Possible Carbonate Minerals Within an Unnamed Gullied Crater in
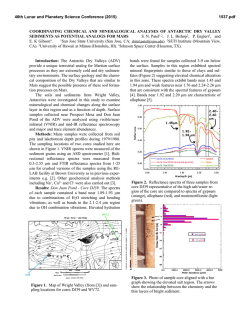
46th Lunar and Planetary Science Conference (2015) 2224.pdf POSSIBLE CARBONATE MINERALS WITHIN AN UNNAMED GULLIED CRATER IN ERIDANIA BASIN, MARS. L. K. Korn and M. S. Gilmore, Department of Earth and Environmental Sciences, Wesleyan University, Middletown, CT, ([email protected]). Introduction: Carbonate minerals have been found in the Martian dust [1], detected in situ at the polar and Gusev Crater landing sites [2,3], and found remotely within the Nili Fossae Region [4], in the Leighton [5], Jezero [6], and McLaughlin [7] craters, in Libya Montes [8,9], in Capri Chasma [10], in the Tyrrhena Terra Region [11], and in the Huygens Basin [12]. Here we describe carbonates in an unnamed crater within the Eridania Basin, part of Terra Sirenum. Geologic Setting: The unnamed ~60km diameter crater excavates Noachian-aged units [13] at ~178°W, 39°S, and is cut by fossae related to Tharsis [e.g., 14]. The crater contains glacial deposits, gullies and chaotic terrain. Carbonates are associated with multiple generations of gullies and depositional fans on the northeast rim of the crater as well as local deposits on the crater floor [15] (Fig. 1). The carbonate signature is also associated with high albedo materials in the crater wall, talus deposits, and boulders weathering from these deposits. This wall rock has been exposed by the formation and headward retreat of some of the gullies. Carbonate found within the depositional fan below is believed to have been transported via the gully’s channels. Thus, the carbonates are localized Noachian-aged deposits that have been exposed by the formation of the crater and modified by gullies. Methods: Long and short wavelength data (0.354.0µm) for two full-resolution CRISM images (Fig. 1), were processed [16] in ENVI using CAT 7.2.1 [17,18], including radiometric and atmospheric correction and noise reduction [19]. Spectral summary products [16,20] were subsequently generated for each image. Regions of interest (ROI) were located based on a RGB summary product combination of BDI1000IR, D2300 [20] and BD2500 [21], which looks for 1.0µm, 2.3µm, and 2.5µm features, respectively. ROIs containing 10s to 100s of pixels were chosen based on the brightest pixels within individual summary products. Derived spectra were ratioed with relatively spectrally neutral areas of the same width and ROI size (preferably located within the same data column). Spectra with the same shape and absorption minima were averaged when applicable. Carbonate Composition: Carbonate minerals have prominent overtone and combination absorptions within the VNIR at 2.35µm, 2.55µm, 3.4µm, and 3.9µm [22,23]. The latter two absorptions are not always present in carbonates [4]. The positions of the 2.3µm and 2.5µm minima shift depending on the size of the associated cations [22]. Ratioed and averaged spectra from the crater contain 1.0µm, 1.9µm, 2.3µm, 2.5µm, and sometimes 3.9µm features, indicating that carbonate minerals are present (Fig. 1). A 1.4µm feature is sometimes visible in continuum removal. The 1.4µm and 1.9µm features are attributed to water [24], while 1.0µm corresponds to ferrous iron [22]. Both water and iron may be mineral components or impurities. High calcium-pyroxene (HCP) minerals with ~1.0µm and ~2.0µm absorptions [24] are also detected within the crater (Fig. 1). Figure 1: The top image contains HiRISE BD_013222_1406, CTX B09_013222_1406, and CRISM data; boxes indicate mineral locations. Select spectra from the crater are shown in Graph (A) with their corresponding library mineral [25, 26] matches in Graph (B). Apparent absorptions are indicated by grey lines. Graphs (C) and (D) show a range of specific carbonate absorptions in continuum removal. Keys in (C) and (D) match (A) and (B), respectively. Pink spectra are HCP minerals outside of the crater (A) and library Clinopyroxene/augiteLAPP95 (B). Yellow spectra also represent ankerite. 46th Lunar and Planetary Science Conference (2015) The locations of the minima for the 2.3µm and 2.5µm features, and the overall shapes of the spectra including the inter-absorption features show that the carbonate in the crater best matches library spectra of ankerite (Ca(Fe2+,Mg)(CO3)2), siderite (FeCO3), and/or [25], and brugnatellite aragonite (CaCO3) (Mg6Fe3+(CO3)(OH)13!4H2O) [26]. The shape of the Eridania carbonate spectra differs from library spectra in that the 2.5µm is shallow relative to the 2.3µm absorption, and the “hump” between them is shifted towards longer wavelengths. The 2.3µm minima are usually in the same location as those in the library spectra, however, the top of the 2.3µm absorption widens towards the longer wavelengths. Some of the carbonate spectra display a relatively shallow slope from ~0.8µm to ~1.88µm. In continuum removal, this feature appears bowl-shaped and has relatively the same depth as the longer wavelength features. The position of this feature is reasonably matched with ankerite or siderite. Alternatively, it could be due to a mixture of carbonates and Fesilicates, as has been interpreted elsewhere on Mars [4]. Spectral Linear Mixing: A mixture between multiple minerals [e.g., 27] within the ROIs may be causing the shallow 2.5µm and the shifted “hump” feature. Here we present linear mixtures between library Ankerite KACB01A and endmember minerals from the entire CRISM library [25] as well as hydrous carbonates [26]. We find that the CRISM spectra can be matched by mixtures of ankerite with small volumes of pumpellyite, chamosite, chlorite, vermiculite, kaolinite-serpentine, and augite (Fig. 2). Also, these mixtures replicate the shallow 1.0µm feature that is apparent in the ROI spectra. No hydrous carbonate mixtures with ankerite were found to produce analogous spectra. Although olivine has been detected with other carbonates on Mars [4], mixtures between library olivine and ankerite did not match our spectra. Implications: Terrestrial formation mechanisms and locations where ankerite and one or more of the silicate minerals can coexist are numerous. The most Mars-relevant environments include diagenetic systems within sandstones and shales [28] at temperatures from ~117 to ~200ºC, in geothermal regimes in siltstones and sandstones with brines containing 250,000 ppm dissolved solids and at temperatures between 80 and ~360ºC [29], and within hydrothermally altered oceanitic basalts [30]. The minerals can also be found together in serpentinites [31] at temperatures less than an estimated 50ºC, as alteration halos around basaltic dykes within dolomitic marbles [32], and potentially in conjunction with CO2-rich brines at 95ºC and 10mPa [33]. Thus, it seems that the minerals favor low to moderate temperature regimes possibly akin to the 2224.pdf Figure 2: Linear mixing results between library [25] ankerite and silicate minerals. The North Wall carbonate spectrum is in red. low-grade metamorphism in the Mordenite to PrehnitePumpellyite facies [34] or as a result of diagenetic processes [28]. Water is also a major factor in these scenarios and acts predominantly as a weathering agent [e.g., 28-30]. Conclusions: Carbonates have been identified within an unnamed crater in the Eridania Basin. The spectra are best matched by linear mixtures dominated by ankerite (or potentially siderite, brugnatellite, and aragonite) mixed with modest amounts of tectosilicates, phyllosilicates, or pyroxenes. On Earth, these mineral assemblages form together in low to moderate temperature hydrothermal systems. Therefore, the Eridania carbonates may have formed under similar circumstances as those that have been previously detected on Mars [4,5]. References: [1] Bandfield J. L. et al. (2003) Science, 301, 1084. [2] Boynton W. V. et al. (2009) Science, 325, 61. [3] Morris R. V. et al. (2010) Science, 329, 421. [4] Ehlmann B. L. et al. (2008) Science, 322, 1828. [5] Michalski J. R. and Niles P. B. (2010) Nat. Geosci., 3, 751. [6] Ehlmann B. L. et al. (2008) Nat. Geosci., 1, 355. [7] Michalski J. R. et al. (2012) LPSC 43, Abstract #1431. [8] Bishop J. L. et al. (2010) LPSC 41, Abstract #2147. [9] Bishop J. L. et al. (2013) JGR, 118, 487. [10] Jain N. et al. (2014) LPSC 45, Abstract #1821. [11] Carrozzo F. G. et al. (2013) LPSC 44, Abstract #2241. [12] Wray J. J. et al. (2011) LPSC 42, Abstract #2635. [13] Tanaka K. L. et al. (2014) Geologic Map of Mars, U.S. Geol. Surv. Sci. Invest. Map 3292. [14] Anderson R. C. and Dohm J. M. (2011) LPSC 42, Abstract #2221. [15] Gilmore M. S. et al. (2014) 8th Internat. Conf. on Mars, Abstract #1388. [16] Murhcie S. et al. (2007) JGR, 112, E05S03. [17] Seelos F. and the CRISM Team (2009) MRO/CRISM Data Users’ Workshop. [18] Mustard J. F. et al. (2008) Nature, 454, 305. [19] McGuire P. C. et al. (2009) Planet. Space Sci., 57, 809. [20] Pelkey S. M. et al. (2007) JGR, 112, E08S14. [21] Ehlmann B. L. et al. (2009) JGR, 114, E00D08. [22] Hunt G. R. and Salisbury J. W. (1971) Modern Geo., 2, 23. [23] Jouglet D. et al. (2007) 7th Internat. Conf. on Mars, Abstract #3153. [24] Hunt G. R. and Salisbury J. W. (1970) Modern Geo., 1, 283. [25] CRISM - PDS Geoscience Spectral Library. [26] Harner P. L. and Gilmore M. S. (2015) Icarus, 250, 204. [27] Bishop J. L. et al. (2013) JGR, 118, 635. [28] Boles J. R. and Franks S. G. (1979) J. Sed. Pet., 49, 55. [29] Muffler L. J. P. and White D. E. (1969) Geo. Soc. Am. Bul., 80, 157. [30] Dudoignon P. et al. (1989) Chem. Geo., 76, 385. [31] Streit E. et al. (2012) Contrib. Min. Pet., 164, 821. [32] Fondriest M. et al. (2012) J. Struc. Geo., 45, 52. [33] Luquot L. et al. (2012) Chem. Geo., 294-295, 75. [34] Seki Y. (1969) J. Geo. Soc. Japan, 75, 255.
© Copyright 2026