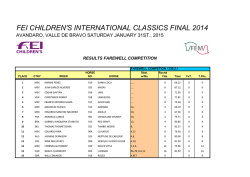
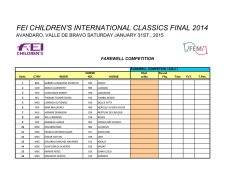
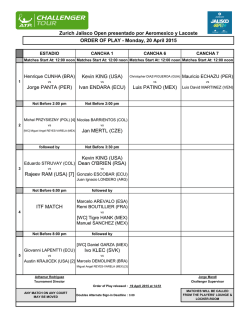
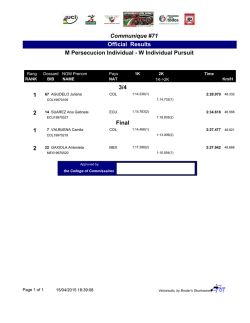
Exosomes Mediate the Cytoprotective Action of
DOI: 10.1161/CIRCULATIONAHA.112.114173 Exosomes Mediate the Cytoprotective Action of Mesenchymal Stromal Cells on Hypoxia-Induced Pulmonary Hypertension Running title: Lee et al.; Exosomes as paracrine vectors of MSC action Changjin Lee, PhD1,2; S. Alex Mitsialis, PhD1,2*; Muhammad Aslam, MD1,2; Sally H. Vitali, MD3,4; Eleni Vergadi, MD1,5; Georgios Konstantinou, MD1; Konstantinos Sdrimas, MD1; Angeles Fernandez-Gonzalez, PhD1,2; Stella Kourembanas, MD1,2* * 1 Equal contributors to this work as senior authors Div off Newborn New wbo b rn n Medicine; Med edic i in ic ne; 3Di Div D v of Critical Cri riti tica ti cal Care Ca Medicine, Med edic icin ic ne, Ch Chil Children’s ildr dren dr e ’ss H en Hospital ospi os pita pi tall Boston; Bost Bo stton on;; 2 Dept p off P Pediatrics; ediaatrrics;; 4D Dept ept pt ooff An Anaest Anaesthesia, theesiaa, H Harvard arrvaard M Medical eddicaal Sc School, cho ooll, B Boston, ostoon,, MA MA; 5 Present Pres Pr esen es en nt Ad Addr Address: d esss: U dr University niive vers rsiity rs ity of C Crete, rete re tee, He Hera Heraklion, r kl ra klio ion, n C n, Crete, rete re te,, Gr Greece ree eece ce Address Add Ad dress for for Correspondence: Correspondence: d Stella Kourembanas, MD Division of Newborn Medicine Children’s Hospital Boston 300 Longwood Avenue Boston, MA 02115 Tel: 617-919-2355 Fax: 617-730-0260 E-mail: [email protected] Journal Subject Codes: [18] Pulmonary circulation and disease; [130] Animal models of human disease; [147] Growth factors/cytokines 1 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 Abstract: Background—Hypoxia induces an inflammatory response in the lung manifested by alternative activation of macrophages with elevation of pro-inflammatory mediators that are critical for the later development of hypoxic pulmonary hypertension (HPH). Mesenchymal stromal cell (MSC) transplantation inhibits lung inflammation, vascular remodeling and right heart failure, and reverses HPH in experimental models of disease. In this study, we aimed to investigate the paracrine mechanisms by which MSCs are protective in HPH. Methods and Results—We fractionated mouse MSC-conditioned media to identify the biologically-active component affecting in vivo hypoxic signaling and determined that exosomes, secreted membrane microvesicles, suppressed the hypoxic pulmonary influx of macrophages and y and ppro-proliferative p g monocyte y the induction of ppro-inflammatory mediators,, including chemoattractant protein-1 and hypoxia-inducible mitogenic factor, in the murinee model mo odeel of HPH. HPH PH. ntravenous delivery of MSC-derived exosomes (MEX) inhibited vascular remodeling and HPH, Intravenous whereas MEX-depleted MEX-depl p eted media or fibroblast-derived exosomes had no effect. MEX suppressed he hypoxic hyppoxi hy xicc activation actiiva vati tion o of of signal sign si g al transducer tran nsdduccer e andd activator actiivatorr ooff transc sccript ptio ionn 3 (S STA AT3)) an andd th tthee the transcription (STAT3) uupregulation pre regu eg lation ooff tthe he mi miRR--17 ssuperfamily uper up erfa f mi fa m ly of microR m i RNA cclusters, lusster lu st rs, w hereaas itt in here ncrea creassedd lu lungg llevels ev vels miR-17 microRNA whereas increased off miR-204, miR iR-204,, a key key microRNA miccrooRNA mi oRNA A whose who hosse expression expres exp presssiion is is decreased deccre de crease easeed inn human huuman uman n PH. PH.. MEX MEX produced prodduced uced d by by huma mann umbilical um mbi b lica cal co cord rd M SCss in SC inhi hibi bited ST STAT AT33 si signal lin ingg in iisolated solate so tedd hu huma mann pu pulm lmonar aryy artery arrte tery ry human MSCs inhibited STAT3 signaling human pulmonary endothelial cells cell ce llss de ll demo mons mo n tr ns trat atin at ingg a di in dire rect ctt effect eff ffec e t of MEX ec MEX on on hypoxic hypo hy poxi po xic vasc vvascular asc scul ular ul ar ccells. ells el l. ls demonstrating direct Conclusions—This study indicates that MEX exert a pleiotropic protective effect on the lung and inhibit PH through suppression of hyperproliferative pathways, including STAT-3 mediated signaling induced by hypoxia. Key words: hypertension, pulmonary; inflammation; hypoxia; signal transduction 2 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 The lung’s response to low levels of environmental oxygen is multifactorial. Diverse signaling pathways, activated or impaired by alveolar hypoxia, converge on endothelial and vascular smooth muscle cells to perturb pulmonary vascular homeostasis. Chronic hypoxia results in pulmonary vascular remodeling, a key pathological feature of pulmonary hypertension (PH). Inflammation plays a prominent detrimental role in most types of human PH and also in animal models of the disease, such as the monocrotaline- and hypoxia-induced PH (HPH) in rodents. The early component of hypoxia-induced lung inflammation, peaking during the first two to three days of hypoxic exposure1, is characterized by alternative activation of alveolar macrophages and appears to be causal to the subsequent vascular remodeling and the development of HPH2. Despite the significant progress in our understanding of the pathophysiology of PH as well we ll as as the the treatment treeatm tr ment en of its symptoms, there is noo cure cuure for this disease dissea e see and and no single therapy has bbeen een n proven ef effe effective. fecttiv fe ve. G Given iven tthe iven he ccomplex he omppleex ppathways om athwaays in involved nvolv nvo olved in th the he ppathogenesis he athhogeene nesi siss off P PH, H H, therapies her erap apie ap i s aimed ie aime ai m d at more me mor oree than th n one one pathway pat athw hw way and andd perhaps perh hap apss more morre than mo tha hann one one cellular cell ce llul ullar a target tarrge gett may mayy prove prrov ve too be more efficacious. efffic icac accio ious us.. Stem us S em cell-based St cellll ba base s d therapeutic th herrap peu e ti t c approaches appr ap prroa oach ches ch es hhold olld su such ch a ppromise romi ro mise mi se as as they may y simultaneously target multiple signaling pathways and have long lasting effects. The therapeutic potential of mesenchymal stromal cells (MSCs; also referred to as mesenchymal stem cells or multipotent stromal cells) derived from the bone marrow, adipose, and other tissues, has been recognized in several animal models of lung disease3. In models of pro-inflammatory lung diseases, such as bleomycin or endotoxin-induced lung injuries4-6 as well as the neonatal murine model of bronchopulmonary dysplasia (BPD)7, 8, MSC delivery ameliorated lung injury, decreased lung inflammation and fibrosis, and increased survival. MSC delivery was reported to inhibit PH induced by monocrotaline in the rat9 and HPH in the mouse10. However, although a 3 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 robust protection against lung injury upon MSC treatment was observed in most of the above animal models, only a small fraction of donor cells were retained in the recipient lung. This observation suggested that engraftment and direct tissue repair was not the sole mechanism of MSC therapeutic function and paracrine mechanisms were contemplated. In support of this, we observed that injections with culture media conditioned by MSCs can efficiently inhibit parenchymal injury, vascular remodeling and right ventricular hypertrophy (RVH), completely supplanting MSC treatment on the neonatal murine model of BPD7, 11. In vitro experiments demonstrated anti-proliferative properties of the MSC secretome on pulmonary vascular smooth muscle cells10, again suggesting that MSC paracrine factors can play a major role in preventing lung ung injury and vascular remodeling. Concordant with our observations, paracrin paracrine ne aand ne ndd immunomodulatory mmunomodulatory paradigms have been recently proposed to account for enhanced MSC therapeutic herrap apeeuti euti ticc fu func function cti tioon on in the context of a number of ddisease isease modelss12. We ha have ave pre previously revi viou vi ouusl slyy pe perf performed rfor orrmed med pproteomic roteeom eomic aanalysis nalys ysis ys is ooff MS MSC-conditioned SC-co C-cond n itio nd io one nedd m medium edi d um di m7, wh w which ich in n aaddition ddit dd i io it ionn to iimmunomodulatory mm mun unom omod om odullat a or oryy fa fact factors, ctor ors, or s, rrevealed e ea ev eale leed th tthee pr pres presence esen es nce c ooff a num nnumber umber mber ooff pr pro proteins ot ins otei ns including ncluding CD63, CD D63 63,, CD81, CD81 CD 81,, moesin, moes mo esin es n, lactadherin l ct la ctad ad dhe heri r n (MFGE8), ri (MFG (M FGE8 FG E ),, hheat-shock E8 eatea t shhoc tockk pr prot protein otei ot einn 90 ei 9 ((hsp90), hsp9 hs p90) p9 0), and 0) hsp70, reported to be associated with secreted vesicles known as exosomes13, 14. Secreted membrane microvesicles, especially the better-defined subclass represented by exosomes15, have been recognized as important mediators of cell-to-cell communication and as participants in immunomodulatory mechanisms16. Exosomes are small heterogeneous microvesicles, 30~100 nm in diameter, that are stored within multivesicular bodies (MVB) and released into the environment upon fusion of the MVB with the plasma membrane. Exosomes and microvesicles have been isolated and characterized from various cell types including dendritic cells17, macrophages18, tumor cells19, and embryonic stem cells20 and information is rapidly 4 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 accumulating on their diverse biological function and their cell type-specific molecular composition. The physiologic relevance of MSC-derived exosomes (MEX) has not yet been evaluated in lung diseases, even though their cellular origin and the recently recognized paracrine function of MSCs may imply a promising therapeutic potential for secreted microvesicles in lung injury. To address the above questions, we fractionated MSC-conditioned media (CM) through size-exclusion chromatography to identify the biologically-active component protecting against hypoxia-induced lung inflammation and HPH. Using the murine model of HPH, we demonstrate here that MEX are the critical vectors of MSC action: MEX delivery in vivo suppressed HPH and vascular remodeling. Moreover, pro-proliferative pathways were also blocked bby y ME MEX X treatment, reatment, as evidenced by the suppression of signal transducer and activator of transcription (STAT3) phosphorylation miR-204, STA TAT3 T3)) ph T3 pho osphhor oryl y ation resulting in increased d llung unng levels off m iR R-204 04,, a microRNA enriched 04 in experimental n distal dis istal pulmonary pulm mon onar a y arterioles ar arrteri riol ri oles ol es that thatt iiss ddown-regulated own-rreg gulaated inn both both h hu hhuman mann PH aand ma nd d iin n ex exp peri peri rim menntal ntall models disease mo ode dels ls ooff di dis sease2211. We found seas foun fo unnd that that hypoxia hypox ypox oxia ia upregulates upr p egu egulat attess members memb memb mbeers ers of o the the miR-17 miR iR-1 - 7 fa -1 fami family milly ly ooff microRNA cclusters lu ust ster errs in lung lun u g tissue, tiiss s ue ue,, microRNAs m crroR mi oRNA NAss shown NA shhow ownn too bbee un unde derr th de thee re regu gula gu lato la to ory ccontrol ontr on t ol of tr under regulatory STAT3, and show that MEX treatment efficiently suppresses this pro-proliferative signal. Combined, our findings point to MEX as the key effectors of MSC paracrine function with the potential to serve as vehicles of lung-targeted therapy. Methods Animal model and hypoxic exposure. The HPH mouse model has been well-established and used by our group extensively in previously-published work 1, 2, 22. The hypoxic exposure and treatment protocols used in this 5 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 study are described in the Supplemental Material. All animal experiments were approved by the Boston Children’s Hospital Animal Care and Use Committee. Preparation of exosomes Isolation of mouse bone marrow-derived MSCs and MSCs from human umbilical cord Wharton’s Jelly (hUC-MSCs) followed by immunoselection (Supplemental Figure 1) and collection of conditioned media (CM) is outlined in the Supplemental Material. Concentrated conditioned media were applied on a column of 16/60 Hiprep Sephacryl S400 HR (GE Healthcare, Piscataway, NJ) that was pre-equilibrated with a buffer containing 20 mM sodium phosphate (pH 7.4) and 300 mM NaCl using an ÄKTA purifier liquid chromatography system (GE Healthcare, Piscataway, NJ). Fractions (1 ml) weree co collected oll l eccte tedd at a flow rate of 0.5 ml/min. Polystyrene nanospheres of 50 nm diameter (Phosphorex, Fall River, MA) fractions corresponding MA A) were weree uused seed as a size reference and elution fr rac a tions correspo ond n in ng to this standard’s retention analyzed. eteention volume volu umee were wer eree pooled pool pool oled ed d and andd further furt urthe ther an nalyzeed. nal For isolation fibroblasts, serum-free For th thee is sollat atio io on of eexosomes xoso some mess from me from hhUC-MSCs UC-M UC -M MSC SCss aand nd hhuman uman um an n ddermal errma mall fi fibr brrob obla lassts, la sts, se erum erum m-ffre r e culture medium filtered (0.2 concentrated mediium cconditioned ondi on d ti di t on o ed ffor orr 224 4 ho hhours urss wa ur wass fi filt ltterred (0. 0.22 μm 0. μm)) an aand d co conc ncen nc entr en t atted bby tr y ultrafiltration device with 100 kDa cut-off (Millipore). Exosomes in CM were precipitated with 1/3 volume of polyethylene glycol (PEG) buffer (33.4% PEG 4000, 50 mM HEPES (pH 7.4), 1 M NaCl) overnight at 4°C followed by centrifugation at 12,000 xg for 5 min and resuspension in PBS (pH7.4). Exosomes in PEG-precipitated fraction were further purified by S200 sizeexclusion chromatography. Seventy-five μl sample was applied on a S200 column (Clontech, Mountain View, CA) preequilibrated with PBS by spinning at 700 xg for 5 min and the exosomal fraction was subsequently eluted in the flow-through by centrifugation at 700 xg for 5 min. In some experiments, exosomes were isolated by ultracentrifugation at 100,000 xg for 2 6 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 hours and the pellet was subsequently washed with PBS followed by repeat ultracentrifugation for 2 hours at the same speed. Exosome pellet resuspended in PBS was measured for protein concentration by Bradford assay (Bio-Rad, Hercules, CA). Expression of exosomal markers between the two preparations was similar, as shown in Supplemental Figure 2. Statistical Analysis All values are expressed as mean ± standard deviation (SD). All comparisons between experimental and control groups were performed by One-way ANOVA (analysis of variance) with Tukey-Kramer post-test using PRISM 5 statistical software (GraphPad software, San Diego, CA) unless otherwise indicated. A value of p < 0.05 was considered to indicate statistically groups. tatistically significant differences. Student’s t-test was used to compare two grou ou upss. See Supplemental Material for a detailed description of further experimental methods. Results R essults su Factors Fa act c or orss secreted secr se cret cr eted ed d by by MSCs MSC can can n prevent pre reve veentt hypoxia-induced hypox ypox oxia ia a-iind ducced pulmonary pul u mo mona n ry inflammation. na inffla l mm mmat atiion at n. To determinee if if hypoxic hypo hy poxi po x c lung xi lu ung inflammation inf nfla nf lamm la m at mm atio io on responds reesppon onds ds too MSC MSC paracrine par araccri r ne signals, sig igna n ls na ls,, we injected inj n ected micee with concentrated serum-free culture media conditioned by either mouse MSCs (MSC-CM) or by mouse lung fibroblasts (MLF-CM) and exposed the animals to normobaric hypoxia (8.5% O2) for 48 hours. In the control group injected with vehicle (serum-free culture media), hypoxia resulted in pulmonary influx of macrophages, as assessed in bronchoalveolar lavage fluid (BALF) and this response was blocked in animals treated with MSC-CM but not in the group treated with MLF-CM (Figure 1A). We also assessed, in cell-free BALF, levels of monocyte chemoattractant protein-1 (MCP-1), a cytokine transiently upregulated in the lung by early hypoxia2, 23 and levels of hypoxia-induced mitogenic factor (HIMF), a pleiotropic factor with 7 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 pro-inflammatory, mitogenic and chemokine-like properties24. In animals injected with either vehicle or MLF-CM, BALF levels of both MCP-1 and HIMF were highly increased by hypoxia and this increase was effectively suppressed by MSC-CM treatment (Figure 1B). These results indicate that, as we have previously reported on the model of hyperoxia-induced BPD7, 11, the protective effects of MSC treatment in HPH10 involve mainly paracrine mechanisms. The anti-inflammatory activity in MSC-CM is associated with exosomes In order to identify the biologically-active component of MSC-CM, we fractionated concentrated conditioned media through size-exclusion chromatography. Polystyrene nanospheres of 50 nm diameter served as a hydrodynamic radius standard to identify the exosomal fraction, and of the thee standard sta tand ndar nd ard fractions in a protein peak eluting with a retention volume corresponding to thatt of were pooled (Figure 2A, Fraction I). Negative staining electron microscopic analysis revealed Frrac acti tioon ti on I to to contain cont nttain ain heterogeneous microvesicles es tthat hat were absent n inn fractions nt fraactions fr ac corresponding to Fraction he retention re volu vo l me lu me ooff mo moie ieti ie ties ti es ooff sm maller ssize izze (Fi Figure Fi re 22B, B, Fr Fra acttion II). II)). Fr raccti tion onn I w as hhighly as ig ighl ghl hlyy the volume moieties smaller (Figure Fraction Fraction was en nri rich ch hed e iin n microvesicles m cr mi crov ovves esiiclees 30 0-1 - 00 nnm m iin n ddiameter iame ia metter ex me xhiibit ibittin ingg bi bico co onccav a e morp m orp phoolo logy gy,, a di gy ist stin in nct enriched 30-100 exhibiting biconcave morphology, distinct morphologica caal fe ffeature atur at u e of ur o eexosomes xoso xo s me so mess ((Fig Fiig 2C 2C,, ar arro rows ro ws). ws ) Ex ). Exos osom os omes om es w eree pr er pres esen es entt in bboth othh MSC- andd ot morphological arrows). Exosomes were present MLF-CM and, in this report, MSC exosome preparations are termed MEX, whereas MLF exosome preparations are termed FEX. Both MEX and FEX contain diverse mature microRNAs (see below) and also Dicer (Figure 2D), a component of the cytoplasmic microRNA maturation complex. However, relative abundance of each exosomal marker differs depending on the cellular origin of the microvesicles. MEX preparations were efficacious in suppressing hypoxic inflammation when injected into animals, whereas the MSC-CM fraction depleted of exosomes (ExD-CM) had no significant effect and FEX had a partial inhibiting effect (Figure 2E). Concordantly, levels of pro- 8 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 inflammatory mediators in cell-free BALF of hypoxic animals were suppressed only by MEX but not by FEX or ExD-CM treatment (Figure 2F), indicating that the ability to suppress early hypoxia-induced pulmonary inflammation is associated specifically with exosomes of MSC origin. Dose response effects of MEX on lung inflammation We have previously reported that suppression of the entire period of the inflammatory response to early hypoxia is required to protect animals from later development of PH 2. This inflammatory response is transient in the murine model, peaking within 2-3 days of hypoxic exposure and subsiding by day 7 (Figure 3A, left panel). We therefore assessed the effect of MEX treatment on the temporal profile of hypoxic lung inflammation, and we fo foun undd th un that at a llow ow found dose of MEX (0.1 μg/animal, via jugular vein) was able to delay but not to completely suppress he pu pulm lmon lm onar on aryy in nfl fluux of macrophages, resulting iin n a shift of the inflammatory inf n lamm nf mmaatory peak towards later mm the pulmonary influx imes mees (Figuree 33A, A, mi idd ddle le ppanel). aneel). an el). T h ob he bserrveed te empooral al pprofile rofi ro file lee ooff pu pulm lmon lm onar aryy ma ar acrropphaage times middle The observed temporal pulmonary macrophage nfl flux ux w as pparalleled aralllelled aral led by tthe h ttemporal he em mpo pora rall prof pprofile rof ofil ilee off iinduction il nduction ndu tion ooff th thee pr proo-in oin nflaamma amma mato t ry m to arrkeers rs,, influx was pro-inflammatory markers, MCP-1, inter rle leuk u in uk in-6 -6 ((IL-6), I -6 IL 6), G alec al e ti ec tinn-3, 3 aand 3, nd H IM MF, in ccell-free ellel l fr lfree ee B ALF, AL F, w h ch hi h aalso lsoo sh ls shifted to a interleukin-6 Galectin-3, HIMF, BALF, which later time (4 to 7 days, Figure 3B). In contrast, a treatment consisting of two sequential injections of MEX, one prior to exposure to hypoxia and a second injection at day 4, just prior to the delayed inflammatory peak (Figure 3A, middle panel), efficiently suppressed pulmonary influx of macrophages over the entire period of inflammatory responses to early hypoxia (Figure 3A, right panel). However the peak of pro-inflammatory markers in BALF, although delayed by the first dose, was not affected by the second injection of MEX (Figure 3B). Multiple administrations of low doses of MEX, but not FEX, ameliorate pulmonary hypertension, right ventricular hypertrophy, and lung vascular remodeling 9 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 The physiologic consequences of partial or complete abrogation of the early inflammatory response to hypoxia are seen in Figure 3C and 3D. A single low dose of MEX did not protect against the elevation of RVSP or the development of RVH after three weeks of hypoxic exposure, whereas the double injection regimen significantly improved both variables. These results mirror the physiologic response we had observed using pulses of heme oxygenase-1 (HO1) overexpression to completely or partially suppress the early hypoxic lung inflammation 2 and suggest a dose- and time-sensitive window for anti-inflammatory treatments to confer protection from HPH. Importantly, FEX treatment using the double injection protocol did not have any physiologic effect, buttressing the assertion that the function(s) protecting against HPH reside pecifically with exosomes produced by MSCs. Furthermore, animals treated w ithh tw it twoo do dose sess of se specifically with doses MEX and exposed to three weeks of hypoxia did nott develop vascular remodeling as determined Į-ssmoot moot oth h mu musc scle sc le actin (Į–SMA) staining, wh her e eas the same tr rea e tm men entt protocol with FEX byy Į-smooth muscle whereas treatment esuult l ed in me edi dial a w all hy al hype pert rtrroph rt rophhy si simi milar to veh hiccle--tr -treat reatted d ccontrols onttrol trolss (F Figu gu ure re 44). ). resulted medial wall hypertrophy similar vehicle-treated (Figure sing ngle ng le h igh ig h do osee M EX X ttreatment reeatm tmen entt in en nhi hibi bits bi ts h ypooxicc iinflammation, yp nfla nf lamm la mmat mm atio ion, io n,, va ascu ascu ula ar re rem mode mode deli ling ng aand n nd A si single high dose MEX inhibits hypoxic vascular remodeling HPH. The incomplete protection from HPH by two sequential low doses of MEX could be related to the failure in completely suppressing early hypoxic inflammation. To test the efficacy of higher MEX dosages on early hypoxic inflammation and HPH, 10 μg MEX were injected through the tail vein and mice were exposed to hypoxia for 2 and 7 days. A higher dose of MEX prevented pulmonary influx of macrophages similarly with two sequential injections of MEX (Figure 5A) and importantly, also completely abrogated the elevation of pro-inflammatory marker FIZZ1/HIMF in the lung for the entire period of early hypoxic responses. An equivalent dose of FEX had no effect (Figure 5B). Next, to examine the efficacy of higher dose of MEX on vascular 10 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 remodeling and HPH, 10 μg of MEX were injected and mice were exposed to chronic hypoxia for three weeks. Compared to vehicle-injected (PBS) controls, the MEX-treated group had significantly (p < 0.001, One-way ANOVA) decreased RVSP (Figure 6A) and did not develop RVH in response to chronic hypoxia (Figure 6B). MEX treatment prevented pulmonary vascular remodeling, as assessed by D-SMA staining (Figure 6C). Morphometric analyses on small arterioles revealed a significant effect on the medial wall thickness index, with values in the MEX-treated group approximating those of the minimally-muscularized normoxic vessels (Figure 6D). Taken together, the above results strongly suggest that the protective mechanism of MEX action is through blocking inflammatory lung responses to early hypoxia, which when left eft unchecked, activate pro-proliferative pathways in the vascular wall thereby in iincreasing ncr creaasi cr sing ngg medial wall thickness and altering vascular cell phenotype. MEX STAT3 ME EX inhibit inhi inhi hibi bitt ST bi TAT3 AT activation by hypoxia Early STAT3 the mouse E arl rlly hypoxiaa resulted reesuultted d in in activation acttiv ac tivat atio io on off S TA AT3 3 in th he mo mous usee lung lung g tthrough hrou ou ughh pphosphorylation hosp ho spho hory ho ry yla lati t on on aatt Tyr-705 without total STAT3 This activation was Ty Tyrr-70 r7055 an 70 and d wi with thou th outt any ou any ef eeffect fect fe c oon ct n the the to tota tall llevels ta eve vels ve lss ooff STAT S TAT AT33 pr pprotein. otei ot eiin. n T hiis ac ctiiva vati tioon ti on w ass suppressed treatment, FEX STAT3 transcription efficiently su upp ppre ress re ssed ss ed bby y ME MEX X tr trea eatm ea t en tm ent, t bbut t, ut nnot ot F E ((Figure EX Figu Fi gure gu re 77A). A). S TAT3 TA T iiss a tr T3 tran ansc an s ription factor integral to signaling pathways of many cytokines and growth factors and its activation plays a critical role in respiratory epithelial inflammatory responses25, 26. Importantly, persistent ex vivo STAT3 activation, has been linked to the hyperproliferative and apoptosis-resistant phenotype observed in pulmonary artery endothelial cells (PAECs) 27 and pulmonary artery smooth muscle cells (PASMCs)28 from patients with idiopathic pulmonary arterial hypertension (IPAH). Therefore, to determine whether MEX regulate STAT3 activation on lung vascular cells, we exposed primary human PAECs (hPAECs) to hypoxia and assessed pY-STAT3 levels. As depicted in Figure 7B, exposure of hPAECs to hypoxia results in robust activation of STAT3 11 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 by Tyr-705 phosphorylation. Treatment with mouse MEX or MEX derived from MSCs isolated from human umbilical cord stroma29 completely abrogated this response. In contrast, neither mouse FEX, human FEX, nor the fraction of human umbilical cord MSC-CM depleted of microvesicles (hUC-ExD-CM) had any effect. Besides demonstrating that suppression of STAT3 activation is a property shared by MEX of both human and mouse origin, these results strongly suggest that direct suppression of hypoxic signaling in pulmonary vascular cells is a primary function underlying the protection conferred by MEX treatment. In concordance, we found that MEX dose-dependently inhibited PASMC proliferation rate in response to serum-derived mitogens (Supplemental Figure 3), confirming that MEX have direct effects on lung vascular cells. Differential miRNA content in MEX vs. FEX numb nu mber mb err ooff recent rece ceent nt studies report the successfull horizontal horrizontal transfer ho transfe fer off functional fe functional un A number mRNA and 0, 31 1 miR RN specie RNA es from f om fr om exosomes exo xoso some so mess into me in nto o recipient reeci ecipieent nt cells cellls330, . To eevaluate valu va lu uate po potential oteent ntia iall di ia diff differential ffer ff erren e ti t al ssignals ignnals ig miRNA species released ele leas assed e bby y ME M MEX X vs vs F FEX EX X tthat haat co coul could uldd medi ul m mediate edi diat atee th at thei their eirr ttherapeutic herrap peu euti t c ef effects ffe fect ctss in ct in vivo, viv ivoo, we w qquantified uant nttiffie i d relative elative levels levells of o a nnumber um mbe b r of can candidate andi an d da di d tee m miRNAs iR RNA NAss in nM MEX EX aand nd F FEX EX ppreparations. repa re para pa rati ra tion ons. on s We s. W found thatt relative to FEX, MEX contain significantly increased levels of miRNA-16 and miRNA-21. Interestingly, although let7b miRNA levels were comparable within these two types of exosomes, let7b pre-miRNA was significantly enriched in MEX vs FEX (>10 fold) (Supplemental Figure 4). These findings point to distinct and potentially important microRNAmediated regulatory signals delivered to the lung by MSC-derived exosomes. MEX treatment suppresses the hypoxic induction of the miR-17 microRNA superfamily and increases levels of anti-proliferative miR-204 in the lung. STAT3 (activated by either vascular endothelial growth factor or IL-6) has been reported to 12 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 directly regulate the transcription of the miR-17~92 cluster of microRNAs in PAECs, resulting in decreased levels of bone morphogenetic protein receptor-2 (BMPR2), a target of miR-1732. Therefore, we assessed the effect of hypoxia and MEX treatment on the miR-17~92 cluster of microRNAs and its conserved paralog clusters, miR-106b~25 and miR-106a~363. These microRNA clusters have been postulated to be pro-proliferative and to target an array of genes involved in the G1/S phase transition33. We found that select microRNAs representing all three clusters of the miR-17 superfamily were upregulated by hypoxia in the lung, and this transcriptional activation was efficiently suppressed by MEX treatment (Figure 8A). Interestingly, levels of microRNAs involved in hypoxic signaling networks, such as miR-199a5p, a microRNA reported to stabilize HIF1Į in cardiac myocytes34, miR-214, whi which hich hi ch h sha shares hare ha ress th re thee same ame host gene with miR-199 35, or miR-210, a hypoxamir under direct hypoxia-inducible fa acttor or-1 -1 1 ((HIF1Į HIF1 HI F Į rregulation egu ation36, were not affected by M egul MEX EX treatment nt (Fi (Figure Figu gure gu r 8B), pointing to factor-1 targeted arg get e ed effects ts ooff ME MEX X on sspecific peci pe cifi ficc hy fi hypoxia-regulated ypoxiaa-rregu ulaated d ssignaling ignnali ig l ng li g ppathways. athw at hwaays. T hw Treatment reat re attme ment nt w with ithh an it equivalent FEX inhibitory with members eq equi u va ui vale l nt ddose le ose of F ose EX hhad ad nno o in inhi hibbito hi bito ory eeffect ffec ectt on ec on tthe hee hhypoxamirs ypox yp oxxam amirs rss eexamined xaamine mineed wi w th oonly nlly me m mber mb es of the miR106b/25/93 miR10 06b 6b/2 / 5/ /2 5/93 93 cluster clu l st ster err being bei eing ei ng moderately mode mo dera de rate ra tely te ly affected afffeccte t d by FEX FEX (Fi (Fig Figg 8A Fi 8A). ). Importantly, we observed that MEX treatment, but not FEX, resulted in the increase of lung levels of miR-204 (Figure 8C), a microRNA enriched in distal pulmonary arterioles that is transcriptionally suppressed by STAT3 but also inhibits the activation of STAT3 in a feed-forward regulatory loop21. The proliferative and anti-apoptotic phenotype of PASMCs isolated from patients with IPAH is inversely related to the level of miR-204 and delivery of exogenous miR-204 to the lungs of animals with PH ameliorated established disease21. Therefore, we interpret these results as an indication that MEX treatment, by suppressing STAT3 activation at the early stages of hypoxic exposure, prevents the hypoxic induction of the pro-proliferative miR-17 superfamily in the lung 13 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 vasculature and blocks the STAT3-miR-204-STAT3 feed-forward loop in distal pulmonary vessels. Discussion This report, demonstrates that the protective functions of MSCs are mediated by secreted microvesicles. Thus, our work provides an explanation for the paradox of the consistently observed significant physiologic effect of MSC treatment despite very low retention of donor cells in the lung. Secreted membrane microvesicles, of which exosomes represent the better characterized subclass, have been recognized as important mediators of intercellular communication, especially in the immune system. It has been proposed that such microvesicles, called exosomes, can act as a vector for the transfer of genetic information (mRNA (mRN NA and and microRNAs) or the shuttling of effector proteins to recipient cells15, 16. Heat-shock protein 72 frrom m tumor-derived tumor umor or-d -deriv -d iv ved ed exosomes mediates immunosuppressive immunoosu s ppressive function funccti t onn to myeloid-derived myeloid-derived from suppressive uppressive ppp cells ceellls through thro rouugh ugh activation acti ac tivvati ti vation on of of STAT3 STA AT319. T Through hro oug ughh pu puta putative tati tiivee ttransfer raansfe nsferr of m microRNAs icro ic ro oRN RNAs As aand ndd mR mRNAs, RNA NAs, s, microvesicles mic icro rovvessicl siclees ssecreted ecreete ec t d fr from om ttumor-initiating um mor or-iini niti tiaatiing ce cells ell llss po positive osi sittive ffor or tthe or he m mesenchymal e en es nch chym ym mal al m marker arke ar k r ke CD105 havee bbeen eenn re ee repo reported port po r ed rt d tto o co confer onf n er ang angiogenic ngiiog ng ogen en nicc pphenotype heno he n ty type pe tto o no norm normal rm mal en endo endothelial doth do t el th elia iaal ce cell cells l s37. ll Supporting our observations here, microvesicles released from MSCs have been recently reported to improve recovery in animal models of experimentally-induced renal failure38 and myocardial ischemia/reperfusion injury39, however, the underlying mechanisms mediating these protective effects were not characterized. Our results show that treatment with MSC-derived exosomes prevents the activation of hypoxic signaling that underlies pulmonary inflammation and the development of PH in the murine model. MCP-1 and HIMF/FIZZ1 are highly upregulated by hypoxia in the lung and both factors are potent proinflammatory mediators. Moreover, both MCP-1 and HIMF/FIZZ1 have 14 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 been linked to the development of PH in murine models of disease40, 41 as well as in human PH42. Egashira et. al. demonstrated that administration of exogenous recombinant MCP-1 resulted in prominent medial wall thickening of pulmonary arterioles and MCP-1 receptor blockade prevented monocyte recruitment as well as the subsequent vascular remodeling 41. HIMF/FIZZ1, a marker of alternative activated macrophages43, is also induced by hypoxia in the respiratory epithelium and plays a critical role in the development of HPH in a murine model and in scleroderma-associated PH. 24, 44, 45. The knockdown of HIMF/FIZZ1 partially blocked increases in pulmonary artery pressure, RVH, and vascular remodeling caused by chronic hypoxia. The important role of HIMF in the development of PH was further confirmed through intrapulmonary demo demonstrate monsstrrat mo atee here here he r gene transfer of HIMF/FIZZ1 which recapitulated the findings of HPH40. We de that hat MEX administration inhibited the hypoxic induction of both MCP-1 and HIMF/FIZZ1 in the lung ung and and this thi hiss was waas associated as d with prevention off HPH. HPH. HPH Directl Directly tlyy related r la re late tedd to ooff th thee an ant anti-inflammatory ti-inf ti-i nfflam mmatorry act action tion tio on ooff ME MEX EX tr trea treatment eaatm men nt is is tthe he oobserved bserrved bs ved prevention pathways. Examining lung pr prev even ev enti en tion on of of hypoxia-activated hy ypo oxi xiaa-a a-acti tivaate t d pro-proliferative propr o-pr prrollif ifer erattiv er ivee pa ath hwaays ys.. Ex Exa amin amin inin ingg tota ttotal otaal lu ung ttissue is ue we issu found that, inn addition add d it itio ionn to MCP-1, io MCP C -1 1, hypoxic hyypooxi x c levels leve le v ls ve l of of IL-6 I -66 were IL wer eree also als suppressed sup uppr pres pr esse es s d by M se MEX E EX administration. IL-6 is a proinflammatory cytokine known to activate STAT346 and, in a number of studies, was associated with PH32, 47. It is thus possible that STAT3 may be a key mediator of hypoxic, pro-inflammatory signaling leading to PH in the in vivo lung. Indeed, phosphorylation of STAT3 at the 705 tyrosine residue is required for STAT3 dimerization and subsequent nuclear translocation48, 49, and this was markedly increased in both lung and PAECs in response to hypoxia but significantly suppressed by MEX treatment. Importantly, persistent ex vivo STAT3 activation, has been linked to the hyperproliferative and apoptosis-resistant phenotype observed in PAECs27 and PASMCs28 from patients with IPAH. Mathew et. al. reported a marked 15 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 upregulation of STAT3 phosphorylation in the lungs of rats with monocrotaline-induced PH50. STAT3 directly regulates the transcription of the miR-17~92 cluster of microRNAs in hPAECs, resulting in decreased levels of BMPR2, a target of miR-1732 whose downregulation is recognized as a hallmark of PH. We found that hypoxia induced select microRNAs of the miR17 superfamily in the lung and MEX effectively suppressed this induction whereas MEX did not suppress other microRNAs involved in hypoxic signaling networks, including the hypoxamir miR-210 which is induced by HIF36, pointing to selective, STAT3 targeted effects of MEX action in the lung rather than global suppression of all hypoxamirs. In addition to STAT3 being a central determinant of the hyperproliferative vascular cell phenotype in patients with IPAH, the suppression off miR-204 (a distal pu lmonar aryy ar aartery teery sspecific peci pe cific pulmonary microRNA) correlates with PH severity in human disease and rodent models of PH21. In this tuddy, miR-204 miR iR-2 -2004 w -2 as suppressed by STAT3 and miR-204 as miR R-204 was show ow wn to o iinhibit nhibit the activation of study, was shown STAT3 STA AT3 in a self-regulatory sel elff-reeguula fl to ory loop. loo oop. p A Although l hough du lt duringg tthe he aacute cutee hhypoxic cute ypox yp oxxic ph pha phase asee we w ddid id d nnot ot obs observe bser bs errvee 2 the he decrease decr de c ea cr ease se iin n miR-204 miR R-204 le R-2 leve levels els oobserved bseerve bs erveed un unde under derr ch de chronic hro oni nicc hy hyp hypoxia poxi xiaa in tthe xi h m he mouse ouse ou se21 we we consider connsid id der the the fact that ME EX tr trea eaatm tmen entt in en ncr crea e se sess th the ba bbasal sall le sa eve v l of m iR R-2 204 as a sstrong t on tr ongg in indi d ca di cati tion ti on tthat h t MEX ha MEX treatment increases level miR-204 indication treatment is shifting the balance of the STAT3-miR-204 loop to an anti-proliferative state. A schema of a hypothesis synthesizing the above results with previous work from our group and the work of others is shown in Supplemental Figure 5. We have previously shown that hypoxia shifts the Th1/Th2 balance of immunomodulators in the lung, resulting in alternative activated alveolar macrophages (AA-AMĭ) and this is inhibited by HO-1 overexpression2. Hypoxia also induces the expression of HIMF in the lung epithelium24 and HIMF mitogenic action on the vasculature requires Th2 cytokines, such as IL-444 to result in PH. Consequences of the shift towards proliferation include the hypoxic activation of STAT3 16 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 signaling and the upregulation of the miR-17 family of microRNAs. Treatment with MEX interferes with an early hypoxic signal in the lung, suppressing inflammation, HIMF transcriptional upregulation, and alternative macrophage activation. It addition, MEX treatment may directly suppress STAT3 activation in lung vascular cells and also upregulate miR-204 levels, thus breaking the STAT3-miR-204-STAT3 feed-forward loop and shifting the balance to an anti-proliferative state. The ability to secrete microparticles that contain not only proteins but RNA or miRNA species which can modulate the expression of multiple genes make these packaging vesicles an attractive and quite plausible means for MSCs to regulate multiple pathways and produce a robust endocytic obust therapeutic effect in vivo. Indeed, exosomes are lipid vesicles of endocyti ticc or oorigin ig gin released rel elea e se ea s d by many cell types including vascular cells, dendritic cells, and mast cells13 that function to medi me diat di atee intercellular at inte in terrcelllu te lullar la communication through the th he ex xchange of prot oteinn an ot andd RNA moieties. Of mediate exchange protein possible poss ssib si le physiologic physiiollog ogicc relevance rel elev evan ev ance an ce is is the the ddifferential ifffere ff ential ddistribution istrib ib butio utio on of ttetraspanins ettras trasppannins nss C CD63 D663 (a (abu (abundant b nd bu ndan an nt iin n mouse mo ous usee MEX) ME EX) and and CD81 CD81 (abundant (abu (a bu und dan antt in in mouse mou ouse se FEX). FEX EX).. Although Alth Alth thou ough ou gh h this thiss differential d ffferren di enti tiaal di ti dis distribution stribu str ribu utioon on is is not no apparent betwe between ween we e hhuman en uman um an M MEX E aand EX n hhuman nd um man F FEX EX ((results resu re s ltts not su not shown), shhow own) n),, th n) thes these esee ar es aaree mo mole molecules lecu le c les that cu may play a role in target cell specificity of exosomes and could participate in signaling pathways. Full molecular characterization of exosomal preparations produced by mouse bone marrow MSCs and human umbilical cord stroma MSCs is the focus of ongoing work. Exosomes isolated from a mast-cell line or from primary bone marrow-derived mast cells were reported to contain mRNAs and microRNAs that were transferable to other mast cells, and, in the case of mRNAs, to be translated into new proteins13. The authors identified different miRNAs within exosomes and, in a more recent study, pre- and mature miRNAs, but not larger species, were identified within MSC-derived exosomes51. Given the robust, long-lasting anti-inflammatory and 17 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 cytoprotective effects of MEX demonstrated for the first time in the present study, it is reasonable to postulate that one or more miRNA species that are unique to or highly enriched within MEX, serve as master regulator(s) of several genes and pathways underlying the development of PH. In summary, in this study we isolated, identified, and characterized exosomes from mouse and human MSC-CM and demonstrated a robust biologic effect that is unique to MEX versus exosomes derived from other cells, such as fibroblasts. Importantly, we demonstrate for the first time that MEX are the major paracrine anti-inflammatory and therapeutic mediators of MSC action on the lung, acting, at least in part, through inhibition of hypoxic STAT3 signaling. protein, lipid, Further work is required to identify the critical components of MEX, be they pro otein tein n, li lipi pid, pi d, oorr nucleic acid species. Although the applicability of our findings to a human disease model need to verified, the makes o bbee ve rifi ri ifi fied ed, th ed he ef eefficacy ficacy of this treatment in ppreventing revventing PH mak re kes e tthese hesse he se microvesicles an attractive candidate PH diseases at ttrractive ac candi dida d te for for exploring expplo ori ring ng models mod odel elss off therapeutic thheraapeutiic interventions intterven in nti tion ons in i P H and and othe oother the herr di dis seassess seas with date. wi ith no no definitive defi de fini fi niti tivve ttherapy herrappy he py too da date te. te Acknowledgements: expertise, Sarah Ackn Ac know owle ledg dgem emen ents ts:: Th Thee authors auth au thor orss would woul wo uldd like like to to thank than th ankk Xianlan Xian Xi anla lann Liu Liu for for technical tech te chni nica call ex expe pert rtis isee S arah ar ah h Gately for assistance in preparing the manuscript, and Dr. Georg Hansmann for critical review of the manuscript. Funding Sources: This work was supported by NIH RO1 HL055454 and NIH RO1 HL085446 (SK & SAM). Conflict of Interest Disclosures: None. 18 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 References: 1. Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A. 2001;98:8798-8803. 2. Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early Macrophage Recruitment and Alternative Activation Are Critical for the Later Development of Hypoxia-Induced Pulmonary Hypertension. Circulation. 2011;123:19861995. 3. Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223-272. 4. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrowderived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145-152. DG. 5. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney ey D G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and 2003;100:8407-8411. ameliorates its fibrotic effects. Proc Natl Acad SSci ci U S A. 2003;100:84077 8411. 6. Xu Xu J, J Woods Wooods d CR, CR, R Mora Mor o a AL, A , Joodi AL Jood Jo o i R,, Brigham od Bri righ g am KL, gh KL, Iyer Iy yer S, S, Rojas Rooja jass M. Prevention Pre r ve vent ntio nt ionn of endotoxinio end ndot otox ot oxin ox i induced systemic marrow-derived mesenchymal mice. ndu uce c d system mic rresponse esspo ponnse nse by bbone on ne ma mar rrow-d -deerivvedd mes m esen senchym hymal mal st stem em m ccells ells el ls iin n mi m icce. ce. Am J Physiol Cell P Phys hys ysiol Lung ng g Ce el Mol ell Mo ol Physiol. Phys yssio ol. l 20 22007;293:L131-141. 07;2 07 ;2 293:L L1331-11411. Kourembanas 7. Aslam Asl slam a M, M, Baveja Ba ja R, R, Liang Lian Li a g OD, OD Fernandez-Gonzalez Fernan ande dezz Go Gonzaale lezz A, Lee Lee C, C, Mitsialis Mits Mi tsiali liss SA, SA, Ko K urem ur emba bana nass S. S chronic Bone marrow w stromal stro st rooma m l cells ceellss attenuate atte at tenu nuat nu atte lung lu ung injury inj n ur uryy in a murine mur urin inee model in mode mo d l of nneonatal eona eo n taal ch na chro roni ro n c lung ni disease. dise di seas asee Am J Respir Res espi pirr Crit Crit Care Car aree Med. Medd 2009;180:1122-1130. Me 2009 20 09;1 ;180 80:1 :112 1222-11 1130 30 8. van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thebaud B. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131-1142. 9. Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120-1128. 10. Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99-107. 11. Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, 19 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 Kourembanas S. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170-81. 12. Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913-919. 13. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. 14. Aoki N, Jin-no S, Nakagawa Y, Asai N, Arakawa E, Tamura N, Tamura T, Matsuda T. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology. 2007;148:3850-3862. 15. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-579. cancer-associated 16. Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-as asso soci so ciat ated at ed immunosuppressive mmunosuppressive microenvironments. Semin Immunopathol. 2011;33:441-454. 17. 17 7. Segura Seggura Se guraa E, E, Guerin Guuer erin i C, Hogg N, Amigorena S, Thery Thery C. CD8+ dendritic The d nd de dri riti ticc cells use LFA-1 to ti capture 2007;179:1489-1496. ca aptture MHC-peptide MH HC-pe pept ptid pt idee complexes c mp co mple lexe xees from f om exosomes fr exo xoso some mes inn vivo. viv vo. J Im IImmunol. munol. mun nol. 20 007 0 ;1 ;179 79:1 79 :148 :1 4899-14 14 496 6. 18. derived from M.. Bo Bovis BCG macrophages 18 8. Giri Giri PK,, Schorey Sch horrey JS. JS. Exosomes Exos xosome som s de eriivedd fr rom M Bovi viss B vi CG G iinfected nfeccted m acr phhages activate acro activ vate antigen-specific CD4+ cells vitro and vivo. 2008;3:e2461. an nti tige genge n sp nspec eciific ec ific C D4+ and D4+ and CD CD8+ 8+ T ce ell llss in vit itro it r an ro nd iin n vi viv vo. PL vo. PLoS oSS One. One. ne. 20 008 08;3 ; :ee246 ;3 2461. 19. Chalminn F, Ladoire Lad adoi o re oi r S, Mignot Mign Mi gn not G, G, Vincent Vinc Vi ncen nc entt J, en J Bruchard Bru ruch c ar ch ardd M, M Remy-Martin Rem e yy-Ma Mart Ma rtin rt in n JP, JP, P Boireau Boi oire reau re a W, Rouleau A, S Simon B, La Lanneau D, D Dee Th Thonel A, Mu Multhoff G, Ha Hamman A, Ma Martin F, Ch Chauffert B, Roul Ro ulea eauu A imon im on B Lann nnea eauu D Thon onel el A Mult ltho hoff ff G Hamm mman an A Mart rtin in F Chau auff ffer ertt B Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457-471. 20. Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847-856. 21. Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535-548. 22. Vitali SH, Mitsialis SA, Liang OD, Liu X, Fernandez-Gonzalez A, Christou H, Wu X, McGowan FX, Kourembanas S. Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia. PLoS One. 2009;4:e5978. 20 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 23. Zampetaki A, Minamino T, Mitsialis SA, Kourembanas S. Effect of heme oxygenase-1 overexpression in two models of lung inflammation. Exp Biol Med. 2003;228:442-446. 24. Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065-1067. 25. Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest. 2004;113:2837. 26. Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38:699-706. 27. Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, pulmonary Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathicc pu pulm lmon onar aryy arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548-554.. 28. Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Cote J, Provencher S, S, Sussman Suussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis ax xiss plays pla lays yss a critical cri r tiica call role in the pathogenesis of human hum hu man pulmonaryy arterial a teeri ar rial al hypertension. Circulation. Circ Ci cul ulation. 2011;123:1205-1215. 2011 2011 1;1 ; 23 23:1 :120 :1 20 055 12 1215 15.. 15 29. Helwig 29 9. Mitchell Mitchelll KE, KE,, Weiss Weisss ML, ML L, Mitchell Mittch chel e l BM, el BM Martin M rtinn P, Ma P, Davis Dav avis is D, D, Morales Mo s L, L, H elwi w g B, B, Beerenstrauch M,, A Abou-Easa K,, Hi Hildreth T,, Tr Troyer D,, Me Medicetty Matrix cells Wharton's Beer Be e en er enst stra rauc uchh M uc boou-Ea Easa saa K Hild ldrreth reth T Troy oyyerr D Med dice dice cett ttty S. S. M attri rixx ce ell llss fr from om W harrtoon's ha on jelly glia. Cells. 2003;21:50-60. ellly fo form m nneurons eu uro ronss aand nd gli lia. a St Stem em C ells. 20 2003 03;2 ;21: 1:550-6 60. 0 30. Montecalvo A, Larregina AT, Shufesky WJ, DB, Su Sullivan ML, Karlsson JM, Ba CJ, 30 Mont Mo ntec ecal alvo vo A Larr La rreg egin inaa AT Shuf Sh ufes esky ky W J Stolz Stol olzz DB Sull lliv ivan an M L K arls ar lsso sonn JM Baty ty C J Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756-766. 31. Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. 32. Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184-1191. 33. Cloonan N, Brown MK, Steptoe AL, Wani S, Chan WL, Forrest AR, Kolle G, Gabrielli B, Grimmond SM. The miR-17-5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol. 2008;9:R127. 21 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 34. Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879-886. 35. Watanabe T, Sato T, Amano T, Kawamura Y, Kawamura N, Kawaguchi H, Yamashita N, Kurihara H, Nakaoka T. Dnm3os, a non-coding RNA, is required for normal growth and skeletal development in mice. Dev Dyn. 2008;237:3738-3748. 36. Chan SY, Loscalzo J. MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072-1083. 37. Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346-5356. 38. Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca microvesicles A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived mic icro rove ro vesi sicl cles es protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053-1067. CN, 39. Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial Cell Res. 2010;4:214-222. my yocar ocar ardi dial di al iischemia/reperfusion sche heemia/ mia reperfusion injury. Stem C el R ell es. 2010;4:214 1 -222 14 22.. 22 Angelini DJ, Su Q,, Y Yamaji-Kegan K,, Fa Fan Skinner Champion HC, MT, 440. 0. A ngelini D J, S J, uQ amaj am ajii-Ke aj i-Keegan gan K an C, S an kinn ner er JJT, T C T, ham ha mpio mpio i n HC C, Crow Crow wM T, JJohns ohns oh ns Hypoxia-induced mitogenic factor induces vascular and RA. Hy RA. H poxiiaa-indu ducedd m du ito ogen genicc fa act c or ((HIMF/FIZZ1/RELMalpha) HIMF MF/FIIZZ1//RE MF RELM LMalppha LM pha) in nduuce uces tthe he va ascula ascu larr an nd hemodynamic changes Physiol Lung Mol Physiol. hemo he mody mo dyna dy nami micc ch mi han ngees of of ppulmonary ulmo ul mo ona narry ry hhypertension. ypper erttens nsio ns ion. n. Am mJP hysi hy s ol si ol L u g Cell un Celll M o P ol hy ioll. hysi 2009;296:L582-593. 2009 09;2 ;296 96:L :L58 822-59 93. 41. Eg Egashira K, Ko Koyanagi M, Ki Kitamoto S, Ni W W, K Kataoka C, Mo Morishita R, K Kaneda Y, A Akiyama 41 Egas ashi hira ra K Koya yana nagi gi M Kita tamo moto to S atao at aoka ka C Mori rish shit itaa R aned an edaa Y kiya ki yama ma C, Nishida KI, Sueishi K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy inhibits vascular remodeling in rats: blockade of MCP-1 activity after intramuscular transfer of a mutant gene inhibits vascular remodeling induced by chronic blockade of NO synthesis. FASEB J. 2000;14:1974-1978. 42. Itoh T, Nagaya N, Ishibashi-Ueda H, Kyotani S, Oya H, Sakamaki F, Kimura H, Nakanishi N. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology. 2006;11:158-163. 43. Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597-602. 44. Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol. 22 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 2010;185:5539-5548. 45. Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Teng X, Hassoun PM, Yang SC, Champion HC, Tuder RM, Johns RA. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol. 2009;41:553-561. 46. Bromberg J, Darnell JE, Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468-2473. 47. Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236-244. 48. Yahata Y, Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hanakawa Y, Detmar M, Hashimoto K. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem. 2003;278:40026-40031. 49. Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation difffe f re rent ntia iati tion on and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. Onco ogene n . 2000;19:2548-2556. 50. Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disrup Disruption uption of endothelial-cell caveolin-1alpha/raft development monocrotaline-induced caave veol olin ol in-1 -1al 1al alph p a//ra raft ft scaffolding during developm men nt of monocro ota taline ne-i -iinduced pulmonary hypertension. hyypeerttensiion n. Circulation. Circ Ci rcul rc ulat atio ion. io n. 2004;110:1499-1506. 2 04 20 4;1 ;110 10:1 :149 4999-15 1506 06. 51. Chen Lai RC, Lee MM, Choo AB, CN, Lim Mesenchymal 51. C hen TS, S, La ai R C, L ee M M, C M, hooo A B, Lee Le CN N, Li im SK SK.. Me esenchhym mal sstem mal tem ccell elll ssecretes eccretees es microparticles pre-microRNAs. Acids miicr c op opar arti ticl clees cl es eenriched nriche nri icheed in pre re-m re mic icro roRN ro RN NAs As.. Nucleic Nucl Nu c eicc A cl cid ds Re Res. s. 2010;38:215-224. 2010 2010 10;3 ;388:21 2 5-22 21 5-22 224. 4. Figure Figu Fi gure re Legends: Leg egen ends ds:: Figure 1. MSC-CM but not MLF-CM suppress hypoxia-induced pulmonary influx of macrophages. (A) Macrophage numbers in the BALF of hypoxic mice and littermate normoxic controls. Animals received either serum-free culture media (vehicle), MSC-CM (5 μg protein) or MLF-CM (5 μg protein) prior to hypoxic exposure for 48 hours. Each dot represents the total number of Kimura stain-positive cells in BALF from individual animals. Horizontal lines represent the group mean and vertical brackets the standard deviation. (B) BALF levels of MCP1 and HIMF. Cell-free BALF specimens from all animals in each treated group were pooled and 23 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 an amount corresponding to one animal equivalent was subjected to western blot analysis. IgA levels were assessed as a control for equal loading. n > 6 for all groups. ¶, p<0.001 vs. untreated normoxic controls; §, p<0.001 vs. PBS-treated hypoxic controls (One-way ANOVA with Tukey-Kramer post-test). Figure 2. (A) Size fractionation of concentrated conditioned media by Sephacryl-400 chromatography. The black trace represents elution of protein (A280) and the gray trace represents the elution profile of 50 nm diameter polystyrene nanospheres (A220). The fractions of conditioned media in the retention volume corresponding to the 50 nm diameter range were pooled and termed Fraction I, and fractions representing moieties of smaller sizee we were eree ppooled oole oo ledd as le a Fraction II. (B) Negative staining electron microscopy at 30,000X magnification revealed heterogeneous he eteero roggene gene neou ous microvesicles ou miccro mi crovesicles specifically in Frac Fraction cti t on I. (C) Fracti Fraction ion o I ppreparations repa re p rations from either MEX ME X or FEX were weree enriched en nri rich ched ch ed in in 30-100 300-1 -100 000 nm nm diameter diaametter m microvesicles iccro rovvesi sicl cles es eexhibiting xh hib ibiitin in ng ty typi typical piica call ex exosome xos osom om me morphology (D) Preparations either MSCs MLFs mo orp rpho holo ho logy gy (arrows). (ar arrrows). ws).. (D D) P r pa re para rati ra tion ti on ns of eexosomes xo oso s me mes pr pproduced oduuced od ed bby y ei eith th her M SCss orr M SC LFs ar LFs aree associated with witth typical typi ty pica pi call exosomal ca e os ex osom omal om al markers mar a ke kers rss as as well welll as Dicer. Diccer er.. The The relative reela l ti tive ve levels lev evel ells of tthese hese markerss he depends on the cellular origin of the microvesicles. Density for each band relative to Alix was measured and relative abundance in MEX compared to FEX for each marker is shown on right. (E) Macrophage numbers in the BALF of animals exposed to hypoxia for 48 hours and treated with either vehicle (PBS), MEX, FEX, or the exosome-depleted fraction of MSC-CM (ExDCM). Normoxic animals were used as a control. ¶, p<0.001 vs. normoxic control animals; §, p<0.001 vs. PBS-treated hypoxic animals (One-way ANOVA with Tukey-Kramer post-test). (F) Western blot analysis of MCP-1 and HIMF levels in BALF of hypoxic animals treated with isolated exosomes and controls. 24 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 Figure 3. Repeated administration of a low MEX dose (x2) can suppress pulmonary influx of macrophages and ameliorate HPH. (A) Animals were injected with one dose of PBS (left panel, n=14) or 0.1 μg MEX (Middle panel, n=31) through the jugular vein prior to hypoxic exposure and alveolar macrophages were measured in BALF at sacrifice on the days indicated on the X axis. Animals (Right panel, n=15) received 0.1 μg MEX prior to exposure to hypoxia and 0.1 μg MEX again at day 4 of hypoxia and alveolar macrophages were measured in BALF at sacrifice on the days indicated on the X axis. Each dot represents an individual animal. Macrophage numbers in BALF from normoxic control animals (open circles) are replicated in all three panels for direct comparison. ¶, p<0.001 vs. normoxic control group (One-way ANOVA with TukeyBALF Kramer post-test). (B) Representative Western blot analysis of cytokine levels in nB ALF AL F of animals treated with a single low dose MEX. BALF from 6 animals in each group was pooled for times. fo or immunoblot imm im muno muno nobl b ot aanalysis bl na nalysis and repeated at least 3 ti tim mes. (C) RVSP P and andd (D) (D) Fulton’s Index determined de erm dete r ined after aft fter er 3 weeks weeks of of hypoxic hypo hy poxi xicc exposure. xi exposuuree. The he Fulton’s Fuult ulton’ ton’’s Index Inde In dexx (RV/[LV+S]) (RV/ (R V [L V/ [LV+ V+S] V+ S])) iiss a measurement ratio. RV, meeas a ur urem emen entt of right en rig ght ventricular ventr entriccul ulaar hypertrophy hype hy pert r ro rt roph phyy expressed ph e preesssed as a weight ex weighht ra atioo. R V, rright ight ig ht vventricle; entr entr t ic iclle;; LV, left ventr ventricle; Animals with MEX(x2), trric icle l ; S, sseptum. le ep ptu um. ME MEX X (x ((x1): 1):: An 1) Anim imal im alss we al were re iinjected njec nj ecte ec teed on once ce w itth ME MEX. X M X. E (x2), EX FEX(x2): Animals were injected twice with equivalent amounts of MEX or FEX. Black dots represent values for individual animals, horizontal lines represent the group mean and vertical brackets the standard deviation. ¶, p<0.001 vs. normoxic control group; ‡, p<0.01 vs. normoxic control group; §, p<0.001 vs. PBS-injected hypoxic control group (One-way ANOVA with Tukey-Kramer post-test). Figure 4. Repeated administration of low MEX dose (x2) inhibits lung vascular remodeling. Representative lung sections are shown from animals exposed to the same conditions as described in the legend of Figure 3 and stained for Į-SMA to evaluate medial vessel wall 25 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 thickness. (A) 100X magnification and (B) 400X. Figure 5. A single high dose MEX efficiently suppresses the entire period of inflammatory responses to early hypoxia. (A) Macrophage numbers in the BALF of animals injected with a high dose (10 Pg) MEX prior to hypoxic exposure for 2 and 7 days. Dots represent values for individual animals. Black dots represent values for MEX-treated group. White and gray dots represent values for normoxic group (NRX) and PBS-treated groups, respectively, which are adopted from Fig 3A. Horizontal lines represent the group mean and vertical brackets the standard deviation. ¶, p<0.001 vs. normoxic control group (One-way ANOVA with TukeyBALF Kramer post-test). (B) Representative Western blot analysis of FIZZ-1/HIMF llevels evel ev elss in B el ALF AL F oof animals treated with a single high dose MEX and FEX (10 μg) prior to hypoxic exposure for 7 days. days ys.. Figure single dose RVSP measurements Fi igu ure 6. A si sing glee high hig gh do ose MEX MEX treatment tre reat atm mennt prevents prevvent ventss HPH. HPH HP H. (A)) R VSP Pm eassure r mennts nts aand nd ((B) B) Fulton’s normoxic animals injected with either PBS μg MEX Fult lton on’s ’ IIndex ndex nd x of no norm rmox xic i aanimals, nima ni mals l , andd an anim imal als inje j ct je cted ed w ithh ei it ith ther er P BS S oorr 10 μ gM EX X pr pprior iorr too a three animals, three weekk hypoxic hypoxic i exposure. exposure Black Black k dots dots t representt values vallues for for iindividual ndi divid iduall ani imals l hhorizontal oriizonttall lines represent the group mean and vertical brackets the standard deviation. ¶, p<0.001 vs. normoxic control group; §, p<0.001 vs. PBS-treated hypoxic control group (One-way ANOVA with Tukey-Kramer post-test). (C) Histology of paraffin-embedded lung sections stained for DSMA at 400X magnification, depicting representative pulmonary arterioles. (D) Assessment of medial wall thickness of small pulmonary arterioles (20-40 μm diameter) in the three experimental groups (sections from four animals per group; n>40 vessels measured per animal). ¶, p<0.001 vs. normoxic control group; §, p<0.001 vs. PBS-treated hypoxic group (One-way ANOVA with Tukey-Kramer post-test). 26 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 DOI: 10.1161/CIRCULATIONAHA.112.114173 Figure 7. MEX of either mouse or human origin suppress the hypoxic phosphorylation (activation) of STAT3. (A) Total protein extracts from lungs of individual animals treated with 10 μg MEX. Hypoxia exposure for 2 days resulted in activation of STAT3 through phosphorylation at Tyr-705 (pY-STAT3) and this was prevented by treatment with MEX of mouse origin. Right panel: Quantitation of STAT3 phosphorylation. #, p<0.01 vs. PBS-treated hypoxic group; ns, statistically non-significant (One-way ANOVA with Tukey-Kramer posttest). (B) Primary cultures of hPAECs exposed to hypoxia (1% O2, 6 hours) exhibit robust activation of STAT3 that is efficiently suppressed by MEX secreted by MSCs from mouse (mMEX) mMEX) and human umbilical cord stroma (hMEX). Note that neither the microv microvesicle-depleted ovves esic icle ic lee-d -dep eple ep lete le t d fraction of media conditioned by human umbilical cord MSCs (hUC-ExD-CM), nor hFEX or mFEX, mF FEX X, ha have ve aany nyy eeffect ffect on STAT3 phosphorylati ff phosphorylation. tiion on. Data are represen representative en nta tati t ve of at least 3 ndeepe p ndentt experiments. expperi ex perime ment ntts. independent Figure Figu gure re 88.. ME M MEX X trea treatment eatm tmentt suppresses suupp ppre ress s es the he hypoxic hyp ypox oxic ic indu induction duct ctio ionn of the he m miR-17 iR-177 mi iR micr microRNA croR oRNA NA upe perf rfam amil ilyy an andd increases incr in crea ease sess levels leve le vels ls of of anti-proliferative anti an ti-pro proli liffer erat ativ ivee mi miR R-20 2044 in tthe he llung. ungg M un icee we ic were re ttreated reat re ated ed superfamily miR-204 Mice with either PBS, MEX (10 μg) or FEX (10 μg) and then exposed to hypoxia for 7 days. Another untreated group remained in normoxia as control. MicroRNA levels in total mouse lung were assessed by qPCR and fold changes in the hypoxic groups are presented relative to the mean of the normoxic group. (A) Select miRs representing the miR-17~92, miR-106b~25 and miR106a~363 clusters. (B) Select miRs reported to be involved in hypoxic signaling. (C) Upregulation of basal levels of the pulmonary arteriole-specific miR-204 upon MEX treatment. Dots represent expression levels in individual animals. NRX : Normoxia; HPX : Hypoxia. ¶, p<0.001 vs. normoxia; ‡, p<0.01 vs. normoxia; †, p 27 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 Exosomes Mediate the Cytoprotective Action of Mesenchymal Stromal Cells on Hypoxia-Induced Pulmonary Hypertension Changjin Lee, S. Alex Mitsialis, Muhammad Aslam, Sally H. Vitali, Eleni Vergadi, Georgios Konstantinou, Konstantinos Sdrimas, Angeles Fernandez-Gonzalez and Stella Kourembanas Circulation. published online October 31, 2012; Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2012 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/early/2012/11/02/CIRCULATIONAHA.112.114173 Data Supplement (unedited) at: http://circ.ahajournals.org/content/suppl/2012/10/30/CIRCULATIONAHA.112.114173.DC1.html http://circ.ahajournals.org/content/suppl/2012/11/02/CIRCULATIONAHA.112.114173.DC2.html Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/ Downloaded from http://circ.ahajournals.org/ by guest on July 24, 2016 SUPPLEMENTAL MATERIAL Expanded Methods Animal model and hypoxic exposure Eight to 10- week old mice (FVB strain) were housed in large plexiglass chambers and the FiO2 regulated by an OxyCycler controller (BioSpherix, Redfield, NY). Hypoxic exposures were performed at 8.5±0.5 % O2 and ventilation was adjusted to insure that CO2 levels did not exceed 5,000 ppm (average range 1,000-3,000 ppm). Ammonia was removed by ventilation and activated charcoal filtration through an air purifier. Under these conditions, the ammonia concentration is less than 2.5 ppm, the limit of detection of Gastec passive dosimetry tubes (Sigma). Animals were anesthetized with pentobarbital (50 mg/kg, I.P.) and injected through the left jugular vein with concentrated conditioned media (5 g protein in 50 l) or exosome preparations (0.1 g protein in 50 l PBS). A higher dose of exosomes (10 g protein in 50 l PBS) was delivered through the tail vein. Table 1 lists the number of cells used and the exosomal protein recovery from each cell type. Approximately 2% of secreted proteins in the conditioned media of both mMSCs and MLFs are associated with the exosomal fraction and roughly 3-fold more MLFs were required to extract equal amounts of exosomes for the injections. An equal volume of PBS or serum-free -MEM media was injected in control experimental groups. Mice were allowed to recover for 3 hours before placement in hypoxic chambers. In certain time-course and dose-dependent studies, a second injection of MEX (0.1 g protein in 50 l PBS) was performed on the contralateral jugular vein after 4 days of hypoxic exposure. 1 Isolation of human MSCs from umbilical cord Wharton’s Jelly Human umbilical cord Wharton’s jelly derived MSCs (hUC-MSCs) were isolated according to published methods1, 2 with minor modifications. Cord was rinsed twice with cold sterile PBS, cut longitudinally, and arteries and vein were removed. The soft gel tissues were scraped out, finely chopped (2-3 mm2) and directly placed on 100 mm dishes (15 pieces per dish) with DMEM/F12 (1:1) (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), 2 mM L-glutamine, and penicillin/streptomycin, and incubated for 5 days at 37°C in a humidified atmosphere of 5% CO2. After removal of tissue and medium, the plates were washed 3 times with PBS, the attached cells were cultured and fresh media replaced 3 times per week. At 70-80% confluence, cells were collected and stained with PE conjugated antibodies for CD34 (Miltenybiotec, Auburn, CA) and CD45 (Miltenybiotec, Auburn, CA). Immunodepletion was performed using the anti-PE-microbeads (Miltenybiotec, Auburn, CA) and MSCS column (Miltenybiotec, Auburn, CA) according to manufacturer’s instructions. The CD34 and CD45 negative populations were further propagated and selected for the expression of MSC markers (CD105, CD90, CD44, and CD73) and the absence of CD11b, CD19, and HLA-DR by using a set of fluorescently-labeled antibodies designed for the characterization of human MSCs (BD Biosciences, San Diego, CA) and a MoFlo flow cytometry (Beckman Coulter). Cell culture and collection of conditioned media Bone marrow-derived MSCs, isolated from the femurs and tibiae of 5-7 week old male FVB mice, were selected and their differentiation potential assessed as previously described3. Briefly, after 3-4 passages, plastic adherent cells were immunoselected using mouse specific antibodies (BD Biosciences Pharmingen, San Diego, CA) and a MoFlo fluorescence-activated cell sorter (FACS) (Dakocytomation, Fort Collins, CO), as 2 we reported previously3, 4 in compliance with published MSC criteria5. Cells were negatively selected for CD11b, CD14, CD19, CD31, CD34, CD45, and CD79α antigens, and positively selected for CD73, CD90, CD105, c-kit and Sca-1 antigens. Primary MLF cultures were derived according to standard methods6, 7. To exclude contamination from serum-derived microvesicles, serum used for propagation of cell cultures and the collection of CM was clarified by ultracentrifugation at 100,000 x g for 18 hours. MSCs were cultured in -MEM media supplemented with 10% (v/v) FBS (Hyclone), 10% (v/v) Horse Serum (Hyclone), 2 mM L-glutamine (GIBCO), and antibiotics. MLFs were cultured in DMEM (Invitrogen) supplemented with 10% (v/v) FBS and 2 mM L-glutamine. Cultures at 70% confluence were washed twice with PBS and incubated with serum-free media supplemented with 2 mM L-glutamine for 24 hours under standard culture conditions. Conditioned media were collected and cells and debris were removed by differential centrifugations at 400 x g for 5 mins, at 2,000 xg for 10 mins, and at 13,000 xg for 30 mins. The clarified CM were subsequently filtered through a 0.2 m filter unit and concentrated using an Ultracel-10K (Millipore) centrifugal filter device, to a protein concentration range of 0.1-0.5 mg/ml. Protein levels in the CM were determined by Bradford assay (Bio-Rad, Hercules, CA). Bronchoalveolar lavage Animals were anesthetized with Avertin (250 mg/Kg i.p.) and their trachea cannulated with a blunt-ended 20 gauge Luer Stub Adapter (Becton Dickinson). BALF was collected via sequential administration of PBS supplemented with 5 mM EDTA (0.8 ml, 0.8 ml, 0.8 ml, and 0.9 ml) and approximately 3.0 ml (+/- 0.1 ml) of BALF was recovered 3 per animal. Cells in BALF were collected by centrifugation at 400 xg for 10 min and leukocytes stained with Kimura solution8 for counting. Right ventricular systolic pressure measurements Mice were anesthetized with 60 mg/kg of pentobarbital and remained spontaneously breathing. A small incision was made in the abdominal wall, and the translucent diaphragm exposed. A 23-gauge butterfly needle with tubing attached to a pressure transducer was inserted through the diaphragm into the right ventricle and pressure measurements were recorded with PowerLab (ADInstruments, Colorado Springs, CO) monitoring hardware and software. Animals with heart rates less than 300 beats per minute were considered over-anesthetized and their RVSP measurements were excluded. Mean RVSP over the first ten stable heartbeats was recorded. Right ventricular weight measurements Hearts and pulmonary vasculature were perfused in situ with cold 1X PBS injection into the right ventricle; hearts were excised and used for Fulton’s Index measurements (ratio of RV weight over left ventricle plus septal weight, RV/[LV+S]). Both ventricles were weighed first, then the right ventricular free wall was dissected and the remaining LV and ventricular septum was weighed. Pulmonary histology Lungs were inflated by tracheotomy and perfused with 4 % paraformaldehyde, excised, and fixed in 4 % PFA overnight at 4oC followed by paraffin embedding. Sections (two per animal) from 4 individuals in each group ( group n > 7) were analyzed for pulmonary histology. For pulmonary vascular morphometry, paraffin-embedded lung sections were stained with hematoxylin and eosin. For immunohistochemical analysis, 5 m lung 4 tissue sections were deparaffinized in xylene and rehydrated. Tissue slides were treated with 0.3% H2O2 in methanol to inactivate endogenous peroxidases and blocked with horse serum for 1 hour. After incubating with monoclonal anti-mouse -SMA antibody (Sigma) at a dilution of 1:125 overnight at 4oC, secondary antibodies and peroxidase staining was applied according to manufacturer's instructions (Vector Laboratories, Burlingame, CA). Vessel wall thickness was assessed by measuring α-SMA staining in vessels (20-40 m in diameter) within each field (40-50 fields per section) captured at 400X magnification with a microscope digital camera system (Nikon, Tokyo, Japan), and using Metamorph image analysis program (Molecular Devices, Sunnyvale, CA). The medial wall thickness index was calculated by the following formula: Wall thickness (%) = 100 x (area[ext] – area[int]) / area[ext] where area[ext] and area[int] denote the areas bounded by the SMA layer. In vitro hypoxia Human PAECs were purchased from GIBCO and cultured in M200 medium (Invitrogen) supplemented with LSGS (Invitrogen). At 80% confluence, cells were exposed to 1% O2 for 6 hours in an inVivo2 workstation (Ruskin Technology, Bridgend, UK) in the presence or absence of exosomal fraction (1 g/ml), or the exosome-depleted fraction of hUCMSC conditioned media (1 g/ml). Cells were lysed and proteins in whole cell lysates were separated on 8% SDS-polyacrylamide gel electrophoresis followed by western blot analysis using rabbit monoclonal antibody for phospho-STAT3 (Y705) and mouse monoclonal STAT3 antibody (Cell Signaling). 5 Electron microscopic analysis EM analysis was performed at the Harvard Medical School electron microscope facility. Exosome preparations were adsorbed to a carbon coated grid that had been made hydrophilic by a 30 second exposure to a glow discharge. Excess liquid was removed and the samples were stained with 0.75% uranyl formate for 30 seconds. After removing the excess uranyl formate, the grids were examined in a JEOL 1200EX Transmission electron microscope and images were recorded with an AMT 2k CCD camera. Protein extraction and immunoblotting BALF (3 ml) was centrifuged at 420 x g for 10 min and cell-free BALF supernatants were used for protein analysis. Equal volumes of BALF specimens from individual animals in the same group were pooled (1 ml) and proteins precipitated overnight by 20% trichloroacetic acid (Sigma). A fraction equivalent to 30% of each protein pellet was dissolved in 1x sodium lauryl sulfate (SDS)-loading buffer was separated on a denaturing 15% polyacrylamide gel. After transfer to 0.2 m PVDF membranes (Millipore), blots were blocked with 5% skim milk and incubated with 1:1,000 diluted rabbit polyclonal MCP-1, galectin-3, or HIMF/FIZZ1 antibody (Abcam) for overnight at 4C. To detect mouse Immunoglobulin A, 1:5,000 diluted goat anti-mouse IgA antibody (Abcam) was used. Peroxidase-conjugated anti-rabbit secondary antibody (Santa Cruz Biotech) was used in 1:20,000 dilution to visualize immunoreactive bands either by the enhanced chemiluminescence reagent (Pierce) or Lumi-LightPLUS (Roche). For analysis of proteins from whole lung, frozen lung tissues were homogenized for 5 seconds with Polytron in cold PBS containing 2 mM phenylmethanesulfonyl fluoride 6 (Sigma) and centrifuged at 3,000 xg for 3 mins. Tissue pellets were washed twice with cold PBS containing 2 mM PMSF followed by centrifugation at 3,000 xg for 3 mins and lysed in RIPA buffer containing protease inhibitor (Roche) and phosphatase inhibitor cocktails (Thermo). Forty g of lung tissue extracts were separated on 10-20% gradient gel (Invitrogen). Antibodies for MCP-1, HIMF, IL-6, STAT3, and phospho-STAT3 (Y705) were used for immunoblotting. For loading control, mouse monoclonal -actin antibody (Sigma) was used. Proteins in exosome preparations were separated on 12% polyacrylamide gel and then transferred onto 0.45 m PVDF membrane (Millipore). Goat polyclonal anti-CD63 antibody (Santa Cruz Biotech), mouse monoclonal Alix and TSG101 antibodies (Santa Cruz Biotech), rabbit polyclonal CD81, CD9, hsp90, and flotillin-1 antibodies (Santa Cruz Biotech), and rabbit polyclonal Dicer (Abcam) antibody were used for immunoblotting. Isolation and Quantification of microRNAs Total lung RNA was extracted by the method of Chomczynski & Sacchi9 and 750 ng was used as a template for reverse transcriptase with specific primers for each target microRNA (TaqMan Reverse Transcription Kit, Applied Biosystems, Foster City, CA). Each reverse transcription reaction included also the primer for the small nuclear RNA sno202, which was used as an internal control. 37.5 ng cDNA was used for each 20 l qPCR reaction with TaqMan universal master mix II with no UNG (Applied Biosystems) in the presence of probes specific for the indicated microRNAs and the internal control (TaqMan microRNA assay, Applied Biosystems). Amplification was performed at 50oC for 2 min, 95oC for 10 min, followed by 40 cycles of 95oC for 15 sec, 60oC for 1 min, on a StepOne Plus platform (Applied Biosystems). 7 RNAs from isolated exosomes were extracted by Trizol reagent (Invitrogen). Briefly, 30 µg exosomal protein was mixed with 0.5 ml Trizol reagent per manufacturer’s recommendation. 20 µg RNase-free glycogen (Ambion) was applied as a carrier prior to RNA precipitation with isopropyl alcohol and samples were placed at -80 °C for overnight. 150 ng exosomal RNAs were used as a template in reverse transcription reactions with specific primers for target microRNAs (TaqMan Reverse Transcription Kit, Applied Biosystems). To quantify pre-let7b, 300 ng of exosomal RNAs were reverse transcribed by High Capacity RNA-to-cDNA kit (Applied Biosystems) per manufacturer’s recommendations. 7.5 ng of cDNA for each microRNA assay and 11.5 ng of cDNA for pre-let7b (TaqMan gene expression assay, Applied Biosystems) were used for qPCR reaction in the presence of specific probes. Amplification was performed as described above. Let7a was used as an internal control. Smooth Muscle Cell Proliferation Assay Primary rat PASMCs were inoculated at a concentration of 2 x 103/well on a 96 well plate in DMEM containing 5% FBS and incubated for 24 hours under standard culture conditions. After serum starvation for 2 days in 0.1% FBS/DMEM, cells were pretreated either with vehicle or varying doses of mMEX (16, 31.5, 62.5, 125 ng/ml) for 30 min then FBS was added at 5% (v/v) to each well. After incubation for 48 hours, cell proliferation reagent WST-1 (Roche), which is cleaved by mitochondrial dehydrogenases in metabolically active cells to form formazan dye, was directly applied to the cells followed by further incubation for 3 hours. Intensity of solubilized dark red formazan was determined at 440 nm using a microplate reader. 8 Supplemental References 1. Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50-60. 2. Penolazzi L, Mazzitelli S, Vecchiatini R, Torreggiani E, Lambertini E, Johnson S, Badylak SF, Piva R, Nastruzzi C. Human mesenchymal stem cells seeded on extracellular matrix scaffold: Viability and osteogenic potential J Cell Physiol. 2011;[published online ahead of print August 9, 2011] doi: 10.1002/jcp.22983. 3. Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122-1130. 4. Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, FernandezGonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99-107. 5. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. 6. McGowan SE. Extracellular matrix and the regulation of lung development and repair. FASEB. 1992;6:2895-2904. 7. McGowan SE. Influences of endogenous and exogenous TGF-beta on elastin in rat lung fibroblasts and aortic smooth muscle cells. Am J Physiol. 1992;263:L257-263. 9 8. Kimura I, Moritani Y, Tanizaki Y. Basophils in bronchial asthma with reference to reagin-type allergy. Clin Allergy. 1973;3:195-202. 9. Chomczynski P, Sacchi N. Single step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156-159. 10 11 Supplemental Figure Legends Supplemental Figure 1 Flow cytometric analysis of surface-markers for human MSCs. Human MSCs from umbilical stroma (hUC-MSCs) were cultured in DMEM/F-12(1:1) supplemented with 10% FBS. Human UC-MSCs at passage 5 express CD90, CD105, CD73, CD44, but lack expression of CD19, CD34, CD45, CD11b, and HLA-DR in flow cytometric analysis. Isotype-matched IgG controls are shown with non-shaded dotted curves, and hUCMSCs curves are shown in red shaded area. Supplemental Figure 2 Comparison of exosomes from two methods of isolation. Exosomes in the medium conditioned by mMSCs were isolated by ultracentrifugation (UCF) or S200 sizeexclusion chromatography (SEC). Two g of proteins from each preparation were loaded onto 12% SDS-PAGE and total proteins stained by SimplyBlue (Invitrogen) (left panel). Exosomal markers, HSP90, flotillin-1, and CD63 were detected by immunoblotting and are comparable in both preparations (right panel). Supplemental Figure 3 Dose-dependent inhibition of SMC proliferation by MEX. Cultured rat PASMCs were serum-deprived for 48 hours followed by treatment with mMEX (16 to 125 ng/ml) in the presence of 5% FBS and their proliferation rate was quantified relative to the treatment with FBS alone. Data are expressed as mean values ± SD. *, p < 0.001 vs. FBS-alone (One-way ANOVA with Tukey-Kramer post-test). 12 Supplemental Figure 4 MicroRNA content in mouse MEX compared with FEX. RNAs extracted from equivalent amount of MEX and FEX were subjected to RT-qPCR analysis. Levels of the indicated microRNAs relative to let7a in MEX and FEX are presented on the left panel and comparisons between the level of pre-let7b and let7b relative to let7a in MEX and FEX are shown on the right. Data are presented as mean values ±SD. *, p < 0.001 MEX vs. FEX (Student’s t-test). Supplemental Figure 5 Schema of a hypothesis synthesizing the results of this study with our previous work and published literature. Hypoxia shifts the Th1/Th2 balance of immunomodulators in the lung, resulting in alternative activated alveolar macrophages (AA-AM) and, in the early phase, induces the expression of IL-6, MCP-1, and HIMF in the lung epithelium. HIMF mitogenic action on the vasculature requires Th2 cytokines, such as IL-4. Consequences of the shift towards proliferation include the hypoxic activation of STAT3 signaling and the upregulation of the miR-17 family of microRNAs. Treatment with MEX interferes with an early hypoxic signal in the lung, suppressing both inflammation and HIMF transcriptional upregulation. It addition, MEX treatment may directly upregulate miR-204 levels, thus breaking the STAT3-miR-204-STAT3 feed-forward loop, and shifting the balance to an anti-proliferative state. 13 14 15 16 17 18 Correction In the article by Lee et al, “Exosomes Mediate the Cytoprotective Action of Mesenchymal Stromal Cells on Hypoxia-Induced Pulmonary Hypertension,” which was published ahead of print on October 31, 2012, an error occurred. In the initial publication of the article, Figure 6 was incorrect. The error has been corrected in the current online version of the article. The Editorial Office regrets the error.
© Copyright 2026