Unrelated Donor Bone Marrow Transplantation for
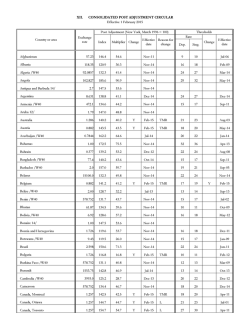
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. Unrelated Donor Bone Marrow Transplantation for Correction of Lethal Congenital Immunodeficiencies By Alexandra H. Filipovich, Ralph S. Shapiro, Norma K.C. Ramsay, Tae Kim, Bruce Blazar, John Kersey, and Philip McGlave Unrelated donor marrow transplantation was undertaken in eight infants with severe combined immunodeficiency (SCID) and two children each with Wiskott-Aldrich syndrome (WAS) and Chediak-Higashi syndrome (CHS) who did not have histocompatible siblings. Donors for three patients were phenotypically matched at all HLA-A, B, Dr, and Dw loci, whereas nine donors were mismatched from the recipients at one of the HLA-A or B loci but phenotypically identical at evaluable D loci. All but one patient received conditioning chemotherapy and/or radiotherapy before infusion of donor marrow, which was not T-cell depleted. Prophylaxis for graft-versus-host disease (GVHD) consisted of methotrexate and prednisone combined with either cyclosporine A (six patients), antithymocyte globulin (five patients), or anti-CD5 ricin A chain immunotoxin (one patient). All patients engrafted with donor cells, and only 4 of 12 experienced any GVHD (1of 8 SCID, 1of 2 WAS, 2 of 2 CHS). Two children who developed grade II and two who developed grade 111 GVHD were successfully treated and all are now alive, off immunosuppressive therapy, with no evidence of chronic GVHD greater than 18 months after transplant. Ten patients are alive with excellent immunoreconstitution h l year t o 2 3 years after transplant; actuarial survival is predicted t o be 83% with a median follow-up of 2 years. Two children with SCID succumbed t o pre-existing opportunistic infection early posttransplant. We conclude that closely matched unrelated donor bone marrow transplantation can correct congenital immunodeficiencies including variants of SCID, WAS, and CHS, with an acceptably low incidence of transplant-related complications, principally GVHD. 0 1992b y The American Society of Hematology. T without malignancies; (2) it has been associated with severe graft-versus-host disease (GVHD)12; and (3) it has an alarming incidence of posttransplant Epstein-Barr virus (EBV)-associated B-cell lymphoproliferative disorder (especially in WAS).13 The time course for immunoreconstitution following T-depleted haploidentical bone marrow transplantation (BMT) in many reported cases has been markedly prolonged9J4 and recovery of specific antibody synthesis has not been achieved in some cases?Jo For the rare cases of congenital immunodeficiency where the genetic defect is known, enzyme replacement therapyI5 and/or gene therapy16has been attempted, as in the case of SCID secondary to adenosine deaminase (ADA) deficiency. However, the long-term benefits of these approaches remain to be proven, and the genetic defects for the majority of prematurely lethal congenital immunodeficiencies are unknown. For these reasons we initiated a study of unrelated donor (URD) marrow transplantation to treat children who had the types of prematurely lethal immunodeficiencies that had been clearly demonstrated to benefit from marrow transplantation from HLA-matched siblings, but who lacked such histocompatible sibling donors. Twelve children who received URD transplants for lethal immunodeficiencies are the subject of this report. H E FIRST REPORTS of successful marrow transplantation for the correction of congenital immunodeficiencies appeared in 1968, involving patients with severe combined immunodeficiency (SCID) and Wiskott-Aldrich syndrome During the following 15 years marrow transplantation from histocompatible siblings became accepted as curative treatment for children with SCID? WAS,4s5 Chediak-Higashi syndrome (CHS),6 as well as other congenital immunodefi~iencies.~ Unfortunately, the percentage of infants with congenital immunodeficiencies (eg, SCID) who have healthy histocompatible sibling donors has been estimated at approximately lo%? For some children without matched siblings successful reconstitution of both T- and B-lymphocyte immune function has been achieved during the past decade through the use of T-celldepleted transplantation from haploidentical, generally parental, family members. Immunoreconstitution has been achieved in a significant proportion of SCID patients9-” and less frequently in WAS.12 T-cell-depleted haploidentical transplantation has several disadvantages: (1) it often requires intensive pretransplant conditioning therapy including the use of total body irradiation (TBI) in patients From the Bone Marrow Transplant Program and the Department of Pediatrics, Divisions of Immunology and BMT; the Divisions of HematologylOncology and BMT; the Department of Medicine, Division of Hematology; the Department of Therapeutic Radiology; and the Department of Laboratory Medicine and Pathology, University of Minnesota Hospital and Clinic, Minneapolis. Submitted November 12, 1991; accepted March 5, 1992. Supported by National Institutes of Health Grant No. CA 21737. Address reprint requests to A .H. Filipovich, MD, University of Minnesota Hospital and Clinic, Box 261 Mayo, 420 Delaware St SE, Minneapolis, MN 55455. The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact. 0 1992 by The American Society of Hematology. 0006-4971l92l8001-0013$3.00/0 270 MATERIALS AND METHODS Patients. All consecutive patients with SCID, WAS, or CHS referred to our institution for BMT between January 1987 and March 1990 who lacked histocompatible siblings or closely matched related donors were referred to the National Marrow Donor Program (NMDP) for an unrelated donor search. This included 11 infants with SCID, two with WAS, and two with CHS. Histocompatibility testing. Lymphocytes from all patients were tested by HLA-A, B, C, and Dr typing using standard serologic techniques.” Dw typing was performed with homozygous typing cells.I8 Mixed lymphocyte cultures between recipients and potential donors were performed as previously reported.19 In some cases DNA analyses using restriction fragment length polymorphisms were used to confirm Dr identity between patients and potential marrow donors.20 Blood, VOI 80, NO1 (July 1). 1992: pp 270-276 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. UNRELATED DONOR TRANSPLANTS FOR IMMUNODEFICIENCIES BMT, All patients were hospitalized in private HEPA filtered rooms on the acute marrow transplant unit from the time that pretransplant conditioning was initiated until an acceptable absolute neutrophil count was achieved posttransplant. Careful hand washing precautions were used for all patients. Infants with SCID were maintained in gown, glove, and mask isolation and provided with sterile formula (when fed enterally) until relevant laboratory assays showed evidence of donor type engraftment and functional immuno-reconstitution (typically 2 to 3 months post grafting). All patients received trimethoprin/sulfamethaxozole 2 days/week as Pneumoqsfis carinii prophylaxis and weekly intravenous (IV) IgG until day +180 (because all donors were EBV and/or cytomegalovirus [CMV] seropositive). Acyclovir 10 mg/kg t.i.d. was administered IV while patients were hospitalized. Blood products were CMV-negative and irradiated. All blood products administered to SCID patients were leukopoor, infused through a PALL filter. Conditioning therapy was assigned according to the underlying diagnosis as outlined (see the footnote to Table 3). For the patients in this report it consisted of either: (1) no pretransplant therapy (one SCID patient); (2) busulfan 2 mg/kg p.0. bid x 4 days followed by cyclophosphamide 50 mg/kg/d IV X 4 days and antithymocyte globulin 15 mg/kg bid IV on days -2 and -1 and 15 mg/kg/d IV on day +1 and +2 (nine patients: seven SCID, two WAS); (3) cyclophosphamide 60 mg/d IV X 2 days followed by fractionated TBI (165 cGy bid x 4 days, total 1,350 cGy [one CHS patient]); or (4) cyclophosphamide 60 mg/d IV X 2 days on days -7, -6, etoposide (VP-16) 500 mg/(mol/L)* IV X 3 days on days -7 to -5, fractionated TBI (165 cGy x 4 days) and antithymocyte globulin (ATG) 15 mg/kg bid IV on days -2 and -1 and 15 mg/kg/d IV on days +1 and +2 (one CHS patient). All patients were infused with 3 x 108 donor bone marrow mononuclear cells/kg recipient weight. GVHD prophylaxis was administered according to the pilot protocols in effect for all unrelated donor transplants conducted at our institution during that particular time period. MCP: methotrexate 15 mg/kg IV on day +1, 10 mg/kg IV on day +3, +6, +11, cyclosporine A 1.5 mg/kg bid IV day -1 to day +30 then 5 to 6 mg/kg bid p.0. until day 180, prednisone 5 mg/d until day 30 and then tapered; MAP as previously published?' or MXP-"short course" methotrexate: 15 mg/kg/d IV on day + 1 , l O mg/kg/d on d. +3, +6, +11, anti-CD5 monoclonal antibody-ricin A chain conjugate 0.1 mg/kg IV qd. on days +1 to + 10 then q.0.d. on days +I2 to +20 and prednisone 40 mg/(mol/L)2 qd on day + I to day +20 then tapered over 1 week. Engrajhent studies. Documentation and quantitation of donor cell engraftment was performed using restriction fragment length polymorphisms (RFLP) for discriminating DNA markersz2 on bone marrow aspirates or blood at approximately day +28, day 100, and later time points in the majority of patients. Immune function studies. Evaluations of serum Ig levels, specific antibody titers, T-cell phenotypes, T-cell mitogen, and antigen proliferation were sequentially performed by standard methods. Statistical anaZysis. Clinical data were retrieved from the University of Minnesota Bone Marrow Transplant Database which contains systematically and prospectively collected data on all bone marrow transplant patients. The end points of survival, mean day of engraftment, and acute GVHD were determined using the Kaplan-Meier product limits methods.23 RESULTS Patient characteristics. Characteristics of the 12 children with SCID, WAS, or CHS who received URD BMT are described in Table 1. Median age at BMT for SCID patients was 8.5 months (range 3 months to 23 months). Two infants 271 Table 1. Patients With Lethal CongenitalImmunodeficiencies Treated With Unrelated Donor BMT UPN Immunodeficiency 807 SClD .1B, .1 Tcells, t NK cells SClD .1T, hypogammaglobulinaemia SClD Omenn's syndrome SClD ADA deficiency SClD .1 8, .1T cells, t NK cells SClD Absent CD8+ T cells, nonfunctional CD4+ cells SClD Absent CD8+ T cells, nonfunctional CD4+ cells 7moF SClD Absent CD8+ T cells, nonfunctional CD4+ cells WAS 3moM WAS CHS CHS 8moM 4yrF 8yrM 937 1024 1049 1091 1107 1265* 1289' 966 1204 818* 1118' AgelSex 10 mo M Significant Pretransplant Complications PCP, Parainfluenza pneumonia PCP 4moF 21 m o F Transfusion acquired GVHD and aplasia PCP Adenoviral hepatitis 23 mo F PCP 27 mo M Severe pulmonary compromise (2" to prolonged ventilator course for PCP and CMV pneumonia) 6moF 30 mo M Pure red blood cell aplasia Accelerated phase Accelerated phase Abbreviation: PCP, Pneumocystiscarinii pneumonia. *Siblings. were identified as very high risk secondary to (1) pretransplant transfusion-acquired GVHD and aplastic anemia with opportunistic infection in one case (unique patient number [UPN] 1024), and (2) a prolonged pretransplant ventilator course secondary to pneumonitis with pneumocystis carinii and cytomegalovirus resulting in chronic oxygen dependence in another case (UPN 1265). One patient with WAS (UPN 966) developed pure red blood cell aplasia 2 months before BMT and both children with CHS were in the accelerated phase at the time of BMT. Donor selection. Results of histocompatibility antigen testing for donors and recipients are shown in Table 2. Acceptable donors were found for 8 of 11 infants with SCID, 2 of 2 patients with WAS, and 2 of 2 patients with CHS. (The three infants with SCID for whom closely matched unrelated donors could not be identified received T-depleted BMT from parental donors.) Three patients had phenotypically identical donors (all with SCID) while the rest of the patients had donors mismatched at one A (six patients) or B (three patients) locus. BMT. Regimens of pretransplant conditioning and GVHD prophylaxis are shown in Table 3, as are outcomes with respect to engraftment, GVHD, and current status. Three patients with SCID (in the group 1 diagnostic category (footnote, Table 3) UPN 807,937, and 1049) were initially infused with donor marrow without any preconditioning. No evidence for engraftment of donor cells was found after 6 weeks of observation in two of three patients: From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 272 FlLlPOVlCH ET AL Table 2. Histocompatibility Typing of Unrelated Donors for BMT of Lethal Congenital Immunodeficiencies MLC (relative response) % Donor UPN Immunodeficiency Recipient Typing 807 SClD A2.32 87.62 937 SClD A2,2 860,62 1024 SClD Al,28 88.44 1049 SClD A1.3 88,7 1051 SClD A2,2 844.35 1107 SClD A2.32 844.62 1265 SClD Al,11 88 1289 SClD Al.11 88,57 966 WAS A1.3 87.57 1204 WAS A26,33 817.38 A2,3 CHS 818 A1,3 CHS 1118 87,44 88.7 Dr2.4 Dw2.4 Dr4,5 Dw4.5 Dr2,4 Dw2,4 Dr2.3 Dw2.3 Dr1.6 Dw1,18 Dr2.4 Dw2.4 Dr3 Dw17 Dr3.7 Dwl7.7 Dr5,7 Dw5,7 Dr6 Dw6 Dr8 Dw18.8 Dr3,8 Dw3,8 Donor Mismatch RDx DM 31yM A3 v A32 0 0 M YF 827 v 860 0 3 47yM Identical 1 0 50yM Identical 2 0 29YF A28 v A2 14 8 (SI = 1.6) 39YM A1 vA32 1 1 53yM Identical 0 0 36yM 817 v 857 0 0 47yF 862 v 857 0 4 36yM A1 v A33 32 19 (SI = 14) 38YF A1 v A 2 5 18 (SI = 2.1) 38YF A26 v A3 5 8 (SI = 7.4) Abbreviations: MLC, mixed lymphocyte culture; RDx, relative response of recipient cells responding against irradiated donor cells; DRx, relative response of donor cells responding against irradiated recipient cells; SI, stimulation index of RDx/RRx. UPN 937 and 1049. These two patients subsequently underwent chemotherapy as outlined for group 2 diagnoses (footnote, Table 3), and second grafts of cryopreserved marrow from the same donor. The five remaining SCID patients who had unusual variants (not described in the World Health Organization classification) received chemotherapy conditioning for the first BMT. Three of the patients (UPN 1107,1205, and 1289) had a variant form of SCID characterized by lack of CD8+ T cells,24a unique activation defect, and a reduced capacity to respond to allogeneic stimuli. UPN 1024 with Omenn’s syndrome had acquired GVHD from an unirradiated erythrocyte transfusion pretransplant.z UPN 1091 had low numbers of T cells but demonstrated a slight degree of responsiveness to allogeneic cells, although mitogen responses were markedly decreased. The two boys with WAS were treated with a conditioning protocol patterned after Kapoor et a15 and identical to the one that has been used successfully for transplantation of WAS patients from histocompatible siblings at our institu- Table 3. Unrelated BMT for Lethal Congenital Immunodeficiencies UPN Disease1 Phenotype AgelSex Pretransplant Conditioning* GVHD Prophylaxis RFLP ( O h ) 807 937 1024 1049 1091 1107 1265 1289 966 1204 818 1118 SClD SClD SClD SClD SClD SClD SClD SClD WAS WAS CHS CHS 7moF 10moM 6moF 4moF 21 moF 23moF 27mo M 3moM 2.5yr M 8moM 4yrF 8yrM 1 1.2 2 1.2 2 2 2 2 2 2 3 3 MCP MCP MCP MCP MAP MAP MXP MAP MCP MAP MCP MAP 51-75 76-99 100 51-75 76-99 76-99 76-99 76-99 100 100 100 100 Day 28 Current RFLP I%) 25-50 51-75 NE 51-75 100 51-75 NE 51-75 100 100 100 100 GvHD Acute Chronic Ill 0 NE 0 0 0 0 0 111 0 II Ill 0 0 NE 0 0 0 0 0 0 0 0 0 Current Status Well at home > 47 mo Well at home > 33 mo Died d 20 aspergillosis Well at home > 30 mo Well at home > 27 mo Well at home > 25 mo Died d 65 CNS hemorrhage Well at home > 18 mo Well at home > 33 mo Well at home > 20 mo Well at home > 23 mo Well at home > 23 mo Abbreviations: MCP, methotrexate, CSA, prednisone; MAP, methotrexate, ATG, prednisone; MXP, methotrexate, anti-CD5 immunotoxin (Xoma-zyme),prednisone; MC, methotrexate, CSA; NE, not evaluable. *1, none; 2, ATG-l5mg/kg IV bid on d -2, -1,15 mg/kg/d I V o n d +1, +2, busulfan-2 mg/kg bid PO x 4d,cyclophosphamide-50mg/kg IV x 4d; 3, pre 1989 cyclophosphamide-60 mg/kg/d IV x 2 d. Fractionated TBI-165 cGy bid x 4 d. Post 1989 cyclophasphamide-60 mg/kg/d IV x 2 d. Etoposide-500mg/m2/dIV x 3d. FractionatedTBL165cGybid x 4d.ATG-l5mg/kg/bidIVond -2, -1,15mg/kg/dIVond +1, +2. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. UNRELATED DONOR TRANSPLANTS FOR IMMUNODEFICIENCIES 273 (UPN 818, 1118). Three of six patients who developed G W D had received prophylaxis with MCP while one of six patients who had MAP prophylaxis developed GVHD. These differences were not statistically significant. Grade 11, I11 GVHD were easily controlled in three of four patients who developed this complication. Moderate dose systemic steroids were used in two cases and antLCD5 ricin A chain immunotoxin (Xoma-zyme) infusion in UPN 807 (who was eligible for a multi-institutional pilot protocol28of this agent as primary therapy of GVHD at the time she developed this complication). UPN 1118 with CHS, the oldest child in this series, developed an acutelchronic GVHD overlap syndrome along with severe pancreatitis while on prednisone therapy. He subsequently received a course of ATG and was maintained on cyclosporine A for 9 months. He is currently off all immunosuppressive therapy greater than 20 months post-BMT with no evidence of chronic GVHD, normal Ig levels, and normal T-cell responses to mitogens (Table 4). Kaplan-Meier estimate of survival for this patient series is 83% with a median follow-up of 2 years (Fig 1). Reconstitution of hematologic and immune function. The 11 patients who received conditioning therapy achieved 3 consecutive days of white blood count of greater than l,000/mm3 by a median of 24 days. All patients have recovered normal blood counts post BMT including persistently normal platelet counts in two of two patients with WAS and absence of Chediak-Higashi granules in peripheral blood lymphocytes and bone marrow in two of two tion. Patients with CHS in accelerated phase received doses of cyclophosphamide and fractionated TBI standardly administered for BMT of leukemias at our center.26UPN 1118 also received VP16 because this agent has been reported to be effective in the transplantation of hemophagocytic syndromes*’ and had been added along with ATG to the protocol for transplantation of patients with CHS (with matched related or unrelated donors) at our institution during the intervening period (footnote, Table 3). GVHD prophylaxis was assigned sequentially according to the protocol that was being used at our institution for all unrelated donor BMT during a given period of time. Thus, the first six patients were treated with the combination of MCP: methotrexate, cyclosporine A, prednisone; the next four with MAP: methotrexate, ATG, prednisone; the subsequent patient (UPN 1265) with MXP: methotrexate, antiCD5 ricin A chain immunotoxin (Xoma-zyme), and prednisone. The most recent patient (UPN 1289) again received MAP secondary to parental refusal of the MXP protocol. Ten patients demonstrated donor engraftment within the first month after their initial transplant. As previously mentioned, two SCID patients did not show evidence of donor cells for 6 weeks after marrow infusion without prior conditioning, but engrafted rapidly after a second transplant following chemotherapy (Table 4). GVHD was clinically apparent in four patients: one 7-month-old infant with SCID (UPN 807, the only child who did not receive pretransplant conditioning) and the three oldest patients (ages 2.5 to 8 years) with either WAS (UPN 966) or CHS Table 4. Immunologic and Hematologic Reconstitution After Unrelated Donor BMT for Lethal CongenitalImmunodeficiencies Mitogen Responses* 1%) LymphocyteSubsets ( O h ) Igs ID Pre/Post ALC G A M E CD3 CD4 CD8 CD19 CD16 PHA ConA PWM 807 SClD 1091 SClD SClD SClD 1289 SClD 966 WAS 1204 WAS 818 CHS 1118 CHS 9 31 33 36 55 4 29 7 37 65 35 65 18 55 30 8 47 49 41 49 37 37 35 18 34 70 27 21 8 30 30 40 0 34 10 24 8 35 28 39 15 39 25 49 19 51 46 24 7 4 16 13 23 58 8 1 8 8 14 7 10 47 7 17 3 3 4 19 4 97 8 0 99 7 102 0 88 0 ND 0 119 68 113 82 45 27 74 53 95 118 3 0 127 7 152 1 132 1265 62 60 36* 68 59 6 65 9 75 64 90 66 36 55 65 20 85 59 78 72 84 58 87 0 34 39 25 4 12 2 10 19 1107 12 99 <2 <2 126 8 <2 <2 7 ND 8 53 ND 4 24 27 32 23 <2 3 2 223 4 15 68 SClD SClD 50 206 26 209 7 34 37 168 54 95 91 173 ND 14 65 77 49 27 54 86 103 89 84 22 112 1024 1049 20 99 <7 72 14 <7 23 114 11 119 39 160 ND <7 28 129 47 83 52 68 47 231 46 6 109 SClD 6,000 1,400 500 2,560 4,800 200 1,290 1,540 1,584 5,610 1,008 5,760 400 4,440 2,100 2,490 2,565 2,200 3,480 5,000 2,205 3,500 1,650 226 540 937 Pre 24 mo post Pre 12 mo post Pre Pre 12 mo post Pre 12 mo post Pre 6 mo post Pre 2mopost Pre 12 mo post Pre 12 mo post Pre 12 mo post Pre 12 mo post Pre 12 mo post 66 1 0 43 11 99 1 66 3 ND 0 91 30 51 85 29 15 39 58 101 UPN 948 366 633 505 1,000 1,580 858 601 1,870 ND 781 991t 5,800 1,140 625 1,410 804t 719 1,040 938 Abbreviation: ND, not done. *Percent normal control responses. tReceived IvlgG. *Only 8% of lymphocytes expressed a PTCR. 20 19 24 5 41 12 23 13 17 17 24 17 20 2 1 ND 0 89 39 105 131 43 16 72 76 114 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 274 FlLlPOVlCH ET AL 12= - L 0.8 0 1 2 3 3.8 Survival (years) Fig 1. Actuarial immunodeficiency free survival for 12 children undergoing unrelated donor BMT for lethal immunodeficiencies. CHS patients. Both patients with WAS and both patients with CHS continue to demonstrate 100%donor cell engraftment. A summary of immune function studies performed at most recent time points post-BMT are shown in Table 4. Total absolute lymphocyte counts, Igs M, A, E, and phytohemagglutinin (PHA) blastogenesis are normal in all patients. Although mixed chimerism has been apparent in five of six SCID patients who received BMT more than 1 year ago (Table 3) all children show persistent and improving immunocompetence. UPN 1049 has had normal white blood cell ADA levels since first measured at day 27 post BMT. The three children with SCID with the absence of CD8+ T cells and a T-cell activation defect have demonstrated CD8+ CD3+ T cells postgrafting and normal proliferation to PHA in two of two surviving patients (Table 4). Nine patients have been immunized at 1 year with a single dose of the pediatric diphtheria Tetanus vaccine. The six patients tested post-immunization all showed evidence of immune response to one or both antigens: 2 of 6 made antibody to diphtheria, 5 of 6 made antibody to tetanus, 5 of 5 developed a proliferative response to tetanus in vitro, and 3 of 3 showed positive delayed type hypersensitivity reactions (3 of 3 tetanus, 2 of 3 diphtheria). UPN 807, the first patient to undergo unrelated donor BMT in this series, showed an increase in antibody titers to parainfluenza type 3 within 5 weeks post BMT. This virus had been the cause of recurrent pneumonitis before transplantation. UPN 807 experienced an uneventful primary infection with varicella zoster at 24 months post BMT. Over the past 3 years, she has been immunized with a pediatric diptheria Tetanus booster x 2, injectable polio x 2 and the measles, mumps, rubella (MMR) vaccines. She now demonstrates protective antibodies to all these agents as well as positive delayedtype hypersensitivity skin tests to diphtheria, tetanus, mumps, candida, and streptococcus. All surviving patients are well at home and do not receive IVIgG infusions. There is no evidence for acquisition of CMV infection associated with BMT in any of the children, although two patients were CMV seropositive pre-BMT. To date no patient has developed symptoms suggestive of EBV-associated B-cell Iymphoproliferative disease. DISCUSSION Opportunistic infections and malignancies, respectively, are the major causes of death in children with premature, lethal congenital immunodeficienciesincluding SCID, WAS, and CHS. Successful immuno-reconstitution with marrow transplantation effectively reduces the long-term risks of both of these complication^.^^ In the present era transplantation of SCID and WAS with whole marrow from histocompatible sibling donors is associated with disease-free survival rates of greater than 90% and 85%, r e ~ p e c t i v e l y . ~ ~ J ~ Unfortunately, the great majority of children with prematurely lethal immunodeficiencies lack histocompatible sibling donors. O’Reilly et aI3O undertook the first unrelated bone marrow transplant for SCID in 1977. Partial engraftment was finally achieved after six attempts with the subsequent development of extensive chronic GVHD contributing to death from squamous cell carcinoma 5 years later. In the early 1980s, with the development of a physical method of marrow T-cell depletion involving soybean lectin agglutination and sheep erythrocyte rosetting,3I children with leukemia and SCID began to receive marrow transplants from haploidentical parental donors. Initial reports indicated that nearly half of SCID patients experienced improvement in T-cell immunity because of partial engraftment of parental cells, such that they could be removed from strict protective isolation, but continued to require Ig replacement indefinitely. More recently, with the use of pretransplant combinations of busulfan, cyclophosphamide, and/or cytosine arabinoside and ATG the rate of successful engraftment of T-depleted marrow in classical forms of SCID has increased to 86%.11 However, recovery of T- and B-cell function remain variably delayed and the reconstitution of SCID variants such as Omenn’s synd r ~ m ise still ~ ~problematic. It has been even more difficult to achieve hematolymphopoetic engraftment in WAS with T-depleted haploidentical donors with some exceptions,12 and the rate of posttransplant EBV-associated B-cell lymphoproliferative disorder (BLPD) is prohibitively high (nearly 50%).13 In early attempts at our institution to use T-depleted haploidentical transplantation, we encountered several discouraging consequences including EBV-associated BLPD,33 transfusion-acquired CMV infection, and engraftment failure in non-SCID immunodeficiencies despite the use of TBI.34For those reasons, we initiated a pilot study of URD BMT for patients with lethal immunodeficiencies who lacked histocompatible siblings or closely matched related donors. With the exception of two children with SCID (one with no laboratory evidence of allogeneic reaction who accepted a third-party skin graft [UPN 9371 and the other with ADA deficiency [UPN 10491) who did not engraft after marrow infusion alone, all other conditioning protocols used for this series of patients resulted in acceptable engraftment and reconstitution of all previously deficient immunologic and hematologic functions. These results were achieved with doses and regimens of chemotherapy and radiation therapy that are commonly used in children undergoing histocompatible sibling transplantation, and were not associated with any unacceptable toxicities. Graft rejection has been documented in phenotypically identicaP5 and HLAmismatched36 unrelated donor transplants in conjunction with T-cell depletion. While our series of patients is From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 275 UNRELATED DONOR TRANSPLANTS FOR IMMUNODEFICIENCIES relatively small, our experience suggests that stable engraftment can be achieved despite mismatching at class I antigens if T-replete grafts are used. In contrast to the eight of eight patients with SCID who engrafted after unrelated donor bone marrow transplant, two of three children with SCID variants who underwent T-depleted haploidentical BMT during the same time period with the same conditioning protocol used for URD BMT did not engraft with donor cells and ultimately died (A.H.F., unpublished data). One of these children went on to develop EBV-associated B-cell lymphoproliferative disease. The rate of GVHD in this series was lowest for infants with SCID (one of eight patients), comparable to that expected with histocompatible transplantation and T-depleted parental graft^.^,^ While three older children developed grade I1 to I11 GVHD, all symptoms of GVHD were successfully reversed with conventional doses of prednisone used for treatment of GVHD. This rate of 2 grade I1 GVHD (actuarial rate = 35%, 95% confidence limits c 28%) is significantly lower than that reported from series of adults treated with URD BMT with and without T-cell depletion3’ and the experience at our institution (actuarial GVHD rate for patients > 18 years old = 73%, 95% confidence limits, f 15%.) Because the methods of GVHD prophylaxis for URD BMT were the same for children and adults at our institution, we can speculate that young recipient age, and possibly the SClD background, contributed to lowering the risk of GVHD in URD BMT as it does in histocompatible BMT.38Because GVHD prophylaxis was administered in several sequential protocols to small numbers of patients, no clear conclusion can be reached regarding the most effective regimen. However, nephrotoxicity and hypertension requiring medical intervention was observed in two of six children who received cyclosporine A as part of their prophylaxis, and the rate of GVHD was not lower than that observed with the combination of methotrexate, ATG, and prednisone. The recovery of nonspecific and specific immune function has been monitored sequentially in all patients. Be- cause of donor seropositivity for CMV and EBV all patients received IVIgG infusions weekly until at least 6 months post BMT, and those who were still receiving immunosuppression for GVHD, for longer periods of time. All but one patient have now discontinued IVIgG therapy and demonstrate efficient endogeneous synthesis of Igs as well as specific antibody formation (partial data shown in Table 4 and described in the Results section). In summary, marrow transplantation using phenotypically identical or one HLA A or B antigen mismatched unrelated donors was undertaken without the use of T-cell depletion or unusually intensive pretransplant conditioning protocols in a group of 12 children with lethal immunodeficiencies. Ten children are well at home, and have documented T- and B-cell immunoreconstitution with a median follow-up of 2 years posttransplant. More detailed analysis of the tempo of immune recovery and comparison with results achieved with T-depleted haploidentical BMT will be necessary to identify the potential advantages of the transfer of unmanipulated marrow with immunocompetent T cells from unrelated donors as treatment of lethal immunodeficiencies. NOTE ADDED IN PROOF A third patient with WAS was treated with URD BMT after completion of this report. UPN 1463 was an 8-year-old boy who had suffered from refractory thrombocytopenia after splenectomy. He had been treated with daily steroids for most of 7 ?hyears and developed liver dysfunction 8 months before BMT. Pretransplant evaluation showed oligoclonal EBV-associated BLPD apparently confined to the liver. This was treated with surgical resection and a-interferon for 3 weeks. The early posttransplant course was unremarkable and complete donor engraftment was documented before day 28. Unfortunately, UPN 1463 developed severe venoqcclusive disease of the liver and died of disseminated aspergillosis 55 days post BMT. At autopsy there was no evidence of GvHD or BLPD. This case illustrates the need for ongoing careful1 evaluation of the role of marrow transplantation with nonsibling donors in WAS and better definition of pretransplant criteria warranting referral for nonsibling BMT. REFERENCES 1. Gatti RA, Meuwissen HJ, Aller HD, Hong R, Good RA: Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 2:1366,1968 2. Bach FH, Albertini RJ, Joo P, Anderson JLY, Bortin MM: Bone marrow transplantation in a patient with Wiskott-Aldrich syndrome. Lancet 2:1364,1968 3. Bortin MM, Rimm AA: Severe combined immunodeficiency disease. Characterization of the disease and results of transplantation. JAMA 238591,1977 4. Ochs HD, Lum LG, Johnson FL, Schiffman G, Wedgwood RJ, Storb R: Bone marrow transplantation in the Wiskott-Aldrich syndrome. Complete hematological and immunological reconstitution. Transplantation 34:284, 1982 5. Kapoor N, Kirkpatrick D, Blaese RM, Oleske J, Helgartner MH, Chaganti RSK, Good RA, O’Reilly RJ: Reconstitution of normal megakaryocytopoiesis and immunologic functions in Wiskott-Aldrich syndrome by marrow transplantation following myeloablation and immunosuppression with busulfan and cyphosphamide. Blood 57:692, 1981 6. Kazmierowski JA, Elin RJ, Reynolds HY: Chediak-Higashi syndrome; reversal of increased susceptibility to infection by bone marrow transplantation. Blood 47:555,1976 7. Neudorf SML, Yanig GA, Pietryga D W Bone marrow transplantation for correction of primary immunodeficiencies, in Johnson FL, Pochedly C (eds): Bone Marrow Transplantation in Children. New York, NY, Raven, 1990, p 165 8. Levinsky RJ, Davies EG, Butler M, Abai A, Linch DC, Goldstone AH: Problems of mismatched bone marrow transplantation for severe combined immunodeficiency after soybean lectin fractionation. Birth Defects 19:147, 1983 9. O’Reilly RJ, Keever CA, Small TN, Brochstein J: The use of HLA-non-identicalT-cell-depleted marrow transplants for correction of severe combined immunodeficiencydisease. Immunodefic Rev 1:273,1989 10. Friedrich W, Goldmann S, Ebell W, Blutters-Sawatzki R, Gaedicke G, Raghavachar A, Peter HH, Belohradsky B, Kreth W, Kubanek B, Kleihauer E: Severe combined immunodeficiency; treatment by bone marrow transplantation in 15 infants using HLA-haplo-identicaldonors. Eur J Pediatr 144:125,1985 11. Fischer A, Landais B, Friedrich W, Morgan G, Gerritsen B, From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 276 Fasth A, Porta F, Criscelli C, Goldman SF, Levinsky R, Vossen J: European experience of bone marrow transplantation for SCID. Lancet 3365350,1990 12. Rumelhart SL, T r i g ME, Horowitz SD, Hong R: Monoclonal antibody T-cell-depleted HLA-haploidentical bone marrow transplantation for Wiskott-Aldrich syndrome. Blood 57:1031,1990 13. Fischer A, Friedrich W, Fasth A, Blanche S, Le Deist F, Girault D, Veber F, Vossen J, Lopez M, Griscelli C Reduction of graft failure by a monoclonal antibody (anti-LFA-1 CDlla) after HLA nonidentical bone marrow transplantation in children with immunodeficiencies, osteopetrosis, and Fanconi’s anemia: A European group for immunodeficiency/European group for bone marrow transplantation report. Blood 77:249,1991 14. Buckley R, Schiff S, Sampson H, Schiff RI, Markert L, Knutsen AP,Hershfield MS, Huang AT, Mickey GH, Ward FE: Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol 136:2398,1986 15. Hershfield MS, Buckley RH, Greenberg ML, Melton AL, Schiff R, Hatem C, Kurtzberg J, Markert ML, Kobayashi RH, Kohayashi AL, Abuchowski A Treatment of adenosine deaminase deficiencywith polyethylene glycol-modified adenosine deaminase. N Engl J Med 316:589,1987 16. Hirschhorn R: Adenosine deaminase deficiency. Immunodefic Rev 2175,1990 17. Filipovich AH: The histocompatibility barrier in bone marrow transplantation, in Johnson FL, Pochedly C (eds): Bone Marrow Transplantation in Children. New York, NY,Raven, 1990, P27 18. Yunis EJ,Awdeh Z, Raum D, Alper CA, Rappeport J: The (Complotypes) MHC in human bone marrow (allo-)transplantstion. Transplant Proc 17440,1985 19. Hansen JA, Clift RA,Thomas ED, Buckner CD, Mickelson EM, Storb R: Histocompatibility and marrow transplantation. Transplant Proc 11:1924,1979 20. Whitehead AS, Woods DE, Fleischnick E, Chin JE, Yunis EJ, Katz AJ, Gerald PS, Alper CA, Colten HR: DNA polymorphism of the C4 genes. A new marker for analysis of the major histocompatibility complex. N Engl J Med 31088, 1984 21. Ramsay NKC, Kersey JH, Robison LL, McGlave PB, Woods W, Krivit W, Kim TH, Goldman A, Nesbit M: Prevention of acute graft-versus-host disease: A randomized study demonstrating the influence of treatment regimen and age. N Engl J Med 306:392, 1982 22. Blazar BR, Orr HT, Arthur DC, Kersey JH, Filipovich AH: Restriction fragment length polymorphisms (RFLPs) as markers of engraftment in allogeneic marrow transplantation. Blood 66:1436, 1985 23. Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457,1958 24. Roifman CM, Hummel D, Martinez-Valdez H, Thorner P, Doherty PJ, Pan S, Cohen F, Cohen A Depletion of CD8+ cells in human thymic medulla results in selective immune deficiency. J Exp Med 170:2177,1989 25. Blazar, Filipovich AH: Identification of transfused blood cells in severe combined immunodeficiency syndrome by analysis of multiple cell lineages using restriction fragment length polymorphisms. Bone Marrow Transplant 5:327,1990 FlLlPOVlCH ET AL 26. Weisdorf DJ, McGlave PB, Ramsay NKC, Miller WJ, Nesbit ME, Woods WG, Goldman AI, Kim TH, Kersey JH: Allogeneic bone marrow transplantation for acute leukemia: Comparative outcomes for adults and children. Br J Haematol69:351,1988 27. Fischer A, Cerf-Bensussan N, Blanche S, Le Deist F, Bremard-Oury C, Leverger G, Schaison G, Durandy A, Griscelli C: Allogeneic bone marrow transplantation for erythrophagocytic lymphohistiocytosis. J Pediatr 108:267, 1986 28. Byers VS, Henslee PJ, Kernan N, Blazar BR, Gingrich R, Phillips GL, Le Maistre CF, Gilliland G, Antin JH, Martin P, Tutscha PA, Trown P, Ackerman SK, O’Reilly RJ,Scannon PJ: Use of an anti-pan T-lymphocyte ricin A chain immunotoxin in steroid-resistant acute graft-versus-host disease. Blood 75:1426, 1990 29. Neudorf SML, Filipovich AH, Kersey JH: Immunoreconstitution by hone marrow transplantation decreases lymphoreticular maignancies in Wiskott-Aldrich and severe combined immune deficiency syndromes, in Purtilo DT (ed): Immune Deficiency and Cancer: Epstein-Barr Virus and Lymphoproliferative Malignancies. New York, NY, Plenum, 1984, p 471 30. O’Reilly RJ, Dupont B, Pahwa S, Grimes E, Smithwick EM, Pahwa R, Schwartz S, Hansen JA, Siegal FP, Sore11 M, Svejgaard A, Jersild C, Thomsen M, Platz P, L‘Esperance P, Good RA: Reconstitution in severe combined immunodeficiency by transplantation of marrow from an unrelated donor. N Engl J Med 297:1311, 1977 31. Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Dupont B, Good RA, O’Reilly RJ: Transplantation for acute leukemia with HLA-A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells. Lancet 2:327, 1981 32. Omenn G: Familial reticuloendotheliosis with eosinophilia. N Engl J Med 273:427,1965 33. Shapiro RS, McClain K, Frizzera G, Gajl-Peczalska KJ, Kersey JH, Blazar BR, Arthur DC, Patton DF, Greenberg JS, Burke B, Ramsay NKC, McGlave P, Filipovich AH: Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 71:1234,1988 34. Filipovich AH: Progress in broadening the uses of marrow transplantation: Availability of donors. Vox Sang 51(S2):95, 1986 35. Fleischhauer K, Kernan N, OReilly RJ, Dupont B, Yang SY: Bone marrow-allograft rejection by T lymphocytes recognizing a single amino acid difference in HLA-B44. N Engl J Med 323:1818,1990 36. Ash RC, Casper JT, Chitambar CR, Hansen R, Bunin N, Truitt RL, Lawton C, Murray K, Hunter J, Baxter-Lowen LA, Gottschall JL, Oldham K, Anderson T, Camitta B, Menitove J: Successful allogeneic transplantaibh of T-cell-depleted hone marrow from closely HLA-matched unrelated donors. N Engl J Med 322:485,1990 37. McGlave PB, Beatty P, Ash R, Hows JM: Therapy for chronic myelogenous leukemia with unrelated donor bone marrow transplantation: Results in 102 cases. Blood 75:1728, 1990 38. Weisdorf D, Hakke R, Blazar B, Miller W, McGlave P, Ramsay N, Kersey J, Filipovich A Risk factors for acute graftversus host disease in histocompatible donor bone marrow transplantation. Transplantation 51:1197,1991 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1992 80: 270-276 Unrelated donor bone marrow transplantation for correction of lethal congenital immunodeficiencies AH Filipovich, RS Shapiro, NK Ramsay, T Kim, B Blazar, J Kersey and P McGlave Updated information and services can be found at: http://www.bloodjournal.org/content/80/1/270.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026