systolic blood pressure (SBP) and the degree of proteinuria.1,13-20 In the AASK study, patients with proteinuria below 0.3g/24 hours, who had received ramipril, an angiotensin-converting enzyme inhibitor (ACEI) from the start had slower progression after an 11 year follow-up. This same study showed that old age (over 70 years) was inversely correlated with kidney failure.20,21 Only some cases of whites, perhaps those who are genetically predisposed, have an unfavourable clinical evolution. The progression of the disease has been associated with the concomitant presence of atherosclerotic lesions in the aorta and main renal arteries, and with processes such as Type 2 diabetes mellitus, hyperuricaemia and dyslipidaemia.3,5,10,19 In the Norwegian HUNT 2 population study, progression to end-stage CKD was associated with the estimated glomerular filtration rate Nefrologia 2010;30(6):687-97 B. Diez Ojea et al. NEFROSEN study (eGFR) and basal microalbuminuria in the multivariate analysis after 10 years of follow-up.22 In the last decade, the disease is being diagnosed mainly in patients older than 65-70 years of age and with vascular disease in other parts of the body. In these cases, nephrosclerosis is observed as a diffuse atherosclerosis of the renal arterioles.19,23,24 Recently, a lesser-known fact has been pointed out: the presence of concomitant cardiovascular disease is a factor for progression to kidney failure.25-29 Levin et al studied a group of 313 patients with kidney failure (eGFR of 10-75ml/min) and a mean followup of 23 months. They found that patients with cardiovascular diseases (ischaemic heart disease, cerebrovascular disease, peripheral artery disease or heart failure) increased the risk of progression to end-stage CKD (relative risk [RR] 1.58, P=.047).25 Hillegas et al’s study of 298 patients who had an acute myocardial infarction (AMI), found that the eGFR had declined 5.4ml/min after the first year, much higher than the usual 1ml/min/year reported for the general population.28 Elsayed et al studied 13,826 subjects, of which 18.5% were African American, enrolled in the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study trials. They found that after 9 years of follow-up, patients with cardiovascular disease at baseline were significantly more likely to develop CKD (odds ratio [OR] 1.75, P<.001).29 In Spain there is an ongoing prospective study supported by the Spanish Society of Nephrology (SEN), the “ESTUDIO PRONEFROS”, which aims to determine the proportion of patients with nephrosclerosis showing progression to kidney failure. “Historical” nephrosclerosis cases have been excluded and only incident cases (n=430) over one year have been included. After 2 years of follow-up, preliminary data show that there is impaired renal function in only 3.9% of cases. Progression markers include the presence of higher baseline SBP and a higher rate of associated cardiovascular events.30 The explanation is complex and probably multifactorial: the deterioration of myocardial function implies a reduction of renal blood flow that would be added to the presence of microvascular and macrovascular renal injury. Furthermore, until just a few years ago, the presence of renal failure has led to underuse of cardioprotective drugs or more conservative application of coronary revascularisation or valvular prostheses.31 For years, the relationship between hyperuricaemia and CKD in patients with essential hypertension has been widely debated. While it is true that up to 40% of individuals with gout develop CKD, the fact remains that almost all have hypertension and arteriolosclerosis and glomerulosclerosis lesions similar to those seen in patients with nephrosclerosis. There is no evidence that Nefrologia 2010;30(6):687-97 research protocols hyperuricaemia is a risk factor for CKD. However, two recent studies have offered new perspectives: Iseki et al have shown that hyperuricaemia is an independent marker of renal failure for subjects at baseline who had normal renal function, especially women.32 Furthermore, Siu et al studied 54 patients and found that when uric acid is decreased with allopurinol progression to CKD is postponed.33 It seems necessary to promote studies specifically designed to clarify whether hyperuricaemia may be an independent risk factor for vascular and renal risk.34 The lack of correlation between the degree of hypertension control and the prevention of disease progression, from the clinical and histological standpoint suggests that this process may be an intrinsic and early renal disease. In some cases the clinical context maintains some similarity with focal segmental glomerulonephritis (FSGP). For many years, genetic markers have been sought that could explain the onset and progression of the disease. Arterial hypertension, hyperuricaemia, dyslipidaemia and metabolic syndrome were frequently associated phenotypic factors, but not the cause of the process.2,3 Some studies carried out a decade ago verified a direct relationship between nephrosclerosis and ACE DD genotype. The D allele appears to be predominant in hypertensive patients with nephrosclerosis and could be a progression marker. Although the proportion of patients was small, studies were conducted on Caucasians, and included histological support and control groups of hypertensives without kidney disease and subjects from the general population.35,36 American nephrosclerosis genetic studies have been conducted primarily in African-Americans because the disease is more frequent and aggressive in this race. 37-42 Two independent studies published recently have led to a new approach to the pathogenesis of nephrosclerosis. The study by Kao et al, which included 1372 patients, revealed a close relationship between the presence of end-stage CKD secondary to hypertensive nephrosclerosis in nondiabetics, and some polymorphisms of the MYH9 gene, located on chromosome 22, which encodes the non-muscle myosin heavy chain IIA. 37 The study by Kopp et al compared 190 black individuals with 222 healthy controls. It found the same association with the presence of FSGP of idiopathic origin or secondary to infection with human immunodeficiency virus (HIV), but no relationship was found with progression.38 Furthermore, a study by Freedman et al confirmed the presence of the MYH9 gene polymorphisms in 696 AfricanAmerican subjects with hypertensive kidney disease and end-stage CKD, compared with 948 control subjects without kidney disease, of whom 34% were hypertensive. However, not all individuals who were homozygous 689 B. Diez Ojea et al. NEFROSEN study research protocols dominant for MYH9 risk alleles developed the disease, which suggests that other factors, such as environment or interaction with other genes, add to individual genetic susceptibility.39 Meanwhile, the same group recently reported an association between the MYH9 E1 haplotype and the presence of microalbuminuria in 1458 African-American patients with essential hypertension but no kidney disease. Increased risk has been observed for MYH9 E1 haplotype, which consists of the polymorphisms rs4821480, rs2032487, rs4821481 and rs3752462. However, the strength of the association is weaker than in subjects with FSGP and terminal CKD of hypertensive origin.40 Further complicating the issue, an association of that gene has been found in 751 diabetic black end-stage CKD patients compared with 227 diabetic controls without kidney disease and 925 healthy controls, but there is no clear correlation with the development of diabetic kidney disease because there is no histological confirmation. It has even been suggested that it may coincide with kidney disease related to baseline MYH9 polymorphisms.41 HYPOTHESIS Hypertensive nephrosclerosis is a CKD that rarely progresses. 1 Clinical progression markers would be related to: a) The presence of associated cardiovascular disease, especially cardiovascular events appearing on a recurring basis. b) The degree of proteinuria. c) Initial renal function. d) The patient being less than 70 years old. 2 Histological progression markers would be related to: a) The degree of global and segmental glomerulosclerosis. It appears that during the early stages, myosin IIA is primarily situated at the podocyte level and induces structural alterations. Recently, the role of podocyte loss and dysfunction has been reported as part of disease development. Wang et al conducted a study on 41 patients with biopsy diagnosis of hypertensive nephrosclerosis, finding less podocytes and a reduced intrarenal expression of protein genes such as nephrin, podocin and synaptopodin, which is also related to the decrease in eGFR, and inversely proportional with the degree of renal fibrosis.43 A bold new hypothesis on this issue states that polymorphisms of this gene would be markers of various kidney diseases that fall into one histological group, FSGP, and any of its variants, such as nephrosclerosis, which would be a disease akin to primary kidney disease with idiopathic FSGP and with the collapse observed in HIV.44 A renal disease associated with macrothrombocytopaenia has even been reported. Lastly, there are some rare entities associated with mutations of that gene, which are transmitted by autosomal dominant inheritance, known as MYH9-related disorders. These include the May-Hegglin anomaly and the Sebastian, Epstein and Fechtner syndromes, which are in many cases present with kidney disease, although its mechanism and that of MYH9-related hypertensive nephrosclerosis polymorphisms in blacks is currently unknown.45 However, not all cases of nephrosclerosis are associated with MYH9 haplotypes.46 For all these reasons, further studies are needed to provide a better understanding of its pathogenesis, and the potential role of detections of MYH9 polymorphisms in the diagnosis and evolution of CKD secondary to nephrosclerosis (Table 1). 690 b) The degree of interstitial fibrosis. 3. Genetic markers would be related to: a) MYH9 gene polymorphisms. b) I/D polymorphisms of the ACE gene. OBJECTIVES Primary Objective To determine if any MYH9 gene polymorphism is associated in Caucasians: a) With the association of essential hypertension and nephrosclerosis. b) With progression of the disease. Secondary Objectives 1. To compare the clinical characteristics of nephrosclerosis patients with essential hypertension and hypertensives with no kidney disease, and to determine clinical progression markers. 2. To recognise the importance of proteinuria, associated cardiovascular disease, sex, age (over and under 70 Nefrologia 2010;30(6):687-97 B. Diez Ojea et al. NEFROSEN study research protocols years), the presence of hyperuricaemia or gout, and concomitant treatments (antihypertensives, statins and antiplatelet agents). study, healthy controls from the general population with similar age and sex as the cases are also included. PATIENTS METHOD Cases Study Design Multicentre retrospective study, which involves four hospitals in Asturias (Central Asturias University Hospital, Valle del Nalón Hospital, Cabueñes Hospital, and San Agustín Hospital), and the Marques de Valdecilla University Hospital in Cantabria. A basic study comparing patients with essential hypertension with nephrosclerosis patients versus hypertensive patients without kidney disease (control group). Furthermore, patients with nephrosclerosis and impaired kidney function are compared with those who are stable. For the genetic The cases were selected between October 2009 and October 2010 from nephrology, kidney transplantation, haemodialysis and peritoneal dialysis units of the participating hospitals. Patients recruited have eGFR <60ml/min/1.73m2 (measured by the MDRD formula) in stages 3-5, attributed to nephrosclerosis according to standard clinical criteria (Table 2), with or without histological documentation. Diagnosis of CKD of unknown origin will not be valid, and patients with secondary, renovascular or accelerated hypertension will be excluded. Patients with stage 5, on dialysis or to undergo kidney Table 1. Main studies on the polymorphisms of the MYH9 gene and the presence of kidney disease Authors, journal and year Kao et al, Nat Genet 200837 n 1372 ESRD cases, 806 controls Kopp et al, Nat Genet 200838 891 CKD cases, 1024 controls Freedman et al, Kidney Int 200939 871 ESRD cases, 948 controls Freedman et al, Am J Nephrol 200940 2903 individuals (HyperGEN study) Freedman et al, Nephrol Dial Transplant 200941 751 ESRD diabetics, 227 diabetic patients without kidney disease, 925 controls Behar et al, Hum Mol Genet 201042 997 ESRD cases, 448 controls Pattaro et al, Kidney Int 200949 2859 individuals Nefrologia 2010;30(6):687-97 Type of patient African-Americans Results Association with ESRD in all non-diabetic subjects, particularly in hypertensive nephrosclerosis, FSGP and nephropathy secondary to HIV 1569 African-Americans Association with African Americans and 346 Caucasians with idiopathic FSGP or secondary to HIV, and hypertensive nephrosclerosis, but not in diabetic kidney disease Non-diabetic African-Americans Association with ESRD in all patients without diabetes, and hypertensive nephrosclerosis E1 haplotype With essential hypertension E1 haplotype association without kidney disease and the presence of microalbuminuria in African-Americans African Americans Association with ESRD in diabetic patients compared to healthy controls and diabetics without kidney disease There does not seem to be a clear relationship with diabetic kidney disease because there is no histological confirmation. African-Americans and Association with ESRD those of Hispanic origin in non-diabetics. The Hispanic population studied had varying degrees of African descent. Europeans without Association with serum creatinine kidney disease levels, primarily in non-diabetics 691 B. Diez Ojea et al. NEFROSEN study research protocols Table 2. Inclusion-exclusion criteria Inclusion criteria Age 18-80 years Caucasian BP > _140/90mm Hg or on antihypertensive medication Creatinine > _1.5mg/dl in men Creatinine > _1.4mg/dl in women eGFR <60ml/min/1.73m2) There are no other identifiable causes of kidney disease, including ischaemic kidney disease Exclusion criteria BP <140/90mm Hg without treatment Diabetes mellitus based on fasting glucose > _126mg/dl Proteinuria > _3.0g/24 hours, except in cases in which there is histological confirmation by kidney biopsy One kidney Malignant or accelerated arterial hypertension (opthalmoscopy grade III or IV) Secondary arterial hypertension, including renovascular Serious systemic disease Morbid obesity Adapted from the AASK study: Agodoa et al JAMA 2001,13 Wright et al JAMA 2002.14 transplant, may be admitted, provided that developmental data are available from their first contact with the hospital. The start of replacement therapy marks the end of follow-up. Cases younger than 80 years will be valid for the study. However, the selection will be made mainly of patients younger than 60 years, to minimise the effect of age-related gene denaturation. Controls The control group is from Central Asturias University Hospital and Valle del Nalón Hospital, to simplify data collection, given that there are few differences between the subjects as they are from the same geographic area. Patients with essential hypertension are included with eGFR>60ml/min/1.73 m2 and microalbuminuria <300mg/g. Laboratory Analysis Routine laboratory tests shall be performed by the usual methods in the laboratory of each hospital. Blood samples for genetic studies will be submitted to the Laboratory of Molecular Genetics, Central Asturias University Hospital. DNA was obtained from leukocytes from 10ml of EDTA anticoagulated peripheral blood. The samples will be stored for 5 years. Major MYH9 gene polymorphisms to be identified are those which showed an association with hypertensive nephrosclerosis in non-diabetics (rs4821480 and rs3752462, belonging to E-1 haplotype), and with the ACE gene, by the polymerase chain reaction (PCR) test. DEFINITIONS Progression of Kidney Disease Data for Each Patient There will be two sections of the study, one for the first hospital visit and one for the last («current visit»). Clinical data are to be collected from all patients including socio-demographic variables, comorbidity and cardiovascular risk, medical treatment and physical examination, weight in kilograms, height in centimetres, systolic (SBP) and diastolic (DBP) blood pressure in millimetres of mercury, and heart rate. Analytical data will be recorded including renal function by serum creatinine, eGFR, creatinine clearance, microalbuminuria in urine alone (mg/g creatinine) and proteinuria in urine for 24 hours and also glucose, uric acid, cholesterol and calciumphosphorus metabolism. Furthermore, the performance of imaging techniques (ultrasound, CT angiography, MR angiography or arteriography), and renal biopsy diagnosis (Table 3) are also included. 692 Doubling of baseline creatinine, eGFR decline >50% above baseline or a reduction of 25ml/min/1.73m2 or onset at end-stage CKD (defined by the need for renal replacement therapy). Arterial Hypertension SBP>140mm Hg and/or DBP>90mm Hg, or treatment with diet or antihypertensive agents. The percentage of patients with SBP>130mm Hg and/or DBP>80mm Hg will also be analysed. Diabetes Mellitus Fasting glucose>126mg/dl or >200mg/dl 2 hours after oral glucose or antidiabetic treatment (diet, oral antidiabetic agents or insulin). Nefrologia 2010;30(6):687-97 B. Diez Ojea et al. NEFROSEN study research protocols Table 3. Clinical and analytical data sheet Vital statistics: Local No.: Initials: Clinical history no.: D.O.B.: Centre: Sex: Comorbidity and cardiovascular risk factors: Family history kidney disease: Smoker: High cholesterol: Gout: Heart disease: Heart failure: Atrial Fibrillation: Degenerative valve disease: Left ventricular hypertrophy: Stroke: Peripheral artery disease: CKD stage: Renal replacement therapy: Type: Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes 3 Yes Haemodialysis Baseline data. Date: Creatinine (mg/dl): Proteinuria (g/24h): Glucose1 (mg/dl): Ccr (ml/min): Microalbuminuria (mg/g): Uric acid1(mg/dl): eGFR (ml/min/1.73m2): Hb1 (g/dl): LDL-cholesterol1 (mg/dl): Final visit. Date: Weight (kg): Systolic BP (mm Hg): Creatinine (mg/dl): Proteinuria (g/24 h): Glucose (mg/dl): Calcium2 (mg/dl): Height (cm): Diastolic BP(mm Hg): Ccr (ml/min): Microalbuminuria (mg/g): Uric acid (mg/dl): Phosphorus2 (mg/dl): Heart rate (bpm): EGFR (ml/min/1.73m2): Hb (g/dl): LDL-cholesterol (mg/dl): Albumin2 (mg/dl): No No No No No No No No No No No 4 No Peritoneal dialysis Treatment at the final visit: ACEI: ARB: DRI: Calcium agonists: Diuretics: Beta blockers: Alpha blockers: Alpha-beta blockers: Other anti-hypertensives: Yes Yes Yes Yes Yes Yes Yes Yes Yes No No No No No No No No No Number of anti-hypertensive drugs: Statins: Antiplatelet therapy: Anticoagulants: Erythropoietin3: Yes Yes Yes Yes No No No No Renal morphology study and diagnosis4: Renal ultrasound: Hyperechogenic kidneys Kidneys <9cm Renal arteriography Angio-CT: Angio-MR: Renal biopsy: Yes Yes Yes Yes Yes Yes Yes No No No No No No No Unknown Ex-smoker Unknown 5 Transplantation Unknown Blood sample for genetic study. Date: In the hypertensive controls, only initial data concerning renal function were recorded. Baseline metabolic or anaemia data were not collected. In hypertensive controls, data were not collected on calcium-phosphorus metabolism at the final visit. 3 In the hypertensive controls, administration of erythropoietin was not recorded. 4 In the hypertensive controls, no diagnostic studies were recorded. 1 2 Nefrologia 2010;30(6):687-97 693 B. Diez Ojea et al. NEFROSEN study research protocols Hypercholesterolaemia LDL cholesterol>100mg/dl, or treatment with lipid lowering agents. hypertensive controls with no kidney disease and 200 general population subjects (healthy controls). We will attempt to control the sample for sex and age (Figure 1). Smoker STATISTICAL ANALYSIS Tobacco consumption (cigarettes, cigars, pipe) during the last month. Former smoker: absence of tobacco use for a consecutive year. Left Ventricular Hypertrophy There will be a descriptive analysis of continuous variables, giving the mean, median, standard deviation and range, and in case of discrete variables, the frequency distribution and percentages. When necessary, 95% confidence intervals are calculated. The description of the main variables are based on age, sex, level of baseline renal failure and proteinuria, degree of hypertension control and dyslipidaemia and associated cardiovascular risk factors. The association between qualitative variables will be assessed with the chisquare or Fisher’s exact test and quantitative variables using parametric tests (t-test, Pearson correlation coefficient and ANOVA). If a normal distribution cannot be assumed, nonparametric tests will be used such as Mann-Whitney U or Kruskal-Wallis. Comparison tests will be bilateral and considered significant when P<.05. A logistic regression model will be used to evaluate the association of those variables in which the result of the P comparison in the “raw” analysis is less than 0.15. Statistical analysis of the study will be conducted with SPSS for Windows, version 15.0 (SPSS, Chicago, IL). Diagnosis made by echocardiography, which is a more specific method than the electrocardiogram. FINANCIAL SUPPORT Obesity BMI>30kg/m2. Cardiovascular Comorbidity Must be properly documented. Presence of one or more of the following: ischaemic heart disease by acute myocardial infarction (AMI), unstable angina or stable angina, atrial fibrillation, heart failure, degenerative mitral or aortic valve disease, ischaemic, hemorrhagic, or transient stroke, or peripheral arterial disease. ETHICAL ASPECTS The investigator must inform the patient about his or her participation in the study, which is voluntary and will not cause any change in the treatment or medical care to be received. Subsequently, the investigator must obtain the patient’s informed and voluntary consent. The highest levels of confidentiality must always be maintained, as well as compliance with national legislation on data protection. The study protocol was approved by the Research Ethics Committee of the Regional Clinical Research of Asturias. SAMPLE SIZE It is estimated that the size of the sample needed to observe the effects of MYH9 gene polymorphisms according to their allele frequencies for an alpha error of 0.05 and a beta error of 0.2, will be 500 cases with CKD and 500 controls. The subjects will be distributed as such: 500 cases, 300 694 The NEFROSEN Study is a typical clinical practice study that does not involve extra costs. It is being carried out with the sponsorship of the Spanish Society of Nephrology (SEN) through its “Kidney and Hypertension” Work Group. The cost for each case analysed in the genetic substudy of the MYH9 gene polymorphisms is about 10 euros. It is being financed with funds from the Fundación Renal Iñigo Álvarez de Toledo (Iñigo Alvarez de Toledo Kidney Foundation) in the Molecular Genetics Group. DISCUSSION Following the publication of genetic studies on nephrosclerosis, some documents have indicated that it should no longer be regarded as a disease that is secondary to essential hypertension. At least in the black race, it seems to be a genetically based disease closely related to FSGP, and it is estimated that there may be an association with MYH9 gene polymorphisms in 43% of African-American individuals who progress to end-stage CKD.47 Of these, control of hypertension does not stop progression, while in Nefrologia 2010;30(6):687-97 B. Diez Ojea et al. NEFROSEN study research protocols FULFILLING INCLUSION EXCLUSION CRITERIA Nephrology and hypertension external consultation KIDNEY transplantationa Haemodialysisa Peritoneal dialysisa 300 hypertensive controls 500 CKD cases 200 healthy controls Genetic analysis Clinical and analytical data collection Last visit First visit CKD CASES compared with hypertensive controls a “PROGRESSING” CASES compared with “STABLE” CASES In stage 5 cases, the start of replacement therapy will be the end of follow-up. Figure 1. Case and control recruitment. the white race it seems more effective, so it is speculated that the treatment of hypertensive nephrosclerosis should be approached from a different perspective that not only includes blocking RAS and strict control of blood pressure.48 However, much is still unknown about these findings. The referred studies have been conducted on patients with nephrosclerosis not supported by renal biopsies. This indicates the opportunity to reassess the cases of the AASK study (n=1094 patients), which is the only one that has been conducted with histological confirmation.13,14 In the white race there are no studies on whether these or other MYH9 polymorphisms might be involved in the disease. Only the Pattaro et al group found an association of this gene with serum creatinine values in Nefrologia 2010;30(6):687-97 2859 European individuals without kidney disease, coming from three different populations, while the genes related to renal function showed great heterogeneity. 49 The proposed study is both clinical and genetic. Their relationship seems logical and complementary. The limitations are those related to a retrospective study design, together with the lack of a centralised laboratory for routine analysis, because the data are historic. Nephrosclerosis cases that maintain periodic visits over a variable time period, and patients that have progressed to end-stage CKD are included. In this respect, we have significant selection bias when calculating the percentage of patients whose disease has progressed, since many will be obtained from dialysis and transplant units. We will probably not be able to draw clear conclusions about disease progression but, despite these 695 research protocols limitations, we believe a study such as this will be interesting because there are very few publications to date on this topic. It seems necessary to design prospective genetic studies to determine the relationship with this gene, both in terms of diagnosis and the possibility that it could be a progression marker. REFERENCES 1. Luft FC. Hypertensive nephrosclerosis: update. Curr Opin Nephrol Hypertens 2004;13:147-54. 2. Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis 1995;25:207-21. 3. Marín R, Gorostidi M, Fernández-Vega F, Álvarez-Navascués R. Systemic and glomerular hypertension and progression of chronic renal disease: the dilemma of nephrosclerosis. Kidney Int Suppl 2005;(99):S52-S56. 4. Luke RG. Hypertensive nephrosclerosis: pathogenesis and prevalence. Essential hypertension is an important cause of end-stage renal disease. Nephrol Dial Transplant 1999;14:2271-78. 5. Marcantoni C, Ma LJ, Federspiel C, Fogo AB. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int 2002;62:172-80. 6. Hill GS, Heudes D, Jacquot C, Gauthier E, Bariéty J. Morphometric evidence for impairment of renal autoregulation in advanced essential hypertension. Kidney Int 2006;69:823-31. 7. Keller G, Zimmer G, Mall G, Ritz E. Amann K. Nephron number in patients with primary hypertension. N Engl J Med 2003;348:101-8. 8. Hsu CY. Does non-malignant hypertension cause renal insufficiency? Evidence-based perspective. Curr Opin Nephrol Hypertens 2002;11:267-72. 9. Sarnak JA, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 2005;142:342-51. 10. Siewert-Delle A, Ljungman S, Andersson OK, Wilhelmsen L. Does treated primary hypertension lead to end-stage renal disease? A 20year follow-up of the Primary Prevention Study in Göteborg, Sweden. Nephrol Dial Transplant 1998;13:3084-90. 11. Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005;165:923-8. 12. Zarif L, Covic A, Iyengar S, Sehgal AR, Sedor JR, Schelling JR. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant 2000;15:1801-7. 13. Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, et al, for the African American Study of Kidney Disease and Hypertension Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis. A randomised controlled trial. JAMA 2001;285:2719-28. 14. Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, et al, for the African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. JAMA 2002;288:2421-31. 696 B. Diez Ojea et al. NEFROSEN study 15. Toto RB. Hypertensive nephrosclerosis in African Americans. Kidney Int 2003;64:2331-41. 16. Marin R, Fernández-Vega F, Gorostidi M, Ruilope LM, Díez M, et al. Blood pressure control in patients with chronic renal insufficiency in Spain: a cross-sectional study. J Hypertens 2006;24:395-402. 17. Vikse BE, Aasarød K, Bostad L, Iversen BM. Clinical prognostic factors in biopsy-proven benign nephrosclerosis. Nephrol Dial Transplant 2003;18:517-23. 18. Verhave JC, Hillege HL, Burgerhof JG, Gansevoort RT, De Zeeuw D, De Jong PE; PREVEND study group. The association between atherosclerotic risk factors and renal function in the general population. Kidney Int 2005;67:1967-73. 19. Tracy ER, Strong JP, Newman WP III, Malcom GT, Oalmann MC, Guzman MA. Renovasculopathies of nephrosclerosis in relation to atherosclerosis at ages 25 to 54 years. Kidney Int 1996;49:564-70. 20. Appel LJ, Wright JT Jr, Greene T, Kusek JW, Lewis JB, et al. Longterm effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med 2008;168:832-9. 21. Norris KC, Greene T, Kopple J, Lea J, Lewis J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006;17:2928-36. 22. Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 2009;20:1069-77. 23. Segura J, Campo C, Gil P, Roldán C, Vigil L, Rodicio JL, et al. Development of chronic kidney disease and cardiovascular prognosis in essential hypertensive patients. J Am Soc Nephrol 2004;15:1616-22. 24. Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant 2001;16:765-70. 25. Levin A, Djurdjev O, Barrett B, Burgess E, Carlisle E, et al. Cardiovascular disease in patients with chronic kidney disease: getting to the heart of the matter. Am J Kidney Dis 2001;38:1398-407. 26. McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease has a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol 2004;15:1912-9. 27. Menon V, Sarnak MJ. The epidemiology of chronic kidney disease stages 1 to 4 and cardiovascular disease: a high-risk combination. Am J Kidney Dis 2005;45:223-32. 28. Hillege HL, Van Gilst WH, Van Veldhuisen DJ, Navis G, Grobbee DE, et al. Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ACE inhibition: the CATS randomized trial. Eur Heart J 2003;24:412-20. 29. Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med 2007;167:1130-6. 30. Marín R, Fernández-Vega F, Díez Ojea B, Alcoy E, Palencia A, et al. Marcadores de progresión de enfermedad renal crónica en pacientes con con nefroesclerosis. Datos evolutivos. Estudio PRONEFROS. Nefrologia 2008;28(Supl 4):48. 31. Hostetter TH. Chronic kidney disease predicts cardiovascular disease. N Engl J Med 2004;351:1344-6. 32. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance Nefrologia 2010;30(6):687-97 B. Diez Ojea et al. NEFROSEN study 33. 34. 35. 36. 37. 38. 39. 40. of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004;44:642-50. Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006;47:51-9. Feig DI, Kang DH, Johnson J. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811-21. Fernández-Llama P, Poch E, Oriola J, Botey A, Coll E, et al. Angiotensin converting enzyme gene I/D polymorphism in essential hypertension and nephroangiosclerosis. Kidney Int 1998;53:1743-7. Mallamaci F, Zuccalà A, Zoccali C, Testa A, Gaggi R, et al. The deletion polymorphism of the angiotensin-converting enzyme is associated with nephroangioesclerosis. Am J Hypertens 2000;13:433-7. Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 2008;40:1185-92. Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, et al. MYH9 is a major-effect risk gene for focal segmental glomeruloesclerosis. Nat Genet 2008;40:1175-84. Freedman BI, Hicks PJ, Bostrom MA, Cunningham ME, Liu Y, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 2009;75:736-45. Freedman BI, Kopp JB, Winkler CA, Nelson GW, Rao DC, et al. Polymorphisms in the nonmuscle myosin heavy chain 9 gene (MYH9) are associated with albuminuria in hypertensive African Americans: research protocols the HyperGEN study. Am J Nephrol 2009;29:626-32. 41. Freedman BI, Hicks PJ, Bostrom MA, Comeau ME, Divers J, et al. Non-muscle myosin heavy chain 9 gene MYH9 associations in African Americans with clinically diagnosed type 2 diabetes mellitus-associated ESRD. Nephrol Dial Transplant 2009;24:3366-71. 42. Behar DM, Rosset S, Tzur S, Selig S, Yudkovsky G, et al. African ancestry allelic variation at the MYH9 gene contributes to increased susceptibility to non-diabetic end-stage kidney disease in Hispanic Americans. Hum Mol Genet 2010;19:1816-27. 43. Wang G, Lai FM, Kwan BC, Lai KB, Chow KM, Li PK, Szeto CC. Podocyte Loss in Human Hypertensive Nephrosclerosis. Am J Hypertens 2009;22:300-6. 44. Murea M, Freedman BI. Essential hypertension and risk of nephropathy: a reappraisal. Curr Opin Nephrol Hypertens 2010;19:235-41. 45. Singh N, Nainani N, Arora P, Venuto RC. CKD in MYH9-Related Disorders. Am J Kidney Dis 2009;54:732-40. 46. Rao M, Balakrishnan VS. The genetic basis of kidney disease risk in African Americans: MYH9 as a new candidate gene. Am J Kidney Dis 2009;53:579-83. 47. Divers J, Freedman BI. Susceptibility genes in common complex kidney disease. Curr Opin Nephrol Hypertens 2010;19:79-84. 48. Freedman BI, Sedor JR. Hypertension-associated kidney disease: perhaps no more. J Am Soc Nephrol 2008;19:2047-51. 49. Pattaro C, Aulchenko YS, Isaacs A, Vitart V, Hayward C, Franklin CS, et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int 2009;76:297-306. Sent for Review: 6 Jul. 2010 | Accepted: 9 Jul. 2010 Nefrologia 2010;30(6):687-97 697 letters to the editor A) BRIEF PAPERS ON RESEARCH AND CLINICAL EXPERIMENTS Cadaveric donor procurement units faced with living donation Nefrologia 2010;30(6):698-9 doi:10.3265/Nefrologia.pre2010.Sep.10590 To the Editor, There are not enough organ donations to cover the needs of transplants. For this reason, it is important to promote living donation, to try to meet these needs.1,2 In Spanish-speaking countries, living donation is still in its infancy. In this respect, cadaveric donor procurement units have played a key part in promoting organ transplants, and could possibly do the same for developing living donation.3 The aim of this study is to analyse the attitude towards living kidney (LKD) and liver (LLD) donation among hospital professionals in the cadaveric donor procurement units of Spain and Latin America, and to analyse the variables that bring about this attitude. From the International Donor Collaboration Project, seven hospitals with cadaveric donor procurement units (intensive care unit, surgical resuscitation unit, and neurosurgery unit) were selected: 2 in Spain, 3 in Mexico, and 2 in Cuba. Random sampling stratified by professional category was performed in these units. The field study was carried out in 2006. Attitude was assessed with a validated survey 4-6 which was selfadministered and completed anonymously. A descriptive analysis was performed, and the student T and the Chi-square tests were applied. The survey was completed by 283 professionals from the procurement units. Of these, 90% (n=254) were in favour of related LKD, 6% (n=16) against, and 4% (n=13) undecided. By countries, 95% (n=71) of Cubans were in favour, 92% 698 (n=44) of Spaniards, and 87% (n=139) of Mexicans (P>.05). No significant differences were observed with regard to sociopersonal or occupational variables, except for employment conditions. The medical professionals with a permanent post were more in favour of this kind of donation than those with a temporary post (95% vs 85%; P=.007) (Table 1). Among the rest of the variables, a more positive attitude is seen among: those who are in favour of cadaveric donation (P<.001); those who would accept a kidney from a living donor if it were necessary (P<.001); those in favour of LLD (P<.001); those whose partner has a positive attitude toward donation and transplantation (P=.001); and those who perform or have performed prosocial activities (P=.013) (Table 1). As for related LLD, 84% (n=237) were in favour, 11% (n=32) against, and 5% (n=14) undecided. By countries, 87% Table 1. Living donation in donor procurement units in Spain and Latin America LKD VARIABLE Demographic variable - Country IN FAVOUR 90% DVR LLD NOT IN FAVOUR 10% – – P IN FAVOUR 84% DVH NOT IN FAVOUR 16% NS – – NS P Sociopersonal variables - Mean age (35 [10] years) – – NS – – NS - Sex – – NS – – NS - Marital status – – NS – – NS Occupational variables - Occupational category - Occupation status - Health personnel Variables of knowledge and attitude towards organ donation and transplants - Personal experience with donation and/or transplants – – NS - – NS Permanent post Temporary post 0.007 Permanent post Temporary post 0.043 – – NS - – NS – – NS Yes No 0.032 - Attitude towards cadaveric donation In favour Not in favour <.001 In favour Not in favour 0.001 - Belief in the likelihood of needing a transplant themselves – – NS – – NS - Attitude towards living kidney donation – – - Accept a live kidney donation if it were necessary Yes Do not know <.001 – – - Attitude towards living liver donation In favour Not in favour <.001 – – - Accept a living kidney donation if it were – – – Yes Do not know <.001 – – NS – – NS Yes, in favour No partner 0.010 – – NS No, but would do No, never 0.013 No, but would do No, never <.001 NS Social interaction and prosocial conduct variables - Family attitude towards donation and transplants - Attitude of partner towards donation and transplants - Performs social or voluntary help activities Religious variables - Religion of interviewee In favour Not in favour <.001 – – NS – – Know the attitude of their religion towards donation and transplants - – NS – – NS Attitude towards the body variable - Concern about mutilation after donation – – NS – – - – NS Nefrologia 2010;30(6):698-713 letters to the editor (n=65) of Cubans were in favour, 85% (n=41) of Spaniards, and 82% (n=131) of the Mexicans (P>.05). No significant correlations were observed between sociopersonal and occupational variables. Among the rest of the variables, a more positive attitude is seen among: those with personal experience with donation and transplants (P=.032); those in favour of cadaveric organ donation (P=.001); those in favour of LKD (P<.001); those whose would accept a liver from a living donor if necessary (P=.001); and those who perform or have performed prosocial activities (P<.001) (Table 1). Hospital staff from cadaveric donor procurement units in Spain and Latin America have a very positive attitude towards both living kidney and liver donation. Their attitude is more favourable than that observed in other studies using the same questionnaire.4 The data obtained by our group3 in 2003 showed that 86% had a favourable attitude towards LKD and 68% towards LLD. Therefore, expectations for this kind of donation are becoming more and more positive and optimistic. LKD is generally more accepted than LLD, possibly due to the lower risk6,8 for the donor. Attitudes towards living donation have been shown not to be influenced by sociopersonal or religious factors, or attitudes towards the body.7,9 However, there is a significant association between attitudes towards LKD and LLD. Therefore, it seems clear that the main problem of living donation is accepting it. It is worth noting that no differences exist with regard to occupational category, nor between healthcare professionals and those working in other settings.6,9 In conclusion, we can declare that the attitude of the staff of cadaveric donor procurement units in Spain and Latin America towards living donation is very favourable. Thus, they could play an important role in its promotion in these times of a desire to develop living donation, provided the socioNefrologia 2010;30(6):698-713 political and economic conditions are right for it. 1. Organización Nacional de Trasplantes. Memoria de actividades ONT 2009. Rev Esp Traspl 2009;14(1). (Monográfico). 2. Ríos A, López-Navas A, Ayala-García MA, Sebastián MJ, Abdo-Cuza A, et al. Attitudes toward living kidney donation in transplant hospitals: a Spanish, Mexican, and Cuban multicenter study. Transplant Proc 2010;42:228-32. 3. Ríos A, Ramírez P, Rodríguez MM, Parrilla P. Las unidades generadoras de donantes de órganos de cadáver ante la donación de vivo. Nefrologia 2007;27:230-1. 4. Conesa C, Ríos A, Ramírez P, Rodríguez MM, Parrilla P. Socio-personal factors influencing public attitude towards living donation in south-eastern Spain. Nephrol Dial Transplant 2004;19:2874-82. 5. Ríos A, Ramírez P, Rodríguez MM, Martínez L, Rodríguez JM, et al. Attitude of hospital personnel faced with living liver donation in a Spanish center with a living donor liver transplant program. Liver Transpl 2007;13:1049-56. 6. Ríos A, Ramírez P, Rodríguez MM, Martínez L, Montoya MJ, et al. Attitude of ancillary personnel faced with living kidney donation in a hospital with a living donor kidney transplant program. Transplantation 2007;83:336-40. 7. Ríos A, Cascales P, Martínez L, Sánchez J, Jarvis N, et al. Emigration from the British Isles to south-eastern Spain: a study of attitudes toward organ donation. Am J Transplant 2007;7:2020-30. 8. Hashikura Y, Ichida T, Umeshita K, Kawasaki S, Mizokami M, et al. Donor complications associated with living donor liver transplantation in Japan. Transplantation 2009;88:110-4. 9. Ríos A, Ramírez P, Martínez L, Montoya MJ, Lucas D, et al. Are personnel in transplant hospitals in favor of cadaveric organ donation? Multivariate attitudinal study in a hospital with a solid organ transplant program. Clin Transplant 2006;20:743-54. A. Ríos, A.I. López-Navas, P. Ramírez, P. Parrilla, Proyecto colaborativo internacional donante* General and Gastrointestinal Surgery Department. Virgen de la Arrixaca University Hospital. El Palmar. Murcia. Correspondence: A. Ríos Unidad de Cirugía General y del Aparato Digestivo. Hospital Universitario Virgen de la Arrixaca. Avda. de La Libertad. 208, 30007 El Palmar. Murcia. [email protected] [email protected] *Group comprised of: : L. Martínez-Alarcón. General Surgery Department, Virgen de la Arrixaca University Hospital. M.A. Ayala-García, E.J. Ramírez, G. Muñoz, J.S. Rodríguez, M.A. Martínez, A. Nieto, Regional Specialist Hospital of Bajío and University of Guanajuato, Guanajuato, Mexico. M.J. Sebastián, A. Camacho, Donation and Transplantation Coordinators, UMAE Specialist Hospital N.º 25 IMSS, Monterrey, Mexico. A. Abdo-Cuza, R. Castellanos, MedicalSurgical Research Centre, Cuba. J. Suárez-López, Hermanos Ameijeiras Hospital, Cuba. Internal jugular vein access in a semi-seated position for catheterisation to enable haemodialysis in orthopnoeic patients Nefrologia 2010;30(6):699-700 doi:10.3265/Nefrologia.pre2010.Aug.10598 To the Editor, Vein access is commonly used to carry out diagnostic and therapeutic procedures, but the right preparation is essential due to the complications. With vein access the patient’s position is crucial, dorsal decubitus being the most commonly used. Haemodialysis requires a permanent vascular access. However, the use of transitory catheters is an alternative as they provide immediate access to the bloodstream and enable effective dialysis to be performed in an emergency. They are used when it is impossible to use arteriovenous (AVF) 699 letters to the editor or prosthetic fistulas because of an urgent need for dialysis or due to dialysis failure,1 or in acute kidney failure. In this last case, the access of choice is the femoral vein,2 followed by the jugular and subclavian veins. Occasionally, inserting a catheter into these veins in not possible due to clinical conditions (obesity, vein stenosis, generalized oedema, local infection, etc.), or orthopnoea in the case of thoracic veins. These situations may delay or hinder the procedure.3 In these cases a median approach to the internal jugular vein with the patient in a semi-seated position is proposed. We describe here the technique, duration of catheterisation, complications, and outcome of patients who had a catheter inserted for haemodialysis with this approach between 1 September 2007 and 1 September 2008. Insertion protocols and medical histories were analysed. The technique used was as follows: the patient was semi-seated, head turned away from insertion side. Then under local anaesthetic, the needle was inserted at a 45º angle with the skin at the apex of the triangle formed by the sternal and clavicular heads of the sternocleidomastoid muscle and the clavicle, and is directed toward the ipsilateral nipple. It then followed the classic Seldinger technique. An analysis was performed of 25 accesses in patients with AKF or acute on CKF4 requiring emergency haemodialysis. The right internal jugular vein was the most used, in 18 cases (72%), and was chosen in accordance with the doctor’s experience. The 700 duration of catheterisation was 25 (5) days; five (20%) were removed when treatment finished after kidney function was restored, 10 (40%) as the permanent vein access was started to be used, and 10 (40%) due to death secondary to the outcome of the underlying disease. The complications observed were prolonged bleeding at the site of insertion in 3 patients (12%) and increased vascular resistance in one patient (4%). There were no severe complications. The femoral vein approach is recommended for emergency haemodialysis due to its low rate of complications. However, it has the disadvantage that catheterisation must not exceed 8 days, mainly due to the risk of infection-related complications or the patient has absolute contraindications such as thrombosis or local infection, or relative contraindications such as obesity, burns, etc. The internal jugular vein is the route of choice for inserting catheters for longer than 8 days, as this access can be used for long periods with a lower incidence of complications. Subclavian veins are no longer used as their cannulation is associated with many serious complications (haemothorax and pneumothorax) and because of the risk of vascular stricture.5 Looking at the literature, vein access techniques to the subclavian and internal jugular vein require the patient to be in dorsal decubitus. Sometimes this position is difficult due to dyspnoea or orthopnoea, which is not uncommon, especially if we consider that many patients with acute kidney failure have a pulmonary oedema. Thus, the femoral vein is contraindicated, and the semi-seated position is opted for, inserting catheters quickly and safely, guided by the anatomy of the neck. This could be an alternative technique with patients needing emergency dialysis or suffering from orthopnoea, as our experience shows that it results in fewer complications. 1. Canaud B, Leray-Moragues H, Garred LJ, Turc-Baron C, Mion C. Wats is the role of permanent central vein acces in hemodialysis patients? Semin Dial 1996;9(5):397-400. 2. Schwab SJ, Quarles LD, Middleton JP. Hemodialysis- associated subclavian vein stenosis. Kidney Int 1988;33:1156-9. 3. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med 2003;348: 12-20. 4. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, and the ADQI workgroup: Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Critical Care 2004;8:R204-R212. 5. Clar DD, Albina JE, Chazan JA. Subclavian vein stenosis and trombosis: a potencial serious complication in chronic hemodialysis patients. Am J Kidney Dis 1990;15:265-8. R. Karatanasopuloz, V. Balbuena, M. Paiz, G. Levy, C. Martín Intensive Care and Nephrology Department. J.R. Vidal Hospital. Corrientes, Corrientes (Argentina). Correspondence: R. Karatanasopuloz Servicio de Terapia Intensiva y Nefrología, Hospital J.R. Vidal, NECOCHEA 1050, 3400, Corrientes, Corrientes, Argentina. [email protected] Nefrologia 2010;30(6):698-713 letters to the editor B) BRIEF CASE REPORTS Granulomatous interstitial nephritis in the absence of extrarenal sarcoid Nefrologia 2010;30(6):701-2 doi:10.3265/Nefrologia.pre2010.Jul.10504 To the Editor, Sarcoidosis is a multisystemic disease which affects the kidneys in between 4% and 20% of cases. Disorders of calcium metabolism such as hypercalcaemia and/or hypercalciuria, nephrolithiasis, and tubular dysfunctions are the most common kidney disorders.1 Glomerular disorders associated with sarcoidosis have also been reported, among which membrane glomerulonephritis is the most common.2 Interstitial granulomatous infiltration is usually silent and rarely manifests as acute kidney failure, and if this does happens, damage to other organs is usually evident. Kidney failure caused by granulomatous interstitial nephritis (GIN) in the absence of extrarenal sarcoidosis is an extremely rare clinical condition. We present the case of a patient with acute renal failure caused by sarcoidosis affecting only the kidneys. The patient was a 56-year-old woman with no allergies or substance abuse and with no medical history of interest or regular medical treatment. She was referred to emergency services by her family doctor after an abnormal blood test (creatinine 8mg/dl), carried out to study a toxic syndrome of 6 to 7 months evolution with a weight loss of 10 kg. She had an analysis carried out 5 months earlier in a check-up at work which highlighted a Hb level of 11 g/dl and creatinine level of 1.7 mg/dl. The patient had normal blood pressure in the physical examination, and pale mucous membranes; everything else was normal. Analyses showed results of Hct/Hb 28 %/9.6 g/dl, urea/creatinine 192/9.4 mg/dl (estimated GFR 4ml/min), calcium 10 mg/dl, FENa 10. Proteinuria 0.6 g/24 h, and sediment was Nefrologia 2010;30(6):698-713 normal. PTHi and vitamin D levels were normal. Patient was negative for HbsAg, HCV and HIV. Immunological study: ANA, anti-DNA, ANCA, serum complement and immunoelectrophoresis were normal or negative. The Rose Bengal test for anti-Brucella antibodies was negative. Urine culture in Löwenstein-Jensen medium was negative. The PPD skin test was negative. ACE levels were 189 U/l (8-52 U/l). The chest x-ray, abdominal ultrasound and CT scan of chest, abdomen and pelvis were normal. No significant changes were found in the eye and ENT examinations. A jugular catheter was inserted and haemodialysis was begun. A percutaneous kidney biopsy was performed showing 10 glomeruli, 2 of them sclerotic, the rest with normal appearance. Large amount of granulomas comprised of epithelioid cells and Langhans multinucleated giant cells without necrosis (Figure 1) were observed in the interstitium. The histochemical techniques to detect fungi and acid-alcohol resistant bacilli were negative. Treatment began with prednisone at 1 mg/kg/day. The patient had conserved diuresis and haemodialysis was well tolerated. After four weeks of corticosteroid treatment, a progressive improvement in kidney function was observed, enabling her to stop the replacement treatment. At present, 4 months after starting treatment and with a dose of 0.3 mg/kg/day, she has a plasma creatinine level of 2.8 mg/dl (GF 19 ml/min) and a normal level of ACE. Figure 1. Kidney cortical. GIN is a very rare histological diagnosis, found in between 0.5% and 0.9% of native kidney biopsies.3,4 It has been associated with drugs (mainly NSAID, allopurinol and antibiotics), infections (tuberculosis, brucellosis and fungal infections, among others), Wegener’s granulomatosis, crystalinduced nephropathy, paraproteinemia, TINU syndrome, idiopathic infections, and sarcoidosis. Joss et al. reviewed the cases of kidney biopsies performed in Glasgow over a 15-year period and identified a total of 18 GIN (in less than 1% of the total biopsies). Of these, 5 were due to sarcoidosis, 2 were associated with TINU syndrome, 2 were secondary to medication, and 9 were classified as idiopathic.5 Acute renal failure in sarcoidosis is mainly associated with hypercalcaemia, and in its chronic form with nephrocalcinosis. 6 GIN is another cause of kidney disease in sarcoidosis, present in a third of patients with sarcoidosis and evidence of kidney disease. In 1987, Ford described the first association of GIN in a patient with sarcoidosis who developed acute renal failure.7 GIN in the absence of extrarenal sarcoidosis is very rare. Robson et al. presented 7 cases of GIN in the absence of extrarenal sarcoidosis.8 Of note is the predominance of men (71%), the mean age of 69, the severe renal failure at the time of presentation (calculated creatinine clearance of 14 ml/min), the need for temporary haemodialysis at onset in one case, minimal proteinuria (mean 0.4 g/day), high ACE levels in 3 patients, and good response to corticosteroid treatment in 5 cases (prednisolone at between 20 and 60 mg/day). Two patients needed to begin periodic haemodialysis at 3 and 15 months after diagnosis. The common histological characteristics were normal glomerular levels and the presence in the interstitial fluid of in the interstitial fluid composed of 701 letters to the editor epithelioid cells and multinuclear giant cells. It is worth highlighting the importance of kidney biopsies in cases of nonjustified kidney failure, and in cases of suspected sarcoidosis in particular, given the variety of lesions it can cause. Performing a biopsy can optimize the choice of clinical treatment. 1. Casella FJ, Allon MJ. The kidney in sarcoidosis. Am Soc Nephrol 1993;3:1555-62. 2. Peces R, Laurés AS, Navascués RA, Baltar J, Seco M, Ortega F, et al. El espectro de afectación renal en la sarcoidosis: presentación de tres casos. Nefrologia 1998;18(2):161-4. 3. O’Riordan E, Willert RP, Reeve R, Kalra PA, O’Donoghue DJ, Foley RN, et al. Isolated sarcoid granulomatous interstitial nephritis. Review of five cases at one center. Clin Nephrol 2001;55:297-302. 4. Mignon F, Mery JP, Mougenot B, Ronco P, Roland J, Morel-Maroger L. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984;13:219-45. 5. Joss N, Morris S, Young B, Geddes C. Granulomatous Interstitial Nephritis. Clin J Am Soc Nephrol 2007;2:222-30. 6. Picazo M, Cuxart M, Ballarín JA, Huerta MV. Diagnóstico de sarcoidosis a partir de un caso de insuficiencia renal aguda. Nefrologia 2006;26(5):631-2. 7. Ford MJ, Anderton JL, MacLean N. Granulomatous sarcoid nephropathy. Postgrad Med J 1978;54:416-7. 8. Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003;18:280-4. M. Cuxart1, M. Picazo1, R. Sans Lorman1, M.J. Muntané2 1 Nephrology Department. Figueres Hospital. Figueres, Girona, Spain. 2 Department of Pathologic Anatomy. Figueres Hospital. Figueres. Girona, Spain. Correspondence: M. Cuxart Servicio de Nefrología. Hospital de Figueres. Ronda Rector Arolas, s/n. 17600 Figueres. Girona. Spain. [email protected] [email protected] 702 Acute phosphate nephropathy after bowel cleansing: still a menace Nefrologia 2010;30(6):702-4 doi: 10.3265/Nefrologia.pre2010.Jul.10544 To the Editor, Colonoscopy is critically dependent on adequate pre-procedural bowel cleansing and oral sodium phosphate bowel purgatives (OSP) have been used with good acceptance and efficacy for this purpose. 1Among others metabolic and clinical disturbances described after the procedure, acute kidney injury may be a serious complication.2,3 We present two cases of sodium phosphate induced acute renal failure. Case 1 A 84 year-old male with a past history of stage 3 obstructive chronic renal failure, prostatic hypertrophy and hypertension, medicated with losartan presented with complaints of six months weigh loss and changed bowel habits. A colonoscopy was performed after preparation with oral sodium phosphate solution (Fleet phosphosoda®) with standard dose and its result was normal. A week later, the patient reported pedal and orbital oedema and was observed on the emergency department. The physical examination was unremarkable except for hypertension (180/80 mmHg) and lower limbs oedema. Laboratory results showed haemoglobin 11.1 g/dl, serum urea 346 mg/dl, serum crea- Figure 1. Kidney biopsy. Von Kossa staining (40x). tinine 9.2 mg/dl, serum sodium 130 mEq/L, serum potassium 6.5 mEq/L, serum phosphorus 6.6 mg/dl, normal serum calcium, serum bicarbonate 15 mEq/L and mild proteinuria. Serum and urine immunoelectrophoresis and immunologic study were normal. Renal ultrasound showed increased cortical echogenicity. Haemodialysis was initiated. Kidney biopsy showed minimal mesangial expansion. The tubules were mildly dilated and focal interstitial fibrosis was present. Von Kossa stain positive deposits were observed within the cytoplasm of tubular epithelial cells, tubular lumen and interstitium (figure 1 and figure 2). Immunofluorescence was negative for immunoglobulin or complement. We made the diagnosis of acute phosphate nephropathy secondary to administration of a sodium phosphate purgative. Renal dysfunction didn’t improve after 7 months and the patient continues on regular haemodialysis. Case 2 A 88 year-old male with a past history of prostatic hypertrophy and marginal zoneB cell lymphoma IV-B stage (treated with vincristine, cyclophosphamide and prednisolone for two cycles followed by second line therapy with rituximab and chlorambucil with disease progression) and stage 4 obstructive chronic renal failure. A virtual colonoscopy was performed after bowel preparation with Fleet phosphosoda®. Colonic diverticulosis was diagnosed. Five days latter the patient reported lethargy and anuria and was admitted on Figure 2. Kidney biopsy. Von Kossa staining (100x). Nefrologia 2010;30(6):698-713 letters to the editor the emergency department. He presented with drowsiness and was hypotensive, apyretic and oliguric. He presented inspiratory crackles on chest exam, distended and painful abdomen without guarding and lower limb oedema. Abnormal test results were hemoglobin 9.7 g/dL, leucocytes 17.1 x 109/L (87% neutrophils), platelets 70 x 109/L, serum creatinine 3.45 mg/dL, serum urea 106 mg/dL, serum calcium 4.5 mg/dl, serum phosphorus 18.3 mg/dL, lactate dehydrogenase 3,028 U/L, C-reactive protein 28.2 mg/dL, pH 7.3, bicarbonate 13.3 mEq/L and lactate 7.5 mmol/L; Urine dipstick was positive for blood, leucocytes and proteins. Renal ultrasound showed kidneys with enhanced echogenicity. Chest radiograph showed interstitial oedema and abdominal radiograph was normal. Suspected severe urinary sepsis and acute kidney injury secondary to sepsis and phosphate nephropathy were assumed. Intravenous fluid was started, samples were obtained for culture and broad spectrum antibiotics were started. There was no clinical improvement and conservative measures were adopted. DISCUSSION Acute phosphate nephropathy (AphN) is a form of kidney injury that occurs after the use of bowel purgatives that contain oral sodium phosphate (OSP).2 OSP bowel solution (Fleet phosphosoda®) is a hyperosmotic purgative that has been used with good acceptance and efficacy in bowel cleansing before colonoscopy.1 Both patients made the standard regimen (two 45 ml doses taken 10-12 h apart). Each dose contains monobasic and dibasic sodium phosphate providing the equivalent of 5,8 g of elemental phosphorus and 5 g of sodium.3 Intestinal absorption occurs and transient hyperphosphatemia and hypocalcemia are found in all patients.2 However, severe hyperphosphatemia, symptomatic hypocalcemia, hypernatremia, symptomatic hyponatremia, hypokalemia, anion-gap acidosis and acute kidney injury have been described after the procedure.3-6 Two different Nefrologia 2010;30(6):698-713 clinical patterns of OSP-induced acute kidney injury have been described: early symptomatic and late insidious.2 The earlier form consists in an acute illness that manifests as changes in mental status, tetany, or cardiovascular collapse, usually in hours of bowel preparation, and patients present with severe hyperphophatemia and hypocalcemia. The second patient presented fit in this category. These patients require urgent fluid resuscitation, rapid correction of electrolyte disturbances, and sometimes dialysis. Despite aggressive fluid replacement and resuscitation our patient died. Some patients with this presentation survive and show renal function recovery.2 The second form is due to AphN with a more insidious onset (days to months) and is generally irreversible.1 At the time of diagnosis, serum phosphorus and calcium levels are normal, unless measured within 3 days of bowel preparation. This was in fact the case of the first patient. As we found, the main pathologic finding in kidney biopsy is nephrocalcinosis demonstrated with the Von Kossa stain.1 Following reports of AphN Fleet phosphosoda® was voluntary withdrawn from US market in 2008.10 However OSP solution is still in use in some countries, as Portugal. The pathophysiology of APhN involves transient hyperphospatemia, volume depletion exacerbated by concurrent ACEI, ARB and diuretics, and elevated distal tubular phosphate and calcium concentrations.1,8,10 Risk factors include advanced age, chronic kidney failure, dehydratation, female gender, diuretics, a history of colitis, and, probably, diabetes mellitus and non-steroidal anti-inflammatory drugs.1,10 Data indicate a high risk for chronic renal failure.1,9,10 Our first patient needed long term haemodialysis. In Markowitz series none patient returned to their baseline creatinine levels and 19% progressed to ESRD at mean of 13.8 months after colonoscopy.9 In conclusion, these cases highlight the importance of AphN because such OSP are still used in clinical practice. Clinical presentation may assume two forms and, in any of these, consequences are serious and sometimes fatal. Strategies to prevent the development of APhN should be adopted and include avoidance in highrisk patients, adequate hydratation, dose minimization, increasing the interval between doses and possibly not administering ACE-I, ARB, diuretics and NSAID on the day before and the day after colonoscopy procedure.6,10 It is also advisable to perform serum biochemistry tests after the procedure, in order to detect any renal or electrolyte abnormalities.7 1. Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy-a meta-analysis. Colorectal Dis 2006;8(4):247-58. 2. Lien YH. Is bowel preparation before colonoscopy a risky business for the kidney? Nat Clinl Pract Nephrol 2008;4(11):606-14. 3. Heher EC, Their SO, Rennke H, Humphreys BD. Adverse renal and metabolic effects associated with oral sodium phosphate bowel preparation. Clin J Am Soc Nephrol 2008;3(5):1494503. 4. Bennouna M, Anaya S, De la Nieta DS, Rivera F. Enema in a patient with renal failure: a cause of severe hyperphosphatemia. Nefrologia 2008;28(6):657-9. 5. Hurst FP, Boehn EM, Osgard EM, Oliver DK, Das NP, Gao SW. Association of oral sodium phosphate purgative use with acute kidney injury. J Am Soc Nephrol 2007;18(12):3192-8. 6. Frizelle FA, Colls BM. Hyponatremia and seizures after bowel preparation: Report of three cases. Dis Colon Rectum 2005;48(2):393-6. 7. Desmeules S, Bergeron MJ, Isenring P. Acute Phosphate Nephropathy and renal failure. N Eng J Med 2003;349(10):1006-7. 8. Markowitz GS, Nasr SH, Klein P, Anderson H, Stack JI, Alterman L et al. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleasing. Human Pathol 2004;35(6):675-84. 9. Markowitz GS, Stokes BM, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol 703 letters to the editor 2005;16(11):3389-96. 10. Markowitz GS, Perazella MA. Acute phosphate nephropathy. Kidney Int 2009;76(10):1027-34. P. Santos1, A. Branco1, S. Silva1, A. Paiva1, J. Baldaia1, J. Maximino1, A. Loureiro1, R. Henrique2 1 Nephrology Unit. Internal Medicine Department. Pedro Hispano Hospital. Matosinhos (Portugal). 2 Department of Pathology. Portuguese Institute of Oncology. Porto (Portugal). Correspondence: P. Santos Nephrology Unit. Internal Medicine Department. Pedro Hispano Hospital. Matosinhos. Portugal. [email protected] Parvovirus B19 infection: diagnosis and treatment in a kidney transplant patient Nefrologia 2010;30(6):704 doi:10.3265/Nefrologia.pre2010.Jul.10429 To the Editor, Parvovirus B19 infection has an incidence of 2% after solid organ transplants. Parvovirus B19 is an erythrovirus belonging to the Parvoviridae family, which usually replicates in human erythrocyte precursor cells. Clinical symptoms include fever, joint pains, and a rash in 25%, 7%, and 6% of cases, respectively. Anaemia is the most common expression of the virus (99%). Diagnosis of the infection by serology (IgM and IgG) is not reliable due to the delayed and inadequate antibodymediated immune response in immunocompromised patients. Using polymerase chain reaction (PCR) improved the detection of parvovirus B19 infection, and the precision rate for positive PCR in immunocompromised hosts is higher when associated with red cell aplasia. If serology and PCR tests are negative, a bone marrow study with in situ hybridization and immunohistochemical techniques may 704 also clarify the diagnosis. At present, no treatment with anti-viral drugs has been described. However, high doses of immunoglobins (IVIg therapy) were shown to have a beneficial effect on the infection in patients receiving kidney transplants. The optimal dose and duration of treatment have not been established as some patients require additional courses of drugs. Furthermore, reducing immunosuppression is a priority. We present the case of a 36-year-old male diagnosed with chronic kidney failure of unknown aetiology. He was admitted for haemodialysis in 2008 and then in January 2010 he received a kidney transplant from a 9-year-old cadaveric donor with a history of cranio-encephalic trauma (CET). He was given thymoglobulin induction therapy, corticosteroids and mycophenolate sodium. Tacrolimus, deltisone and mycophenolate sodium were given as maintenance treatment. He received ganciclovir prophylaxis for cytomegalovirus (CMV), and trimethoprim-sulfamethoxazole (TMPSMX) for PCP. The patient had urinary fistula on the third day after the transplantation, which was corrected with surgery. Two weeks later, he suffered diarrhoea associated with mycophenolate sodium, and severe normocytic anaemia (Hct 19%), normochromic anaemia with reticulocytopenia, without changes in the series of white blood cells, and negative Coombs’ test on platelets. He needed multiple transfusions due to symptomatic anaemia. In February 2010, he had a fever syndrome and a urinary infection due to Acinetobacter sp., and was given treatment with amikacin. The patient continued with anaemia with the same characteristics described above, associated with joint and muscle pain. A ferrokinetic study was requested, showing normal parameters. The maximum dose of erythropoietin was increased with no response. A study of blood in stools and serial parasitological examinations of faecal samples were negative. We performed an upper and lower endoscopy with no signs of haemorrhages; a video capsule endoscopy: s/p, blood smear analysis with PAMO: erythroid hypoplasia, with normal white blood cell and platelet counts. Parvovirus B19 IgG and IgM antibodies tests were positive, and PCR test for parvovirus B19 was positive. Treatment with IVIg at 400 mg/kg/day (30 g/day) was prescribed for 5 days. Mycophenolate sodium treatment was stopped. Kidney function remained stable (average creatinine 1.4 mg/dl); Hct levels increased 10 (35%) and 14 (38%) days after treatment, as did the number of reticulocytes. The follow-up parvovirus PCR (8 weeks after treatment) was negative. Immunosuppression treatment with tacrolimus and deltisone was continued. DNA PCR testing will continue on a regular basis. 1. Eid A, et al. Parvovirus B19 in Solid Organ Transplant Recipients. Am J Transplant 2009;9(Suppl 4):S147-S150. 2. Manaresi E, et al. Diagnosis and quantitative evaluation of parvovirus B19 infections by realtime PCR in the clinical lab. J Med Virol 2002;67:275-81. L.R. León, D. Curcio, D. Casadei Nephrology and Renal Transplant Department. Nephrology S.A. Caba. Buenos Aires (Argentina) Correspondence: L.R. León Servicio de Nefrología y Trasplante Renal, Nephrology S.A., Cabello 3889, 1425, Caba, Buenos Aires, Argentina. [email protected] Disseminated tuberculosis with splenic abscesses during haemodialysis Nefrologia 2010;30(6):704-6 doi:10.3265/Nefrologia.pre2010.Aug.10617 To the Editor, Haemodialysis patients are more likely to develop infections due to changes in their immune response Nefrologia 2010;30(6):698-713 letters to the editor associated with kidney failure. The use of some immunosuppressor drugs and pathologies such as diabetes also make infections more likely. 1-4 In recent years we have witnessed an increase in the incidence of tuberculosis (TB) in the general population, and as a result in the haemodialysis population as well. 1 The diagnosis of TB in these patients is hindered by its insidious and unspecific medicial symptoms. Also, some diagnostic tests and screening fail to identify the disease in this group of patients.3,5 We present the case of a 67-year-old male patient in a haemodialysis program with a history of high blood pressure, a positive blood test for hepatitis C virus, and chronic kidney disease due to nephroangiosclerosis. He began haemodialysis in 1989, had a kidney transplant in 1990; he has post-transplant diabetes mellitus; he began dialysis again in September, 2007. He has been in treatment for 3 months with prednisone at 30 mg/day due to an initial clinical suspicion of kidney graft rejection. He was admitted with week-long fever and night sweats without apparent source. The case history of the patient and physical examination were not specific, only revealing asthenia and weight loss in the last few months. Blood, urine, pleural liquid and sputum cultures were negative, as were x-rays of the chest and abdomen. He began broad-spectrum antibiotic therapy. The study was continued to find the source of the fever. The results of transthoracic and transoesophageal echocardiography were normal. Staphylococcus aureus was isolated from only one of the subsequent blood cultures, so the antibiotic treatment was changed and transthoracic and transoesophageal echocardiograms were performed again. These ruled out endocarditis. In view of the persistent fever, antifungal treatment was added. Tests revealed: PCR 13 U, total protein 5.1 g/dl, albumin 2.3 g/dl, leukocytes 3600 mm3 (80% neutrophils), Hgb 12.4, hematocrits 37%, platelets 102 000 x 10.9 Abdominal ultrasound showed splenomegaly with multiple small, well-defined hypoechoic Nefrologia 2010;30(6):698-713 areas of up to 1cm in diameter which could correspond to multiple splenic microabscesses. The patient underwent a thoracoabdominal CT scan which showed multiple mediastinal lymphadenopathies of up to 2cm in diameter in the retrocavalpretracheal, para-aortic, upper and lower right paratracheal, and subcarinal spaces. Bilateral pericardial and pleural effusion. Mosaic attenuation of lung parenchyma. CT scan showed at least 3 pseudonodular focal masses of around 1cm in diameter in a peripheral, subpleural location in an anterior segment of the right upper lobe, and 2 masses in the left upper lobe. Irregular interstitial and acinar infiltrates at the base of the abdomen and pelvis, splenomegaly with multiple undefined hypodense focal areas of less than 1cm compatible with splenic microabscesses; at least 2 small hypodense punctiform masses in the liver (Figure 1). This suggested a differential diagnosis between microcytic pulmonary carcinoma and lymphoma. A bone marrow biopsy showed hypoplasia, with no evidence of neoplastic cells. Gallium-67 scintigraphy revealed no source of inflammatory/infectious activity. PET/CT scan revealed hypermetabolic adenopathies in cervical, supraclavicular, axillary and mediastinal regions, and multiple hypermetabolic lung nodules in subpleural regions. Splenomegaly showed multiple hypermetabolic nodules, and hypermetabolic focal lesions in the C3, C4, and D8 vertebral bodies, and the fourth costal arch. Despite broad-spectrum antibiotic and antifungal treatment, the patient still suffered fever spikes with Figure 1. CT scan with contrast at the level of the spleen with multiple lesions. significant constitutional syndrome and weight loss. All the cultures, blood tests, the intradermal PPD test, bacilli sputum cultures, pleural fluid, and urine, as well as pleural fluid adenosine deaminase were repeatedly negative. A mediastinoscopy was performed with biopsies of 2 adenopathies. The pathologic diagnosis was described as necrotic granulomatous lymphadenitis highly indicative of mycobacterial infection. No acid-alcohol-resistant bacilli or fungi were found in the histochemical stains. In view of this, and the patient’s symptoms, with a strong suspicion of tuberculosis infection, we decided to begin treatment with tuberculostatic drugs (rifampin, isoniazid, and ethambutol). The patient then progressed favourably; the fever remitted, his general condition improved and he gained weight, even enabling physiotherapy and mobility. A control CT scan 2 months after beginning treatment showed a significant reduction in the size and number of mediastinal adenopathies, fewer paratracheal adenopathies, a decrease in the size and density of the parenchymal lesions in the lungs, and the disappearance of the splenic masses. Only a hypodense lesion remained at the anterior pole of the spleen which was smaller than in the previous CT scan (Figure 2). Therefore, the diagnosis was disseminated TB probably due to TB reactivation in the patient in dialysis under chronic corticosteroid treatment. The diagnosis was made using anatomical pathology and the patient’s clinical manifestations. In this type of cases, diagnosing the infection is difficult because the detection and laboratory tests are usually negative. It is also difficult because patients have unspecific clinical symptoms, and due to its extrapulmonary location; thus, the diagnosis and onset of treatment are usually delayed.5 We agree with other authors that levels of suspicion need to be maintained high and early empirical treatment needs to be administered which reduces mortality. 705 letters to the editor Cocaine use, high blood pressure and chronic kidney disease Nefrologia 2010;30(6):706-7 doi: 10.3265/Nefrologia.pre2010.Sep.10594 Figure 2. CT scan with contrast at the level of the spleen after 2 months of treatment with tuberculostatic drugs. 1. Caminero JA, Caylà JA, Lara N, and the working group on the current Status of TBC in Spain. Evolution of TBC trends in Spain, 1991-1999. Int J Tuberc Lung Dis 2000;7:1-7. 2. Resic H, Dizdarevic Z, Coric A, Avdic E, Kukavica N, Mesic E. Evaluation of clinical presentation and prognosis of tuberculosis in patients undergoing hemodiálysis. Acta Med Croatica 2008;62(1):65-8. 3. Aladrén MJ, Vives PJ, Celorrio JM. Detección y prevención del desarrollo de tuberculosis en pacientes en hemodiálisis ¿Un antiguo problema actual? Nefrologia 2004;25(3):253-60. 4. Klote MM, Agodoa LY, Abbott KC. Risk factor for Mycobacterium tuberculosis in US chronic dialysis patients. Nephrol Dial Transplant 2006;21(11):3287-92. 5. Christopoulos AI, Diamantopoulos A, Dimopoulos P, Goumenos D, Barbalias G. Risk factors for tuberculosis in dialysis patients: a prospective multi-center clinical trial. BMC Nephrology 2009;10:36. B. Moragrega1, R. Dolz2, I. López Alejandre3, A. Núñez Sánchez1 1 Nephrology Department. Obispo Polanco Hospital. Teruel.Spain. 2 Internal Medicine Department. Hospital Obispo Polanco. Teruel. Spain. 3 Nephrology Department. San Juan de Dios Hospital. Zaragoza. Spain. Correspondence: B. Moragrega Sección de Nefrología. Hospital Obispo Polanco. Ruiz Jarabo, s/n. 44002 Teruel. Spain. [email protected] 706 To the Editor, Cocaine use is associated with multiple complications, the most common being cardiovascular and neurological disorders. 1 In recent years, knowledge about the role of cocaine in acute and chronic kidney injuries has increased. Regular and continual use can lead to severe arterial hypertension (AH) and terminal chronic renal failure.2-5 We present the case of a 26-year-old man, a habitual user of inhaled cocaine for 6 years, seen at the emergency department with dyspnoea on minimal exertion and headaches. He had a blood pressure of 200/110 mm Hg, a chest x-ray with butterfly pattern and a plasma creatinine level of 10 mg/dl. Creatinine kinase levels were normal, and neither microhematuria nor proteinuria were found. The patient denied having taken cocaine in the last month due to his worsening general health, but a urine sample was not taken to rule it out. Haemodialysis was begun via a right jugular catheter with evident clinical improvement. Of note in his medical history were 2 admissions to another hospital. The first was 2.5 years earlier due to a hypertensive emergency with acute pulmonary oedema requiring oral tracheal intubation and mechanical ventilation. He was also suffering from acute renal failure with a plasma creatinine level of 5 mg/dl in the context of rhabdomyolysis after inhaling cocaine. Doppler echocardiography showed severe concentric hypertrophy of the left ventricle and moderately depressed systolic function. The Doppler echocardiogram and catecholamine levels were normal. Kidney function recovered completely without dialysis. The second admission, 6 months before this one, was due to chest pain after consuming cocaine; the level of troponin enzymes was normal, but level of plasma creatinine of 2.3 mg/dl remained high. Therefore, the patient continued to take irbesartan, amlodipine, carvedilol, and torsemide to control his blood pressure. Later, the patient stopped having medical checkups. Six months after beginning dialysis, with no improvement in renal function, a kidney biopsy was performed which showed that 10% of the glomeruli had global sclerosis, and the rest showed varying degrees of mesangial expansion, 12% with segmental hyalinosis, and 40% with moderate fibrosis of Bowman’s capsule. The medium and small-sized arteries showed significant lumen reduction with hyalinization of the intima and media, and intimal proliferation (Figure 1). Immunofluorescence was negative. After 8 months without using cocaine, the patient had stable blood pressure of 135/85 mm Hg and needed treatment with 5 antihypertensive drugs and haemodialysis 3 times per week. The most common kidney complications due to cocaine abuse are rhabdomyolysis or severe AHT.2 The mechanism linking cocaine with rhabdomyolysis is unclear, but it could involve ischaemia due to vasoconstriction and vasospasm caused by cocaine’s sympathomimetic action. This triggers tissue hypoxia with myocytic necrosis, direct muscle toxicity, hyperpyrexia, and an increase in muscle activity with repeated trauma due to the agitation after consumption.2,6 Chronic cocaine use sets in motion chronic and haemodynamic changes mediated by the increase in oxidative stress and the stimulation of the reninangiotensin system, which lead to an increase in mesangial expansion, tubulointerstitial fibrosis, and greater atherogenesis.4,5 Furthermore, chronic cocaine use can cause a permanent circle of vasoconstriction, AHT, and kidney failure.7 Blood pressure can be extremely high and often related to the degree of kidney disease, and it can be resistant to treatment.2 Nefrologia 2010;30(6):698-713 letters to the editor 5. 6. Figure 1. Arteries with significant lumen reduction due to the hyalinization and proliferation of the intima and media. This is the first case in our hospital of chronic kidney failure with the need for replacement therapy due to cocaine use. It is also worth highlighting in the patient’s medical history a proven episode of acute kidney failure due to rhabdomyolysis and severe, hard-to-control AHT. He also suffered from heart disease in the form of severe hypertrophy of the left ventricle with a depressed ejection fraction; a pathology described in and related with cocaine consumption. Besides, cocaine is known to lead to ischaemic cardiomyopathy in the form of angina pectoris, acute myocardial infarction, and episodes of cardiac arrest due to arrhythmias, even occurring in sporadic or one-off cocaine users.8 As nephrologists, it is necessary to understand the extensive range of diseases that cocaine can produce in the kidneys, taking into consideration its high, ever-increasing rates of consumption. 1. Furaz K, Bernis C, Cirugeda A, Pérez A, Sánchez JA. Infarto renal e insuficiencia renal aguda por consumo de cocaína. Nefrologia 2008;3:347-9. 2. Van der Woude FJ. Cocaine use and kidney damage. Nephrol Dial Transplant 2000;15:299-301. 3. Van der Woude FJ. Severe renal arterioarteriolosclerosis after cocaine use. Nephrol Dial Transplant 1999;14:434-5. 4. Norris KC, Thornill-Joiynes M, Robinson Nefrologia 2010;30(6):698-713 7. 8. Ch, Strickland T, Alperson BL, Witana SC, et al. Cocaine use, Hypertension, and End-Stage Renal Disease. Am J Kidney Dis 2001;38(3):523-8. Jaffe JA, Kimmel PL. Chronic Nephropaties of Cocaine and Heroin Abuse: A critical Review. Clin J Am Soc Nephrol 2006;1:655-67. El-Hayek BM, Nogué S, Alonso D, Poch E. Rabdomiólisis, síndrome compartimental y fracaso renal agudo asociados a consumo de cocaína. Nefrologia 2003;5:469-70. Amodedo ML, Craver L, Marco MP, Fernández E. Cocaine-induced acute renal failure without rhabdomyolysis. Nephrol Dial Transplant 1999;14:2970-1. Lange LA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med 2001;345(5):351-9. M. Picazo Sánchez1, M. Cuxart Pérez 1, F. Martín Romero2, R. Sans Lorman1 1 Nephrology Department. Fundació Salut Empordà. Figueres Hospital. Figueres. Girona. Spain. 2 Anatomical pathology Department. Fundació Salut Empordà. Figueres Hospital. Figueres. Girona. Spain. Correspondence: M. Picazo Sánchez Servicio de Nefrología. Fundació Salut Empordà. Hospital de Figueres. Figueres. Girona. Spain. [email protected] Delayed spontaneous rupture of the kidney graft Nefrologia 2010;30(6):707-9 doi:10.3265/Nefrologia.pre2010.Aug.10591 To the Editor, Spontaneous ruptures of kidney grafts are an uncommon but potentially severe complication of kidney transplants nowadays. They can lead to the loss of the graft or even to death.1 Prior to calcineurin inhibitors, this complication was quite common, and it was mainly caused by acute rejection (AR). After the introduction of cyclosporine, the most common aetiologies are renal vein thrombosis 2 and acute tubular necrosis (ATN). 3,4 Cases associated with trauma to the graft have also been described. 5 Most cases of graft rupture occur in the first 3 weeks after the transplant. 6 It rarely occurs later. The most frequent symptoms are the triad of pain over the graft, oliguria and hypotension 7 and the diagnosis can be confirmed with ultrasound and CT scans. 8 In the past, an emergency nephrectomy was the most common treatment in unstable patients, but more recently it has been possible to save up to 80% of the grafts 9 using aggressive haemodynamic stabilization and emergency surgical repair. 10 We present the case of a 50 year old male kidney transplant recipient of 9 months of evolution who went to hospital after suffering anuria for 12 hours and back pain on the same side as the graft. Of note in his medical history was CKD, probably secondary to nephroangiosclerosis, treated with peritoneal dialysis for 2 years before the transplant, secondary hyperparathyroidism under treatment with cinacalcet, controlled chronic AHT, dyslipemia, and a tobacco habit prior to the transplant. He received his first kidney transplant in May 2007 from a 55 year old cadaveric donor, receiving immunosuppressor treatment with 2 doses of basiliximab, tacrolimus, mycophenolate sodium, and steroids. Renal function began immediately, and he progressed well and reached a basal creatinine level of around 1.5 mg/dl a month after the transplant. He underwent periodic check-ups as an outpatient, complying with his medication regime. A week before admission he had attended his programmed 9-month check-up, presenting with stable kidney function (creatinine 1.5 mg/dl, MDRD 60 ml/min, and haemoglobin 15.8 g/dl), with no signs of proteinuria or haematuria, and blood pressure of 136/80 mm Hg. An ultrasound scan 2 months before admission showed a renal graft of 122 mm in length, with normal echostructure and morphology 707 letters to the editor with no evidence of collections, RF 0.62-0.64. On the day of admission the patient presented with anuria, a mild fever of 37.5 ºC, no apparent signs of infection, blood pressure of 148/89 mm Hg, heart rate of 76 bpm. He was suffering left lumbar pain, and moderate pain in the left iliac fossa above the graft on palpation, with no other significant findings. Blood test: haemoglobin 13.6 g/dl, hematocrit 39.7%, total leukocytes 14,500/µl, neutrophils 85%, creatinine 7.7 mg/dl, urea 145 mg/dl, sodium 139 mEq/l, potassium 4.7 mEq/l, pH 7.34, sodium bicarbonate 21.2 mmol/l, INR 1,1, thromboplastin time 31 s. Urine analysis: 375 erythrocytes/µl and proteins 1000 mg/dl. Blood levels of tacrolimus: 8.5 ng/ml. The patient reported no trauma to the graft and had not undertaken strenuous physical activity. There were no changes in his medication and the patient assured his compliance with the treatment. Treatment until the day of admission included: prednisone 5 mg/24 h, mycophenolate sodium 720 mg/12 h, tacrolimus 1 mg/12 h, omprazole, atorvastatin, amlodipine, irbesartan and sodium bicarbonate. An emergency Doppler echocardiogram was performed showing the graft of 121 mm in the left iliac fossa (LIF) with increased cortical echogenicity, good perfusion, RF: 1. An organized perirenal haematoma of 93 x 37 x 60 mm was identified. To complete the study, a CT scan with contrast was performed showing enhancement of all the kidney with 2 hypoechoic linear lesions. These were interpreted as a fracture of the anterior pole of the graft (Figures 1 and 2). No aneurysm or kidney tumours were observed. In view of the patient being haemodynamically stable and the diagnosis of advanced kidney rupture, emergency surgery was ruled out. An initial session of haemodialysis was performed; diuresis began again after 48 h with progressive improvements in the analytical parameters. The diagnosis of AR was ruled out given the patient’s good 708 progress without having taken other therapeutic measures and the fact that his immunosuppressor levels were within the normal range. During this time we have not managed to characterise the aetiology of the episode, having ruled out the most common causes of graft ruptures such as AR, 11 renal vein thrombosis, 2 or trauma to the graft. 5 Subsequent ultrasound scans and 2 CT scans, 6 and 12 months after admission, showed no signs of ectasia, perirenal collections, angiomyolipoma, or tumours. We believe that the situation of anuria and acute kidney failure could be justified by the presence of acute tubular necrosis secondary to compression on the parenchyma by the haematoma. This was probably present on the days prior to admission. This case is of interest in that it involves a delayed spontaneous rupture of the graft in a patient with a stable kidney transplant with an evolution of 9 months which can not be attributed to the most common causes. 1. García Sánchez de la Nieta, MD, SánchezFructuoso AI, Alcázar R, Pérez-Contin MJ, Prats D, Grimalt J, et al. Graft Salvage Rate in Renal Allograft Rupture Associated With Acute Tubular Necrosis. Trasplant Proc 2004;36:3016-8. 2. Richardson AJ, Higgins RM, Jakowski AJ. Spontaneous rupture of renal allograft, the importance of renal vein thrombosis in cyclosporine era. Br J Surg 1990;77:558. 3. Chan YH, Wong KM, Lee KC, Li CS. Spontaneous renal allograft rupture attributed to acute tubular necrosis. Am J Kidney Dis 1999;34(2):355-8. 4. Busi N, Capocasale E, Mazzoni MP, Benozzi L, Valle RD, Cambi V, et al. Spontaneous renal allograft rupture without acute rejection. Acta Biomed 2004;75(2):131-3. 5. Ahmed S, Batiuk TD. Broken kidney: traumatic fracture of a renal allograft. Am J Kidney Dis 2001;37(4):E33. 6. Holchleimer WB, Kafka R, Spechtenhauser B, et al. Renal allograft rupture is associated with rejection or acute tubular necrosis, but not with renal vein thrombosis. Nephrol Dial Transplant 2001;16:124. 7. Lord RSA, Effeney DJ, Hayes JM, Tracy GD. Renal allograft rupture: cause, clinical features and management. Ann Surg 1973;177(3):268-73. 8. Soler R, Pérez-Fontán FJ, Lago M, Moncalián J, Pérez-Fontán M. Renal allograft rupture: diagnostic role of ultrasound. Nephrol Dial Transplant 1992;7(8):871-4. 9. He B, Rao MM, Han X, et al. Surgical repair of spontaneous renal allograft Figure 1. CT scan of the graft showing a perirenal collection of the graft compatible with fracture of the inferior pole (thin arrow). Figure 2. CT scan of the graft showing hypoechoic linear lesions compatible with fracture of the anterior pole (thin arrows). On the eighth day, after having no new complications, he was discharged with a serum creatinine level of 2.6 mg/dl. He then showed gradually improving renal function until reaching a serum creatinine level on day 14 close to the baseline level (1.7 mg/dl). Since then (2 years) his renal function has remained stable with no proteinuria or haematuria. Nefrologia 2010;30(6):698-713 letters to the editor rupture: a new procedure. ANZ J Surg 2003;73(6):381-3. 10. Shahrokh H, Rasouli H, Zargar MA, et al. Spontaneous Kidney allograft rupture. Transplant Proc 2005;37:3079-80. 11. Guleira S, Khazanchi RK, Dinda AK, et al. Spontaneous Renal Allograft Rupture: Is Graft Nefrectomy an Option? Trasplant Proc 2004;36:3016-8. J. Kanter Berga1, C. Cáceres Borrero1, T. Ripollés González2, A. Ávila Bernabeu1, E. Gavela Martínez 1, L. Pallardó Mateu1 1 Nephrology Unit. Dr. Peset. Hospital. Valencia. Spain. 2 Radiology Unit. Dr Peset Hospital. Valencia. Spain. Correspondence: J. Kanter Berga Unidad de Nefrología. Hospital Dr. Peset. avda., Gaspar Aguilar, 90. 46017 Valencia. [email protected] [email protected] Coexistence of anti-GBM antibodies and MPO-ANCA in a patient with systemic vasculitis and crescentic glomerulonephritis Nefrologia 2010;30(6):709-10 doi: 10.3265/Nefrologia.pre2010.Aug.10563 To the Editor, Anti-glomerular basement membrane (GBM) antibodies are sometimes detected in patients with sera that contain antineutrophil cytoplasmic antibodies (ANCAs), especially in those with specificity for myeloperoxidase (MPO).1-5 Double positive patients may have a clinical course and response to treatment more typical of vasculitis than of anti-GBM disease, and renal function recovery may be more likely if ANCAs are present. 1 Recent observations 3-5 however, have failed to detect the differences described in early reports. Here, we present a case of a 62 years-old Caucasian female with rhinoNefrologia 2010;30(6):698-713 sinusitis and asthma diagnosed 2 years before was admitted at our Department with renal failure requiring dialysis. She had anorexia, weight loss (15% of body weight), and weakness over the last 7 months. One month before the hospitalization she developed fever, persistent cough, dyspnoea, myalgias, arthralgias, numbness and weakness of lower limbs. Physical examination revealed pale skin, blood pressure of 135/78 mmHg, heart rate of 68 beats per minute, respiratory rate of 18 cycles per minute, body temperature of 36.3 °C, oliguria (350 ml/day). Cardiac, pulmonary and abdominal examination did not reveal any changes. There was no ocular inflammation, joint tenderness or effusion, and rash. Neither uedema nor tenderness of lower limbs, nor peripheral lymphadenopathies was present. Neurologic examination disclosed asymmetrical motor and sensory compromise of lower limbs. Laboratory disclosed microcytic hypochromic anemia (hemoglobin, 4.8 g/dl; mean globular volume, 76.9 fl; mean globular hemoglobin, 24.1 pg), leucocytosis (14.460/mm 3), eosinophilia (2.300/mm 3), thrombocytosis (880.000/mm 3), elevation of erythrocyte sedimentation rate (142 mm/hour) and of C-reactive protein (20 mg/dl), renal failure (uremia, 199 mg/dl; creatinemia, 6.1 mg/dl), and hiperkalemia (8.2 mEq/l). Urinalysis showed proteinuria of 100 mg/dl and 200 erythrocytes/microliter. Serum protein electrophoresis revealed IgG/K monoclonal gammopathy. Hepatic function tests, lactate dehydrogenase, calcemia, and phosphatemia were on the normal range. Renal ultrasound revealed normal sized kidneys, cortical hyperechogenicity, normal parenquimatous differentiation, and no hydronephrosis. Computed tomography (CT) of the face disclosed mucous thickening of the frontal, ethmoid, sphenoid and maxillary sinuses lining, and CT of the chest revealed ground-glass opa- cities widely spread across both lungs. Lower limbs electromiogram revealed severe multiple mononeuritis. Bone marrow biopsy disclosed eosinophilic hypercellularity and no morphologic abnormalities. A renal biopsy was performed and showed CGN with linear deposition of IgG along the glomerular capillaries (Figures 1 and 2). Serology for lupus (antinuclear, anti-double strand deoxyribonucleic acid, anti-Smith, extractable nuclear and anti-ribonucleoprotein antibodies) was negative. Serum C3, and C4 were on the normal range. Serology for human immunodeficiency virus types 1 and 2, hepatitis B, hepatitis C was also negative. Indirect immunofluorescence assay detected perinuclear ANCA (pANCA) (90 U/ml) and enzyme-linked immunosorbent assay (ELISA) revealed MPO specificity. Anti-GBM antibodies in serum (169 U/ml) were detected by direct ELISA. According to these, the diagnosis of CGN with double-positivity for anti-GBM anti- Figure 1. Kidney biopsy showing crescentic glomerulonephritis (Masson trichrome, x100). Figure 2. Immunofluorescence microscopy showing linear deposition of IgG along the glomerular capillaries (x400). 709 letters to the editor bodies and MPO-ANCA was established. She underwent intermittent hemodialysis, and immunosupressive therapy with metilprednisolone (15 mg/kg/day, 3 days, IV) followed by oral prednisone (1 mg/kg/day), cyclophosphamide (750 mg/m 2, monthly, IV), and plasma exchange with daily exchange of one volume of plasma for 5% human albumin for 14 days was initiated. She also received red blood cell transfusions. Two weeks later, the patient was asymptomatic, recovered diuresis, and improved renal function. At hospital discharge (day 38), uremia and creatinemia were 148 mg/dl and 2.5 mg/dl, respectively, and hemoglobin was 10.9 g/dl. She had no clinical or laboratorial evidence of disease relapse, and she remains out of dialysis. This case illustrates several interesting points. The patient presented with symptoms and signs in other organs suggesting systemic vasculitis. Serologic tests revealed coexistence of anti-GBM antibodies and MPOANCA, and histology showed CGN with linear deposition of IgG along the glomerular capillaries. Although the patient presented with renal failure requiring dialysis, there was renal function recovery after immunosupression with plasma exchange, without evidence of disease relapse. In a substantial proportion of patients with CGN double-positivity for anti-GBM antibodies and ANCAs (mostly MPO-ANCA) is detected. 1-5 Double-positive patients may have a clinical course and response to treatment more typical of vasculitis than of anti-GBM disease, and have possibly developed anti-GBM antibodies secondary to vasculitic glomerular damage. Some patients have symptoms and signs in other organs suggesting systemic vasculitis, and renal function recovery may be more likely if ANCAs are present.1 Recent observations 3-5 however, have failed to detect the differences described earlier. Rutgers et al. 4 reviewed 46 MPO-ANCA-positive, 10 double-positive and 13 anti-GBM-positive pa- tients with CGN. Creatinemia was lower in ANCA-positive patients compared to double-positive or antiGBM-positive patients (5.0, 10.3, 9.6 mg/dl, respectively; P = .01), and renal survival was different among the 3 groups (65%, 10%, and 15% of dialysis at 1 year, respectively; P = .04). Levy et al. 3 analyzed 27 patients with CGN and double-positivity for anti-GBM antibodies and ANCAs (mostly MPO-ANCA), and described patient and renal survival rates of 52% and 26%, respectively, at one year. Sixty-eight percent of patients were dialysis-dependent at presentation, and none of these recovered renal function, despite immunosuppression with or without plasma exchange. Although patients with CGN and double-positivity for anti-GBM antibodies and ANCAs may have a poor prognosis when presenting with severe disease, behaving more like anti-GBM disease than vasculitis, and recovery from severe renal failure may be rare, this case highlights that immunosupressive therapy with plasma exchange can improve patient and renal outcome in such patients. 1. Bolton WK. Goodpasture’s syndrome. Kidney Int 1996;50:1753-66. 2. Jannette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int 2003;63:1164-77. 3. Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int 2004;66:1535-40. 4. Rutgers A, Slot M, Van Paassen P, Van Breda Vriesman P, Heeringa P, Tervaert JW. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis 2005;46:253-62. 5. Yang G, Tang Z, Chen Y, Zeng C, Chen H, Liu Z, et al. Antineutrophil cytoplasmic antibodies (ANCA) in Chinese patients with anti-GBM crescentic glomerulonephritis. Clin Nephrol 2005;63:423-8. S. Gonçalves1, S. Jorge1 Nephrology and Renal Transplantation Department. Hospital de Santa Maria. Lisboa (Portugal) 1 Anatomical Pathology Department. Hospital de Santa Maria. Lisboa (Portugal) Correspondence: J.A. Lopes Nephrology and Renal Transplantation Department. Hospital de Santa Maria. Av. Prof. Egas Moniz, 1649-035 Lisboa. Portugal. [email protected] 2 Treatment with rituximab for a patient with p-ANCA glomerulonephritis, alveolar bleeding and multiple relapses on haemodialysis Nefrologia 2010;30(6):710-12 doi:10.3265/Nefrologia.pre2010.Aug.10535 To the Editor, The treatment of vasculitis associated with ANCA is still a topic of interest. Corticosteroids and cyclophosphamide are still the cornerstone in the treatment of vasculitis associated with ANCA. We must also point out the use of rituximab, a chimeric anti-CD20 antibody which has already been studied in several series of patients and which seems to have a beneficial effect on refractory patients or those with intolerance to the first-line therapy. It has also been used with other types of primary glomerulonephritis. Several outbreaks seem to appear during the course of vasculitis associated with ANCA. In patients with an established chronic disease on dialysis or kidney transplant recipients, relapse from the baseline disease i s u s u a l l y u n l i k e l y, b u t n o t i m possible. P. Fernandes1, J.A. Lopes1, L. Correia2, 710 Nefrologia 2010;30(6):698-713 letters to the editor We present the case of a patient diagnosed with vasculitis associated with ANCA with lung and kidney disease being treated with renal replacement therapy with haemodialysis. He suffered multiple outbreaks despite treatment with cyclophosphamide, corticosteroids and mycophenalate sodium. However, on beginning treatment with rituximab, he achieved full remission from the disease and ANCA levels returned to normal. A male of 65 years of age, ex-smoker, diabetic was admitted to hospital in 2005 due to minor haemoptysis, oedemas on lower limbs, polyneuropathy, deterioration in renal function, and creatinine levels of 6 mg/dl. He was found to have severe anaemia together with impaired renal function, proteinuria of 1.5 g/day, haematuria with dysmorphic red blood cells; he was positive for mpoANCA with a titre of 68.34 U/ml. A kidney biopsy was performed showing extracapillary glomerulonephritis with fibrinoid necrosis and crescent formations with negative immunofluorescence. In view of the diagnosis of pauci-immune extracapillary glomerulonephritis with pulmonary involvement, treatment was begun with 3 bolus of methylprednisolone at 1 g/day and monthly cyclophosphamide bolus. The patient was discharged with creatinine level of 2.9 mg/dl, and continued the treatment with monthly bolus cyclophosphamide and corticosteroids. A year after the diagnosis, in 2006, still in treatment with cyclophosphamide and corticosteroids, he had a new outbreak of vasculitis with a deterioration in renal function (Cr of 10.4 mg/dl), an increase in proteinuria and p-ANCA levels (188 U/l), with no evidence of alveolar bleeding. He was treated with methylprednisolone bolus. In view of the severity of the outbreak and the lack of renal function improvement, he was given renal replacement therapy with haemodialysis. Nefrologia 2010;30(6):698-713 During the follow-up, the patient was diagnosed with bronchiectasis, and was admitted on several occasions with superinfections of the lung. In 2007, a year after the onset of haemodialysis and in remission from the disease, he was re-admitted with a new outbreak of vasculitis with an episode of haemoptysis, muscle pain, asthenia, and worsening of the anaemia. ANCA levels were 100 U/l. He was treated with corticosteroids and i.v. cyclophosphamide, with a remission in the outbreak. Six months later, still in treatment with cyclophosphamide, he had a new outbreak characterized by fever, asthenia, haemoptysis and ANCA of 193 U/l. Treatment was begun with corticosteroids and cyclophosphamide, and mycophenolate was added as a maintenance therapy but was badly tolerated due to a digestive disorder and was abandoned. In view of the patient’s history of outbreaks of vasculitis despite the standard immunosuppressor treatment with cyclophosphamide, and the fact that he was undergoing renal replacement therapy with haemodialysis, it was decided to begin treatment with 4 doses (375 mg/m 2) of rituximab. Later, the patient continued treatment with 4 bolus of cyclophosphamide per month, remaining in remission and with ANCA negativisation 21 months after beginning treatment with rituximab, without any secondary complications to date. The pathogenesis of glomerulonephritis associated with ANCA is not totally clear. ANCA have been related with the pathogenesis of vasculitis, although the levels of ANCA in circulation do not always correlate with the level of activity of the disease. The generation of ANCA is determined by the activation of autoreactive B lymphocytes through the chronic stimulation of T lympho- cytes, which explains the relapsing nature of the disease. Furthermore, ANCA produce the liberation of free radicals and proteolytic enzymes which leads to damage of the vascular endothelium in adjacent tissues.1 Immunosuppressor treatment of the outbreaks in patients on dialysis does not differ from the normal treatment for patients with a generalised disease. Regarding the maintenance therapy, various options have been attempted to reduce the toxicity associated with cyclophosphamide, such as azathioprine, mycophenolate mofetil, and leflunomide.2 In refractory cases, rituximab has been shown to be effective in achieveing remission from the disease in several series of patients. Rituximab is a chimeric anti-CD20 antibody which depletes B lymphocytes, so it has been proposed as a rescue therapy in refractory disease, as the aetiopathogenetic importance of B cells in the generation of ANCA is well known. 3 As we have mentioned above, relapse of vasculitis is relatively uncommon in patients on haemodialysis, and it is even rarer to find patients such as ours with multiple relapses in spite receiving standard immunosuppressor therapy and haemodialysis. Little experience has been acquired into the use of rituximab as a rescue therapy in haemodialysis patients. However, reviewing the bibliography, rituximab treatment was assayed in a patient with a history of terminal chronic disease on haemodialysis as a lymphoma B cell treatment, showing that therapeutic levels of the drug remain in the blood despite haemodialysis, so it is not necessary to adjust the treatment doses. 4 Another patient with nonHodgkin lymphoma requiring haemodialysis was treated with rituximab with no evidence of an increase in side effects.5 711 letters to the editor In our case, treatment began with 4 doses of rituximab accompanied with a monthly bolus of cyclophosphamide, with which remission from the vasculitis outbreaks was achieved together with the reduction and later negativisation of the levels of ANCA. This suggests that rituximab may be an effective drug as a rescue therapy in patients on chronic haemodialysis with a vasculitis relapse. 1. Csernok E, Ai M, Gross WL, et al. Wegener autoantigen induces maturation of dendritis cells and licences them for Th1 priming via the protease - activated receptor-2 pathway. Blood 2006;107(11):4400-8. 2. Jayne DR, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003;349(1):36-44. 3. Wong C. Rituximab in refractory antineutrophil cytoplasmic antibodyassociated vasculitis: what is the current evidence?. NDT 2007;22:32-6. 4. Jillella AP, Dainer PM, Kallab AM, Ustun C. Treatment of a patient with end-stage renal disease with Rituximab: pharmacokinetic evaluation suggest Rituximab is not eliminated by hemodialisis. Am J Hematol 2002;71(3):219-22. 5. Feldman G, Nattermann J, Gerhart T, Nähle CP, Spengler U, Woitas R. Partial remission of a newly diagnosed diffuse large B-cell Non Hodgkin’s lymphoma in a hemodialysis patient after administration of inmunochemotherapy with Rituximab-CHOP. Int J Lab Hematol 2007;29(6):469-73. M.A. Azancot, I. Agraz Pamplona, J. Fort Ros, A. Marín Valencia, I. Gil Carballeira, J. Camps Domenech Nephrology Department. Vall d’Hebron Hospital. Barcelona, Spain. Correspondence: I. Agraz Pamplona Sección de Nefrología. Hospital Vall d’Hebron. Barcelona. Spain. [email protected] 712 Kidney failure and diabetes. Diagnostic inertia? Nefrologia 2010;30(6):712-3 doi:10.3265/Nefrologia.pre2010.Oct.10679 To the Editor, Diabetes mellitus (DM) is a common cause of kidney failure (KF), but this pathology is not the only aetiology in diabetic patients presenting with renal failure. We present the case of a 62 year old man with a history of arterial hypertension (AHT), duodenal ulcer, acute myocardial infarction (AMI) and type 2 DM, diagnosed in 2007. In February 2008, he was admitted to the internal medicine department with pyelonephritis with acute KF (MDRD 21 ml/min), which were interpreted in the context of the infection which was made worse by taking non-steroidal anti-inflammatory drugs (NSAIDs), and continued on discharge. In September 2008, he was referred to the nephrology department presenting with grade 4 KF (MDRD 13.83 ml/min), proteinuria of 5 g/24 h, and persistent microhematuria (negative urine cultures and negative cytology for malignancy). Of note in the rest of the analysis were: Negative ANA, ANCA, and Anti-GBM. Immunoglobulins, light chains, complements, and proteinogram were normal. Blood cultures for HCV, HBV, and HIV were negative. Abdominal ultrasound: kidneys of normal size with moderately cortical echogenicity with no evidence of dilation of the excretory tract. Normal ocular fundus. In view of the rapid evolution of the KF, without a clear aetiology being known, a kidney biopsy was performed which revealed 21 glomeruli, 6 of them sclerotic and the rest with glomerular mesangial expansion. A crescent formation was observed in one of these. Thickening of the tubular basal membrane and moderate in- Figure 1. E.M: Postinfectious GN. Presence of subepithelial electron-dense deposits (humps). terstitial inflammatory infiltrates was seen, with a predominance of plasma cells, polymorphonuclear neutrophils, and lymphocytes. Immunofluorescence was negative. An electronic microscope showed diffuse thickening of the glomerular basal membrane with the presence of subepithelial electron-dense deposits forming the typical humps (Figure 1). This finding lead to a diagnosis of post-infectious glomerulonephritis (PIGN) complicating a nephropathy. 1. Pham TT, Sim JJ, Kujubu DA, et al. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 2007;27:322. 2. Fingerhut D. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007;49(Suppl 2). 3. Coppo R, Gianoglio B, Porcellini MG, Maringhini S. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant 1998;13:293. 4. Haas M. Incidental healed postinfectious glomerulonephritis: a Nefrologia 2010;30(6):698-713 letters to the editor study of 1012 renal biopsy specimens examined by electron microscopy. Hum Pathol 2003;34(1):3-10. 5. Nasr SH, Markowitz GS, Stokes MB, Said SM, Valeri AM, D’Agati VD. Acute postinfectious glomerulonephritis in the modern era: experience with 86 adults and review of the literature. Nefrologia 2010;30(6):698-713 Medicine (Baltimore) 2008;87(1):2132. R. Blanco García 1, J.J. Bravo López 2, A. Pérez 3, M. Moreiras Plaza 1 1 Nephrology Department. Hospital Xeral de Vigo. Spain 2 Nephrology Department. Complexo Hospitalario de Ourense. Spain. 3 Servicio de Anatomía Patológica. Hospital Xeral de Vigo. Spain. Correspondence: Raquel Blanco García Servicio de Nefrología. Hospital Xeral. Vigo. Spain.. [email protected] 713 In memory of Professor Saulo Klahr E.J. Fernández Ruiz Nephrology Department. Hospital Universitario de Puerto Real. Cadiz, Spain Nefrologia 2010;30(6):714 doi:10.3265/Nefrologia.pre2010.Oct.10654 Dear colleagues, allow me to describe to you a famous nephrologist, a maestro of maestros, whose biography is humble yet brimming with work and teaching deserving of our most heartfelt admiration and gratitude. I am talking about our friend Professor Saulo Klahr who died on 3rd of June at home in St. Louis, capital of the north American state of Missouri, after a long and terrible illness, and surrounded by his beloved family. A few days ago I read this simple yet powerful headline in an online Columbian newspaper: “Cauca nephrologist dies, a leading figure in United States”. These few words captured who he was – a nephrologist doctor who was born in his beloved Colombia and who ended his days in the most advanced country in the world, deservedly considered a leading figure, or “sublime”, “outstanding among his peers”. Saulo Klahr was born in 1935 in Santander de Quilichao, in the Cauca region to the South of the Republic of Colombia. From a young age he wanted to become a doctor, and so he moved away to attend the Universidad Nacional de Bogotá, where he achieved his Bachelors Degree at the Faculty of Medicine in 1961. Straight after this he applied to complete his residency and specialism at the Washington University School of Medicine in St. Louis and was accepted that same year. It was there, 2 years later, where he met Eduardo Slatopolsky, who says “from then on he was my friend, my closest friend”. Also, at that time he fell head over heels in love with Carol de Clue, a beautiful nurse at the Metabolic Unit, who he married in 1965. Carol was an adorable friend and loyal wife who Saulo has leaned on throughout his life; they worked side by side, and had two sons, James and Robert. Neither followed the same profession as their father, they studied law. Both married and gave their parents four lively little grandchildren to fill the grandparents’ home with happiness. Regarding scientific matters, we can categorically state that the professional life of Professor Klahr, which he developed entirely at the Renal Division of Washington University Hospital, St. Louis was brilliant and spectacular. Saulo was a brilliant man who had a great ability to work, he was a workaholic with a photographic memory, very meticulous and as Eduardo Slatopolsky says, he was “always prepared”. We should remember that when the young Saulo left his home land and arrived at this prestigious and enormous American hospital, the Director of the Renal Division was none other than Neal S. Bricker, a great researcher who developed the “intact nephron hypothesis”, which is prestigiously recognised. In only 10 years Saulo Klahr advanced his career from resident doctor to Professor of Medicine and then in 1972 714 he became Director of this Renal Division, a position he held for 20 years. In 1986 he was named the Joseph Friedman Professor of Renal Diseases in Medicine and in 1991 he became Chair of Medicine at Jewish Hospital in St. Louis. We would need a lot more time and space to be able list Dr. Klahr’s scientific accomplishments. Throughout his life he published more than 500 articles, most of which came from his research investigations, and he wrote around 20 books, most of these dedicated to making it easier to learn about the specialism of first generation of nephrologists in the world. Therefore, when some of us were just beginning our compulsory selfdirected study on dialysis and alterations in hydroelectrolytic metabolism the early seventies, faced with the uncertainty of a specialism yet to be born, it was Saulo Klhar’s books which helped us most. For some, he was a “long distance maestro”. I had the honour and pleasure of meeting Professor Saulo Klhar in 1980 in Seville. It was during the “Endocrinological Conference” one of the annual meetings arranged by the then Head of Medical Pathology at the Universidad de Sevilla, Professor Antonio Aznar. Our good friend Francisco Llach took part in these meetings, who at the time was at Oklahoma University; every year he would come with very prestigious nephrologist colleagues from the United States to give us very interesting talks on new aspects of the new specialism. I fondly remember Eduardo Slatopolsky, Morton H. Maxwell, Charles R. Kleeman, Saul Massry, Solomon Papper, Jack W. Coburn, Robert Narins, and others. I have a nice anecdote to share. With a surname like Klahr, working in St. Louis in the State of Missouri, and having studied his text book in English “Renal and Electrolyte Disorders”, which was in the series “Differential Diagnosis” from Arco Publishers, I was convinced that the author ‘was’ an American professor. I purposely took the copy I used so much with me to Seville, because I really wanted him to sign it. So, when he was pointed out to me, I approached him and asked him (in the best English I could) if he would sign it for me. Imagine my surprise when he answered me in Spanish with his unique unaffected grace, “¡Pues claro que sí, cómo no, mi amigo, con gran placer!” (“Well, of course, without a doubt my friend, with pleasure!”). Right there in the corridor he wrote me an affectionate message in his book and he also let me know that he was from Colombia. Then he stepped onto the platform and with his calm, kind voice which he was known for he asked for “his first slide”. Four years later, in 1984, he edited his book “The Kidney and Body Fluids in Health and Disease”, which is still of huge value to nephrology residents today, and in 1995 he collaborated with Dr. Saulo Klahr, 1935-2010. R. Jacobson and Gary E. Striker on his recognised work The Principles and Practice of Nephrology. Since then we met at different conferences and events on our growing specialism, our friendship developed and in April 1993 when we organised our Andalusian Nephrology Society Meeting in Cádiz, he was kind enough to accept our invitation to participate. At that time, Saulo was principal researcher of the already classic Study “Modification of Diet in Renal Disease (MDRD)” in which attempts were being made at evaluating the impact of a low protein intake diet on the possibility of slowing the progression of renal failure. The work had started almost 2 years earlier and was in its final stages. We suggested that his main conference be based on these results. Of course he accepted without thinking twice. However, when he was about to begin his presentation he explained that because of the contract with The New England Journal of Medicine, he could not show any images of his results before they were published. So, without turning a hair, he went on to give a detailed explanation of all of his results, for around 45 minutes. Without showing a single slide! Professor Saulo Klahr received various prizes throughout his life and held positions of great responsibility. He was the 20th President of the American Society of Nephrology in 1985 and President of the National Kidney Foundation in 1988, taking the role of Editor-in-Chief of publications such as The Journal of Clinical Investigation, The American Journal of Kidney Diseases and Kidney International. In 1998 he received the John P. Peters prize from the American Society of Nephrology and in 2002 he was given the National Torchbearer Award from the American Kidney Foundation and the Edward N. Gibbs Award from the New York Academy of Medicine. In recent years we even had the opportunity to hear this gifted man speak at one of the great meetings named “Kidney and Hypertension” organised firstly by Fernando Valderrábano, and then by José Luño. On the last occasion, I noticed some subtle changes in him which gave us a sense of foreboding that the onset of Alzheimers had taken hold of this brilliant maestro. I want to end with the words of his soul mate Eduardo Slatopolsky: «Saulo, I miss you! Until we meet again…” Nefrologia 2010;30(6):714
© Copyright 2026