1-s2.0-S1569905614604577-main
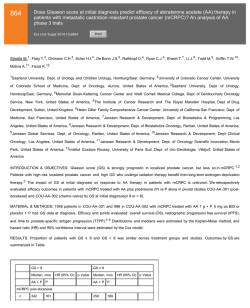
463 The efficacy and safety of repetitive intravesical onabotulinumtoxin-A in the treatment of interstitial cystitis/bladder pain syndrome: Long-term follow up Eur Urol Suppl 2014;13;e463 Print! Print! Lee C-L., Jiang Y-H., Jhang J-F., Kuo H-C. Buddhist Tzu Chi General Hospital and Tzu Chi University, Dept. of Urology, Hualien, Taiwan INTRODUCTION & OBJECTIVES: Currently, there is no cure for interstitial cystitis/ bladder pain syndrome (IC/BPS) and the treatment is limited to symptomatic relief. Intravesical onabotulinumtoxin-A (BoNT-A) injection is a safe and effective therapeutic option, although its long-term sustainability after single injection remains unsatisfactory. This study evaluated long-term efficacy and safety of repeated intravesical BoNT-A injections. MATERIAL & METHODS: From July 2006 to Aug 2012, consecutive patients with IC/BPS who failed conventional treatments were prospectively enrolled. All subjects received an initial 100U of intravesical BoNT-A injection followed by repeated injections every 6 months for up to 4 times or until he/she wished to discontinue. O’Leary-Sant symptom indexes (ICSI) and problem indexes (ICPI), visual analogue score (VAS) for pain, urodynamic study (VUDS) parameters, 3-day voiding diary, global response assessment (GRA), adverse events were recorded. All baseline values were compared at each treatment time point. Long-term successful results were analysed. RESULTS: Of these 104 patients, there were 45 subjects who had completed all 4 injections and available for follow-up. ICSI, ICPI, VAS, functional bladder capacity (FBC) and GRA all showed significant improvement after repeated BoNT-A injections (Table 1). The occurrence of adverse events did not increase with increasing number of BoNT-A injections (Table 2). Kaplan-Meier survival curves revealed better success rate in those whom received repetitive injections (Fig.1). Table 1. Changes of parameters in the 45 patients receiving all 4 repeated BoNT-A injections N=45 BoNT-A 1 BoNT 2 BoNT 3 BoNT 4 ICSI 12.3±3.38 8.67±4.24 8.53±3.84 8.04±3.91 <0.0001 ICPI 11.5±2.91 7.89±4.38 7.62±4.29 6.31±4.59 <0.0001 OSS 23.7±85.9 16.6±8.22 16.2±7.92 14.3±8.31 <0.0001 VAS 5.36±2.24 3.67±2.28 3.33±2.43 2.78±2.34 <0.0001 FBC 135±74.6 169±82.3 204±96.1 Frequency 13.8±4.96 10.5±5.65 11.1±6.08 10.1±5.31 <0.0001 Noturia 3.84±1.99 3.09±1.69 3.02±2.29 3.16±2.59 0.072 Qmax 14.0±4.76 13.3±6.37 13.3±5.03 12.6±5.55 0.647 Volume 259±106 281.9±125 296±132 PVR 15.3±7.87 31.4±8.56 44.3±8.05 57.9±9.08 0.039 CBC 276±106 319±112 340±114 223±110 274±139 338±141 P value <0.0001 0.297 0.012 MBC 686±206 739±209 742±206 758±199 0.030 GRA 0 1.39±1.04 1.65±0.82 1.87±1.15 <0.0001 Gr 1.73±1.03 1.64±0.93 1.32±0.92 1.24±0.96 0.010 Table 2. Adverse events reported at each treatment time point None UTI Dysuria CISC AUR Hematuria Total BoNT-A 1 62 6 34 2 104 BoNT-A 2 44 7 36 1 1 89 BoNT-A 3 30 10 22 1 1 64 BoNT-A 4 28 5 12 45 CONCLUSIONS: In this study, we have shown that repetitive intravesical injections of BoNT-A is safe, effective and sustainable in the treatment of IC/BPS who were refractory to conventional treatment.
© Copyright 2026