Mode of Action of Iron (111) Chelators as Antimalarials: I
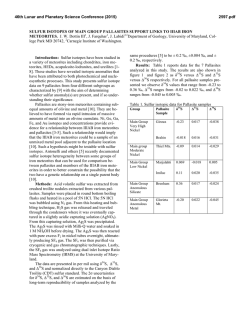
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. Mode of Action of Iron (111) Chelators a s Antimalarials: I. Membrane Permeation Properties and Cytotoxic Activity By Simon D. Lytton, Brenda Mester, lzac Dayan, Hava Glickstein, Jacqueline Libman, Abraham Shanzer, and Z. loav Cabantchik W e have designed two subfamilies of lipophilic iron (111) chelators previouslytermed reversed siderophores(RSFs). The agents display physicochemical properties that favor extraction of iron beyond membrane barriersof Plasmodium falciparum-infected red blood cells. W e studied the in vitro antimalarial potency of RSFs and their relationship to the membrane permeation properties of these agents. The mode of RSF action involves: (1) fast access to intracellular compartments of parasitized cells; (2) selective and high- affinity chelation of iron (111) from parasitized cells; (3)fast exit from cells after iron (111) complexation; and (4)exertion of cell damage on parasites exposed for 3 to 5 hours to drugs, irrespective of the stage of parasite development. These results suggest that on reaching a critical intraerythrocyte target, RSFs induce an iron deficit that parasites in general, and rings in particular, have limited capacity to restore. 0 1993 by The American Society of Hematology. I selected members of that subfamily showed various advantages over DFO as antimalarial. These included IO-fold lower concentrations of inhibitor which reduce growth by 50% (IC5’ values), similar effects on all erythrocytic stages of parasite growth, and considerably faster rates of action.’ Because iron (111) binding to RSFs completely abolished their antimalarial effect, it was implied that the arrest of parasite growth involved sequestration of critical iron and not the formation of intracellular toxic iron (111)-ligand complexes, as proposed for other classes of iron (111) chelators.” To explore the mode of action of RSFs as antimalarials, we studied the molecular properties that confer antimalarial activity to RSFs. The molecular design of these agents is predicated on biomimetic and modular approaches described earlier,’ which allowed systematic substitutions of a prototypic backbone with groups of different defined chemical character. The antimalarial activity of two families of RSFs were studied in in vitro cultures of Pfalciparum in terms of: (1) ICsovalues and speed of drug action, ( 2 ) developmental stage of drug sensitivity, (3) irreversibility of action on the overall biosynthetic capacity of the parasite and (4) drug permeation into infected and uninfected cells. The studies indicate that permeation plays a dominant role in the antimalarial activity of iron chelators and is a major determinant in the irreversible inhibition of parasite growth. NTRAERYTHROCYTE growth and development of human malaria parasites is highly dependent on iron (111) to the extent that it is arrested in the presence of iron (111) chelators.’-4Previous studies suggested that the iron sources affected by desfemoxamine (DF0)1-6and related hydroxamates7 reside inside the parasite and not at the level of host red blood cell (RBC)1,3,7 or serum source^.*^^ Possible mechanisms involved in the antimalarial action of these iron chelators are sequestration of iron (111) from vital sources, such as storage proteins, low molecular weight siderophores, iron centers of key parasite enzymes, such as ribonucleotide reductase,”.” or the scavenging of iron from degraded hemoglobin.” Previously, however, identification of these putative targets had not been accomplished. DFO is an iron chelator with remarkable therapeutic performance,13including antimalarial activity both in ’ and in v ~ v o . However, ~-~ because of its relatively slow and apparently selective permeation into the advanced growth stages of Plasmodium falciparum-infected cells,’3.14its biological activity is slow to develop, ie, it demands relatively long exposures of cells at mature stages of parasite growth.’.’ I We have undertaken the design and synthesis of lipophilic iron (111) carriers, which show requisite metal ion specificity and binding affinity but display fast penetration across membrane bamers so that they can gain rapid access into parasite compartments of essential iron need. In a previous study7we introduced the major prototype of a novel family of antimalarial agents that we termed reversed siderophore (RSF), that is, siderophores with relatively high lipophilic properties that subserve their permeation into cells. By virtue of their hydrophobic side chain and high iron (111)-binding efficiency, From the Department of Biological Chemistry, Hebrew University, Jerusalem, and the Department of Organic Chemistry, Weizmann Institute of Science, Rehovot, Israel. Submitted April 13, 1992; accepted August 26, 1992. Supported by Israel Ministry of Science and Technology, and the Hebrew University Central Fund for Applicative Research. Address reprint requests to Z. Ioav Cabantchik, MD, Department of Biological Chemistry, Hebrew University,Jerusalem, Israel 91904. The publication costs ofthis article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact. 0 I993 by The American Society of Hematology. 0006-4971/93/8101-0006$3.00/0 214 MATERIALS AND METHODS Materials. The synthetic scheme for the preparation ofthe various RSFs and their iron (111) complexes was described previ~usly.~.’~ Synthesis of N-(7-nitrobenz-2-oxa-1,3-diazole)-DFO (NBD-DFO) is given elsewhere.16Desfemoxamine B (DFO), was obtained from CibaGeigy (Basel, Switzerland). All other chemicals were from Sigma Chemical Co (St Louis, MO) or best available grade. Radiochemicals were from the Radiochemical Centre (Amersham, United Kingdom). Parasite cultures. The P falciparum strain ITG2Gl (Brazil, cloned; a gift of Dr L.H. Miller, NIH) was used for all experiments and was maintained in culture flasks of human erythrocytes by a modified version17of Trager and Jensen’s method18as described elsewhere.” Parasitemia values were obtained by differential counting of parasite growth stages on Giemsa-stained smears. Bioassay of RSF antimalarial activity. The antimalarial activity o f RSFs was assayed as described previo~sly.~ The compounds were added from concentrated stock solutions in dimethyl sulfoxide (DMSO) to microcultures (24 wells; Costar, Cambridge, MA) containing infected RBCs (2.5% hematocrit and 2% parasitemia). The cultures at ring stage were synchronized previously by incubation in 300 mmol/L alanine and 10 mmol/L TRIS-C1, pH = 7.4. After the Blood, Vol81, No 1 (January 1). 1993: pp 214-221 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. MODE OF ANTIMALARIAL ACTION OF IRON CHELATORS 215 if YDROSAMATE BINDIiVG CAVITY indicated time of incubation with the indicated drug or after washing twice with 200 volumes of medium, the cells were supplemented with 6 pCi/mL of [3H]-hypoxanthine (Radiochemical Centre) and parasite growth assessed after 24 hours by harvesting the freeze-thaw lysate of labeled cells onto glass fiber filters (Tamar Inc, Jerusalem, Israel). For all experiments the level of hypoxanthine uptake into cultures of noninfected RBCs was measured and counts of parasitized cultures were adjusted according to this background value. Uptake of radioactive carrier-Fe complexes. DFO and the different RSF derivatives were precomplexed to iron by the addition of concentrated stock solutions of 20 mg/mL carriers to 59FeC1,(Amersham) in 0.5 mL of 150 mmol/L NaCI, 10 mmol/L HEPES at 3 to 5 molar excess of carrier for 1 hour at room temperature. To begin the flux, normal or parasitized RBCs that had been washed three times in saline buffer and resuspended in the same buffer supplemented with 5% bovine serum albumin, 2.5 mmol/L citrate, and 2 mg/mL glucose were added to radioactive 59Fe-complexesat a final suspension of 20%hematocrit. After incubation at 37"C, 75 pL suspension was removed in duplicate aliquots for each time point, placed in plastic 15-mL test tubes (Sarstedt, Niimbrecht, Germany) and centrifuged 1 minute at 2,500g. The supematant was removed and the pellet immediately placed on ice. After all sampling was completed the pellets were washed twice in 15 mL of ice cold buffer containing 50 mmol/L EDTA and lysed in distilled water. Radioactivity (gamma emission, 700 to 1,300 keV) was counted and the cell number for each sample determined from hemoglobin absorption of the lysate at 410 nm.I9 Extraction of chelatable iron. Trophozoite-stage parasites were obtained from step gradient of Percoll (Pharmacia, Uppsala, Sweden)3% L-alanine in phosphate-buffered saline (10 mmol/L Na-phosphate, 150 mmol/L NaCI, pH 7.4).*' Cell suspensions of 100%parasitemia were adjusted to 1% hematocrit and placed in RPMI growth medium o m=2 m =I Fig 1. Structure of RSF subfamilies. The basic structure comprises the tripode anchor with the ethyl tail, the amino acid-containing bridges, which extend to the hydroxamatebinding cavity. The t w o subfamilies of RSFs ( m l and m2) differ in the number of methylene groups in their connecting bridge; m l = 1 methylene group and m2 = 2 methylene groups. R group refers to the amino acid substitution shown in Table 1. The upper figures highlight the structural differences in the hydroxamatebinding cavities of the two subfamilies. i CHzCH3 ANCHOR supplemented with 50 mmol/L sucrose and 20 mmol/L glucose. Pretreatment of cells was accomplished with 12 pmol/L of the indicated RSF or DMSO for 2 hours at 37°C at which time the cells were washed twice in X500 volumes of buffer containing 150 mmol/L NaCI, 10 mmol/L HEPES, and 50 mmol/L sucrose, pH = 7.4. Next the cells were incubated for an additional 3 hours in 6.8 pmol/L NBD-DFO and washed to remove fluorescent probe. Measurements of chelatable iron were performed in the trichloroacetic acid (TCA)soluble fraction of freeze-thaw lysates as described previously.'6 RESULTS Molecular properties of the RSFs family of iron (III) chelators. The RSF design is based on a biomimetic approach and modular assembly that allows formation of iron-binding cavities and systematic substitutions that confer the desired chemical character to the This design is achieved in the tripod topology, which consists of a carbon anchor from which extend bridges to the three hydroxamate groups that make up the iron (111)-binding cavity (Fig I). The overall construct enables substitution of amino acids of variable hydrophobicity into the bridges (R group). Figure 1 shows the structures of two subfamilies of R S s , which differ in the number of -CH2- groups, m 1 and m2, respectively, linking the anchor to the amino acid substituted in the bridge. Physicochemical properties and antimalarial activity of RSFs. The antimalarial activity of various derivatives from the two RSF subfamilies are expressed as IC5o values and compared on the basis of their physicochemical properties; iron (111)-binding and partition coefficients (Table 1). Irre- From www.bloodjournal.org by guest on February 6, 2015. For personal use only. LYTTON ET AL 216 Table 1. Physical Properties and Antimalarial Activity of RSFs Compound (R Group of RSFl m Re1 P.&t Re1 Fe Binding$ PdI* Fe-Binding5 0.29 0.298 0.12 1.31 0.16 1.25 1.25 0.23 1 .o 1 .o 1.16 2.4 2.4 0.9 2.8 0.06 0.37 0.37 3.86 9.3 1 .o 0.065 ICs0 (pmollL)~~ ~ (L-ileu) (D-ileu) (L-leu) (L-Val (L-pro) (L-ala) (D-ala) (L-leu) (L-ileu) (L-Val) DFO 2 2 2 2 2 2 2 1 1 1 8.2 8.2 7.4 2.1 0.4 0.3 0.3 17.0 90 1.0 <0.056 3f2 9f3 22 f 4 6f2 >IO0 62f 10 70f 13 5+2 3f 1 4f1 40 f 8 DFO and RSFs with various amino acid substitutions are compared on the basis of: tpartition coefficients (P,& n-octanol/saline); *relative binding efficiencies (determined spectrophotometricallyby competition with EDTA at 0.75mmol/L hydroxamate, 0.15mmol/L EDTA, and 0.15 mmol/L Fe3+ in aqueous methanol'; §the product of relative partition coefficient and iron (Ill)binding; and "the ICso values are the mean of 3 to 4 experiments performed on trophozoites, 40 hours exposure to drugs, of which the last 24 hours are incorporation of 3H-hypoxanthine.'6The , ,P are given relative to those of L-Val-m1, which is 1.7.The values of , , ,P of Free RSF ileumZand of leumzand their respective iron values for (Ill)complexes were essentially the same (not shown). The Fe-binding affinities are relative to DFO, which was given an arbitrary value of 1.16 so that the value for L-val m l is 1 (or 86% that of DFO). spective of the subfamily, the inhibitory potency of the an RSF correlates with the magnitude of its partition coefficient and therefore of its lipophilicity. All the derivatives display iron (111)-binding affinities of the same order of magnitude as DFO, and therefore this parameter would seem a priori to play a secondary role in determining the difference in the antimalarial activity of these series of RSFs. The best correlation between ICs0 values and physicochemical properties was obtained when ICs0 was plotted against the product of relative iron (111)-binding and partition coefficient (Pmff)(Fig 2). Derivatives of m 1 and m2 fall on the curve according to hydrophobicity of amino acid substitution. In the m2 subfamily a minimum value of 2 to 3 for product of relative iron (111) binding *Pcoeffis required for potent antimalarial activity, IC5o < 5, whereas L-val of m l subfamily shows a product of I , which seems sufficient for high activity, ICs0 = 4 (Fig 1). The differences in ICs0 values of L and D isomers of ileumzderivatives indicate that the predominant biological effect on parasites is probably not of the level of specificity as those displayed by siderophore receptors of the plant and bacterial ~ e l l s . ~ ~ ~ ~ ~ Speed of action and stage sensitivity of RSFs. The speed of action and stage sensitivity of RSFs was assessed by measurement of percent parasitemia (Tables 2 and 3) and of total parasite nucleic acid synthesis during exposure to various RSFs and DFO (Fig 3). The RSFs exert their inhibitory effects throughout the parasite asexual cycle as evident by the demonstrable inhibition of growth found at both the trophozoite (Table 3 and Fig 3A) and ring (Table 2 and Fig 3B) stages. On the other hand, DFO acted primarily at the trophozoite stage. Members of RSF m 1 and m2 subfamilies with either ileu or leu substitutions cause marked depression in the rates of nucleic acid synthesis even after short exposure times. The speed of action of RSFs is clearly manifested in the profiles of nucleic acid synthesis that diverge from those of control. The extent of this divergence is apparent in the lower average rates of synthesis for different intervals of drug exposure as compared with control (DMSO) (Fig 3 inset graphs). At trophozoite stage the rank order for RSF speed of action was ileum2was greater than leu,, , which was greater than ileu,, , as determined during early exposure periods (Fig 3A inset graph), and at ring stage, m 1 and m2 were equally effective at inhibition of initial rates (Fig 3B inset graph). After 12 to 13 hours of exposure no increase in the level of hypoxanthine incorporation was detected in either rings or trophozoites, indicating complete shutdown of nucleic acid synthesis. Time dependence for induction of irreversible growth arrest at the trophozoite and ring stages: RSFs versus DFO. In our previous study we showed that RSFl (m2, ileu) arrested parasite growth at both ring and trophozoite stages and that the inhibitory effect prevailed after washing the cells.' To determine if the speed and mode of action (continuous v irreversible) of the various RSFs as compared with DFO were dependent on the molecular properties of RSFs, we tested various amino acid derivatives of both m 1 and m2 subfamilies on parasites at the trophozoite stage. Figure 4 shows that although DFO (100 pmol/L) and ileu and leu members of the ml and m2 subfamilies (at 40 Wmol/L) produced similar inhibition after 24 hours continuous exposure, the subfamilies differed in their speed of action as irreversible inhibitors. Members of the m2 subfamily were as fast acting as members of the m 1 subfamily in the continuous mode of exposure but more effective in the irreversible mode of action. These results would suggest that specific molecular dimensions favor the irreversible mode. On the other hand, although DFO displayed slower speed of action and relatively lower potency than RSFs in the continuous mode, it was as effective in 100 IO I 0.1 1 2 3 4 5 re]. Fe binding *Pme,, Fig 2 . Correlation of antimalarial activity (IC, values) vcombined iron-binding properties and lipophilicity of RSFs. The correlation is shown for different amino acid derivatives of m l (open symbols) and m2 (closed symbols) subfamilies. The IC, and calculated values of Fe binding *P, are listed in Table 1. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. MODE OF ANTIMALARIAL ACTION OF IRON CHELATORS 217 Table 2. Effect of DFO and RSF-lleu,, Time on Maturation of Rings Into Trophozoites Control DFO Hours Rings Trophs 0 24 10f 1 1.1 f0.5 Percent control 10 <0.2 11 f 2 100 Rings lo? 1 3.2f 1.3 32 RSF-lleum2 Trophs Rings Trophs <0.2 8.3f 2.5 83 IO* 1 8.4f 2.8 84 t0.2 2.2 f 0.5 22 Data are given as percent parasitemia at 0 and 24 hours after drug administration to a synchronized culture containing 10% ring forms, and as percent trophozoites and rings present after 24 hours incubation with drug relative to the 10% rings originally present (percent control). producing irreversible effects on trophozoite growth as the leu member of the m2 subfamily. These results are in contrast to those obtained with RSF-Ileum2and DFO on rings (Fig 5). Rings are considerably more susceptible to RSFs than DFO at either mode of inhibition (continuous or irreversible). This property is particularly evident after 5 hours exposure to RSF, at which point DFO hardly affected nucleic acid synthesis. The differential susceptibilities of rings and trophozoites manifested either during continuous exposures to RSFs and DFO or by remaining after removal of the drugs (irreversible inhibition) can also serve as an indication that the growth assay based on nucleic acid synthesis provides a reliable measure for assessing stage dependence of drug susceptibility. This is of importance because of the likelihood of minor (undetectable) contaminations (1% to 5%) of seemingly synchronized cultures of rings with metabolically active trophozoites (1% to 5%).Nonetheless, determination of stagerelated parasitemia of drug-treated cultures by light microscopy (Tables 2 and 3) confirmed the results obtained by measuring nucleic acid synthesis: with continuous exposure to drugs for 24 hours, trophozoites were arrested by either DFO or RSFs, whereas rings were susceptible primarily to RSFs. However, irreversible arrest was obtained primarily by DFO action on trophozoites and by RSF-ileum2action on rings (Tables 2 and 3). Permeability of RSFs versus DFO into normal versus infected RBCs and removal of chelatable iron. In a previous study14 Fritsch and Jung showed that 14C-DF0was taken up by RBCs and that after 5 hours exposure it reached intracellular levels that were threefold to fourfold higher than those attained in normal RBCs. Assuming the entire RBC space is drug accessible, the estimated levels attained intracellularly correspond to less than 5% of the extracellular concentration of DFO. Because of the fact that RSFs have markedly greater lipophilicity than DFO, we predicted faster penetration of these compounds and therefore greater capacity for chelation of intracellular iron after short exposure times. Penetration of free RSFs into normal and infected RBCs was estimated by monitoring uptake of the R S F S - ~ (111) ~ F ~complexes into infected and uninfected cells. To confirm actual uptake of Fe-RSF ileum2rather than adsorption to the outer membrane, cells were thoroughly washed and lysates were subjected to 80,OOOg centrifugation for 20 minutes. More than 90% of the total counts were found in the cytosol fraction (results not shown). Figure 6 shows rapid penetration of RSF-ileum, iron (111) complexes into both normal and infected RBCs with tl,Z = 2 to 3 minutes. Maximum uptake was attained after 10 to 15 minutes at which point the level of 4 nmol/ 10" cells is approximately equal to the equilibrium concentration of 5 pmol/L external Fe (Fig 6A). Identical uptake kinetics were obtained with RSF-leuml. Similar profiles were also obtained for efflux of complex preloaded cells (not shown). In contrast, the more hydrophilic RSF 1-alam2,whose relative PcoeK is considerably smaller than those of the leu or ileu congeners (Table l), was hardly taken up as iron (111) complex by infected cells during the first hour of exposure. Even after 8 hours incubation it attained an intracellular level of less than one tenth the external concentration (Fig 6A). Figure 6B compares DFO-Fe uptake into normal and infected RBCs over an 8-hour time course. The uptake trend into infected RBCs is approximately twice that of normal RBCs yet the concentration of intracellular Fe-DFO after 8 hours indicates an uptake level that is 20-fold lower than that attained by hydrophobic RSFs after 10 minutes (Fig 6A). To the extent that the values of uptake obtained in this work for the DFO-iron (111) complexes are comparable to those obtained by others for free I4C-DF0,l4 we assume that permeation of the complexes provides a reliable approximation for free chelator permeation. The assumption that permeation of iron (111) complexes provides a measure for free ligand permeation is probably applicable to the rapidly penetrating iron (111)-RSF complexes because the values of the partition coefficients of both the free RSF ligand and the respective iron (111)-RSF complexes Table 3. Effect of DFO and RSF-Ileum, on Maturation of Trophozoites and Formation of New Rings Control Time DFO RSF-lleu, Hours Trophs Rings Trophs Rings Trophs Rings 0 24 1.8f 0.5 1.2f 0.4 <0.5 8f1 100 1.8f 0.5 1.5f 0.6 <0.5 0.7 k 0.4 1.8? 0.5 1.6 f 0.6 <0.5 0.5 k 0.3 Percent control 9 6 Data are given as percent parasitemia at 0 and 24 hours after drug administration to a synchronized culture containing 1.8% trophozoites (trophs), and as percent formation of new rings after 24 hours incubation relative to control (percent control) From www.bloodjournal.org by guest on February 6, 2015. For personal use only. LYTTON ET AL 218 - A TROPHS DMSO 0 $-I 4-11 0 ,B 7-24 20 10 RINGS RSPs - 30 Time (hrsj DMSO 15 - tated by their capacity for rapid penetration into parasitized cells, chelation of iron (III), and its extraction from infected cells. Because the iron (111)-drug complexes are apparently inert to RBCs or the RSFs must exert their antimalarial activity via an iron deprivation me~hanism.~.~,',~ We have assumed in this work that the permeation of RSFsiron (111) complexes across the membranes of parasitized cells provides a measure for permeation of the free chelators. This assumption holds for the most hydrophobic RSFs, which display high and comparable values of partition coefficient (octanol/water) for either the free or the complexed forms. Even for the hydrophilic DFO, it is remarkable that uptake of iron (111)-DFO into infected cells (Fig 6B) is qualitatively and quantitatively similar to that obtained with free DF0.14 In contrast to DFO, the highly lipophilic RSFs attain rapid chemical equilibrium (t1,2of minutes) with the entire RBC ' TROPHS- - - - ->scH/ZoNs 0 5-7 4-I3 10 - 0 10 20 30 Time (hrs) Fig 3. Time dependence of RSF-induced growth arrest during ring and trophozoite stages. Parasitegrowth at trophozoite (A) and ring ( 6 )stages was assessed in the presence of RSFs. Parasites were preexposed to different RSF derivatives at 40 pg/mL or dimethyl sulfoxide (DMSO) control for 2 hours and then labeled with [3H]-hypoxanthineas indicatedby arrow. For each time point shown the amount of incorporation was determined as described in Materials and Methods. Parasitemia in ring-stagecultures was adjusted to 3%to 4%. were similar and relatively high (above 10-in absolute scale for leu and ileu congeners of m 1 and m2 subfamilies, Table 1). On this basis, the results shown in Fig 6A reflect the permeability of the free RSF ligands. Therefore, we might presume that permeation of free ligand into cells should also lead to iron (111) extraction from the cells and its delivery in the medium. We have followed the depletion of chelatable iron from infected RBCs after 2-hour RSF treatment (Table 4). The assay of chelatable iron involved fluorescence iron quenchingtacid-EDTA dequenching using the fluorescent probe NBD-DF0.I5,I6Table 4 shows the results of experiments in which pretreatment of trophozoites with RSF ileum2 reduced the amount of chelatable iron by 5- to 10-fold. The amount of chelatable iron detected in noninfected RBCs was approximately 50-fold lower than in parasitized RBCs and was hardly affected by pretreatment with RSF. If anything, it slightly increased after RSF treatment. DISCUSSION Pursuant to our initial study of RSFs as antimalarial agents,' we show that the inhibitory potency of RSFs is dic- Z4hrs I Fig 4. Inhibition of parasite growth during and after exposures of trophozoites to RSFs. RSFs were added at 40 pg/mL and DFO at 100 gg/mL to trophozoite stage cultures for 3, 5, and 24 hours. Parasite growth was assessed by labeling of cultures with 6 pCi/ mL [3H]-hypoxanthine either concomitant with the exposure to drug (solid bars) or after removal of drug by washing with medium and incubation up to 24 hours (hatched bars). Time indicates the hours of exposure to drug. Growth is expressed as ['HI-hypoxanthine incorporation relative to control cultures (<0.5%DMSO, the drug carrier). The graphed values represent the mean and standard deviation of 2 to 3 experiments with counts from 5 to 6 replicate samples at each time point. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. MODE OF ANTIMALARIAL ACTION OF IRON CHELATORS RINGS- - - - ->EARL Y TROPHS 219 A 5.0 I 0.0 20 0 B DFO 40 60. Time (min) 8h ileum, 0.00 - 0 DFO Fig 5. Inhibition of parasite growth during and after exposures of rings to RSF-ileum, and DFO. The experiment was performed essentially the same as described in Fig 4. except that the culture contained predominatly (>95%) rings. Solid bars represent values of either 5-hour or 24-hour nucleic acid synthesis in the presence of either DFO or Ileum2as percent of control (untreated cultures) and hatched bars of 19-hour nucleic acid synthesis after exposure of cultures to drug for 5 hours and removal of extracellular drug. volume, which includes the parasite. This result alone could be sufficient to explain the vast difference in ICso values of the various RSFs and DFO and also their markedly different speed of action. Assuming similar iron extraction capacities, which is the case for various RSFs and DFO, faster access to iron pools might lead to faster biological effects, as previously indi~ated.~'?~~ The subfamilies of RSFs differ in the structure of the hydroxamate-binding cavity and their molecular dimension25 (Fig 1). Although the relative contribution of both physicochemical parameters to the antimalarial potency of RSFs might vary for the different structural congeners (Table l), their combined properties correlate reasonably well with their antimalarial potency (Fig 2). The results of this study supplement our recent findings that show strict dependence of DFO and methylanthramilicDFO antimalarial activity on direct access of hydrophilic drugs to the parasite via an aqueous access route,26recently identified as a parasitophorous This pathway of access for drug entry into parasites by no means circumvents the 2 4 6 Time (hrs) 8 Fig 6. Uptake of Fe"-carrier complexes into normal and infected RBCs. Uptake of 69Fe-RSF(A) and 69Fe-DF0(6) into normal RBCs (NRC, open symbols) and infected RBCs (IRC, 60% parasitemia, >90%trophozoites, closed symbols). The carriers were precomplexedto 69FeC13at 4:l ratio in DMSO with 5 pmol/L external FeCI, (from methanolic stock solutions). The radioactive complexes were then added to 20%hematocrit cell suspensions and flux was measured as described in Materials and Methods. Specific activity was 485,000 cpm/nmol and 369,000 cpm/nmol for the RSF and DFO uptake experiments, respectively. Cell number was determinedfrom hemoglobin absorption of lysate at 410 nm.Ig need for drug to diffuse across the parasite membrane, probably the rate-limiting step in the overall uptake process. Lipophilic RSFs can reach the parasite either directly from the medium or after permeating into the host cell, whereas more hydrophilic RSFs (eg, RSF-ala), in analogy with DFO and Table 4. Extraction of Chelatable Iron From Normal and Infected RBCs Iron (nmol/lO1o cells) Experiment 1 RSF pretreatment NRC IRC No 10.1 4.6 Yes 0.7 0.9 ExDeriment2 No Yes <o. 1 ND 0.3 2.9 Iron extractions were performed on cell suspensions treated (+) or untreated (none) with 12 Mmol/L RSF-lleu,, for 2 hours at 37"C./ Fluorescence assays of chelatable iron in noninfected(NRC)and in parasitized (IRC) RBCs are described in Materials and Methods. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 220 its structural congeners, are probably restricted in their entry into the parasite from the medium. The enhanced membrane permeation properties of lipophilic RSFs shed light on the mode of antimalarial action of hydroxamate iron chelators. Previous studies*,'' claim stagedependence of DFO for the mature trophozoite/schizont and thereby imply that the critical pool or target of chelatable iron of the parasite is available at a specific time during its intraerythrocytic development. These studies2>''did not fully consider the slow penetration of DFO across RBC membrane in general and the limited access of this hydrophilic drug to the parasite. Therefore, the apparently poor inhibitory effect of DFO on rings might merely reflect its poor accessibility to the parasite at early stages of growth. In this study we have clearly shown sensitivity of both ring and trophozoite stages to RSFs. On this basis, the induction of growth arrest by hydroxamate iron chelators seems to result from access to resident iron pools and sequestration of essential iron that is maintained for the survival of the parasite at various stages of metabolic activity or development. The possible use of RSFs as antimalarials depends also on their capacity to exert irreversible damage to parasites. This capacity depends not only on drug properties per se, but also on the potential of the parasite for replenishing iron pools after drug removal. Faster permeating and therefore faster acting drugs, such as some lipophilic RSFs, might act on parasites at relatively faster rates but also in a reversible mode because, on drug removal, metabolically active forms, such as trophozoites, seemingly overcome the growth arrest by mobilizing metabolic iron. However, the fast permeating RSFs have an advantage in that they demonstrably permeate into rings and irreversibly affect their development. On the other hand, slower permeating drugs, such as DFO, exert minor effects on rings but act on trophozoites, although at higher concentrations and with slower rates than RSFs. However, most of the inhibitory effect of DFO on trophozoites is probably caused by its retention in the parasite after external drug removal (Fig 4). This information regarding stage dependence and speed of action of antimalarial drugs has implications for identification of the putative iron-containing targets that are affected by the chelators and their relationship to the mode of antimalarial action. In a subsequent study we further explore RSF mode of action on ring and trophozoite stages and the strong implication that ribonucleotide reductase iron centers are key but not sole targets for RSFs and DFO inhibition of parasite growth.28 In presentation of the significant differences in potency and speed of action of various RSF derivatives we do not rule out the possibility that also the unique structural features of these compounds confer a capacity for chelation and removal of iron from target enzymes, iron carriers (siderophores), and storage sites that are either poorly accessible or not recognized by DFO and hydrophilic iron chelators. For D and L isomers of RSF-ileum2,it is not clear at this stage to what extent the difference in their antimalarial potency might be contributed by a specific (ie, receptor-like) interaction of the chelator with a component of the parasite (eg, a putative siderophore receptor). Such interactions have been amply described in the microbial and plant ~ o r l d ~ as '.~ LYTTON ET AL part of the mechanisms of iron (111) acquisition from extracellular sources.29For the m 1 and m2 congeners of RSF-ileu, the difference in their speed of action might stem from conformational differences of their iron (111) complexes (Fig I). Further structure-activity relationship studies and chemical analysis of drug fate within cells should provide further information on those questions. Several iron-binding compounds presented in this study were also tested on various mammalian cell lines in culture and were shown to cause no short-term toxic effects.' Although RSF-ileum2also has demonstrable in vivo antimalarial activity with no apparent adverse reactions,30the information presently available is insufficient for assessing its therapeutic potential and toxicity. REFERENCES 1. Raventos-Suarez C, Pollack S, Nagel RL: Plasmodium falci- parum: Inhibition of in vitro growth by desfemoaxmine. Am J Trop Med Hyg 31:919, 1982 2. Whitehead S, Pet0 TEA: Stagedependent effect of deferoxamine on growth of Plasmodium falciparum in vitro. Blood 76: 1250, 1990 3. Hershko C, Pet0 TEA: Deferoxamine inhibition of malaria is independent of host iron status. J Exp Med 168:375, 1988 4. Fritsch G, Treumer J, Spira DT, Jung A: Plasmodium vinckei: Suppression of mouse infectionswith desfemoxamine B Exp Parasitol 60:171, 1985 5. Traore 0, Carnevalle P, Kaptue-Noche L, MNBede J, Desfontaine M, Elion J, Labie D, Nagel R: Preliminary report on the use of desfenioxamine in the treatment of Plasmodium falciparum malaria. Am J Hematol 37:206, 1991 6. Pollack S, Rossan RN, Davidson DE, Escajadillo A: Desferrioxamine suppresses Plasmodium falciparum in Aoutus monkeys. Proc SOCExp Biol Med 184:162, 1987 7. Shanzer A, Libman J, Lytton SD, Glickstein H, Cabantchik Z1: Reversed siderophores act as antimalarial agents. Proc Natl Acad Sci USA 88:6585, 1991 8. Pet0 TEA, Thompson JL: A reappraisal of the effects of iron and desfemoxamine on the growth of Plasmodium falciparum in vitro: The unimportance of serum iron. Br J Haematol 63:273, 1986 9. Pollack S, Schnelle V: Inability to detect transferrin receptors on P. ,falciparum parasitized red cells. Br J Haematol 68:125, 1988 10. Scheibel LW, Rodriguez S: Antimalarial activity of selected aromatic chelators. V. Localization of 59Fein Plasmodium falciparum in the presence of oxines, in Brewer G (ed): Malaria and the Red Cell. New York, NY, Liss, 1989, p 119 1 I . Carter T, Bayne MT, Gordeuk VR, Brittenham GM, Aikawa M: Stage-specific ultrastructural effects of desfemoxamine on Plasmodium falciparum in vitro. Am J Trop Med Hyg 45593, 1991 12. Slater AFG, Swiggard WJ, Orton BR, Flitter WD, Goldberg DE, Cerami A, Henderson GB: An iron-carboxylate bond links the heme units of malaria pigment. Proc Natl Acad Sci USA 88:325, 1991 13. Hershko C, Wheatherall DJ: Iron chelating therapy. CRC Critical Reviews in Clinical Laboratory Sciences(vol26). Boca Raton, FL, CRC, 1988, p 303 14. Fritsch G, Jung A: I4C-Desfemoxamine B: Uptake into erythrocytes infected with Plasmodium falciparum. Z Parasitikd 72:709, I986 15. Lytton SD, Mester B, Libman J, Shanzer A: Monitoring of iron (111) removal from biological sources using a novel fluorescent ~siderophore. ,~~ Anal Biochem 205:326, 1992 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. MODE OF ANTIMALARIAL ACTION OF IRON CHELATORS 16. Lytton SD, Cabantchik ZI, Libman J, Shanzer A Reversed siderophores act as antimalarial agents: 11. A fluorescent derivative of desferal, NBD-desferal, selectively scavanges iron (111) from parasitized red cells. Mol Pharmacol 40584, 1991 17. Jensen J B Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg 27: 1274, 1978 18. Trager W, Jensen JB: Continuous culture of human malaria parasites. Science 193:673, 1976 19. Kutner S, Breuer WV, Ginsburg H, Aley JB, Cabantchik ZI: Characterization of permeation pathways in plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: Association with parasite development. J Cell Physiol 125: 521, 1985 20. Rivadeneira EM, Wasserman M, Espinal C: Separation and concentration of schizonts of Plasmodium falciparum by percoll gradients. J Protozol 30:367, 1983 2 1. Shanzer A, Libman J: Biomimetic siderophores, in Winkelmann G (ed): Handbook of Microbial Iron Chelators. Boca Raton, FL, CRC Press, 1991, p 309 22. Shanzer A, Libman J, Lazar R, Tor Y, Emery T Synthetic femchrome analogues with growth promotion activity for Arthrobacterflavescens. Biochem Biophys Res Commun 157:389, 1988 22 1 23. Porter JB, Gyparaki M, Huenns ER, Hider RC: The relationship between lipophilicity of hydroxypyrid-one iron chelators and cellular iron mobilization, using a hepatocyte culture model. Biochem SOCTrans 14:1180, 1986 24. Porter J: Oral iron chelators: Prospects for future development. Eur J Haematol 43:271, 1989 25. Jurkevitch E, Hadar Y , Chen Y , Libman J, Shanzer A: Iron uptake and molecular recognition in Pseudomonas putida: Receptor mapping with femchrome and its biomimetic analogs. J Bacteriol 1457, 1992 26. Loyevsky M, Lytton SD, Mester B, Libman J, Shanzer A, Cabantchik ZI: The antimalarial action of Desferral involves a direct access route to erythrocytic (Plasmodium falciparum) parasites. J Clin Invest 1992 (in press) 27. Pouvelle B, Spiegel R, Hsiao L, Howard RJ, Moms RL, Thomas AP, Taraschi TF: Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature 353:73, 1991 28. Lytton, et al: (manuscript in preparation) 29. Winkelmann G, van der Helm D, Neilands JB (eds): Iron Transport in Microbes, Plants and Animals. Weinheim, Germany, VCH Verlagsgesellschaft mbH, 1987 30. Lytton, et al: (submitted for publication) From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1993 81: 214-221 Mode of action of iron (III) chelators as antimalarials: I. Membrane permeation properties and cytotoxic activity SD Lytton, B Mester, I Dayan, H Glickstein, J Libman, A Shanzer and ZI Cabantchik Updated information and services can be found at: http://www.bloodjournal.org/content/81/1/214.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026