Petrologic and Isotopic Classification of Ungrouped Achondrite NWA
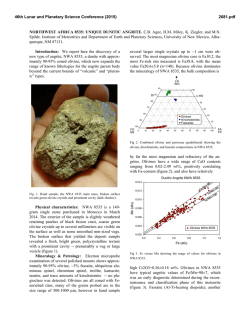
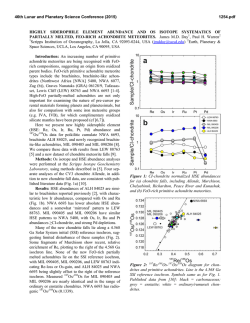
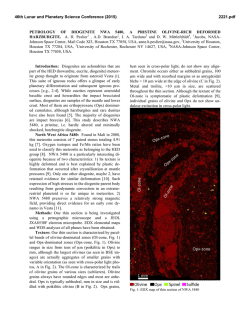
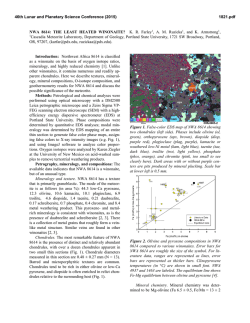
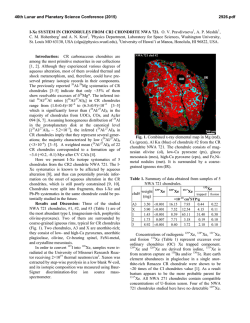
46th Lunar and Planetary Science Conference (2015) 1472.pdf PETROLOGIC AND ISOTOPIC CLASSIFICATIONS OF UNGROUPED ACHONDRITE NWA 8186: IMPLICATIONS FOR A CK/CV ASTEROIDAL ORIGIN. P. Srinivasan1, F. M. McCubbin1, C. B. Agee1, K. Ziegler1, M. E. Sanborn2, Q.-Z. Yin2. 1Institute of Meteoritics and Dept. of Earth and Planetary Sciences, University of New Mexico, Albuquerque, NM 87131, USA. 2Dept. of Earth and Planetary Sciences, UC Davis, CA 95616, USA. Email: [email protected]. Introduction: The discovery of primitive achondrites and models of partially differentiated chondrite parent bodies have reintroduced the hypothesis of a common origin for chondrites and achondrites. This single-body hypothesis indicates that both groups could coexist on the same parent body in radial layers of increasing thermal grade towards the core [1]. Thermal evolution models on early accreting bodies predict the formation of an unmelted chondritic crust as a consequence of melting by radiogenic heating [2-7]. Therefore, achondritic components would form within the inner, hotter regions of the parent body, and chondritic components would remain on the outer, cooler regions. CK and CV chondrites share a resemblance in mineralogy, oxygen and chromium isotopes [8-10], and recent discoveries of CV-like (e.g. NWA 3133 [11-13]) and CK-like [14] metachondrites and achondrites indicate a compellingly similar provenance that fits with the single-body hypothesis. NWA 8186 is an ungrouped achondrite showing geochemical and isotopic similarities to CK-like carbonaceous chondrites. This meteorite plots precisely on the CCAM line for oxygen isotopes, and in the region where CK, CV, and CO chondrites plot for Cr isotopes (Figure 1). Preliminary analyses showed NWA 8186 is dominated by olivine and plagioclase, with minor amounts oxides, augite, and Cl-apatite. No traces of iron metal or troilite were identified [14]. We have further examined textures and compositions in this meteorite in order to obtain a refined petrologic classification of this sample, and to assess the likelihood that this achondrite is linked with the CK/CV parent body. Methodology: For the analysis of NWA 8186, a ~1 x 0.5 cm thin section was used that was carbon coated for quantitative electron-beam procedures. A FEI Quanta 3D FEG SEM was used to produce BSE and EDS X-ray maps, and EPMA analyses were completed on all observed phases using a JEOL 8200 superprobe. Figure 2. BSE mosaic of NWA 8186 overlain by a sulfurmap (blue) and phosphorus-map (red). This sample is roughly 1x0.5 cm, primarily composed of olivine, and is highly fractured. Apatite (light red) and merrillite (dark red) are found clustered in the left region of the section. Sulfides tend to be larger in size towards the right region of the section. Figure 1. Plot of NWA 8186 and other achondrite and chondrite meteorite groups in 17O-54Cr space. Literature data are from [15,16] and references therein. Results: Examination of the meteorite sample showed significant fracturing in all mineral phases. Many 120° triple junctions were also observed, typically occurring between olivine and plagioclase feldspar. Olivine is the dominant phase, comprising >80% of the sample (Figure 2), and has a Mg# of ~0.65, Fe/Mn = ~127, and NiO ranges from 0.5-1.5 wt.%. Nanometer-sized Ni-rich metal (or Ni-rich oxide) blebs were also found in the olivine. Plagioclase feldspar, ~10% of the sample, is An51, and augite, <5% of the sample, is Fs11En39. Modal abundances indicate that this meteorite is a dunite, in accordance with IUGS classification protocols [17-19]. Four oxides phases were observed totaling about ~5% of the meteorite. The dominant oxide phase is magnetite, and some of the magnetite grains display thick exsolution lamellae of hercynitic spinel (FeAl2O4), thin ilmenite (FeTiO3) lamellae, and micrometer-sized areas of titanomagnetite (Figure 3). Sulfides were also identified from SEM X-ray maps, which had previously not been recognized in NWA 8186 [14]. The modal abundance of sulfides is 46th Lunar and Planetary Science Conference (2015) Figure 3. BSE image of an exsolved magnetite (Mag) grain with thick Fe-rich spinel (Sp) lamellae, thin ilmenite (I) lamellae, and blocky areas of titanomagnetite (TM). Ol=olivine, P=pyroxene, F= feldspar, Me = merrillite, Sf = sulfide. <1% of the sample, and they typically occur in close relation to the oxides. Figure 2 displays an S X-ray map overlain on a BSE image. These micrometer-sized sulfides are monosulfide solid solution, mss, (Fe,Ni)1xS, with x values ranging from 0.02-0.11, and an average composition of Fe=26.2%, Ni=29.6%, S=33.3%, and Co=0.4%. Pentlandite solid solution, Fe4.27Ni4.73S7.84, with composition of Fe=27.4%, Ni=32.0%, S=33.3%, and Co=0.377% was also found coexisting with mss. The P X-ray map overlain on Figure 2 indicates the phosphate distribution in NWA 8186. Cl-rich apatite (Ca5(PO4)3Cl), composed of ~53.4% CaO, 41.5% P 2O5, 4.8% Cl, and 0.43% F, was seen surrounding olivine grains. Figure 4 is a plot of X-site occupancy (mol.%) in apatite grains. Apatite in NWA 8186 mainly consists of chlorine in the X site with minor F and a missing structural component that is likely OH. Merrillite (Ca18Na2Mg2(PO4)14) was also found associated with apatite, and composed of ~46.5% CaO, 46.7% P2O5, 3.5% MgO, 2.78% Na2O. Figure 4. Truncated ternary plot of X-site occupancy (mol.%) in apatite grains. Apatite is Clrich, and depleted in OH(?) and F. 1472.pdf Discussion: In addition to NWA 8186 (this study and [14]), previous studies have noted the potential association of heavily metamorphosed chondritic samples (above petrologic grade 6) to CV and CK chondrites [11-13]. Thermal evolution models have been used to suggest that the CK/CV parent body could have radially evolved into a layered body with a metallic core, silicate magma ocean, and undifferentiated chondritic crust [2-7]. The crust of the CK/CV parent body would increase in metamorphic grade towards the mantle and might include reduced CVs in the outer crust, oxidized CVs in the mid-crust, and CK chondrites in the lower crust [2]. CK and oxidized-CV meteorites are some of the most oxidized carbonaceous chondrites known. Ilmenite-magnetite pairs in CKs indicate an fO2 of QFM+3 – QFM+5 [20-22]. Exsolution of magnetite, spinel, and ilmenite phases, as well as the presence of NiO-rich olivine and absence of Fe-rich metal indicates this meteorite also formed at high fO2. Two formation mechanisms are being postulated for CK-like achondrites. 1) NWA 8186 might have formed as a residue from partial melting of CK chondrite, indicating proximity to the silicate magma ocean, or 2) NWA 8186 might represent a cumulate that formed as the crystallization product of a CK partial melt that erupted onto the surface of the CK/CV parent body. Exploring the link between CK chondrites and CK-like achondrites is currently being probed through high-pressure/high-temperature experimental studies. The connection between these two groups will help constrain the thermal and magmatic evolution of the CK/CV parent body. References: [1] Wood J.A. (1958) Smithson. Astrophys. Obs. Tech. Report, No. 10, Cambridge, Mass. [2] Elkins-Tanton L.T. et al. (2011) EPSL, 305, 1-10. [3] Weiss B.P. et al. (2013) Annu. Rev. Earth Planet. Sci., 41, 529-560. [4] Ghosh A. et al. (1998) Icarus, 134, 187-206. [5] Hevey P. et al. (2006) MAPS, 41, 95-106. [6] Sahijpal S. et al. (2011) JGR, 116, E06004. [7] Sramek O. et al. (2012) Icarus, 217, 339354. [8] Greenwood R.C. et al. (2010) GCA, 74, 1684-1705. [9] Qin L. et al. (2010) GCA, 74, 1122-1245. [10] Trinquier A. et al. (2009) Science, 324, 374-376. [11] Schoenbeck T.W. et al. (2006) LPSC, 37, #1550. [12] Shukolyukov A. et al. (2011) LPSC, 42, #1527. [13] Irving A.J. et al. (2004) AGU, 85, #P31C-02. [14] Agee C.B. et al. (2014) Met. Soc., 77, #5385. [15] Sanborn M.E. et al. (2014) LPS XLV, Abstract #2032. [16] Jenniskens P. et al. (2014) MAPS, 49, 1388-1425. [17] Le Maitre et al. (1989) Black. Sci. Pub. 193 pp. [18] Le Bas et al. (1991) J. Geol. Soc. London 148, 825-833 [19] Le Maitre et al. (2002) Cam. Uni. Press 252 pp. [20] Huber H. et al. (2006) GCA, 70, 4019-4037. [21] Righter K. et al. (2007) Polar Sci., 1, 25-44. [22] Geiger T. et al. (1995) PSS, 43, 485498.
© Copyright 2026