PDF形式 - 医薬品医療機器総合機構
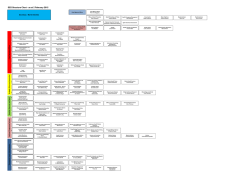
第 1 回 日本-マレーシアシンポジウム 1 Malaysia - Japan Symposium on Pharmaceutical Regulatory System st 要旨: 医薬品の開発・製造・流通・販売はグローバル化が進んでおり、各国の薬事規制当局及び業界は協力・ 連携して規制活動に取り組むことが重要となっております。とりわけ近年、医薬品の臨床開発・製造 の現場としてアジアは重要な地域であり、MHLW/PMDA ではアジア各国の規制当局等との協力関係 の構築に向けた取り組みを強化しております。 本シンポジウムは、医薬品医療機器総合機構(PMDA)とマレーシア国保健省薬品管理局(NPCB) が主催となり日本・マレーシア間で初めて開催されるシンポジウムであり、両国の薬事の相互理解を 深め、医薬品規制や開発のよりよき発展を目指す事を目的としております。今回のシンポジウムでは、 医薬品の薬事規制の視点から分野別テーマについて掘り下げた発表および討論が行われます。 Purpose: Globalization of development, manufacturing, trade, and marketing of drugs has been progressing, and cooperation of regulatory activities amongst pharmaceutical regulatory agencies of each region has become a necessity. Nowadays, Asian countries have become crucial in clinical development and manufacturing of drugs globally, and therefore, collaborative relationships among the Asian regulatory agencies are highly important. This is the first joint conference being hosted by National Pharmaceutical Control Bureau (NPCB) and Pharmaceutical and Medical Devices Agency (PMDA), with the aim to enhance Malaysia and Japan’s mutual understanding of each other’s regulatory system, and to promote advancement of pharmaceutical regulations and development. This symposium will offer in-depth presentations and discussions in each area from the pharmaceutical regulatory perspective. 主催 (Host) 独立行政法人医薬品医療機器総合機構 Pharmaceuticals and Medical Devices Agency(PMDA), Japan マレーシア国保健省薬品管理局 National Pharmaceutical Control Bureau (NPCB), Malaysia 後援 (Support): 日本製薬工業協会 Japan Pharmaceutical Manufacturers Association(JPMA) マレーシア業界 Malaysian Pharmaceutical Society (MPS) 1.日程 (Date) 平成 27 年 3 月 10 日~11 日(AM) (March 10th and 11th (AM)、2015) 2.会場 (Venue) ALOFT KUALA LUMPUR SENTRAL No 5, Jalan Stesen Sentral, 50470 Kuala Lumpur, Malaysia. Phone: +60 3 2723 1188 Fax: +60 3 2723 1588 www.aloftkualalumpursentral.com 3.参加者数 (Attendees) 約 150 名 (150 attendees) Registration will be closed when application reaches 150 4. 参加登録 (Registration) (1) 参加登録をされる際は、以下の URL より登録フォームへ記入の上、ご送付ください。 To attend the symposium, kindly download the registration form which can be obtained from URL link: http://portal.bpfk.gov.my/view_file.cfm?fileid=2635 (2) 記入済みの登録フォームと参加費のお支払いのレシートは [email protected] までお送り ください。 The completed registration forms as well as proof of payment / bank-in receipt must be sent via e-mail to [email protected] 5.参加費 to confirm your registration. (Registration Fee) 600 リンギット (RM 600 / person) 6.参加費のお支払い方法 (Payment Method of Registration Fee) ※4の登録フォーム内に詳細の記載がありますので、そちらをご参照ください。 * Please refer to the Registration form in item 4 for details on payment methods. お支払いに関するご質問につきましては、以下へご連絡ください (For enquiries on fee payment, kindly contact the following): Malaysian Pharmaceutical Society (MPS), Wisma MPS, 16-2, Jalan OP 1/5, 1-Puchong Business Park, Off Jalan Puchong, 47160 Puchong, Selangor, Malaysia. Phone: +60-3-8079 1861 Fax: +60-3-8070 0388 E-mail: [email protected] 7.宿泊 (Accommodation) 参加費に宿泊費は含まれません。シンポジウム会場が Aloft Kuala Lumpur Sentral となっております。便 宜を考慮し、会場で宿泊される参加者の方はホテルへ直接ご連絡ください。 The registration fee does not include accommodation. As the event will be held at Aloft Kuala Lumpur Sentral, participants may wish to choose accommodation at this hotel for convenience. Please contact the hotel directly for Hotel Reservation ALOFT KUALA LUMPUR SENTRAL https://www.starwoodmeeting.com/StarGroupsWeb/res?id=1501050145&key=18A5C5A1 他のオプション OTHER OPTIONS: Participants may also choose accommodation at various nearby hotels that are within walking distance, some of which are listed below. Kindly contact the chosen hotel directly for Hotel Reservation. HILTON KUALA LUMPUR 3 Jalan Stesen Sentral, 50470 Kuala Lumpur, Malaysia. Phone: (603) 2264 2264 Fax: (603) 2264 2266 http://www3.hilton.com/en/hotels/malaysia/hilton-kuala-lumpur-KULHIHI/index.html LE MERIDIEN KUALA LUMPUR 2 Jalan Stesen Sentral, Kuala Lumpur Sentral, 50470 Kuala Lumpur, Malaysia. Phone: (603) 2263 7888 Fax: (603) 2263 7222 www.lemeridienkualalumpur.com 8.お問い合わせ先 事務局 1st (Inquiries) The Secretariat: Malaysia – Japan Symposium on Pharmaceutical Regulatory System (Attn: Ms. Sha Yee Vonne) c/o Centre for Organisational Development National Pharmaceutical Control Bureau (NPCB), Ministry of Health Malaysia, Lot 36, Jalan Universiti, 46200 Petaling Jaya, Selangor. Phone : (603) 7801 8489 Fax : (603) 7958 2960 E-mail : [email protected] 9.通訳 同時通訳 (Translation) 日本語⇔英語 10.プログラム案 (Simultaneous translation to be provided for Japanese and English) (Tentative Programme) As below: 1st Malaysia – Japan Symposium on Pharmaceutical Regulatory System Day 1 (10 March 2015) 8:30-9:00 9:00-10:00 Registration of Participants Symposium Narration: (NPCB) Welcoming Remarks/Officiating Speech YBhg. Dato' Eisah A. Rahman Senior Director of Pharmaceutical Services, Ministry of Health, Malaysia (20 min) Day 1 (10 March 2015) Updates/Keynote NPCB “Role and Vision of PMDA -Promoting Global Public Health-“ Taisuke Hojo, Senior Executive Director, PMDA (20 min) PHOTO SESSION (10 min) 10:00-10:30 Morning Break Session 1: Regulatory Review & Updates Chair: (NPCB) 10:30-12:30 “Japan regulatory system for pharmaceutical products and Active Pharmaceutical Ingredients “(tentative) Hiroaki Yamada, Office Director, Office of New Drug II, PMDA “Malaysia regulatory system for pharmaceutical products” (NPCB) Discussion 12:30-14:00 Lunch Session 2: Guidelines & References Chair: (PMDA) “MS 2424:2012 - Halal pharmaceuticals: General guidelines” (NPCB) 14:00-16:00 “Malaysia herbal monograph” (NPCB) “Japanese Pharmacopoeia” (tentative) Seiko Miyazaki, Office Director, Office of Standards and Guidelines Development, PMDA Discussion Day 1 (10 March 2015) 16:00-17:30 Afternoon Break & Networking Session End of Day 1 Day 2 (11 March 2015) Session 3: NPCB Sessions Chair: (NPCB) 9:00-10:15 “Regulatory control for herbal/traditional medicines and health supplements” (NPCB) “Regulatory control for generics” (NPCB) Discussion 10:15-10:45 Morning Break Session 4: PMDA Sessions Chair: (PMDA) “GMP Inspection (Risk Based Assessment & Product Recall)” (tentative) Kentaro Hara, Principal Inspector, Office of Manufacturing/Quality and Compliance, PMDA 10:45-12:00 “Regulatory framework for biotherapeutic products including similar biotherapeutic products” Yasuhiro Kishioka, Principal Reviewer, Office of Cellular and Tissue-based Products PMDA Discussion Day 2 (11 March 2015) Closing Remarks 12:00-12:30 Teruyoshi Ehara, Office Director, Office of Int’l Programs, PMDA End of Symposium 12:30-14:00 Lunch
© Copyright 2026



![Lafite Menu-web [Converted] - Shangri](http://s2.esdocs.com/store/data/000465114_1-4e14a74a3e0c4224bd0b1bfb84a62f58-250x500.png)