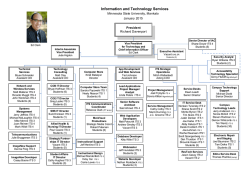
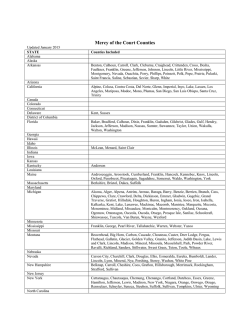
DELIMITACIÓN DE ESPECIES Y POSICIÓN FILOGENÉTICA DEL
DELIMITACIÓN DE ESPECIES Y POSICIÓN FILOGENÉTICA DEL GÉNERO DE BAMBÚ AMERICANO Otatea (POACEAE: BAMBUSOIDEAE) TESIS QUE PRESENTA EDUARDO RUIZ SÁNCHEZ PARA OBTENER EL GRADO DE DOCTOR EN CIENCIAS DIRECTORES DE TESIS VICTORIA SOSA Y MARÍA TERESA MEJÍA SAULÉS SISTEMÁTICA Xalapa, Veracruz, México 2009 Aprobación final del documento final de tesis de grado: Delimitación de especies y posición filogenética del género de bambú Americano Otatea (Poaceae: Bambusoideae) Nombre Firma Director Dra. Victoria Sosa ________________________ Co-director Dra. Teresa Mejía Saulés ________________________ Comité Tutorial Dr. Francisco Lorea ________________________ Dr. Aarón Rodríguez ________________________ Ph.D. Lynn G. Clark ________________________ Jurado 2 RECONOCIMIENTOS La realización de la presente tesis fue posible gracias a la participación y apoyo de colaboradores, amigos e Instituciones al CONACYT (19006) e Instituto de Ecología, A.C. Mis más sinceros agradecimientos a: A las Dras. Victoria Sosa y Teresa Mejía por su excelente dirección del trabajo de investigación, por todas las facilidades, apoyos y consejos brindados durante la realización de ésta tesis. A los miembros del comité tutorial. Dr. Aarón Rodríguez y Dr. Francisco Lorea, por sus comentarios, sugerencias y revisión de ésta tesis. A la Dra. Lynn Clark, por sus comentarios, sugerencias y participación activa en cada uno de los capitulos de éste trabajo. A la Dra. Helga Ochoterena, por sus valiosos comentarios. A Ximena Londoño y el Dr. Jaime Eduardo Muñoz, por su amistad y colaboración con las colectas de Colombia. Al Dr. Philip Silverston, por la logística para el intercambio de material entre el INECOL y la Universidad del Valle en Calí, Colombia. Al Gilberto Cortés y Ana Paula SantosGonçalves por su valiosa colaboración con ejemplares de donados de bambues y la bonita amistad bambusera que nos une. Al Dr. Jerzy Rzedowski, por su ayuda con el latín de las especies nuevas. A Edumuno Saavedra, por sus excelentes ilustraciones incluidas en esta tesis. A Bianca Delfosse, por su ayuda en la edición de la versión en inglés de los manuscritos. A Cristina Barcenas, por su ayuda en las secuencias a Gloria Martínez, por su valiosa ayuda en la parte administrativa al Posgrado y todo su personal. A mis profesores duante del doctorado, en especial a los Drs. Alejandro Espinosa y Francisco Ornelas por el conocimiento y motivación que me brindaron. A todos aquellos que me acompañaron y/o colectaron material de campo: Pablo Carrillo, Arturo de Nova, Flor Rodríguez, José Luis Martínez, Nelly Jiménez, Jaime Pacheco, Xochitl Galarza, Diego Ángulo, Etelvina Gándara, Eva Piedra y Fernando Nicolalde. A todos mis amigos y compañeros de mi generación y de generaciones pasadas y posteriores del INECOL, a todos mis hermanos académicos y amigos fuera del INECOL. A la Red Latinoamericana de Botáncia (RLB07-ATP01), Bamboos of the Americas e International Association for Plant Taxonomists, por las becas obtenidas para las colectas de material botánico. 3 DEDICATORIA A las tres F´s Para la “Flor” mas bella de mi ejido Flor Con todo mi amor Mi Familia Con gran respeto, cariño y amor y Filo (Filogenio Parsimonio Bayes del Likelihood) 4 DECLARACIÓN Excepto cuando es explícitamente indicado en el texto, el trabajo de investigación contenido en esta tesis fue efectuado por (nombre completo del alumno) como estudiante de la carrera de (Maestría y/o Doctorado) en Ciencias (Ecología y Manejo de Recursos Naturales / Manejo de Fauna Silvestre o Sistemática) entre (mes) de (año) y (mes) del (año), bajo la supervisión del (nombre del director de tesis). Las investigaciones reportadas en esta tesis no han sido utilizadas anteriormente para obtener otros grados académicos, ni serán utilizadas para tales fines en el futuro. Candidato: Eduardo Ruiz Sánchez _____________________________ Director de tesis: Dra. Victoria Sosa Dra. Teresa Mejía Saulés _____________________________ _____________________________ INDICE 5 RESUMEN……………………………………………………………………………………...13 CAPITULO I. Introducción general…………………………………………………………..15 LITERATURA CITADA (Capítulo I)………………………………………………………...21 CAPITULO II. Filogenia de Otatea inferida por morfología y secuencias del ADN del cloroplasto y recircunscripción de Guaduinae (Poaceae: Bambusoideae)………………….29 Resumen…………………………………………………………………………………………31 Introducción…………………………………………………………………………………….32 Materiales y Métodos……………………………………………………………………...……34 Resultados……………………………………………………………………………………….36 Discusión………………………………………………………………………………………...39 Literatura citada…………………………………………………………………..……………43 CAPITULO III. Delimitando especies en el bambú neotropical Otatea (Poaceae: Bambusoideae) usando datos morfológicos, moleculares y ecológicos……………………...67 Resumen…………………………………………………………………………………………69 Introducción…………………………………………………………………………………….70 Materiales y Métodos……………………………………………………………………...……73 Resultados……………………………………………………………………………………….78 Discusión………………………………………………………………………………………...83 Literatura citada…………………………………………………………………..……………91 CAPITULO IV. Cuatro especies nuevas en Otatea (Poaceae: Bambusoideae) y revisión taxonómica………………………………………..……………………………………………117 Resumen………………………………………………………………………………….……119 6 Introducción……………………………………………………………………………..…….120 Materiales y Métodos……………………………………………………………………….…122 Resultados……………………………………………………………………………….….….122 Discusión………………………………………………………………………………...……..126 Tratamiento Taxonómico……………………………………………………………………..129 Literatura citada…………………………………………………………..………..…………159 CAPITULO V. Conclusiones generales…………………...…………………………………177 LITERATURA CITADA (Capítulo V)………………….…………………………………..182 7 LISTA DE APENDICES, FIGURAS Y TABLAS CAPITULO II APPENDIX 1. Taxa used in the phylogenetic study of Otatea, selected specimens for morphological analysis, and vouchers, GenBank accession numbers for sequences obtained in this project as well as for the previously published sequences of the DNA sequences used in this paper. GenBank accessions correspond to rpl16 and trnH-psbA; if there is only one accession it corresponds to rpl16……………………………………...49 APPENDIX 2. Morphological characters used in analyses based in the Bamboo Phylogeny Group. Only characters marked with * were based in Londoño and Clark (2002)……………...54 APPENDIX 3. Morphological data matrix of 61 characters. “?” = character not observable, “-” = inapplicable. Polymorfism: A = (0,1); B = (1,2); C = (2,3); E (0,1,2); G (0,2); I (0,3) All species names are listed with authorship in Appendix 1. Characters states and descriptions are listed in Appendix 2…………………………………………………….59 FIG. 1. Strict consensus of six most-parsimonious trees inferred from analysis of the morphological data set L = 204 steps, CI = 42, RI = 64). Numbers below branches indicate jackknife/Bremer support. Character numbers are listed in Appendix 2. Filled circles are synapomorphic. Subtribe abbreviations: Gua = Guaduinae; Art = Arthrostylidiinae; Chu = Chusqueinae; Bam = Bambusinae…………………………….62 FIG. 2. Single most parsimonious tree based on rpl16 intron and trnH-psbA intergenic spacer combined sequence data (L = 48 steps, CI = 0.68, RI = 80). Numbers above branches 8 indicate jackknife support, and numbers below indicate Bremer support. Subtribe abbreviations: Gua = Guaduinae; Art = Arthrostylidiinae; Chu = Chusqueinae; Bam = Bambusinae……………………………………………………………………………....63 FIG. 3. Strict consensus of 53 most-parsimonious trees inferred from analysis of the combined morphological and molecular data (L = 261, CI= 45, RI = 67). Numbers below branches indicate jackknife/Bremer support. Character numbers are as listed in Appendix 2. Filled circles are synapomorphies. .Subtribe abbreviations: Gua = Guaduinae; Art = Arthrostylidiinae; Chu = Chusqueinae; Bam = Bambusinae…………………………….64 FIG. 4. Distribution of the southern Otatea species, Olmeca erecta, O. reflexa, and Aulonemia fulgor and A. clarkiae in southeastern Mexico. Numbers indicated the terrestrial protected areas. 1. Pico de Orizaba – Cofre de Perote region in central Veracruz; 2. Los Tuxtlas region in southern Veracruz; 3. Sierra de Juarez in Oaxaca; 4. Uxpanapa region in the junction of Chiapas, Oaxaca and Veracruz; 5. Bosques mesófilos en Los altos, Chiapas; 6. Cañon del Sumidero, Chiapas and 7. El Triunfo, Chiapas…………...……..65 CAPITULO III Table 1. Study populations and their haplotypes. n = number of individuals sampled for cpDNA markers, and numbers below ITS are the individuals sampled to this marker…………102 Table 2. Nineteen climate variables used in GARP and MAXENT analysis and PCA loadings for the four principal components. Values in bold indicate higher loadings……………….103 Appendix 1: Morphological characters……………………………………………………...…104 Appendix 2: Morphological character matrix……………………………………………….…106 Appendix 3: Vouchers and specimens examined……………………………………………...107 9 FIG. 1. Distribution and sampling localities of the previously recognized species of Otatea. Numbers correspond to localities in Table 1. A and B show details of localities in central Mexico and Chiapas respectively…………………………………………..…………..110 FIG. 2. MPT on chloroplast DNA haplotypes for Otatea (left). Population numbers and haplotypes in parenthesis correspond to Table 1. Number below branches indicates Bootstrap values. New species retrieved by the morphological tree are indicated. Strict consensus from parsimony analysis of the ITS (center) and combined cpDNA-ITS (right). Populations numbers correspond to Table 1. Number below branches indicates Bootstrap values. New species retrieved by the morphological tree are indicated……..…………111 FIG. 3. Statistical parsimony network compound cpDNA-ITS genotypes…………….…….…112 FIG. 4. The single most parsimonious tree retrieved from the morphological data set (L = 179; CI = 47; RI = 76). Population numbers and haplotypes in parenthesis correspond to Table 1. Black circles indicate synapomorphies, numbers above and below the circles indicate character number and character state respectively. Numbers below the branches indicate Bootstrap and Bremer support. Ol.recta = Olmeca recta; Oa = Otatea acuminata; Of = Otatea fimbriata; Og = Otatea glauca…………………..……………………………...113 FIG. 5. GARP and Maxent niche-based distribution models for: a. and b. O. acuminata maps, c. and d. O. fimbriata, e and f. O. sp. nov. Transvolcanic maps, g and g. O. sp. nov. Jalisco...............................................................................................................................114 CAPITULO IV Table 1. Diagnostic characters for the new four species of Otatea……………………………..161 FIG. 1. Micromorphology of the abaxial surface of lemma in Otatea. A. Microhair (m) in O. glauca (E. Ruiz-Sánchez 144, XAL) and B. O. mixtecana (J. Panero & F. Calzada 4441, 10 XAL). C. Macrohairs (ma) in O. acuminata (F. J. Santana 2529, XAL) and D. O. mixtecana (J. Panero & F. Calzada 4441 XAL). E. Prickles with extended barb (prd) in O. acuminata (F. J. Santana 2529, XAL). F. Hooks (h) in O. glauca (E. Ruiz-Sánchez 144, XAL)………………………………………………………………………………166 FIG. 2. Micromorphology of the abaxial surface of the lemma in Otatea. A. Silica bodies with an irregular dumb-bell shape (sbd) in O. glauca (E. Ruiz-Sánchez 144, XAL). B. Silica bodies saddle-shaped (sbs) in O. glauca. C. Silica bodies rounded (sbr) in O. acuminata (F. J. Santana 2529, XAL)……………………………………………………..………167 FIG. 3. Micromorphology of the abaxial surface of palea in Otatea. A. Intercostal long-cells with sinuous outline U-shaped (Lc) in O. glauca (E. Ruiz-Sánchez 144, XAL). B. Prickles with extended barb (prd) in O. glauca. C. Prickles with extended barb (prd) in O. acuminata (F. J. Santana 2529, XAL). D. Prickles with extended barb (prd) and prickles with barb not developed (pr) in O. mixtecana (J. Panero & F. Calzada 4441 XAL). E. Silica bodies irregular dumb-bell shaped (sbd) in O. glauca. F. Silica bodies saddle-shaped (sbs) in O. mixtecana……………………………………………………………………………......168 FIG. 4. Culm leaves. A. Otatea carrilloi. B. O. trasnvolcanica. C. O. reynosoana. D. O. mixtecana…………………………………………………………………………………..…169 FIG. 5. Geographical distribution of Otatea acuminata and O. fimbriata…………...…………170 FIG. 6. Otatea carrilloi. a; rhizome. b; culm with persistent culm leaf sheath. c; branch complement. d; foliage leaf complement. e; culm leaf, abaxial apical view. f; ligular area of foliage leaf with fimbriae and oral setae. (a, d-f. based on E. Ruiz-Sánchez & R. Córdoba 147; b-c. based on P. Carrillo-Reyes, D. Cabrera-Toledo y M. A. Perez-Farrera 5144)…...……………….................................................................................................171 11 FIG. 7. Geographical distribution of Otatea carrilloi, O. glauca and O. mixtecana……………172 Fig. 8. Otatea mixtecana. a; rhizome. b; culm with culm leaves. c; branch complement. d; culm leaf apex witn oral setae. e; Synflorescence and foliage leaf complement. f; ligular area of foliage with oral setae. g; spikelet. (a-d, f. based on E. Ruiz-Sánchez, F. Rodriguez & V. Sosa 217; e, g. based on J. Panero & I. Calzada 4441)………………………………173 FIG. 9. Otatea reynosoana. a; culm with overlapping culm leaves. b; apical shoot with culm leaves. c; branch complement. d; synflorescence and foliage leaf complement e; ligular area of foliage leaf with oral setae. f; spikelet with proximal floret remaining. (a-c, e. based on E. Ruiz-Sánchez & F. Rodriguez 130; d, f. based on G.B. Hinton 9879)……174 FIG. 10. Geographical distribution of Otatea reynosoana and O. transvolcanica………...……175 FIG. 11. Otatea transvolcanica. E. Ruiz-Sánchez, Londoño and L.G. Clark. a, branch complement from mid section to culm apex. b, culm middle section with extravaginal branching. c, apical shoot with reflexed culm leaves. d, foliage leaf complement. e, ligular area of foliage leaf with oral setae and outer ligule lobes in an early stage of development. f, ligular area of foliage leaf with connate oral setae and outer ligule lobes at maturity. g, culm leaf abaxial view. i, detail of foliar leaf. (a-i. based on E. RuizSánchez, D. Angulo & E. Gándara 179)……………………………………………......176 12 RESUMEN Esta tesis se realizó con tres objetivos : 1) determinar la posición filogenética del género Otatea en la subtribu Guaduinae con base en caracteres moleculares y morfológicos; 2) delimitar las especies del género Otatea, utilizando caracteres moleculares, morfológicos y ecológicos y 3) describir las especies nuevas detectadas y preparar una monografía del género Para determinar la posición filogenética de Otatea en Guaduinae se secuenciaron un intron y un espaciador del cloroplasto (rpl16 y trnH-psbA), además se codificaron 61 caracteres morfológicos. Los resultados filogenéticos identificaron a Otatea como un grupo monofilético perteneciente a la subtribu Guaduinae, aunque su grupo hermano no pudo ser corroborado debido a la falta de resolución. Sin embargo, resulta inespearada la inclusión de Aulonemia clarkiae y A. fulgor dentro de la subtribu Guaduinae y no en la subtribu Arthrostyliidinae, donde habían sido clasificadas antes, por lo que probablemente tengan que ubicarse bajo una nueva categoría taxonómica. En la delimitación de las especies de Otatea secuencias de ADN del cloroplasto (atpFatpH, psbI-psbK, trnL-rpl32), nucleares (ITS), caracteres morfológicos y la modelación del nicho. Encontramos que las hipótesis filogenéticas resultantes de los análisis moleculares y morfológicos eran incongruentes. Un minucioso análisis de los resultados moleculares nos llevó a sugerir que en la evolución de las especies de Otatea hubo retención de polimorfismos ancestrales, reticulación (flujo genético pasado) o una hibridización, procesos que explican la incongruencia del árbol de genes con el morfológico. Por otra parte, el análisis del nicho ecológico sugiere que nicho divergente es el causante de la especiación de Otatea. 13 Finalmente, reconocemos siete especies para el género Otatea. Sinonimizamos las subespecies de Otatea acuminata y describimos cuatro especies nuevas, todas ellas endémicas de México. 14 CAPÍTULO I. INTRODUCCIÓN GENERAL 15 Los bambúes pertenecen a la subfamilia Bambusoideae, una de las 13 subfamilias reconocidas de la familia Poaceae (GPWG, 2001, Sánchez-Ken et al., 2007). Bambusoideae agrupa de 80 a 90 géneros y de 1000 a 1500 especies (GPWG, 2001; Sungkaew et al., 2008). Están presentes en Asia, África, Australia y las Américas, crecen desde el nivel del mar hasta los 4000 m (Sungkaew et al., 2008). Bambusoideae, está compuesta por dos tribus: Bambuseae (bambúes leñosos) y Olyreae (bambúes herbáceos) y constituye un grupo monofilético con base en caracteres moleculares y morfológicos (Zhang y Clark, 2000; GPWG, 2001; Sungkaew et al., 2008). Sin embargo, estudios filogénéticos de Poaceae y Bambusoideae indican que Bambuseae no es monofilética (Bouchenak-Khelladi et al., 2008; Sungkaew et al., 2008). Bambuseae se subdivide en nueve subtribes: Arthrostylidiinae, Arundinariinae, Bambusinae, Chusqueinae, Guaduinae, Hickeliinae, Melocanninae, Racemobambosinae and Shibataeinae (Judziewicz et al., 1999; Zhang y Clark, 2000; Sungkaew et al., 2008). Pero hasta la fecha solo se ha comprobado y confirmado la filogenia de las subtribus Chusqueinae y Hickeliinae (Clark et al., 2007). Por otra parte, en el estudio filogenético de Bambusoideae realizado por Zhan y Clark (2000), se identificaron tres clados principales de Bambuseae: 1) el clado de los bambúes templados asiáticos, y dos clados de bambúes tropicales, 2) los asiáticos y 3) los americanos. En el más reciente estudio filogenético para los bambúes, éstos tres clados se siguen identificando como monofiléticos, sin embargo Bambuseae resulta parafilética, en otras palabras Bambusoideae fue divida en tres tribus: Arundinarieae, Bambuseae y Olyreae (Sungkaew et al., 2008). Arthrostylidiinae, Chusqueinae, Guaduinae, se agrupan en un clado llamado el clado de bambúes neotropicales que incluyen a 21 géneros y más de 345 especies, distribuidas desde en el sur de Estados Unidos, México, Centroamérica, Sudamérica e islas del 16 Caribe (Judziewicz et al., 1999; Zhang y Clark, 2000; Sungkaew et al., 2008). Guaduinae, está conformada por cinco géneros: Apoclada, Eremocaulon, Guadua, Olmeca y Otatea (Judziewicz et al., 1999). Existen algunos trabajos taxonómicos sobre bambúes americanos, (Guzmán et al., 1984; Londoño y Clark 2002) y algunos análisis filogenéticos (Guala 1995; Guala et al., 2000; Kelchner y Clark 1997), utilizando marcadores moleculares de ADN (ndhF y rpl16) y datos morfológicos (Clark et al., 2007). De los análisis filogenéticos realizados con bambúes asiáticos destacan los elaborados por (Hodkinson, 2000; Guo et al., 2001, 2002; Guo y Li 2004; Sun et al., 2005; Yang et al., 2007; Peng et al., 2008) con marcadores moleculares GBSSI, ITS y trnL-F. El género de estudio en esta tesis es Otatea. Es un grupo que no se ha estudiado desde el punto de vista filogenético. Se distribuye desde el sur de Sonora y Chihuahua en México hasta, Centroamérica (Calderón y Sorderstrom, 1980; Guzmán et al., 1984; Judziewicz et al., 1999). Londoño y Clark (1998) reportaron una población de Otatea fimbriata en el Norte de Santander al noreste de Colombia, lo que marca una clara disyunción de esta especie y del género. En México, las especies de Otatea tienen diversos usos: los culmos o tallos son utilizados en la elaboración de canastos, bastones, mangos para escobas, garrochas para diversos usos, además son utilizados para la construcción de casas donse se utilizan los tallos con una mezcla de lodo (bahereque) para hacer paredes y como vigas para los techos, además las hojas son consumidas por el ganado (Anaya, 1989; Guzmán et al., 1984; Judziewicz et al., 1999; Cortés, 2000; Vázquez, 1995). McClure (1973) describio el subgenero Otatea in Yushania y comprendía dos especies Y. aztecorum y Y. acuminata. Yushania aztecorum McClure fue descrita de una colecta de El Rosario, Sinaloa, México. Y. acuminata fue una combinación basada en Arundinaria acuminata 17 Munro (descrita de Jalcomulco, Veracruz). Calderón y Sodersrom (1980) elevaron a género a Otatea y O. aztecorum fue considerada sinonimo de O. acuminata. En 1983, se describe O. fimbriata Soderstrom de Chiapas. Posteriormente se dividió O. acuminata en dos subespecies O. acuminata subsp. acuminata y O. acuminata subsp. aztecorum Guzmán, Anaya & Santana con base a caracteres morfológicos derivados del tipo de hoja culmea y tipo de floración (Guzmán et al., 1984). En el 2004, se describe O. glauca L.G. Clark & G. Cortés de Chiapas (Clark y Cortés, 2004) y Ruiz-Sánchez et al., (2008) citán una nueva especie no descrita para Chiapas. La distribución de las especies de Otatea es la siguiente; O. acuminata subsp. aztecorum se distribuye por la vertiente del Pacífico, desde Sonora hasta Chiapas y simpátricamente con este taxón encontramos a O. fimbriata, con una población disyunta en Colombia. Otatea acuminata subsp. acuminata se distribuye a lo largo de la Faja Transvolcánica Mexicana y parte del Golfo de México y por último O. glauca se conoce solo de una población en Chiapas. (Guzmán et al., 1984; Beetle et al., 1995; Judziewicz et al., 1999; Londoño y Clark, 1998). Resumiendo, se reconocen tres especies y una subespecie de Otatea. No se ha llevado a cabo una revisión del género, ni se ha realizado algún estudio filogenético. Por otra parte, la posición filogenética de Otatea es incierta. Se ha identificado como grupo hermano de Guadua con base en caracteres moleculares de sitios de restricción y secuencias del ADN ndhF (Soreng y Davis, 1998; Zhan y Clark, 2000). Los resultados de Zhan (2000) sugieren una relación de grupos hermanos entre Otatea y Glaziophyton (Arthrostylidiinae). Alternativamente Guala et al., (2000) indican una relación cercana entre Otatea y el clado Guadua - Apoclada. Sin embargo el número de taxones utilizados en estos estudios filogenéticos ha sido muy pobre. De ahí la importancia en esclarecer su posición filogenética. 18 En esta tesis los objetivos son: 1) identificar grupos monofiléticos ; 2) describir especies y 3) determinar relaciones filogenéticas entre las especies (Sites y Marshall, 2003, 2004; Wiens, 2007) y en este trabajo aplicaremos estos tres en Otatea. Primero investigaresmos si se identifica como un grupo monofilético, posteriormente determinaremos sus relaciones filogenéticas y finalmente delimitaremos y describiremos la o las especies nuevas resultantes. La delimitación de especies es importante en el contexto del entendimiento de muchos procesos y mecanismos evolutivos (Sites y Marshall, 2003). Las especies son utilizadas en estudios de ecología, macroevolución, sistemática, filogeografía, biogeografía y biología de la conservación (Barraclough y Nee, 2001; Wiens y Penkrot, 2002; Sites y Marshall, 2003, 2004; Agapow et al., 2004). Los datos morfológicos han sido utilizados tradicionalmente para delimitar especies y continúan siendo ampliamente utilizados, pero muchos estudios recientes han utilizado las secuencias de ADN, para evaluar la taxonomía tradicional morfológica (Wiens y Penkrot, 2002). Recientemente, la utilización de datos ecológicos como es la modelación del nicho ecológico, ha demostrado ser útil para identificar y diagnosticar especies (Raxworthy et al., 2007; Rissler y Apodaca, 2007; Stockman y Bond, 2007; Bond y Stockman, 2008) y para encontrar potenciales especies nuevas (Raxworthy et al., 2003). El criterio operacional utilizado para la delimitación de las especies, lleva consigo la utilización de algún concepto de especie y a pesar de la larga historia de disputas sobres conceptos de especies, la mayoría concuerdan que las especies son linajes y se considera que todas las otras definiciones son como herramientas “secundarias” para el reconocimiento de las especies (Wiens y Penkrot, 2002; Sites y Marshall, 2003, 2004; de Queiroz 2005, 2007) bajo esta premisa de que las especies son linajes, es el concepto de especie que seguiremos. Por otra parte existen diferentes métodos relacionados con la detección y delimitación de las propiedades de los 19 linajes, los cuales se dividen en dos: métodos no basados en árboles y métodos basados en árboles (Sites y Marshall, 2003, 2004). Entre los criterios operacionales basados en árboles (haplotipos y caracteres morfológicos) donse se asume que no existe flujo génico entre la especie focal a estudio y una o más especies cercanamente relacionadas, además de encontrar caracteres morfológicos diágnosticos, y congruencia geográfica. Éste método es propuesto por Wiens y Penkrot (2002), mismo que se utilizará en este trabajo para la delimitación de las especies del género Otatea. Los objetivos de esta tesis son: 1) determinar la posición filogenética del género Otatea, con base en secuencias de ADN del cloroplasto (intron rpl16, espaciador trnH-psbA) y caracteres morfológicos; 2) delimitar las especies del género Otatea, utilizando haplotipos no recombinantes de los espaciadores atpF-atpH, psbI-psbK, trnL-rpl32 del cloroplasto e ITS nuclear, caracteres morfológicos y ecológicos y 3) describir las especies nuevas resultantes del análisis filogenético del género Otatea. 20 LITERATURA CITADA (CAPITULO I ) 21 LITERATURA CITADA Agapow, P.M., Bininda-Edmonds, O.R., Crandall, K.A., Gittleman, J.L., Mace, G.M., Marshall, J.C., Purvis, A. 2004. The impact of species concept on biodiversity studies. Q. Rev. Biol. 79, 161-79. Anaya, C. 1989. Estudio de la Subfamilia Bambusoideae (Poaceae), con Revisión Taxonómica para el Estado de Jalisco, México. Tesis de Licenciatura. Facultad de Agronomía. Universidad de Guadalajara. Guadalajara, Jalisco, México. Barraclough, T.G., Nee, S. 2001. Phylogenetics and speciation. Trends Ecol. Evol. 16, 391-99. Beetle, A.A, Miranda, S J., Jaramillo, L.V., Rodríguez, R.A., Aragón, M.L., Vergara, B.M., Chimal, H.A., Domínguez, S.O. 1995. Las Gramíneas de México IV. COTECOCA. México, D.F. Bond, J.E., Stockman, A.K. 2008. An integrative method for delimiting cohesion species: finding the population-species interface in a group of Californian trapdoor spiders with extreme genetic divergence and geographic structure. Syst. Biol. 57, 628-646. Bouchenak-Khelladi, Y., Salamin, N., Savolainen, V., Forest, F., van der Bank, M., Chase, M.W., Hodkinson, T.R. 2008. Large multi-gene phylogenetic trees of the grasses (Poaceae): 22 progress towards complete tribal and generic level sampling. Mol. Phylogenet. Evol. 47, 488505. Calderon, C.E., Soderstrom, T. R. 1980. The genera of bambusoideae (Poaceae) of the American Continent: Keys and Comments. Stmith. Cont. Bot. 44: 1-27. Clark, L.G., Cortés, G. 2004. A new species of Otatea from Chiapas, Mexico. Bamboo Science and Culture 18, 1-6. Clark, L.G., Dransfield, S., Triplett, J., Sánchez-Ken, J.G. 2007. Phylogenetic relationships among the one-flowered, determinate genera of Bambuseae (Poaceae: Bambusoideae). Aliso 23, 315-322. Cortés, R.G. 2000. Los bambúes nativos de México. Biodiversitas 30: 12-15. de Queiroz, K., 2005. A unified species concept and its consequences for the future of taxonomy. Proc. Calif. Acad. Sci. 56, 196-215. de Queiroz, K. 2007. Species concepts and species delimitation. Syst. Biol. 56, 879–886. Grass Phylogeny Working Group. 2001. Phyllogeny and subfamilial classification of the grasses (Poaceae). Ann. Miss. Bot. Gard. 88: 373-475. 23 Guala, G.F. 1995. A cladistic Analysis and Revision of the Genus Apoclada (Poaceae: Bambusoideae: Bambuseae). Syst. Bot. 20: 207-223. Guala, G., Bogler, D., Sadle, J., Francisco-Ortega, J. 2000. Molecular evidence for polyphyly in the genus Apoclada (Poaceae: Bambusoideae). Bamboo Science and Culture 14: 15–20. Guo, Z.H. Li, D.Z. 2004. Phylogenetics of the Thamnocalamus group and its allies (Gramineae: Bambusoideae) inferred from the sequences of GBSSI gene and ITS spacer. Mol. Phylogenet. Evol. 30, 1-12. Guo, Z.H., Chen, Y.Y., Li, D.Z., Yang, J.B. 2001. Genetic variation and evolution of the alpine bamboos (Poaceae: Bambusoideae) using DNA sequence data. J. Plant Res. 114, 315-322. Guo, Z.H., Chen, Y.Y., Li, D.Z. 2002. Phylogenetic studies on Thamnocalamus group and its allies (Bambusoideae: Poaceae) based on ITS sequence data. Mol. Phylogenet. Evol. 2, 20-30. Guzmán, R., Anaya, M.C., Santana, F.J. 1984. El género Otatea (Bambusoideae), en México y Centroamérica. Boletín del Instituto de Botánica 5, 2-20. Hodkinson, T.R., Renvoize, S.A., Ni Chonghaile, G., Stapleton, C.M.A., Chase, M.W. 2000. A comparison of ITS nuclear rDNA sequence data and AFLP markers for phylogenetic studies in Phyllostachys (Bambusoideae, Poaceae). J. Plant Res. 113, 259-269. 24 Judziewicz, E.J., Clark, L.G., Londoño, X., Stern, M.J. 1999. American Bamboos. Smithsonian Institution Press. New York. Kelchner, S.A., Clark, L.G. 1997. Molecular Evolution and Phylogenetic Utility of the Chloroplast rpl16 intron in Chusquea and the Bambusoideae (Poaceae). Mol. Phylogenet. Evol. 8: 385-397. Londoño, X., Clark, L.G. 1998. Eight new taxa and two new reports of Bambuseae (Poaceae: Bambuseae) from Colombia. Novon 8, 408-428. Londoño, X., Clark, L.G. 2002. A revision of the Brazilian bamboo genus Eremocaulon (Poaceae: Bambuseae: Guaduinae). Syst. Bot. 27, 703-721. McClure, F. A. 1973. Genera of bamboos native to the New World (Gramineae: Bambuosideae). Smithsonian Contribution to Botany 9: 1-148. Peng, S., Yang, H.Q., Li, D.Z. 2008. Highly heterogeneous generic delimitation within the temperate bamboo clade (Poaceae: Bambusoideae): evidence from GBSSI and ITS sequences. Taxon 57, 799-810. Raxworthy, C.J., Martínez-Meyer, E., Horning, N., Nussbaum, R. A., Schneider G.E., OrtegaHuerta, M.A., Peterson, A.T. 2003. Predicting distributions of known and unknown reptile species in Madagascar. Nature 426, 837-841. 25 Raxworthy, C.J., Ingram, C.M., Pearson, R.G. 2007. Species delimitation applications for ecological niche modeling: a review and empirical evaluation using Phelsuma day gecko groups from Madagascar. Syst. Biol. 56, 907-923. Rissler, L.J., Apodaca, J.J. 2007. Ecological niche models and phylogeography help uncover cryptic biodiversity. Syst. Biol. 56, 924-942. Ruiz-Sánchez, E., Sosa, V., Mejía-Saules, M.T. 2008. Phylogenetics of Otatea inferred from morphology and chloroplast DNA sequence data and recircumscription of Guaduinae (Poaceae: Bambusoideae). Syst. Bot. 33, 277-283. Sánchez-Ken J.G., Clark, L.G., Kellog, E.A., Kay, E.E. 2007. Reinstatement and Emendation of Subfamily Micrairoideae (Poaceae). Syst. Bot. 32: 71-80 Sites, J.W., Marshall, J.C. 2003. Delimiting species: a renaissance issue in systematic biology. Trends Ecol. Evol. 18, 462-470. Sites, J.W., Marshall, J.C. 2004. Operational criteria for delimiting species. Annu. Rev. Ecol. Evol. Syst. 35, 199-227. 26 Soreng, R.J., Davis J.I. 1998. Phylogenetics and character evolution in the grass family (Poaceae): simultaneous analysis of morphological and chloroplast DNA restriction site character sets. Bot. Rev. 64: 1-85. Stockman, A.K., Bond, J.E. 2007. Delimiting cohesion species: extreme population structuring and the role of ecological interchangeability. Mol. Ecol. 16, 3374-3392. Sun, Y., Xia, N., Lin, R. 2005. Phylogenetic analysis of Bambusa (Poaceae: Bambusoideae) based on internal transcribed spacer sequences of nuclear ribosomal DNA. Biochem Genet. 43, 603-612. Sungkaew, S., Stapleton, C.M.A., Salamin, N., Hodkinson, T.R. 2008. Non-monophyly of the woody bamboos (Bambuseae; Poaceae): a multi-gene region phylogenetic analysis of Bambusoideae s.s. J Plant Res. 122, 95-108. Vázquez, J.M. 1995. Estudio Etnológico del Aprovechamiento del Otate (Otatea acuminata (Munro) Cald. & Sod. subsp. aztecorum Guzmán, Anaya & Santana) en el Ejido Platanarillo, Municipio de Minatitlan, Colima. Tesis de Licenciatura. División de Ciencias Biológicas y Ambientales. Universidad de Guadalajara. Guadalajara, Jalisco. Wiens, J.J. 2007. Species delimitation: new approaches for discovering diversity. Syst. Biol. 56, 875-878. 27 Wiens, J.J., Penkrot, T.A. 2002. Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus). Syst. Biol. 51, 69-91. Yang, H.Q., Peng, S., Li, D.Z. 2007. Generic delimitations of Schizostachyum and its allies (Gramineae: Bambusoideae) inferred from GBSSI and trnL-F sequence phylogenies. Taxon 56, 45-54. Zhang, W. 2000. Phylogeny of the grass family (Poaceae) from rpl16 intron sequence data. Mol. Phylogenet. Evol. 15: 135-146. Zhang, W., Clark. L.G. 2000. Phylogeny and Classification of the Bambusoideae (Poaceae). En S. W. L. Jacobs & J. E. Everett. (Eds.), Grasses: systematics and evolution, 35-42. CSIRO Publishing, Collingwood, Victoria. 28 CAPÍTULO II. FILOGENIA DE OTATEA INFERIDA POR MORFOLOGÍA Y SECUENCIAS DEL ADN DEL CLOROPLASTO Y RECIRCUNSCRIPCIÓN DE GUADUINAE (POACEAE: BAMBUSOIDEAE) Publicado en: Systematic Botany 33: 277-283. 2008 29 Phylogenetics of Otatea inferred from morphology and chloroplast DNA sequence data and recircumscription of Guaduinae (Poaceae: Bambusoideae) EDUARDO RUIZ-SÁNCHEZ 1, VICTORIA SOSA1, 2, M. TERESA MEJÍA-SAULES1 1 Departamento de Biologia Evolutiva Instituto de Ecologia, A. C. Apartado Postal 63 91070 Xalapa, Veracruz, Mexico 2 Correspondence: [email protected] 30 ABSTRACT. Representative taxa of the five genera of Guaduinae, a subtribe of Neotropical woody bamboos, were sampled to investigate the phylogenetic relationships of the species of the genus Otatea using morphological and molecular (cpDNA intergenic spacer trnH-psbA and the rpl16 intron) evidence. Phylogenetic analysis of a combined data set retrieved 53 most parsimonious trees in which subtribe Guaduinae is monophyletic if two species of Aulonemia (A. clarkiae and A. fulgor) are included. They were previously classified within subtribe Arthrostylidiinae. Guaduinae is supported by the lack of papillae from the abaxial surface, by an almost solid style, a short rachis extension, and oral setae present in culm and foliage leaves. Monophyly of the genera in Guaduinae (Eremocaulon, Guadua, Apoclada, Otatea and Olmeca) was corroborated. Otatea species formed a monophyletic clade, supported by culms with three subequal ascending branches and pubescent lemmas. Eight species in Guaduinae (the four species in Otatea, Olmeca recta, O. reflexa, Aulonemia clarkiae and A. fulgor) are distributed in southeastern Mexico in areas determined as Pleistocene refuges. Some of them possess baccoid caryopses and long culm necks, and grow in threatened vegetation types such as cloud, tropical and tropical deciduous forests, therefore they are important bamboos to preserve. KEYWORDS, Aulonemia, Olmeca, Eremocaulon, rpl16 intron, trnH-psbA intergenic spacer, woody bamboos. 31 One of the two tribes of subfamily Bambusoideae in the Poaceae is the Bambuseae, an assemblage of woody bamboos comprised of nine subtribes, three of which are exclusive to the Neotropics (Soderstrom and Ellis 1987; Judziewicz et al. 1999). These subtribes, Arthrostylidiinae, Chusqueinae and Guaduinae, were retrieved in a clade by Zhang and Clark (2000) in their molecular phylogeny of the Bambusoideae, with strong support for a sister relationship between Guaduinae and Arthrostylidiinae and weak support for the association of Chusqueinae with these two subtribes. Subtribe Arthrostylidiinae has 13 genera, while subtribes Chusqueinae and Guaduinae have two and five genera respectively (Judziewicz et al. 1999). Subtribe Guaduinae includes Otatea, Olmeca, Guadua, Eremocaulon, and Apoclada. The subtribe is distributed from northeastern Mexico to Uruguay, Argentina and Bolivia and it has been diagnosed by leaf characters. Soderstrom and Ellis (1987) mistakenly indicated that leaves in the subtribe lack abaxial stomates but they correctly noted that the adaxial surface often has unusual papillae, which they called refractive papillae, associated with the stomates. Judziewicz et al. (1999) and Londoño and Clark (2002) showed that Guaduinae indeed usually have abundant stomates on both leaf surfaces, and that the adaxial surface often has refractive papillae associated with the stomates whereas papillae are frequently absent from the abaxial surface. In contrast, the other subtribes of woody bamboos have abundant stomates only on the abaxial surface and papillae are usually found only on the abaxial surface (although small papillae may occur on the adaxial surface, particularly on bulliform cells) and are not usually refractive. We are interested in Otatea, one of the genera in subtribe Guaduinae which has four species: O. acuminata (Mexico to Central America), O. fimbriata (Mexico to Central America and northeastern Colombia), O. glauca (endemic to Chiapas, Mexico) and an undescribed species found in Chiapas, Mexico during field work for this study. Otatea is distinguished from 32 the other genera in Guaduinae by the presence of branch complements with three subequal branches per node (Guzmán et al. 1984; Londoño and Clark 1998, 2002; Judziewicz et al. 1999; Clark and Cortés 2004). The position of Otatea within Guaduinae has not yet been determined. Otatea has been retrieved either as the sister group to Guadua and Apoclada from subtribe Guaduinae or to a clade formed by Rhipidocladum and Glaziophyton from subtribe Arthrostylidiinae (Kelchner and Clark 1997; Soreng and Davis 1998; Guala et al. 2000; Zhang 2000; Zhang and Clark 2000). Furthermore, taxon sampling of Guaduinae in those studies has been incomplete. Guala et al. (2000), in their phylogenetic study of Apoclada, included three genera of Guaduinae, one of them Otatea. However Eremocaulon and Olmeca have never been included in any published phylogenetic studies. Only a few chloroplast DNA regions have been explored in phylogenetic studies of the taxa in Bambusoideae. Among them are the rpl16 intron (Kelchner and Clark 1997; Clark et al., 2007), and the ndhF gene (Guala et al. 2000; Zhang and Clark, 2000). Among nuclear DNA regions, ITS and the GBSSI gene have been studied (Hodkinson et al. 2000; Guo et al. 2001; Guo et al. 2002; Guo and Li 2004; Sun et al. 2005). The trnH-psbA intergenic spacer has never been used for bamboos, though it has been useful in other groups of angiosperms (Holdreggrer and Abbott 2003; Miller et al. 2003; Tate and Simpson 2003; Shaw et al. 2005; Clark et al. 2006). Moreover, due to its high level of species and interspecific divergence it has been proposed as a candidate for DNA barcoding research (Kress et al. 2005). The aims of this study are to determine phylogenetic relationships of the species of Otatea based on morphological characters and cpDNA sequences from the rpl16 intron and the trnH-psbA intergenic spacer considering representative taxa of the five genera of Guaduinae. 33 MATERIALS AND METHODS Taxon sampling. A total of 26 terminal taxa were included in the analyses, 15 as the ingroup and 11 as the outgroup. Ingroup taxa considered representative taxa (type species) of the four genera of subtribe Guaduinae and the fifth, Otatea, included its three species and an undescribed taxon. Currently about 35 or 36 species are included within the Guaduinae, in this study we considered about 40% of them. Selection of the outgroup was based on Zhang and Clark (2000). Representative species of Aulonemia, Arthrostylidium, Atractantha, Glaziophyton and Rhipidocladum from subtribe Arthrostylidiinae; Chusquea and Neurolepis from Chusqueinae and Bambusa from Bambusinae were selected. Bambusa vulagaris was used as the functional root because it represents the most distant taxon according to Zhang and Clark (2000). Taxa and vouchers are listed in Appendix 1. Specimens for this project were collected either in the field or from the Mexican bamboo collection of the “Francisco Javier Clavijero” botanical garden of the Instituto de Ecologia, A.C. Herbarium material analyzed is also recorded in Appendix 1. Morphological data. The morphological matrix included 61 characters (29 vegetative, 21 floral and 11 leaf micromorphological characters; Appendix 2). Selection of characters was based on the list and illustrations of L. Clark in “Bamboo Biodiversity” (http://www.eeob.iastate.edu/research/bamboo) and on Londoño and Clark (2002). Characters were scored by examining live material, herbarium specimens, and when necessary, complemented with literature (McClure 1973; Stapleton 1997; Judziewicz et al. 1999; Londoño and Clark 2002). There are two hypotheses to code bud and branch characters. The first one was proposed by McClure (1973), and considers that only one of the buds is homologous to the single primary bud typical of bamboos, while the two to many additional smaller subsidiary buds are derived 34 separately from meristematic tissue of the nodal region with one usually much larger than the others. In contrast, the second hypothesis was proposed by Stapleton (1997), and considers that an extensive loss or reduction of prophylls was consistent with condensation of a single primary axis as a pathway for the evolution of the bud complement. For coding these characters (characters 9-11), we followed the second hypothesis because it gives the most parsimonious explanation for the evolution of these vegetative attributes (Clark et al. 2007). To observe leaf micromorphological characters in Arthrostylidium ecuadorense, Atractantha radiata, Otatea glauca and Otatea sp. nov we followed the techniques described by Hilu and Randall (1984). The samples were prepared as described in the protocols of the Bamboo Biodiversity website (http://www.eeob.iastate.edu/). For the rest of taxa in our study we examined scanning electron micrographs provided by L. Clark (unpubl. data). Characters are listed in Appendix 2. The morphological and molecular data matrices are available in TreeBASE (study accession number S1860; www.treebase.org/treebase/) and from the authors. The combined data matrix comprised 26 terminal taxa each with 1606 characters; out from the total number of characters in the data matrix 4.48% were scored as missing. DNA, extraction, amplification, and sequencing. DNA was isolated using either the modified 2x CTAB method (Rogers and Bendich 1985; Doyle and Doyle 1987; Cota-Sánchez et al. 2006) or the DNeasy Plant Mini Kit (Quiagen, Valencia, California), according to the manufacturer’s instructions. The rpl16 intron was amplified using primers F71 and R1661 (Zhang 2000) and based on protocols by Kelchner and Clark (1997), and by Shaw et al. (2005). Sequencing was performed using the primers F71 and R1516 (Zhang 2000). The trnH-psbA intergenic spacer was amplified and sequenced using primers trnH2 and psbA (Tate and Simpson 2003) and based on the protocol by Shaw et al. (2005). Amplified double-stranded DNA fragments were purified 35 using QIAquick columns (Qiagen) following protocols provided by the manufacturer and sequenced using Taq BigDye Terminator Cycle Sequencing Kits (Perkin Elmer Applied Biosystems, California) on an ABI 310 automated DNA sequencer (Perkin Elmer Applied Biosystems, California). GenBank accessions are recorded in Appendix 1. Sequences were aligned manually using the program Se-Al v. 2.0a11 (Rambaut 2002). Gaps were coded using the method of Simmons and Ochoterena (2000), in which each gap is treated as a separate character. Phylogenetic analyses. Data matrices were constructed with Winclada (Nixon 2002) and Mesquite v 1.1 (Maddison and Maddison 2006). Three analyses were performed, a morphological analysis, a molecular analysis with rpl16 and trnH-psbA cpDNA sequences, and a total evidence analysis. Parsimony searches were conducted using Nona (Goloboff 1999) including only potentially informative characters. A total of 5,000 random addition sequences in sets of 1,000 seeds were submitted to TBR holding 100 trees, followed by more extensive TBR holding 50,000 trees (5 times: h 50,000 h/100 mu*1,000 max*). The potential incongruence of the molecular and morphological data sets was tested using the incongruence length difference (ILD) test of Farris et al. (1994) as implemented in WinClada (Nixon 2002). Clade support was estimated by jackknife (Farris et al. 1996) as implemented in WinClada (Nixon 2002), resampling 1,000 times with TBR set to 100 replications holding 20 trees, followed by a more extensive TBR holding 5,000 trees, and saving the consensus for each resampling matrix. Bremer support (Bremer, 1994) was calculated using the BS5 option of Nona (Goloboff 1999) on 10,000 trees held in memory. RESULTS 36 Morphological analysis. Parsimony analysis of morphological data retrieved six trees (L = 204 steps, CI = 42, RI = 67). The strict consensus is shown in Fig. 1. Otatea was supported as a monophyletic group with low support (jk = 54%, brs = 1) by two synapomorphic states: a branch complement with three subequal and ascending branches (14, CI = 50) and pubescent lemmas (39, CI = 57). Olmeca is supported as monophyletic (jk = 96%, brs = >5) by a single synapomorphic character state: a baccoid caryopsis (50, CI = 1) (Fig 1). The Chusqueinae clade received support (jk = 97%, brs = >5). There were other clades with jackknife support: the two species of Eremocaulon (jk = 97%, brs >5), Aulonemia patula and A. laxa (jk = 95%, brs = >5), the clade formed by Atracthanta radiata, Arthrostylidium ecuatorense, Aulonemia laxa and A. patula (jk = 82%, brs = >5) and the clade formed by the six species of Guadua (jk = 55%, brs = 2) (Fig. 1). However the majority of the clades did not receive support, moreover tribe Guaduinae is retrieved as paraphyletic (Fig. 1). Molecular analyses. Separate analyses of rpl16 intron and trnH-psbA sequence data retrieved both a single most parsimonious tree (MPT) without resolution (not shown). The data matrix of rpl16 intron and trnH-psbA intergenic spacer sequences included 1545 bp (1014 and 531 respectively), with 30 bp (1.94%) being parsimony informative. An inversion of 16 bp was found in the trnH-psbA intergenic spacer in species such as: Aulonemia clarkiae, A. fulgor, Bambusa vulgaris, Chusquea bilimekii, Eremocaulon asymmetricum, E. aureofimbriatum, Guadua longifolia, G. velutina and Rhipidocladum racemiflorum. A similar inversion has been described for several groups of plants on this cpDNA region (Tate and Simpson 2003; Clark et al. 2006). However, this inversion was omitted from analyses because it has been suggested that it was 37 most likely caused by an intramolecular recombination (Kelchner and Wendel 1996; DumolinLapègue et al. 1998; Kelchner 2000). The parsimony analysis of the two molecular markers retrieved a single MPT (L = 48 steps, CI = 0.68, RI = 80), with the species of Otatea in a weakly supported clade (jk= 63%, brs = >2) (Fig. 2). Two other genera of the subtribe were grouped in clades with weak jackknife and Bremer support: the six species of Guadua (53%, >2) and the two species of Eremocaulon (54%, >2). Olmeca was retreived as paraphyletic. Guaduinae formed a monophyletic clade but without jackknife support (Fig. 2). The clade formed by Aulonemia laxa and A. patula had moderate support as a clade (jk=76%, brs = >2). Subtribes Guaduinae and Arthrostyliidinae were grouped in a large clade with moderate support (jk=80%, brs = >2) (Fig. 2). Total evidence analysis. Out of 1606 characters of the combined analysis, 91 were parsimony informative. The parsimony analysis of combined data retrieved 53 MPT (L = 261, CI= 45, RI = 67) and the strict consensus tree is shown in Fig.3. The ILD results indicated that the morphology and molecular partitions were significantly incongruent (P = 0.010). However when the morphological and molecular data are combined the MPT are more resolved. The strict consensus tree shows tribe Guaduinae as a monophyletic group (jk=87%, brs=>5), and although the position of Otatea and Olmeca was not resolved, species of Guadua, Eremocaulon, Otatea and Olmeca were grouped in their respective subclades with moderate to high support. Two species previously included in subtribe Arthrostylidiinae were grouped within Guaduinae: Aulonemia fulgor and A. clarkiae (in a subclade with low support) (Fig. 3). The relationship between Arthrostylidiinae and Guaduinae was not resolved either. Chusqueinae (jk = 90%, brs = >5) was sister to Arthrostylidiinae and Guaduinae but with no support for this relationship (Fig. 3). 38 DISCUSSION Initially subtribe Guaduinae comprised five genera (Criciuma, Eremocaulon, Guadua, Olmeca and Otatea) (Soderstrom and Ellis, 1987). Judziewicz et al. (1999) included Apoclada with these other five genera in their circumscription of the Guaduinae. Guala et al. (2000), based on molecular phylogenetic analyses, found that Apoclada was polyphyletic and placed Apoclada simplex in Guaduinae and the two other species in the new genus Filgueirasia in subtribe Arthrostylidiinae. Later, in their revision of Eremocaulon, Londoño and Clark (2002) concluded that the genus Criciuma should be synonymized with Eremocaulon and followed Guala et al. (2000) in including Apoclada simplex within the subtribe. Our analyses found Aulonemia clarkiae and A. fulgor nested within the Guaduinae clade; this result has important consequences for the circumscription of the subtribe. These species are the only ones in Aulonemia with a stomate distribution pattern like that of Guaduinae (Judziewicz et al. 1999). When Soderstrom (1988) described A. fulgor, he indicated that this species was morphologically similar to Aulonemia but anatomically similar to Olmeca. Moreover, when Davidse and Pohl (1992) described A. clarkiae they pointed out its similarity with A. fulgor and suggested that these two species might be segregated as a different genus. Our study confirmed the relationship between these two taxa and their inclusion in subtribe Guaduinae. Further studies are needed, extending analyses to include greater sampling of species of Aulonemia, before a decision to describe a new genus can be made. Our results also confirmed that Apoclada simplex forms part of Guaduinae as suggested by Guala et al. (2000) and Guala (2003), and supported the close relationship between Eremocaulon and Guadua, as previously suggested by Londoño and Clark (2002). Therefore we conclude that subtribe Guaduinae should 39 include Apoclada, Eremocaulon, Guadua, Olmeca, Otatea and Aulonemia clarkiae and A. fulgor. Judziewicz et al. (1999) indicated that the diagnostic feature of Guaduinae is the usual presence of abundant stomates on both adaxial and abaxial foliage leaf blade surfaces, often combined with the presence of refractive papillae associated with adaxial stomates whereas papillae are usually absent from the abaxial surface. Our study found the same characters defining the subtribe (characters 51 and, 53). The Guaduinae clade was also supported by having an almost solid style, a short rachis extension, and oral setae present in culm and foliage leaves, which were lost in Guadua. Solid style has not been examined for most other bamboo groups and needs further analysis. Previous phylogenetic studies of subfamily Bambusoideae and Bambuseae have included only Otatea acuminata to represent the genus. This species was sister either to Guadua paniculata, to a clade formed by Guadua-Apoclada, or to the Rhipidocladum-Glaziophyton clade (Kelchner and Clark 1997; Guala et al. 2000; Zhang and Clark 2000). However, Olmeca and Eremocaulon, the other genera in Guaduinae, have not been sampled before. The total evidence analysis retrieved a subclade in which Otatea species is monophyletic but it did not resolve which of the genera in Guaduinae is the most closely related to Otatea. Olmeca recta and O. reflexa resulted in a subclade. Olmeca is an interesting genus that is restricted to a few localities of tropical forests in southeastern Mexico. According to our results the Olmeca subclade is supported by a single synapomorphic character: a baccoid caryopsis. Moreover, the distributions of Olmeca and Otatea coincide in this area of southeastern Mexico. The Otatea clade received moderate jackknife and strong Bremer support, and was diagnosed by the character states of three subequal, ascending branches and pubescent lemmas. 40 The first character was previously recognized as diagnostic when Calderón and Soderstrom (1980) described the genus and our results confirm this. The combination of all three datasets retrieved more resolved trees than the individual analysis of every data set did, stressing the importance of total evidence analysis (Clark et al. 2006; Wortley and Scotland, 2006). Morphological and molecular matrices were combined although the ILD test determined incongruence between the two data sets for two reasons. The first is that it has been pointed out that incongruence is related to the number of variable characters in every matrix (Cunningham, 1997). In this study, the molecular matrix included 30 parsimony informative characters, while the morphological matrix included 61 parsimony informative characters. Therefore the difference in variable characters between the two data sets was enormous. The second reason for merging data matrices is that a combined analysis can improve the estimate by increasing the number of informative characters, revealing groups not seen in the trees from the separate data sets (Chippindale and Wiens 1994). Moreover, secondary phylogenetic signals emerge from both molecular and morphological characters sets when they are combined (Nixon and Carpenter 1996). Southeastern Mexico is a common area of distribution of some populations of the four species of Otatea, of Olmeca recta, O. reflexa and Aulonemia clarkiae and A. fulgor (Fig. 4). Five of the eight species are endemic to Mexico, and A. clarkiae is known only from two populations in Mexico and Nicaragua. The account of terrestrial protected areas of Mexico by Arriaga et al. (2000) included four areas localized in Pleistocene refuges (Toledo 1988; Wendt 1993): the Pico de Orizaba – Cofre de Perote region in central Veracruz, the Los Tuxtlas region in southern Veracruz, the Sierra de Juarez in Oaxaca, and the Uxpanapa region in the junction of Chiapas, Oaxaca and Veracruz (Fig. 4). The eight taxa are found in these areas. Some of these 41 species possess interesting characters such as a baccoid caryopsis and long culm necks, and grow as well in threatened vegetation types such as cloud, tropical and tropical deciduous forests, therefore they are an important group of bamboos to preserve. Future studies including additional plastid and nuclear genomic markers will resolve the phylogenetic position of Otatea within Guaduinae. In addition, analyses considering more taxa from Arthrostylidiinae and Chusqueinae will further test the apparent close relationship between the Arthrostylidiinae and Guaduinae, and clarify their relationship to other tropical woody bamboo subtribes, including the Chusqueinae. These studies are being addressed in the Bamboo Phylogeny Project (Bamboo Phylogeny Group, 2006). ACKNOWLEDGEMENTS. We are grateful to Lynn Clark for providing reviews that improved this manuscript singnificantly as well as unpublished material and micrographs. We are grateful to Aarón Rodríguez for his review. We thank Gilberto Cortés and Ana Paula Santos-Gonçalves for providing plant material. We are grateful to Pablo Carrillo-Reyes, Arturo de Nova, Flor Rodríguez-Gómez, José Luis Martínez, Nelly Jiménez-Pérez, Jaime Pacheco and Xóchitl Galarza for their assistance with field work. Bianca Delfosse edited the English version of the manuscript. We thank Cristina Bárcenas for her help in lab work. We thank the curators of the following herbaria for access to their collections and the loan of specimens: ISC, MEXU, MO, NY, US and XAL. Field work was supported by a graduate student grant of the Instituto de Ecologia, A. C., by a grant of the student assistance program of BOTA “Bamboos of the Americas”, by a grant provided by “Red Lationamericana de Botanica” (RLB07-ATP01) and also by a student grant from the International Association for Plant Taxonomists. A fellowship to ER-S by CONACYT (190069) is also acknowledged. 42 LITERATURE CITED ARRIAGA, L., J. M. ESPINOZA, C. AGUILAR, E. MARTÍNEZ, L. GÓMEZ and E. LOA. 2000. Regiones terrestres prioritarias de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México. BAMBOO PHYLOGENY GROUP. 2006. The Bamboo Phylogeny Project. BAMBOO, The Magazine of the American Bamboo Society, December 2006, Vol. 27 (6): 11-14. BREMER, K. 1994. Branch support and tree stability. Cladistics 10: 295-304. CALDERÓN, C. E. and T. R. SODERSTROM. 1980. The genera of Bambusoideae (Poaceae) of the American continent: Keys and comments. Smithsonian Contributions to Botany 44: 1-27. CHIPPINDALE, P. T. and J. J. WIENS. 1994. Weighting, partitioning, and combining characters in phylogenetic analysis. Systematic Biology 43: 278-287.CLARK, L. G. and G. CORTÉS. 2004. A new species of Otatea from Chiapas, Mexico. Bamboo Science and Culture 18: 1-6. , S. DRANSFIELD, J. TRIPLETT and J. G. SÁNCHEZ-KEN. 2007. Phylogenetic relationships ——— among the one-flowered, determinate genera of Bambuseae (Poaceae: Bambusoideae). Columbus, J. T. et al. (eds.), Proceedings of the 4th International Conference on Grass Diversity and Evolution, Rancho Santa Ana Botanic Garden, Claremont, California. CLARK, J. L., P. S. HERENDEEN, L. E. SKOG, and E. A. ZIMMER. 2006. Phylogenetic relationships and generic boundaries in the Episcieae (Gesneriaceae) inferred from nuclear, chloroplast, and morphological data. Taxon 55: 313-336. COTA-SÁNCHEZ, H. J., K. REMARCHUK, and K. UBAYASENA. 2006. Ready-to-use DNA extracted with a CTAB method adapted for herbarium specimens and mucilaginous plant tissue. Plant Molecular Biology Reporter 24: 161-167. 43 CUNNINGHAM, C. W. 1997. Can three incongruence tests predict when data should be combined? Molecular Biology and Evolution 14: 733-740. DAVIDSE, G. and W. P. POHL. 1992. New taxa and nomenclatural combinations of Mesoamerican grasses (Poaceae). Novon 2: 81-110. DOYLE, J. J. and J. L. DOYLE. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochemical Bulletin 19: 11-15. DUMOLIN-LAPÈGUE, S., M. H. PEMONGE, and R. J. PETIT. 1998. Association between chloroplast and mitochondrial lineages in oaks. Molecular Biology and. Evolution 15: 1321-1331. FARRIS, J. S., M. KÄLLERSJÖ, A. G. KLUGE, AND C. BULT. 1994. Testing significance of incongruence. Cladisticis 10: 315-319. , V. A. ALBERT, M. KÄLLERSJÖ, D. LIPSCOMB, and A. G. KLUGE. 1996. Parsimony ——— jackknifing outperforms neighbor-joining. Cladistics 12: 99-124. GOLOBOFF, P. 1999. NONA (NO NAME) ver. 2 Published by the author, Tucumán, Argentina. GUALA, G. F. 2003. A new genus of bamboo from Cerrados of Brazil (Poaceae: Bambusoideae). Bamboo Science and Culture 17: 1-3. , D. BOGLER, J. SADLE, and J. FRANCISCO-ORTEGA. 2000. Molecular evidence for polyphyly ——— in the genus Apoclada (Poaceae: Bambusoideae). Bamboo Science and Culture 14: 1520. GUO, Z. H., Y. Y. CHEN, D. Z. LI, and J. B. YANG. 2001. Genetic variation and evolution of the alpine bamboos (Poaceae: Bambusoideae) using DNA sequence data. Journal of Plant Research 114: 315-322. 44 , , and D. Z. LI. 2002. Phylogenetic studies on Thamnocalamus group and its allies ——— ——— (Bambusoideae: Poaceae) based on ITS sequence data. Molecular Phylogenetics and Evolution 2: 20-30. , and D. Z. LI. 2004. Phylogenetics of the Thamnocalamus group and its allies (Gramineae: ——— Bambusoideae) inferred from the sequences of GBSSI gene and ITS spacer. Molecular Phylogenetics and Evolution 30:1-12. GUZMÁN, R., M. C. ANAYA, and M. SANTANA. 1984. El género Otatea (Bambusoideae), en México y Centroamérica. Boletín del Instituto de Botánica 5: 2-20. HILU, K. W. and J. L. RANDALL. 1984. Convenient method for studying grass leaf epidermis. Taxon 33: 413-415. HODKINSON, T. R., S. A. RENVOIZE, G. N. CHONGHAILE, C. M. A. STAPLETON, and M. W. CHASE. 2000. A comparison of ITS nuclear rDNA sequence data and AFLP markers for phylogenetic studies in Phyllostachys (Bambusoideae, Poaceae). Journal of Plant Research 113: 259-269. HOLDREGGER, R. and R. J. ABBOTT. 2003. Phylogeography of the Arctic-Alpine Saxifraga oppositifolia (Saxifragaceae) and some related taxa based on cpDNA and ITS sequence variation. American Journal of Botany 90: 931–936. JUDZIEWICZ, E. J., L. G. CLARK, X. LONDOÑO, and M. J. STERN. 1999. American Bamboos. New York: Smithsonian Institution Press. KELCHNER, S. A. 2000. The evolution of non-coding chloroplast DNA and its application in plant systematics. Annals of the Missouri Botanical Garden 87: 482-498. . AND J. F. WENDEL. 1996. Hairpins create a minute inversions in non-coding regions of ——— chloroplast DNA. Current Genetics 30: 259-262. 45 . and L. G. CLARK. 1997. Molecular evolution and phylogenetic utility of the chloroplast ——— rpl16 intron in Chusquea and the Bambusoideae (Poaceae). Molecular Phylogenetics and Evolution 8: 385-397. KRESS, W. J., K. J. WURDACK, E. A. ZIMMER, L. A. WEIGT, and D. H. JANZEN. 2005. Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of. Sciences USA 102: 8369-8374. LONDOÑO, X. and L. G. CLARK. 1998. Eight new taxa and two new reports of Bambuseae (Poaceae: Bambuseae) from Colombia. Novon 8: 408-428. . and ———. 2002. A Revision of the Brazilian bamboo genus Eremocaulon (Poaceae: ——— Bambuseae: Guaduinae). Systematic Botany 27: 703-721. MADDISON, W. P., and D. R. MADDISON. 2006. Mesquite: A modular system for evolutionary analysis. Version 1.1. http://mesquiteproject.org MCCLURE, F. A. 1973. Genera of bamboos native to the New World. Smithsonian Contributions to Botany 9: 1-148. MILLER, J. T., J. W. GRIMES, D. J. MURPHY, R. J. BAYER, and P. Y. LADIGES. 2003. A phylogenetic analysis of the Acacieae and Ingeae (Mimosoideae: Fabaceae) based on trnK, matK, psbA-trnH, and trnL/trnF sequence data. Systematic Botany 28: 558-566. NIXON, K. C. 1999-2002. WinClada ver. 1.0000 Published by the author, Ithaca, NY, USA. . and J. CARPENTER. 1996. On simultaneous analysis. Cladistics 12: 221-241. ——— RAMBAUT, A. 2002. Se-Al Sequence Alignment Editor, v2.0a11. Department of Zoology, University of Oxford, Oxford. ROGERS, S. O. and A. J. BENDICH. 1985. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Molecular Biology 5: 69-76. 46 SHAW, J., E. LICKEY, J. T. BECK, S. B. FARMER, W. LIU, J. MILLER, K. C. SIRIPUN, C. T. WINDER, E. E. SCHILLING, and R. L. Small. 2005. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany 92: 142-166. SIMMONS, M. P. and H. OCHOTERENA. 2000. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49: 369-381. SODERSTROM, T. R. 1988. Aulonemia fulgor (Poaceae: Bambusoideae), a new species from Mexico. Brittonia 40: 22-31. . and R. P. ELLIS. 1987. The position of bamboo genera and allies in a system of grass ——— classification. Pp. 225-238 in: Grass systematics and evolution, eds. Soderstrom, T. R., K. W. Hilu, C. S. Campbell, and M. E. Barkworth. Washington, D. C.: Smithsonian Institution Press. SORENG, R. J. and J. I. DAVIS. 1998. Phylogenetics and character evolution in the grass family (Poaceae): simultaneous analysis of morphological and chloroplast DNA restriction site character sets. The Botanical Review 64: 1-85. STAPLETON, C. M. A. 1997. The morphology of woody bamboos. Pp. 251-267 in: The Bamboos, ed. Chapman, G. P. London: Linnean Society of London Symposium Series. Academic Press. SUN, Y., N. XIA, and R. LIN. 2005. Phylogenetic analysis of Bambusa (Poaceae: Bambusoideae) based on Internal Trascribed Spacer sequences of nuclear ribosomal DNA. Biochemical Genetics. 43: 603-612. TATE, J. A. and B. B. SIMPSON. 2003. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Systematic Botany 28: 723-737. 47 TOLEDO V. M. 1988. La diversidad biológica de México. Ciencia y Desarrollo 14: 17-30. WENDT, T. 1993. Composition, floristic affinities, and origins of the canopy tree flora of the Mexican Atlantic slope rain forests. Pp. 595-680 in: Biological Diversity of Mexico: Origins and Distribution, eds. Ramamoorthy,T. P., Bye R. Lot, A. and J. Fa. Oxford Univ. Press, Oxford. WORTLEY, A. H. and R. W. SCOTLAND. 2006. The effect of combining molecular and morphological data in published phylogenetic analyses. Systematic Biology 55: 677-685. ZHANG, W. 2000. Phylogeny of the grass family (Poaceae) from rpl16 intron sequence data. Molecular Phylogenetics and Evolution 15: 135-146. , and L. G. CLARK. 2000. Phylogeny and classification of the Bambusoideae (Poaceae). Pp. ——— 53-42 in: Grasses: systematics and evolution, eds. S. W. L. Jacobs and J. E. Everett. Collingwood, Victoria: CSIRO Publishing. 48 APPENDICES APPENDIX 1. Taxa used in the phylogenetic study of Otatea, selected specimens for morphological analysis, and vouchers, GenBank accession numbers for sequences obtained in this project as well as for the previously published sequences of the DNA sequences used in this paper. GenBank accessions correspond to rpl16 and trnH-psbA; if there is only one accession it corresponds to rpl16. Apoclada simplex McClure & L.B.Sm., Brazil: L. Clark, X. Londoño & W. de Oliveira 1027 (ISC, MO, US); L. Clark & W. de Oliveira 898 (ISC, US); G. Davidse, T. R. Ramamoorthy & D. V. Vital 10903 (MO); P. Dusén 17506 (MO, NY); J. R. Swallen 8270 (MO, NY); J. Valls, H. Longhi & A. Barcellos 3113 (MO, US); R. M. Klein 4108 (NY, US); X. Londoño & L. Clark 695 (US); R. M. Klein 4657 (US); A. Castellaños 3461 (US); R. M. Klein 7779 (US); R. M. Klein & M. M. Klein 11059 (US). Arthrostylidium ecuadorense E.J.Judziewicz & L.G.Clark, Ecuador: B. Lojtnant, U. Molau & M. Madison 12588 (MO); B. Löjtnant, U. Molau & M. Madison 12469 (MO); S. Lægaard 55168 (MO, NY); L. Clark, S. Lægaard & P. Stern 1101 (US); L. B. Holm-Nielsen, J. Jaramillo, F. Coello & E. Asanza 27037 (US); S. Lægaard 53778 (US); J. Cuatrecasas 11878 US. AY912189 Clark et al (in press). Atractantha radiata McClure, Brazil: A. M. de Carvalho, L. Clark, W. W. Thomas & J. Kallunki 4362 (ISC); A. M. de Carvalho, L. Clark & W.W. Thomas 4387 (ISC); F. O. Zuloaga 2476 (MO, US); C. E. Calderón, T. S. dos Santos & L. de Oliveira 2397 (MO, NY, US); C. E. Calderón, T. S. dos Santos & L. de Oliveira 2452 (MO); W. W. Thomas, J. Pirani, J. Kallunki & I. Cordeiro 10370 (MO); T. S. Santos 2928 (MO, US); C. E. Calderón & T. S. dos Santos 2485 (MO, NY, US); F. O. Zuloaga, G. Marinelli & J. Caruso 2465 (MO, US); T. S. Santos & T. R. Soderstrom & al. 3913 (MO); C. E. Calderón & T. S. dos Santos 2479 (MO, NY); C. E. Calderón 2474 (MO); T. S. Santos & T. 49 R. Soderstrom & al. 3914 (MO, NY); C. E. Calderón 2454 (MO, NY); X. Londoño, T. dos Santos y S. Sant'Ana 723 (US). AY912190 Clark et al. (in press). Aulonemia clarkiae Davidse & R.W.Pohl, Honduras: G. Davidse & R. Zuniga 34651 (ISC, MO). Mexico: D.E. Breedlove 58512 (US); G. Cortés s/n (MEXU) (EF589612, EF589628). Aulonemia fulgor Soderstr., Mexico: L. Clark, M. Cházaro, P. Tenorio & G. Bol 454 (ISC, MO, NY); L. Clark, G. Cortés, P. Tenorio & L. Hornberger 1143 (ISC, MO, NY, US); W. Ruiz 101 (MO). G. Cortés 324 (MO); G. Davidse, M. Souza, O. Téllez, E. Martínez & J. Davidse 30258 (MO, US); L. G. Clark, M. Cházaro, P. Tenorio & G. Bol 458 (MO, NY); Rzedowski 33350a (US); J. Bauml & M. Kimnach 491 (US); A. A. Beetle 5025 (US); A. A. Beetle 5027 (US); S. D. Koch 75549 (US); T. MejiaSaulés. 2018 (XAL) (EF589613, EF589629). Aulonemia laxa (F.Maek.) McClure, Mexico: L. Clark, P. Tenorio & G. Bol 485 (ISC, MO, US); C. Santiz 252 (MO, NY); D. E. Breedlove 28181 (NY); S.D. Koch & P.A. Fryxell 82133 (NY, US); R. Ortega 1113 (US); T. Tateoka 1164 (US); G. N. Ross 144 (US); G. Cortés 311 (MEXU) (EF589614, EF589630). Nicaragua: T.B. Croat 43057 (US). Aulonemia patula (Pilg.) McClure, Colombia: X. Londoño & L. Clark 385 (MO, US); (US); M. L. Bristol 819 (US). Ecuador: L. Clark, C. Calderón & B. Treviño 324 (ISC, MO); L. Clark, C. Calderón & E. Azanza 306 (ISC, NY); L. Clark & P. Asimbaya 1482 (ISC, MO); P.M. Peterson, E. J. Judziewicz, R. M. King & P.M. Jørgensen 9207 (MO); S. Lægaard 52399, 53030 (MO, US); L. Clark, S. Lægaard & P. Stern 1075 (MO, US); L. Clark, H. Navarrete, A. Freire & K. Romoleroux 1135 (MO, US); S. M. Young 121, 122, 127 (MO); P. M. Peterson & C. R. Annable 9010 (MO); L. Clark, R. Townsend, G. Reiners & F. Santiana 1639 (MO); F. A. McClure 21419, 21423(MO). AY912191 Clark et al. (in press). Bambusa vulgaris Schrad. ex J.C. Wendl., Mexico: J. L. Martínez y Pérez s/n (XAL); AY912192 Clark et al. (in press); E. Ruiz-Sánchez 158 (XAL) (EF589631). Chusquea bilimekii E.Fourn., Mexico: L. 50 Clark, M. Cházaro, P. Tenorio & G. Bol 455 (MO, NY); C. Cortés, V. Luna, G. Cooper & J. Mora 345 (MO); N. Santacruz & R. Acosta 901 (MO); U54757 Kelchner and Wendel, 1996; T. Mejia-Saulés 2012 (XAL) (EF589632). Eremocaulon asymmetricum (Soderstr. & Londoño) Londoño, Brazil: A. M de Carvalho, L. Clark, W. W. Thomas, J. Kallunki & S. Sant´Ana 4372 (ISC, US); X. Londonño., A. Carvalho & S. Sant'Ana 740 (MO, US); T. R. Soderstrom & G. Martinelli 3898 (US); Santos-Gonçalves 595 (UEC, XAL) (EF589615, EF589633). Eremocaulon aureofimbriatum Soderstr. & Londoño, Brazil: C. E. Calderón & R. S. Pinheiro 2234 (ISC, US); X. Londoño, T. dos Santos & S. Sant´Ana 734 (ISC, MO); C. E. Calderón, T. S. Santos & L. de Oliveira 2396 (US); Santos-Gonçalves 590 (UEC, XAL) (EF589616, EF589634). Glaziophyton mirabile Franch., Brazil: AF133471 Zhang, 2000. Guadua aculeata Rupr. ex E.Fourn., Guatemala: F.A. McClure 21572 (ISC, US). Honduras: F. A. McClure 21556 (US). Mexico: L. Clark & P. Tenorio 949 (ISC, US). Nicaragua: F. Ortiz 2059 (ISC, MO). Panama: P. H. Allen 300 (US); J. Pale Pale 23 (XAL) (EF589617, EF589635). Guadua amplexifolia J.Presl, Colombia: X. Londoño & L. Clark 473 (ISC, MO, NY). Costa Rica: U. Chavarria 766 (MO, US). Ecuador: F. A. McClure 21382 (MO). Mexico: L. Clark, P. Tenorio & G. Bol 474 (ISC, MO). Nicaragua: F. A. McClure 21479 (US). Panama: P. H. Allen 4039 (ISC, US). Venezuela: G. S. Bunting 9200 (MO, NY, US); J. Pale Pale 24 (XAL) (EF589618, EF589636). Guadua angustifolia Kunth, Colombia: X. Londoño 964 (US). Ecuador: L. Clark, S. Lægaard & P. Stern 1095 (ISC). Peru: F. A. McClure 21464 (MO). Trinidad: C. D. Adams 14710 (NY). Venezuela: R. Liesner & A. González 10663 (MO, US); AY912198 Clark et al. (in press); E. Ruiz-Sánchez 157 (XAL) (EF589637). Guadua longifolia (E.Fourn.) R.W.Pohl, Belice: T. B. Croat 23424 (MO); C. L. Lundell 3870 (MO)Guatemala: A. Molina 15876 (US). Mexico: L. Clark, I. Calzada & D. Farrar 1314 (ISC, MO, US); T. Mejia-Saulés 2020 (XAL) (EF589619, EF589638). 51 Guadua paniculata Munro, Bolivia: W. W. Thomas., P. Betella & A. Centurion 5659 (MO); T. Killeen 752. Brazil: H.S. Irwin & T. R. Soderstrom 7187 (ISC, MO). Costa Rica: R.W. Pohl & R. Pinette 13239 (MO, US). Guatemala: J. A. Steyermark 51248 (US). (MO). Mexico: L. Clark, P. Tenorio & G. Bol 464 (MO). Nicaragua: S. B. Robbins 5827 (MO); E. Ruiz-Sánchez 95 (XAL) (EF589620, EF589639). Guadua velutina Londoño & L.G.Clark, Mexico: L. G. Clark & P. Tenorio 946 (ISC, MO); G. Cortés & F. Aguilar 79 (US); T. Mejia-Saulés 2021 (XAL) (EF589621, EF589640). Neurolepis aperta (Munro) Pilg., Colombia: C. E. Calderon., L. Clark & J. Cavalier 2994 (US, NY). Ecuador: S. Lægaard 55191 (MO, US, NY). Venezuela: L.J. Dorr, E. Briceno, G. Briceno & R. Caracas 8491 (MO, NY). U62793 Kelchner and Clark, 1997. Olmeca recta Soderstr., Mexico: M. Nee 29749 (ISC, MO, US); T.R. Soderstrom 2235 (US); L. G. Clark, G. Cortés, I. Calzada & D. Farrar 1313 (ISC, US); E. Ruiz-Sánchez 132 (XAL) (EF589622, EF589643). Olmeca reflexa Soderstr., Mexico: T. R. Soderstrom 2243 (ISC, US); L. Clark, P. Tenorio & G. Bol 467 (ISC, MO); G. Cortés & W. Sánchez 312 (MO); E. RuizSánchez 117 (XAL) (EF589623, EF589644). Otatea acuminata (Munro) C. E.Calderón & Soderstr., Mexico: Liebmann 127 (US); F. A. McClure 21204 (ISC); D. E. Breedlove 27177 (NY); L. Clark, P. Tenorio, M. Cházaro & G. Bol 450 (ISC, US, NY); R. Guzmán 6122 (ISC, US); AF133473 Zhang, 2000; E. Ruiz-Sánchez 112 (XAL) (EF589645). Otatea fimbriata Soderstr., El Salvador: F. A. McClure 21617 (ISC, US, MO, NY). Colombia: X. Londoño, A. Amaya, J. Jácome y M. V. Forgioni 884 (US, NY). Mexico: L. Clark, P. Tenorio & G. Bol 469 (ISC, US, MO, NY); R. Guzmán 6113 (US); D. E. Breedlove 28085 (US, NY); E. Ruiz-Sánchez 118 (EF589624, EF589641), 130 (XAL). Otatea glauca L.G.Clark & G.Cortés, Mexico: G. Cortés & W. Sánchez 306 (ISC); L. Clark, P. Tenorio & G. Bol 481 (US, NY); E. Ruiz-Sánchez 144 (XAL) (EF589625, EF589642). Otatea sp. nov Mexico: P. Carrillo-Reyes 5144 (XAL) 52 (EF589626); E. Ruiz-Sánchez 147 (XAL) (EF589646). Rhipidocladum racemiflorum (Steud.) McClure, Brazil: L. S. Sarahyba., L. Clark & M. Alves da Silva (1062) (NY). Bolivia: M. Saldias & A. Veliz 4333(NY). Colombia: X. Londoño & L. G. Clark 396 (MO). Costa Rica: R. W. Pohl 15683 (MO, NY). Ecuador: W. H. Camp 3814 (MO, NY). El Salvador: A. Monro, K. Sidwell, G. Davidse & C. Ramirez 1911 (MO). Guatemala: P. C. Standley 77650 (MO). Honduras: A. Molina & A. R. Molina 27900 (MO, NY). Mexico: L. Clark, F. Santana, P. Tenorio & L. Hornberger 1152 (MO). Venezuela: G. Davidse, O. Huber & S.S. Tillet 17119 (MO); E. Ruiz-Sánchez 128 (XAL); J. Pale Pale 15 (XAL) (EF589627, EF589647). 53 APPENDIX 2. Morphological characters used in analyses based in the Bamboo Phylogeny Group. Only characters marked with * were based in Londoño and Clark (2002). Rhizomes and Culms: 1. Culm neck development: 0 = short (neck ≤ 1/2 rhizome length); 1 = long (neck > 1/2 rhizome lenght). 2. Habit: 0 = erect; 1 = apically arching/pendulous; 2 = clambering/scandent; 3 = twining; 4 = decumbent. 3. Culm internodes: 0 = all solid (at least when young); 1 = all hollow; 2 = some proximal internodes (including the basalmost ones) solid, distal internodes hollow. 4. Wall thickness (ratio of 2X wall thickness:culm diameter): 0 = walls very thin (ratio up to 0.15); 1 = walls thin (ratio 0.16-0.30); 2 = walls moderately thick (ratio 0.31-0.45); 3 = walls thick (ratio 0.46-0.60); 4 = walls very thick (ratio 0.61-0.99). Nodes, Buds and Branching: 5. Vegetative culm branching: 0 = present; 1 = absent. 6. Supranodal ridge: 0 = inconspicuous (a line, diameter less than at the nodal line); 1 = conspicuous (a ridge, diameter equal to or greater than at the nodal line). 7. Aerial roots: 0 = absent; 1 = present on the lower nodes only; 2 = present on lower and upper nodes. *8. Infra- and supranodal bands of hairs: 0 = absent; 1 = present. 9. Compression of the proximal internodes of the primary axis: 0 = no compressed internodes present; 1 = one compressed proximal internode present at the basalmost portion; 2 = two to several compressed proximal internodes at the base; 3 = all internodes compressed. 54 10. Relative sizes of secondary branches developing from the primary axis: 0 = secondary axes subequal to the central primary axis; 1 = at least some of the secondary axes no more than one-half the diameter of the central axis. 11. Primary axis size relative to the main culm: 0 = more or less equal in diameter; 1 = primary axis smaller in diameter than the main culm. 12. Thorns developing from the primary axis (or central primary axis): 0 = absent; 1 = present. 13. Bud/branch complement base: 0 = indistinguishable from the adjacent nodal region (promontory absent); 1 = swollen, forming a promontory that bears the bud/branch complement. *14. Branch complement: 0 = 1 divergent branch; 1 = 1 dominant divergent bearing few to several, small 2° branches basally; 2 = 2 divergent, subequal branches; 3 = 3 subequal, ascending branches; 4 = numerous secondary branches. Culm Leaves: 15. Girdle: 0 = absent or poorly developed; 1 = present as a band at least 1 mm wide, no flap, prominent or not; 2 = prominent, with or without a flap covering the bud complement. 16. Abaxial sheath surface: 0 = stiff, dark, irritating hairs present; 1 = only soft hairs present; 2 = glabrous, no hairs present; 3 = scabrous. 17. Sheath apex (or summit or shoulders) indument: 0 = glabrous; 1 = fimbriate. 18. Oral setae: 0 = absent; 1 = present, whether adnate to the inner ligule or not. 19. Culm leaf blade position: 0 = erect to slightly spreading; 1 = reflexed. 20. Culm leaf blade shape: 0 = broadly triangular; 1 = narrowly triangular; 2 = lanceolate (pseudopetiolate). 55 21. Culm leaf blade midrib abaxial development: 0 = indistinguishable; 1 = visible or even prominent toward the apex. 22. Auricle (blade-derived appendage) development: 0 = absent; 1 = present and contiguous with the base of the blade; 2 = present on the sheath apex but not contiguous with the blade. 23. Auricle size: 0 = auricles more or less equal on both sides of the blade base; 1 = strongly unequal, at least 2 times as large (or long) on one side as on the other side. 24. Auricle indument: 0 = glabrous or ciliate; 1 = fimbriate. Foliage Leaves: 25. Blade-derived appendages on the sheath summit: 0 = no true auricles or fimbriae (glabrous); 1 = efimbriate auricles present; 2 = fimbriate auricles present; 3 = fimbriae only present; 4 = cilia (or tufts of cilia) present. 26. Sheath: 0 = rounded on the back; 1 = strongly keeled at least near the apex. 27. Foliage leaf blade: 0 = abaxial marginal green stripe absent; 1 = abaxial marginal green stripe present. 28. Midrib placement: 0 = centric; 1 = excentric (wider side of the blade > 1.3 times as wide as the narrower side). 29. Oral setae: 0 = absent; 1 = present. Synflorescences: 30. Gemmiparous bracts subtending the spikelet proper: 0 = absent; 1 = present, buds developing subsequently or not. 31. Subtending bracts at the base of the first- (lowermost) and/or second-order paraclades: 0 = absent; 1 = present, as a scar/rim or scale-like, blade absent, a few mm long; 2 = present, well developed, with sheath and blade (modified). 56 32. Prophylls at the base of the first- or second-order paraclades: 0 = absent; 1 = present. Spikelets: 33. Compression: 0 = terete; 1 = lateral; 2 = dorsal. 34. Number of glumes (in female-fertile spikelets or spikelets proper): 0 = absent; 1 = one; 2 = two; 3 = three; 4 = four; 5 = five or six. 35. Awns on the lower two glumes: 0 = absent; 1 = present. 36. Number of female-fertile florets per spikelet or spikelet proper: 0 = one; 1 = two or more. 37. Rachis extension (internode only, with or without rudimentary spikelet): 0 = absent; 1 = present and short (< floret); 2 = present and long (> floret). 38. Rachis extension (internode only): 0 = glabrous; 1 = hairy. 39. Lemma indument: 0 = glabrous (glabrescent); 1 = scabrous; 2 = densely hispid; 3 = hispid only near the apex; 4 = pubescent (all or in part). 40. Palea indument (excluding the sulcus): 0 = glabrous; 1 = scabrous; 2 = pubescent; 3 = hispid. 41. Sulcus indument: 0 = glabrous; 1 = pubescent; 2 = scabrous. *42. Palea keel wings: 0 = absent; 1 = present. Flower: 43. Lodicule margin pubescence: 0 = ciliate (or ciliolate); 1 = glabrous (entire). 44. Stamen number: 0 = two; 1 = three; 2 = six; 3 = > 6. 45. Style proper length: 0 = absent (including extremely short, < 0.1 mm); 1 = elongated > 0.1 mm up to the length of the ovary; 2 = elongated and greater than the length of the ovary. 46. Style proper pubescence: 0 = glabrous; 1 = pubescent. 57 47. Style proper core: 0 = hollow; 1 = solid. 48. Stigma number: 0 = three; 1 = two. 49. Stigma branching: 0 = very branched and plumose (2 or more orders of branching); 1 = limited branching/simple, hispid (1 order of branching). Fruit: 50. Fruit type: 0 = dry caryopsis; 1 = baccoid. Foliar Micromorphology: 51. Papillae over long cells in stomatal zone (abaxial): 0 = absent; 1 = present. 52. Papillae over long cells in stomatal zone (abaxial): 0 = simple; 1 = branched; 2 = simple and branched. 53. Papillae over the long cells in interstomatal zone (abaxial): 0 = absent; 1 = present. 54. Papillae over adaxial surface: 0 = absent; 1 = present only on bulliform cells; 2 = present only on long cells; 3 = present on both bulliform and long cells. 55. Papillae over subsidiary cells of the stomatal apparatus: 0 = absent; 1 = present and simple; 2 = present and branched. 56. Stomates on foliage leaf blades: 0 = present and common only on the abaxial surface; 1 = present and common on both surfaces; 2 = present only on adaxial surface. 57 Silica bodies, vertical tall and narrow (abaxial, intercostal): 0 = present; 1 = absent. 58 Silica bodies saddle-shaped (abaxial, intercostal): 0 = present; 1 = absent. 59. Silica bodies vertical tall and narrow (abaxial, costal): 0 = present; 1 = absent. 60. Silica bodies saddle-shaped (abaxial, costal): 0 = present; 1 = absent. 61. Silica bodies horizontal dumbbell-shaped (abaxial, costal): 0 = present; 1 = absent. 58 APPENDIX 3. Morphological data matrix of 61 characters. “?” = character not observable, “-” = inapplicable. Polymorfism: A = (0,1); B = (1,2); C = (2,3); E (0,1,2); G (0,2); I (0,3) All species names are listed with authorship in Appendix 1. Characters states and descriptions are listed in Appendix 2. Apoclada simplex Arthrostylidium ecuadorense Atractantha radiata Aulonemia clarkiae Aulonemia fulgor Aulonemia laxa Aulonemia patula Bambusa vulgaris Chusquea bilimekii Eremocaulon asymmetricum Eremocaulon aureofimbriatum Glaziophyton mirabile Guadua aculeata Guadua amplexifolia Guadua angustifolia Guadua longifolia Guadua paniculata Guadua velutina Neurolepis aperta Olmeca recta Olmeca reflexa Otatea glauca Otatea acuminata Otatea fimbriata Otatea sp. nov Rhipidocladum racemiflorum 0000000001 1234567890 0114000010 020-001011 02G3000011 0112010021 0113010021 0112011011 0112011011 0113010021 000-001021 020-010021 0112010121 A01?1000-0112010121 0112010121 0123010121 0122010021 A113010121 0112000121 000-1010-1111011011 1223011011 0111000020 01A2000020 0113000020 0113000020 0111000031 1111111112 1234567890 1012020001 1011131012 1011120001 0011001111 0011001111 1010121012 1010121012 1001000000 1001020001 1011121111 1111000000 ----?20001 1111000000 1111000000 1111000000 1111020000 1111120000 1111000000 -0--2----0010000101 0010000111 1013000111 1013000A01 1013000101 1013001111 -004020000 59 2222222223 1234567890 00--300000 00--311000 00--301001 00--300010 00--300010 10--311000 10--311000 0100010001 00--000000 0201200111 00--000001 10--301001 00--000001 0210I00001 00--C00001 00--200001 00--300001 0210I00001 ----100000 00--300010 00--300010 00--300010 00--300000 00--300110 00--30001? 00--301000 3333333334 1234567890 0012111110 0012012000 1120-02000 0022111030 0022111000 0012112011 0012112110 1112010000 0024000-01 2110-11100 1110-11100 B11E012001 1110-11100 1110-11110 1110-11100 1110-11100 B110-11100 1110-11120 0024000-01 0012011000 0012011000 0012111141 0012111141 0012111141 ?????????? 0012112011 4444444445 1234567890 2001101110 0001?????? 0001110100 1001101110 1001101110 2001000100 2001110100 0002210010 1001000110 1002201100 1112111010 001110?100 1112111010 0112211010 1112111010 1111111010 0112211010 0112111010 -011000110 1001101011 1001101111 1001101110 1001101110 1001101110 ?????????? 1001210110 5555555556 1234567890 0-02020000 1212000101 1210000101 0-02011010 0-03011010 1210000101 1212000101 1012011010 1011101010 0-02011010 0-02010010 1012010110 0-02010010 0-02010010 0-02010010 0-02010010 1013010010 0-02010010 1010110000 0-02011010 0-02011010 0-02010110 1013010110 0-02010110 1012010110 1213011111 6 1 1 0 1 1 1 1 1 1 0 1 1 1 1 1 1 1 1 1 1 1 1 0 0 0 0 1 FIGURE LEGENDS FIG. 1. Strict consensus of six most-parsimonious trees inferred from analysis of the morphological data set L = 204 steps, CI = 42, RI = 64). Numbers below branches indicate jackknife/Bremer support. Character numbers are listed in Appendix 2. Filled circles are synapomorphic. Subtribe abbreviations: Gua = Guaduinae; Art = Arthrostylidiinae; Chu = Chusqueinae; Bam = Bambusinae. FIG. 2. Single most parsimonious tree based on rpl16 intron and trnH-psbA intergenic spacer combined sequence data (L = 48 steps, CI = 0.68, RI = 80). Numbers above branches indicate jackknife support, and numbers below indicate Bremer support. Subtribe abbreviations: Gua = Guaduinae; Art = Arthrostylidiinae; Chu = Chusqueinae; Bam = Bambusinae. FIG. 3. Strict consensus of 53 most-parsimonious trees inferred from analysis of the combined morphological and molecular data (L = 261, CI= 45, RI = 67). Numbers below branches indicate jackknife/Bremer support. Character numbers are as listed in Appendix 2. Filled circles are synapomorphies. .Subtribe abbreviations: Gua = Guaduinae; Art = Arthrostylidiinae; Chu = Chusqueinae; Bam = Bambusinae. FIG. 4. Distribution of the southern Otatea species, Olmeca erecta, O. reflexa, and Aulonemia fulgor and A. clarkiae in southeastern Mexico. Numbers indicated the terrestrial protected areas. 1. Pico de Orizaba – Cofre de Perote region in central Veracruz; 2. Los Tuxtlas 60 region in southern Veracruz; 3. Sierra de Juarez in Oaxaca; 4. Uxpanapa region in the junction of Chiapas, Oaxaca and Veracruz; 5. Bosques mesófilos en Los altos, Chiapas; 6. Cañon del Sumidero, Chiapas and 7. El Triunfo, Chiapas. 61 62 63 64 65 CAPÍTULO III. DELIMITANDO ESPECIES EN EL BAMBÚ NEOTROPICAL OTATEA (POACEAE: BAMBUSOIDEAE) USANDO DATOS MORFOLÓGICOS, MOLECULARES Y ECOLÓGICOS. Enviado a: Molecular Phylogenetics and Evolution. 2009 66 Delimiting species boundaries within the Neotropical bamboo Otatea (Poaceae: Bambusoideae) using molecular, morphological and ecological data Eduardo Ruiz-Sánchez* and Victoria Sosa Departamento de Biologia Evolutiva, Instituto de Ecologia, A. C., Apartado Postal 63, 91000 Xalapa, Veracruz, México. Apartado Postal 63, 91000 Xalapa, Veracruz, Mexico. Tel. (52) (228) 842-1874 Fax (52) (228) 818-7809 ruizSá[email protected]; [email protected] *Correspondence: Eduardo Ruiz-Sánchez, Departamento de Biologia Evolutiva, Instituto de Ecologia, A. C., Apartado Postal 63, 91070 Xalapa, Veracruz Mexico. E-mail: ruizSá[email protected]; [email protected] 67 Abstract Species delimitation is a task that has engaged taxonomists for more than two centuries. Recently, it has been demonstrated that molecular data and ecological niche modeling are useful in species delimitation. In this paper multiple data sets (molecular, morphological, ecological) were utilized to set limits for the species belonging to the Neotropical bamboo Otatea, because there is disagreement about species circumscriptions and also because the genus has an interesting distribution, with most of its populations in Mexico and a single disjunct population in Colombia. Molecular and morphological phylogenetic analyses recovered trees with conflicting topologies. Tree-based morphological and character-based analyses recognized the same entities. Ecological niche models and PCA/MANOVAS agreed with the recognition of the same entities that resulted from the morphological analyses. Morphological analyses retrieved clades supported by diagnostic characters and coherent geographical distributions. Based on these results seven entities should be recognized in Otatea, instead of the three previously described species. Key words: diagnostic characters, ecological niche modeling, molecular parsimony, morphology tree-based analysis, niche divergence, statistical parsimony network 68 Introduction Traditionally, species delimitation in the plant kingdom has been based on morphological differences. However, the inclusion of molecular and ecological data in recent years has provided more evidence for delimiting species. Sites and Marshall (2003, 2004), in their synopsis of operational criteria for delimiting species boundaries, designated two broad categories: treebased and non-tree-based methods. Among procedures for establishing species boundaries is the novel method proposed by Wiens and Penkrot (WP; 2002), which uses morphological and molecular data and tree-based and character-based analyses simultaneously. The WP method utilizes DNA haplotypes in parsimony analysis assuming a phylogeny of nonrecombining haplotypes which may show the focal species to be either exclusive or not exclusive. The treebased morphological analysis uses populations as terminals rather than individuals to avoid a biased treatment of the polymorphisms shared between populations as homoplasies rather than synapomorphies. Furthermore, the character-based analysis in the WP method involves finding diagnostic character states that represent differences among the putative species. The WP method considers strongly supported set of exclusive and geographically coherent populations to be potentially distinct species. In this paper this concept was expanded by performing niche modeling analyses. Current research considers niche modeling to be useful for identifying and diagnosing species (Raxworthy et al., 2007; Rissler and Apodaca, 2007; Stockman and Bond, 2007; Bond and Stockman, 2008) and for finding potential new species (Raxworthy et al., 2003). Niche modeling is able to provide evidence for geographic isolation among populations (based on either conserved or divergent ecological niches) and takes populations to be separately evolving lineages when gene flow is considered unlikely for the intervening geographic regions (Wiens and Graham, 2005). 69 A number of animal species were defined with the WP method (e.g. Hendrixson and Bond 2005; Leavitt et al. 2007; Mulcahy 2008) yet it has never been utilized with plants. It has been argued that polyploidy, asexual reproduction or hybridization are factors that solely affect evolutionary processes in plants (Rieseberg 1997; Mable 2004; Rieseberg and Willis 2007; Silvertwon, 2008; Soltis et al. 2007). However, Rieseberg et al (2006) have suggested that a lack of congruence between correspondence of plant and animal species could be in relation to these same factors but not by contemporary hybridization. Moreover, Rieseberg et al (2006) generalized that plant species are more likely than animal species to represent reproductively independent lineages. We selected the Neotropical bamboo Otatea (McClure & E. W. Sm) C. Calderón & Soderstr. as a test of the WP for delimiting plant species for a number of reasons. The first reason is that there is controversy on the recognition of two groups within O. acuminata, a variable species with the widest distribution in the genus. Initially Otatea was described with Otatea acuminata (Munro) C. Calderón & Soderstr. Later Otatea aztecorum was described but it is now considered to be a subspecies of O. acuminata (subsp. aztecorum). Therefore O. acuminata is the focal species in our analyses. Two additional species were later described, O. fimbriata Soderstr. and O. glauca L.G. Clark & G. Cortés, and a new, still undescribed species from Chiapas is also known (Guzmán et al., 1984; Judziewicz et al., 1999; Clark and Cortés, 2004; Ruiz-Sánchez et al., 2008). What is more, our intensive fieldwork has discovered an elevated morphological variation in populations from previously unexplored areas, which have not yet been characterized. The second reason to select this taxon, is that species in Otatea have allopatric distribution patterns and occupy different habitats, so that niche modeling could give new 70 insights to identify and diagnose taxa. Otatea acuminata is endemic to Mexico, from Sonora along of the Pacific slopes to Chiapas and in central Mexico along the Transvolcanic Belt in tropical dry forest and xerophytic scrubs. Otatea fimbriata has a disjunct distribution, collected from three areas in Mexico and Central America, and one record from Colombia (Londoño and Clark 1998) in dry pine-oak-juniper or even in dry tropical forests. O. glauca is endemic to Chiapas and only recollected from a single population, on slopes in the ecotone of tropical dry forests and oak forests. The undescribed species from Chiapas is currently only known from a single population in tropical dry forest. The third reason for selecting Otatea is because its plants are monocarpic with a mass flowering. Populations usually flower for two or three years, and then the plants die. Herbarium records indicate that the cycles of mass flowering last 17-30 years. Thus it is difficult to find flowering plants to analyze floral characters. Molecular evidence for specimens lacking inflorescences might increase the number of informative characters used in defining species boundaries, together with vegetative morphological characters. The main objective of this study is to set limits for the species belonging to Otatea using multiple data sets (molecular, morphological, and ecological). Four questions emerge from this objective: Do subspecies in O. acuminata merit species status? Could the disjunct population of O. fimbriata from Colombia be recognized as different? Can recently collected populations be assigned to previously described species or do they need to de described as new taxa? Does ecological niche modeling give new insights to identify and diagnose taxa? How many species should be recognized in the Neotropical bamboo genus Otatea? 71 Materials and Methods Taxon sampling Individuals of Otatea were collected during 2005-2007 throughout the entire range of distribution of the genus (Fig. 1). Olmeca recta Soderstr., a taxon closely related to Otatea, was used as the outgroup (Ruiz-Sánchez et al., 2008). A total of 109 individuals from 28 populations were sampled with three to twelve individuals per population (Fig. 1, Table 1). Fresh leaves were collected from each individual and dried in silica (Chase & Hills, 1991) and the vouchers were deposited at XAL. Morphological data set The morphological matrix included 54 characters (40 vegetative, six from synflorescences and flowers and eight leaf micromorphological characters (Appendix 1; data matrix in Appendix 2). Character selection was based on the list and illustrations by L. Clark in “Bamboo Biodiversity” (http://www.eeob.iastate.edu/research/bamboo) and on Londoño and Clark (2002). Characters were scored by examining live material and herbarium specimens. Vouchers and examined specimens are also listed in Appendix 3. Molecular data set DNA extraction, amplification, and sequencing.- DNA was isolated using the modified 2X CTAB method (Cota-Sánchez et al., 2006). Three chloroplast regions (atpF-atpH, psbK-psbI, trnL-rpl32) were used; the first two were amplified and sequenced using primers and protocols by Lahaye et al. (2008) and the latter following Shaw et al. (2007). In addition, the nuclear DNA region ITS, using the ITS5 and ITS4 primers of White et al. (1990), following the protocol of Shrestha et al (2003) was also sequenced. Amplified products and DNA were purified using the 72 QIAquick PCR purification kit (Qiagen, California, USA) following the protocols provided by the manufacturer. Clean products were sequenced using the Taq BigDye Terminator Cycle Sequencing Kit (Perkin Elmer Applied Biosystems, Foster City, USA) with an ABI 310 automated DNA sequencer (Perkin Elmer Applied Biosystems, Foster City, USA). Electropherograms were edited and assembled using Sequencher 4.1 (Gene Codes, Ann. Arbor, MI). Sequences were manually aligned with Se-Al v. 2.0a11 (Rambaut, 2002). Phylogenetic analyses Two sets of phylogenetic analyses were conducted, molecular and morphological. With molecular data, initially two parsimony (MP) analyses were conducted, one based on the combined chloroplast atpF-atpH, psbK-psbI, trnL-rpl32 intergenic loci and a second with the nuclear ITS. Finally a combined molecular analysis with chloroplast and nuclear DNA loci was performed. Parsimony analyses were run in TNT (Goloboff et al., 2003) using a new technology search approach, the ratchet algorithm with 200 iteration. Parsimony bootstrapping support for internal branches was estimated with one thousand replicates using TBR branch swapping, with 10 random entry orders saving one tree per replicate. The potential incongruence of the molecular and morphological data sets was tested using the incongruence length difference (ILD) test of Farris et al. (1994) as implemented in WinClada (Nixon 2002). In the morphological phylogenetic analysis the 28 populations of Otatea were treated as terminal units (Fig. 1, Table 1). The data matrix included 54 characters, two of which were autapomorphic (Appendix 3), and it was constructed with WinClada (Nixon, 2002). Parsimony and bootstrap support analyses were run in TNT (Goloboff et al., 2003) with the same settings 73 indicated for the molecular data. Bremer support (Bremer, 1994) was calculated using the BS5 option of Nona (Goloboff, 1999) on 10,000 trees held in memory. A statistical parsimony network was obtained with the program TCS v.1.21 (Clement et al. 2000), to understand the genotype relationships of every individual. The network was constructed using concatenated sequences (chloroplast and nuclear DNA sequences). Morphological character-based species delimitation The character-based approach was implemented by comparing the frequencies of qualitative characters and the range of trait values for quantitative continuous and meristic characters across all populations to search for potentially diagnostic characters. Characters were considered to diagnose a species or a set of populations if they were invariant for alternative character states or showed no overlap in trait values as indicated by Wiens and Penkrot (2002). Ecological niche modeling To determine niche dimensions, niche differences and geographic predictions in the studied populations of Otatea, two algorithms that vary in their predictive performance were used (Elith et al., 2006); GARP (DesktopGarp v 1.1.6; http://nhm.ku.edu/desktopgarp/index.html) and Maxent v 3.2.1 (Phillips et al., 2006). The use of niche modeling can help differentiating between two hypothesis (niche conservatism vs niche divergence). Niche conservatism promotes allopatric speciation, by limiting dispersal between populations (e.g., Wiens and Graham, 2005). Niche divergence indicate, adaptation to different climate conditions in allopatric or parapatric populations accelerating the evolution of reproductive isolation (Kozak et al., 2008). 74 A total of 134 georeferenced records were compiled, including 102 for O. acuminata and 37 for O. fimbriata. However, O. glauca and O. sp. nov. Chiapas have each been recorded in only a single locality. Georeferenced locality data were obtained during the fieldwork of this project and from herbarium specimens from the following herbaria: ENCB, F, IBUG, IEB, ISC, MEXU, MO, NY, US and XAL. The dataset was modeled with 19 standard bioclimatic variables derived from modern temperature and precipitation data from WorldClim 1.4 (Hijmans et al., 2005) with a resolution of one square kilometer. Maxent creates species distribution models (DMs) by combining presence-only data with ecological layers using a statistical approach known as maximum entropy. The maximum entropy approach estimates a species’ environmental niche by finding a probability distribution that is based on a distribution of maximum entropy (Rissler and Apodaca, 2007). Following Phillips et al. (2006), we used the default modeling parameters for all species with a logistic output. Binary maps (predicted presence or absence) were created from the Maxent-generated niche distribution models using the lowest presence threshold value (LTP) (Pearson et al., 2007; Stockman and Bond, 2007; Bond and Stockman, 2008). The genetic algorithm for rule-set prediction (GARP) (Stockwell and Noble, 1992; Stockwell and Peters, 1999) like Maxent, reconstructs the potential distribution of species with an evolutionary computing genetic algorithm to search for a nonrandom association between environmental variables and known occurrences of species, as contrasted with environmental factors across the study area (Stockwell and Peters, 1999). GARP was run for species with more than 25 records with the parameters: 50% for training, 50% for testing, runs = 100, convergence limit = 0.01, iterations = 1000. “Best subsets” omission measure = extrinsic, omission threshold = hard and 10% omission, total models under hard omission threshold = 20, commission 75 threshold = 50%). For species with fewer than 20 records GARP analysis was run with the same parameters except for 100% for training and omission measure = intrinsic. Both procedures generated 10 best models. The geographic predictions (binary predictions, 0 = absence, 1 = presence) of the 10 best models were averaged to provide a summary of potential geographic distributions (Anderson et al., 2003). Otatea glauca and O. sp. nov. Chiapas were not considered for ecological niche modeling because there are fewer than five records for each. The resultant Maxent and GARP ASCII file was converted to raster format using ArcView GIS 3.2. To evaluate ecological interchangeability, two evaluations were performed (Stockman and Bond, 2007; Bond and Stockman, 2008). From the closely related lineages we estimated the degree of overlap following Barraclough and Vogler (2000) from the DMs generated by Maxent and GARP. Firstly, from the closed related lineages the degree and significance of overlap between closely related lineages was estimated with D-NOVL v 1.3 (Stockman et al., 2008). This program uses a Monte Carlo algorithm that creates a null model to determine the probability distribution of the degree of overlap for DMs of known sizes (Stockman and Bond, 2007). We then used standard methods of statistical inference to evaluate the amount of overlap observed. The null hypothesis that the DMs are randomly distributed is rejected if the observed overlap has a probability < 0.05. Lineages whose DMs are completely or largely overlapping are considered to be ecologically interchangeable (Stockman and Bond, 2007). For each closed related lineage we performed 1000 simulations following Stockman et al. (2008). Secondly, a PCA was performed using the extracted values of 19 climate variables (WorldClim 1.4; Hijmans et al., 2005) for each unique locality of each clade to examine the overall level of divergence in environmental space among the extant taxa. We then quantified how pairs of sister taxa overlapped in environmental space using the environmental variables. A MANOVA 76 (multivariate analysis of variance) of PC scores was performed to test for significant differences among the PC scores of closely related lineages. The F-statistic was reported, as well as a test of between-subject effects to determine which PCs account for significance in the overall test (Graham et al., 2004; Stockman and Bond, 2007). Results DNA haplotype phylogenetic analyses Sequence lengths for the three chloroplast atpF-atpH, psbK-psbI and trnL-rpl32 intergenic loci were 622, 438 and 847 base pairs (bp) respectively and total length was 1907 bp. All sequences were deposited in GenBank (accession numbers; atpF-atpH; FJ483849-FJ483862; psbK-psbI: FJ483863-FJ483876; rpl32-trnL: FJ483877-FJ483888); twenty parsimonyinformative characters, 36 variable sites and twelve haplotypes were found (Table 1). Sequence length for nuclear ITS, was 587-593 bp; 42 parsimony-informative characters, 49 variable sites and 33 haplotypes were found (accession numbers; GQ384308- CQ384341). The cpDNA data resulted in a single most parsimonious tree (MPT) (L= 21, CI = 95, RI = 99) (Fig. 2). The ITS resulted in eight MPT (L= 95, CI = 64, RI = 88), strict consensus is shown in (Fig. 2). The combined data set resulted in five MPT (L= 124, CI = 65, RI = 91) strict consensus is shown in Fig. 2. The ILD test revealed no incongruence between the chloroplast and the nuclear datasets (p = 0.1517). The single chloroplast MPT shows that the haplotypes of O. acuminata and O. fimbriata are not exclusive and formed two different supported clades. Individuals of the two subspecies of O. acuminata did not form groups. Individuals of Otatea glauca and O. sp. nov. Chiapas are combined and only two individuals of the populations of O. sp. nov. Chiapas formed an exclusive group (Fig. 2). ITS strict consensus tree shows similar 77 topology to the chloroplast single MPT (Fig. 2). Strict consensus topology of the combined data matrix is similar to the chloroplast MPT, showing besides the two main clades an additional supported clade formed by individuals of O. glauca and O. sp. nov. Chiapas (Fig. 2). Statistical parsimony network The network with the 108 Otatea individuals resulted in 34 unique compound (chloroplast + nuclear) genotypes grouped in a single network (Fig. 3). Seventeen genotypes were found in O. acuminata, three in O. fimbriata, two in O. glauca, seven in O. sp. nov. Transvolcanic, and one for O. sp. nov. Oaxaca, O. sp. nov. Chiapas and O. sp. nov. Jalisco, respectively (Fig. 3). The biggest outgroup probability was for population 28 of O. fimbriata from Colombia. Morphological phylogeny Parsimony analysis of morphological data resulted in a single most parsimonious tree (MPT) (L = 179, CI = 0.47 and RI = 0.76), shown in Figure 4. MPT displays populations of O. acuminata (B = 4) (Bootstrap support = BS and Bremer support = B) and O. fimbriata in their own clades, as well as populations of O. glauca and the single population (22) of O. sp. nov. Chiapas. Populations from the subspecies of O. acuminata are combined in the same clade. Three main clades were retrieved, a first clade formed by populations of O. glauca and O. sp. nov. Chiapas; a second clade formed by populations of O. fimbriata, O. sp. nov. Transvolcanic, O. sp. nov. Oaxaca and O. sp. nov. Jalisco; the third clade was formed by populations of the two subspecies of O. acuminata (Fig. 4). Character-based species delimitation 78 The morphological character-based approach supports the recognition of seven different entities, the same entities identified by the morphology tree-based phylogeny. Otatea acuminata is the most morphologically variable species with a widespread distribution (Fig. 1) and is recognized by a single diagnostic character: the lack of oral setae in foliar leaves (Char. 28) and the two subspecies can not be recognized based on morphological characters. O. fimbriata is distributed from Chiapas, through Central America down to South America, and is recognized by three diagnostic characters: nodal line dipping slightly below the buds, brown foliar oral setae, and a patch of brown of cilia on the abaxial foliar surface. O. sp. nov. Transvolcanic has five diagnostic characters: an extravaginal branching pattern, branch complement with one or two divergent branches, a glabrous sheath apex, foliar oral setae connate at the basal third or more, and a patch of yellow cilia on the abaxial foliar surface. O. sp. nov. Oaxaca has two diagnostic characters: purple foliar oral setae with a length of 6 mm. O. sp. nov. Jalisco has three diagnostic characters: papyraceous foliar oral setae, white culm and foliage leaf oral setae. O. glauca from Chiapas has three diagnostic characters: thin culm walls and glabrous foliar oral setae. O. sp. nov. Chiapas has two diagnostic characters: fimbriate culm leaf bases and straight fimbriae of foliar leaves. Ecological niche models and PCA/MANOVA Based on the morphological tree phylogeny, which was coherent with geographic distribution (Fig. 1, 4), GARP and Maxent predictive models were produced based on 19 environmental variables (Table 2) for only the four entities with more than five records (Pearson et al., 2007). These species were Otatea acuminata, O. fimbriata, O. sp. nov. Transvolcanic, and O. sp. nov. Jalisco, none of which are sister taxa (Fig. 4). Distribution models (DMs) of O. acuminata (Fig. 79 5a GARP and 5b Maxent; LTP = 11%) were based on 103 samples, the highest number of records with the widest distribution in Mexico. GARP and Maxent DMs for O. acuminata (Fig. 5a) found the areas of predicted occurrence to be similar. The DMs of O. fimbriata (Fig. 5c GARP and 5d Maxent; LTP = 57%) were based on 19 records and both algorithms predicted almost the same DMs, with suitable areas in Oaxaca, Guerrero, Michoacán, Estado de México, Colima and Jalisco from Mexico and Guatemala, Nicaragua and Venezuela. DMs of O. sp nov 2 Transvolcanic (Fig. 5e GARP and 5f Maxent; LTP = 58%) were based on 10 records. The GARP (Fig. 5e) prediction was better than that of Maxent (Fig. 5f), because it excluded a record from Nayarit. The DMs of O. sp. nov. Jalisco (Fig. 5g GARP and 5h Maxent; LTP = 56%) were based on seven records. In this case the GARP prediction was better than that of Maxent, because the latter overpredicted suitable areas in Nayarit, Jalisco, Michoacán, Estado de Mexico and D.F. GARP DMs for O. sp. nov. Transvolcanic and O. sp. nov. Jalisco, and with 10 or fewer records gave better predictive models than Maxent did (Fig. 5e-h). The PCA based on 19 climate variables found that PC1 = 33%; PC2 = 30%; PC3 = 13.7% and PC4 = 10.3% explained almost 87% of the variability. Table 2 shows that seven temperature variables load negatively on PC1, one temperature and three precipitation variables load on PC2, one temperature and one precipitation variable load on PC3, and finally two precipitation variables load on PC4. The PCA/MANOVAs of the lineages recognized from the morphological phylogeny gave the following results. The comparison between O. glauca with O. sp. nov. Chiapas was not performed because of the low number of sample records for the two species. However a statistically significant difference between PC1 and PC4 (P = 0.007, P = 0.01) was found for these groups, suggesting that they are found in areas with different precipitation and temperature 80 regimes. The overall MANOVA shows a statistically significant difference between O. sp. nov. Oaxaca and O. sp. nov. Jalisco (F1,8 = 841.7, P < 0.0001). This difference occurs along PC2 (F = 18.7, P = 0.004), PC3 (F = 42.07, P = 0.0006) and PC4 (F = 62.7, P = 0.0002), suggesting that these two entities are found in areas with different amounts precipitation, but similar temperatures. The comparison of the groups formed by populations from Oaxaca-Jalisco with populations from the Transvolcanic Belt showed no statistically significant differences (F1,16 = 1.21, P = 0.3526), suggesting that the three entities are found in areas with similar temperature and precipitation. O. fimbriata and (O. sp. nov. Jalisco, O. sp. nov. Oaxaca, O. sp. nov. Transvolcanic) were significantly different (F1,35 = 23.89, P < 0.0001). This difference occurs along PC1 (F = 6.19, P < 0.018), PC2 (F = 43.09, P < 0.0001) and PC4 (F = 14.93, P = 0.0005), suggesting that the entities are found in areas with different precipitation and temperature regimes. The ecological interchangeability models produced by GARP and Maxent revealed that O. sp. nov. Jalisco and O. sp. nov. Transvolcanic showed a large degree of overlap (GARP = 70.8 % and Maxent = 65.4%) and the probability of detecting overlap if the ranges were randomly distributed was < 0.05, suggesting that these two clades display little ecological divergence. This agreed with the MANOVA results. GARP and Maxent DMs for O. fimbriata with O. sp. nov. Transvolcanic and O. sp. nov. Jalisco had minimal or no overlap. O. fimbriata with O. sp. nov. Transvolcanic (GARP = 8.2 % and Maxent = 8.3%) and O. fimbriata with O. sp. nov. Jalisco (GARP = 0 % and Maxent = 3.9%) were consistent with a random distribution (P > 0.05), therefore there is no ecological interchangeability between O. fimbriata with either O. sp. nov. Transvolcanic or O. sp. nov. Jalisco and they have divergent ecological niches. In 81 conclusion, the results of the above combinations indicate that populations of O. fimbriata occur in areas with different precipitation and temperature regimes. Discussion Molecular analyses Instead of chloroplast loci such as trnD-trnT, trnC-rpoB, rps16-trnQ, and the rpl16 intron, which are currently being utilized in the Bamboo Phylogeny Project (http://www.eeob.iastate.edu/research/bamboo), we sequenced atpF-atpH, psbK-psbI, because more variation in a large number of angiosperms has been reported for them (Lahaye et al., 2008), and we used the spacer trnL-rpl32 because it is one of the most variable according to Shaw et al. (2007). The loci of the Bamboo Phylogeny Project were selected to determine the relationships of groups at higher taxonomic levels. The nuclear ITS has been sequenced for temperate woody bamboos and we used it as well (Hodkinson et al., 2000; Guo et al., 2001; Guo and Li, 2002, 2004; Peng et al., 2008; Sun et al., 2005; Yang et al., 2008). Trees retrieved by molecular analyses were mostly unresolved. Low substitution rates have been found in Bambusoideae phylogenetic analyses that used different DNA markers (Kelchner and Clark, 1997; Hodkinson, 2000; Guo et al., 2001, 2002; Guo and Li, 2004; Sun et al., 2005; Yang et al., 2007, 2008; Bouchenak-Khelladi et al., 2008; Peng et al., 2008; RuizSánchez et al., 2008; Sungkaew et al., 2008). In spite of the fact that we selected these previously reported and variable markers, they had low numbers of informative molecular characters in the phylogenetic analyses for Otatea. Tree topologies retrieved by the molecular analyses were similar and individuals of Otatea glauca and O. sp. nov. Chiapas were the only ones to form supported clades. However, 82 according to the WP method, molecular- and morphological tree-based, character-based analysis and geographic coherence should be taken into account to define species, so decisions for Otatea were taken based in all evidence. The statistical parsimony network identified a single network, implying that both markers (chloroplast and nuclear) have congruent histories with low global homoplasy levels (GómezZurita and Vogler 2006). The network exhibits some reticulation, particularly between genotypes of Otatea acuminata and O. sp. nov. Transvolcanic. Morphological analyses Morphological parsimony retrieved only three well supported clades (O. fimbriata populations, O. sp. nov. Transvolcanic populations, and two populations of O. sp. nov. Jalisco). Character-based analysis recognized seven identities, which possess the diagnostic character states indicated above. These are: O. acuminata (including subsp. aztecorum), O. fimbriata (including the Colombian population), O. glauca, and four putative new species: O. sp. nov. Jalisco, O. sp. nov. Transvolcanic, O. sp. nov. Oaxaca and, O. sp. nov. Chiapas. Diagnostic characters were all vegetative. In Otatea, in contrast to many other Neotropical bamboo genera, there appear to be relatively few good characters in the floral structures even when they are present to distinguish reliably among its species, but flowering specimens were not known for some of our populations. Traditionally, a number of morphological characters have been used to differentiate taxa in bamboos (Londoño and Clark 1998, 2002; Judziewicz et al., 1999). The vegetative attribute of a branch complement with three subequal branches per node is the character that has been used to distinguish Otatea from the other genera in subtribe Guaduinae (Guzmán et al., 1984; Londoño and Clark, 1998; 2002; Judziewicz et al., 1999; Ruiz-Sánchez et 83 al., 2008). An additional character is the presence of oral setae at the apex of the culm and foliage leaf sheaths (Ruiz-Sánchez et al., 2008). These characters vary among the taxa recognized by our analyses, but differences in habitats (types of vegetation) and an allopatric distribution could be responsible for this variation. The focal species in our study, Otatea acuminata, displayed an elevated morphological variation. Diameter of stems was variable. The subsp. aztecorum was recognized by having thick stems, more than 3 cm in diameter. Also, pubescence on abaxial sheath surface was another character for differentiating O. acuminata subsp. aztecorum from subsp. acuminata. Yet our results did not find these characters supporting groups of populations in the tree-based nor as diagnostic in the character-based analyses. Oral setae on culm leaves were either present or lacking in populations of O. acuminata, but they were always absent from foliar leaves, and this was a diagnostic attribute according to the character-based analysis. In contrast to vegetative attributes, floral characters were not variable in populations of this species. Qualitative characters, such as color of oral setae or cilia on the abaxial foliar surface or pubescence on sheath apex that resulted diagnostic for entities such as O. sp. nov. Jalisco, O. sp. nov. Transvolcanic and, for O. fimbriata were observed from both wild plants and from new shoots of cultivated plants in the Clavijero Botanical Garden. Ecological niche The principle for projecting ecological niche is that adaptation to different climate conditions in allopatric or parapatric populations might play an important role in speciation by driving phenotypic divergences and accelerating the evolution of reproductive isolation (Kozak et al., 2008). Niche divergence implies that there is no ecological interchangeability while niche 84 conservatism implies the contrary (Graham et al., 2004; Jakob et al., 2007; McGuire et al., 2007; Rissler and Apodaca, 2007; Stockman and Bond, 2007; Bond and Stockman, 2008). The results of GARP, MAXENT and PCA/MANOVAS suggest niche divergence for O. sp. nov. Jalisco, O. sp. nov. Oaxaca, O. sp. nov. Transvolcanic and O. fimbriata. Otatea sp. nov. Jalisco and O. sp. nov. Oaxaca have divergent ecological niches. Morphological results indicate that the allopatric sister species, O. sp. Jalisco and O. sp. Oaxaca, are divergent in ecological niches. The species from Jalisco grows in pine-oak humid forests or cloud forests and the species from Oaxaca grows in pine-juniper-oak forests with differences in precipitation. Populations of Otatea acuminata resulted the sister group to a clade formed by populations of O. sp. nov. Jalisco, O. sp. nov. Oaxaca, O. sp. nov Transvolcanic and O. fimbriata in the morphological tree. Ecological niche models are projected for sister taxa, thus it was not possible to consider O. acuminata, our focal species, in these projections. Moreover, populations of O. acuminata are found from tropical dry forest to even drier habitats, like xerophytic scrubs. In addition this species is found in altitudes from 400 to 2000 m. Conflicting results from molecular and morphological analyses Molecular and morphological analyses retrieved trees with conflicting topologies. Probable causes of incongruence between molecular and morphological data sets include processes like lineage sorting of ancestral polymorphisms, paralogy, lateral gene transfer and hybridization (Brower et al., 1996; Maddison, 1997). Recent research on plants (Jakob and Blattner, 2006; Bänfer et al., 2006; Flagel et al., 2008; van der Niet and Linder, 2008) and animals (Pollard et al., 2006; Carstens and Knowles, 2007; Carstens and Knowles, 2007a; Leaché and Mulcahy, 2007; McGuire et al., 2007; Gray et al., 2008; Trewick, 2008) has found 85 that incomplete lineage sorting is often one of the causes of incongruence between gene and species trees. Furthermore, the effect of lineage sorting persists regardless of whether more and more regions within a genome are added to the analyses or not (Hudson and Coyne, 2002; Degnan and Rosenberg, 2006; Knowles and Carstens, 2007). Recently derived species may reflect the incongruence between species boundaries inferred with genetic markers and the boundaries inferred with morphology (Knowles and Carstens, 2007), which could be the case in Otatea. As mentioned above, in plants, hybridization processess can also cause discordance between morphological and molecular data (e.g. Lihová et al., 2007, Pan et al., 2007). In Bambusoideae natural hybridization has been reported in the Neotropical genus Chusquea (Clark et al. 1989) and in a Japanese lineage formed by genera such as Pleioblastus, Sasamorpha, Phyllostachys, and Sasa, representing intergeneric hybrids (Triplett and Clark 2009). The statistical parsimony network found evidence of gene flow between populations of Otatea acuminata and O. sp. nov. Transvolcanic, but according to Gómez-Zurita and Vogler (2006), proponents of this method, this single network does not suggest potential hybridization. Most of the ancient cases of hybridization appear to be caused by introgression rather than by hybrid speciation (Rieseberg et al., 1997). Rieseberg et al. (2006) elucidated that plant species are more likely than animal species to represent reproductively independent lineages. Conclusions Trees recovered by molecular and morphological analyses retrieved two contrasting hypotheses. Molecular analyses indicated that species of Otatea have diverged recently with gene exchange, hybridization or incomplete lineage sorting. Therefore, we base our conclusions 86 on the tree-based and character-based morphological analyses as suggested by the WP methodology, recognizing four new species plus three currently recognized in Otatea, each with fixed diagnostic characters. Moreover ecological niche modeling agreed that niche divergence could be one of the causes of speciation, because allopatric groups of populations were separated by different mountains and we think that niche divergence could be the responsible for speciation in Otatea species. In Otatea, the morphology data set was more useful for studying recently diverged taxa than the molecular data set. It was advantageous to gather morphological, molecular and ecological data simultaneously in order to detect recently diverged taxa, because the molecular data alone was unable to distinguish recently formed species. Furthermore, the species will be described based on the vegetative characters that supported the new taxa. Niche modeling corroborated that the groups recognized in the morphological analyses occupy different habitats. The molecular statistical parsimony network indicated that the ancestral genotype in Otatea is found in the population from Colombia, corroborating the hypothesis of Kelchner and Clark (1997) that the bamboos originated in South America, migrating to Central and to North America. We conclude that the two subspecies in Otatea acuminata can not be segregated and raised to the level of species. In addition we resolved that the population in Colombia indeed forms part of O. fimbriata. Therefore, based in the morphology tree- and character-based analyzes and corroborated by the modeling of ecological niche we decided to recognize seven entities in Otatea. Further research considering additional DNA markers (AFLP, microsatellites, other nuclear DNA sequences) will allow to date time of origin of Otatea species. Timing will detect if 87 Pleistocene climate fluctuations could be responsible for the process of speciation in Otatea. They will also allow to elucidate if some of the species had an hybrid origin. 88 Acknowledgements We are particularly grateful to two anonymous reviewers which greatly improved the manuscript. To John J. Wiens for his thoughtful review of a preliminary version of the paper, to Ximena Londoño and Jaime Eduardo Muñoz for assistance in field work in Colombia, and for kindly providing DNA sequences for the Colombian Otatea population. We thank Francisco Ornelas for providing reviews that improved this manuscript significantly. We are grateful to Pablo Carrillo-Reyes, Arturo de Nova, Flor Rodríguez-Gómez, José Luis Martínez, Nelly Jiménez-Pérez, Jaime Pacheco, Xóchitl Galarza, Diego Angulo and Etelvina Gándara for their assistance with field work. Bianca Delfosse edited the English version of the manuscript. We thank the curators of the following herbaria for access to their collections and the loan of specimens: ISC, MEXU, MO, NY, US and XAL. Field work was supported by a graduate student grant of the Instituto de Ecologia, A. C., by a grant of the student assistance program of BOTA “Bamboos of the Americas”, by a grant provided by “Red Lationamericana de Botánica” (RLB07-ATP01) and also by a student grant from the International Association for Plant Taxonomists. A fellowship to ER-S by CONACYT (190069) is also gratefully acknowledged. 89 References Anderson, R.P., Lew, D., Peterson, A.T. 2003. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol. Mod. 162, 211-232. Bänfer, G., Moog, U., Fiala, B., Mohamed, M., Weising, K., Blattner, F.R. 2006. A chloroplast genealogy of myrmecophytic Macaranga species (Euphorbiaceae) in Southeast Asia reveals hybridization, vicariance and long-distance dispersals. Mol. Ecol. 15, 4409-4424. Barraclough, T.G., Vogler, A.P. 2000. Detecting the geographical pattern of speciation from species-level phylogenies. Am. Nat. 155, 419-434. Bond, J.E., Stockman, A.K. 2008. An integrative method for delimiting cohesion species: finding the population-species interface in a group of Californian trapdoor spiders with extreme genetic divergence and geographic structure. Syst. Biol. 57, 628-646. Bouchenak-Khelladi, Y., Salamin, N., Savolainen, V., Forest, F., van der Bank, M., Chase, M.W., Hodkinson, T.R. 2008. Large multi-gene phylogenetic trees of the grasses (Poaceae): progress towards complete tribal and generic level sampling. Mol. Phylogenet. Evol. 47, 488505. Bremer, K. 1994. Branch support and tree stability. Cladistics 10, 295-304. 90 Brower, A.V.Z., DeSalle, R., Vogler, A.P. 1996. Gene trees, species trees, and Systematics: A cladistic perspective. Annu Rev Ecol Syst 27:423–450. Carstens, B.C., Knowles, L.L. 2007. Estimating species phylogeny from gene-tree probabilities despite incomplete lineage sorting: an example from Melanoplus grasshoppers. Syst. Biol. 56, 400-411. Carstens, B.C., Knowles, L.L. 2007a. Shifting distributions and speciation: species divergence during rapid climate change. Mol. Ecol. 16, 619-627. Chase, M.W., Hills, H.H. 1991. Silica gel: An ideal material for field preservation of leaf samples for DNA studies. Taxon 40, 215-220. Clark, L.G., Cortés, G. 2004. A new species of Otatea from Chiapas, Mexico. Bamboo Science and Culture 18, 1-6. Clark, L.G., Davidse, G., Ellis, R. 1989. Natural Hybridization in Bamboos: Evidence from [Chusquea] sect. [Swallenochloa] (Poaceae: Bambusoideae). Nat. Geo. Res. 5, 459-476. Clement, M., Posada, D., Crandall, K.A. 2000. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9, 1657-1999. 91 Cota-Sánchez, H.J., Remarchuk, K., Ubayasena, K. 2006. Ready-to-use DNA extracted with a CTAB method adapted for herbarium specimens and mucilaginous plant tissue. Plant Mol. Biol. Rep. 24, 161-167. Degnan, J.H., Rosenberg, N.A. 2006. Discordance of Species Trees with Their Most Likely Gene Trees. PLoS Gen. 2, e68. DOI: 10.1371/journal.pgen.0020068. Elith, J., Graham, C.H., Anderson, R.P., Dudik, M., Ferrier, S., Guisan, A., Hijmans, R.J., Huettmann, F., Leathwick, J.R., Lehmann, A., Li, J., Lohmann, L.G., Loiselle, B.A., Manion, G., Moritz, C., Nakamura, M., Nakazawa, Y., Overton, J.M., Peterson, A.T., Phillips, S.J., Richardson, K., Scachetti-Pereira, R., Schapire, R.E., Soberon, J., Williams, S., Wisz, M.S., Zimmermann, N.E. 2006. Novel methods improve prediction of species' distributions from occurrence data. Ecography 29, 129-151. Farris, J.S., Källersjö, M. Kluge, A.G., Bult, C. 1994. Testing significance of incongruence. Cladisticis 10, 315-319. Flagel, L.E., Rapp, R. A., Grover, C.E., Widrlechner, M.P., Hawkins, J., Grafenberg, J.L., Álvarez, I. 2008. Phylogenetic, morphological, and chemotaxonomic incongruence in the North American endemic genus Echinacea. Am. J. Bot. 95, 756-765. Goloboff, P. 1999. NONA (NO NAME) ver. 2 Published by the author, Tucumán, Argentina. 92 Goloboff, P.A., Farris, J.S., Nixon, K. 2003. TNT: Tree Analysis Using New Technology, Version 1.0. Program and documentation available from http://www.zmuc.dk/public/phylogeny/tnt. Gómez-Zurita, J., Vogler, A.P. 2006. Testing introgressive hybridization hypothesis using statistical network analysis of nuclear and cytoplasmatic haplotypes in the leaf beetle Timarcha goettingensis species complex. J. Mol. Evol. 62, 421-433. Graham, C.H., Ron, S.R., Santos, J.C., Schneider, C.J., Moritz, C. 2004. Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution 58, 1781-1793. Gray, D.A., Huang, H., Knowles, L.L. 2008. Molecular evidence of a peripatric origin for two sympatric species of field crickets (Gryllus rubens and G. texensis) revealed from coalescent simulations and population genetic tests Mol. Ecol.17, 3836-3855. Guo, Z.H. Li, D.Z. 2004. Phylogenetics of the Thamnocalamus group and its allies (Gramineae: Bambusoideae) inferred from the sequences of GBSSI gene and ITS spacer. Mol. Phylogenet. Evol. 30, 1-12. Guo, Z.H., Chen, Y.Y., Li, D.Z., Yang, J.B. 2001. Genetic variation and evolution of the alpine bamboos (Poaceae: Bambusoideae) using DNA sequence data. J. Plant Res. 114, 315-322. 93 Guo, Z.H., Chen, Y.Y., Li, D.Z. 2002. Phylogenetic studies on Thamnocalamus group and its allies (Bambusoideae: Poaceae) based on ITS sequence data. Mol. Phylogenet. Evol. 2, 20-30. Guzmán, R., Anaya, M.C., Santana, F.J. 1984. El género Otatea (Bambusoideae), en México y Centroamérica. Boletín del Instituto de Botánica 5, 2-20. Hendrixson, B.E., Bond, J.E. 2005. Testing species boundaries in the Antrodiateus unicolor complex (Aranae: Megalomorphae: Antrodiaetidae): “Paraphyly” and criptic diversity. Mol. Phylogenet. Evol. 36, 405-416. Hijmans, R.J., Cameron, S.E., Parra, J.L., Jones, P.G., Jarvis, A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965-1978. Hodkinson, T.R., Renvoize, S.A., Ni Chonghaile, G., Stapleton, C.M.A., Chase, M.W. 2000. A comparison of ITS nuclear rDNA sequence data and AFLP markers for phylogenetic studies in Phyllostachys (Bambusoideae, Poaceae). J. Plant Res. 113, 259-269. Hudson, R.R., Coyne, J.A. 2002. Mathematical consequences of the genealogical species concept. Evolution 56, 1557-1565. Jakob, S.S., Blattner, F.R. 2006. A chloroplast genealogy of Hordeum (Poaceae): long-term persisting haplotypes, incomplete lineage sorting, regional extinction, and the consequences for phylogenetic inference. Mol. Biol. Evol. 23, 1602-1612. 94 Jakob, S.S., Ihlow, A., Blattner, F.R. 2007. Combined ecological niche modeling and molecular phylogeography revealed the evolutionary history of Hordeum marinum (Poaceae)—Niche differentiation, loss of genetic diversity, and speciation in Mediterranean Quaternary refugia. Mol. Ecol. 16, 1713-1727. Judziewicz, E.J., Clark, L.G., Londoño, X., Stern, M.J. 1999. American Bamboos. Smithsonian Institution Press. New York. Kelchner, S.A., Clark, L.G. 1997. Molecular evolution and phylogenetic utility of the chloroplast rpl16 intron in Chusquea and the Bambusoideae (Poaceae). Mol. Phylogenet. Evol. 8, 385-397. Knowles, L.L., Carstens, B.C. 2007. Delimiting species without monophyletic gene trees. Syst. Biol. 56, 887-895. Kozak, K.H., Grahahm, C.H., Wiens, J.J. 2008. Integrating GIS-based environmental data into evolutionary biology. Trends Ecol. Evol, 23, 141-148. Lahaye, R., Savolainen, V., Duthoit, S., Maurin, O., van der Bank, M. 2008. A test of psbK-psbI and atpF-atpH as potential plant DNA barcodes using the flora of the Kruger National Park (South Africa) as a model system. Available from Nature Precedings <http://hdl.handle.net/10101/npre.2008.1896.1> 95 Leaché, A.D., Mulcahy, D.M. 2007.Phylogeny, divergence times and species limits of spiny lizards (Sceloporus magister species group) in western North American deserts and Baja California. Mol. Ecol. 16, 5216-5233. Leavitt, D.H., Bezy, R.L., Crandall, K.A., Sites, Jr. J.W. 2007. Multi-locus DNA sequence data reveal a history of deep cryptic vicariance and habitat-driven convergence in the desert night lizard Xantusia vigilis species complex (Squamata: Xantusiidae). Mol. Ecol. 16, 4455-4481. Lihová, J., Kučera, J., Perný, M., Marhold, K. 2007. Hybridization between Two Polyploid Cardamine (Brassicaceae) Species in North-western Spain: Discordance Between Morphological and Genetic Variation Patterns. Ann. Bot. 99, 1083-1096. Londoño, X., Clark, L.G. 1998. Eight new taxa and two new reports of Bambuseae (Poaceae: Bambuseae) from Colombia. Novon 8, 408-428. Londoño, X., Clark, L.G. 2002. A revision of the Brazilian bamboo genus Eremocaulon (Poaceae: Bambuseae: Guaduinae). Syst. Bot. 27, 703-721. Mable, B.K. 2004. Why polyploidy is rarer in animals than in plants? Biol. J. Linn. Soc. 82: 453466. Maddison, W.P., 1997. Gene trees in species trees. Syst. Biol. 46, 523-536. 96 McGuire, J.A., Linkem, C.W., Koo, M.S., Hutchison, D.W., Lappin, A.K., Orange, D.I., LemosEspinal, J., Riddle, B.R., Jaeger, J.F. 2007. Mitochondrial introgression and incomplete lineage sorting through space and time: phylogenetics of crotaphytid lizards. Evolution 61, 2879-2897. Mulcahy, D.G. 2008. Phylogeography and species boundaries of the western North American Nigthsnake (Hypsiglena torquata): Revisiting the subspecies concept. Mol. Phylogenet. Evol. 46, 1095-1115. Nixon, K.C. 1999-2002. WinClada ver. 1.0000 Published by the author, Ithaca, NY, USA. Pan, J., Zhang, D., Sang, T. 2007. Molecular phylogenetic evidence for the origin of a diploid hybrid of Paeonia (Paeoniaceae). Am. J. Bot. 94, 400-408. Pearson, R.G., Raxworthy, C.J., Nakamura, M., Peterson, A.T. 2007. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 34, 102-117. Peng, S., Yang, H.Q., Li, D.Z. 2008. Highly heterogeneous generic delimitation within the temperate bamboo clade (Poaceae: Bambusoideae): evidence from GBSSI and ITS sequences. Taxon 57, 799-810. Phillips, S.J., Anderson, R.P., Schapire, R.E. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231-259. 97 Pollard, D.A., Iver, V.N., Moses, A.M., Eisen, M.B. 2006 Widespread discordance of gene trees with species tree in Drosophila: evidence for incomplete lineage sorting PLoS Gen 2, e173. DOI: 10.1371/journal.pgen.0020173 Rambaut, A. 2002. Se-Al Sequence Alignment Editor, v2.0a11. Department of Zoology, University of Oxford, Oxford. Raxworthy, C.J., Martínez-Meyer, E., Horning, N., Nussbaum, R. A., Schneider G.E., OrtegaHuerta, M.A., Peterson, A.T. 2003. Predicting distributions of known and unknown reptile species in Madagascar. Nature 426, 837-841. Raxworthy, C.J., Ingram, C.M., Pearson, R.G. 2007. Species delimitation applications for ecological niche modeling: a review and empirical evaluation using Phelsuma day gecko groups from Madagascar. Syst. Biol. 56, 907-923. Rieseberg, L.H. 1997. Hybrid origins of plant species. Annu. Rev. Ecol. Syst. 28, 359-389. Rieseberg, J.H., Wood, T.E., Baack, E.J. 2006. The nature of plant species. Nature 440: 524-527. Rieseberg, L.H., Willis, J.H. 2007. Plant speciation. Science 317: 910-914. Rissler, L.J., Apodaca, J.J. 2007. Ecological niche models and phylogeography help uncover cryptic biodiversity. Syst. Biol. 56, 924-942. 98 Ruiz-Sánchez, E., Sosa, V., Mejía-Saules, M.T. 2008. Phylogenetics of Otatea inferred from morphology and chloroplast DNA sequence data and recircumscription of Guaduinae (Poaceae: Bambusoideae). Syst. Bot. 33, 277-283. Shaw, J., Lickey, E.B., Schilling, E.E., Small, R.L. 2007. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare. III. Am. J. Bot. 94, 275-288. Shrestha, S., Adkins, S.W., Graham, G.C., Loch, D.S. 2003. Phylogeny of the Sporobolus indicus complex, based on internal transcribed spacer (ITS) sequences. Aust. Syst. Bot. 16, 165176. Silvertwon, J. 2008. The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. Int. J. Pl. Scs. 169: 157-188. Sites, J.W., Marshall, J.C. 2003. Delimiting species: a renaissance issue in systematic biology. Trends Ecol. Evol. 18, 462-470. Sites, J.W., Marshall, J.C. 2004. Operational criteria for delimiting species. Annu. Rev. Ecol. Evol. Syst. 35, 199-227. 99 Soltis, D.E., Soltis, P.S., Schemske, D.W., Hancock, J.F., Thompson, J.N., Husband, B.C., Judd, W.S. 2007. Autopolyploidy in angiosperms: have we grossly underestimated the number of species?. Taxon. 1, 13-30. Stockman, A.K., Bond, J.E. 2007. Delimiting cohesion species: extreme population structuring and the role of ecological interchangeability. Mol. Ecol. 16, 3374-3392. Stockman, A.K., Danell, R.M., Bond, J.E. 2008. D-NOVL: A program to simulate overlap between two niche-based distribution models. Mol. Ecol. Resour. 8, 290-294. Stockwell, D.R.B., Noble, I.R. 1992. Induction of sets of rules from animal distribution data: a robust and informative method of analysis. Math. Comp. Simul. 33, 385-390. Stockwell, D.R., Peters, D., 1999. The GARP modeling system: problems and solutions to automated spatial prediction. Int. J. Geogr. Inform. Sys. 13, 143-158. Sungkaew, S., Stapleton, C.M.A., Salamin, N., Hodkinson, T.R. 2008. Non-monophyly of the woody bamboos (Bambuseae; Poaceae): a multi-gene region phylogenetic analysis of Bambusoideae s.s. J Plant Res. 122, 95-108. Sun, Y., Xia, N., Lin, R. 2005. Phylogenetic analysis of Bambusa (Poaceae: Bambusoideae) based on internal transcribed spacer sequences of nuclear ribosomal DNA. Biochem Genet. 43, 603-612. 100 Trewick, S.A. 2008. DNA Barcoding is not enough: mismatch of taxonomy and genealogy in New Zealand grasshoppers (Orthoptera: Acrididae). Cladistics 24, 240 254. Triplett, J. K., Clark, L.G. 2009. Molecular phylogenetics of Pleioblastus sensu stricto (Poaceae: Bambusoideae) and allies in the Japanese Archipelago. Botany 2009. Utah. van der Niet, T., Linder, H.P. 2008. Dealing with incongruence in the quest for the species tree: a case study from the orchid genus Satyrium. Mol. Phylogent. Evol. 47, 154-174. White, T. J., Bruns, Y., Lee, S., and Taylor, J. 1990. Amplification and direct sequencing of fungal RNA genes for phylogenetics. In “PCR Protocols: A Guide to Methods and Applications” (M. Innis, D. Gelfand, J. Sninsky, and T. White, Eds.), pp. 315–322, Academic Press, San Diego. Wiens, J.J., Graham, C.H. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Syst. 36, 519-539. Wiens, J.J., Penkrot, T.A. 2002. Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus). Syst. Biol. 51, 69-91. Yang, H.Q., Yang, J.B., Peng, Z.H., Gao, J., Yang, Y.M.,Peng, S., Li, D.Z. 2008. A molecular phylogenetic and fruit evolutionary analysis of the major groups of the paleotropical woody bamboos (Gramineae: Bambusoideae) based on nuclear ITS, GBSSI gene and plastid trn L-F DNA sequences. Mol. Phylogenet. Evol. 48, 809-824. 101 Table 1. Study populations and their haplotypes. n = number of individuals sampled for cpDNA markers, and numbers below ITS are the individuals sampled to this marker. Population 1 2 3 4 5 5a 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 Locality Sonora Sinaloa Durango Nayarit Jalisco Jalisco Jalisco Colima Colima Colima Jalisco Jalisco Guanajuato Michoacán Michoacán Edo. Mexico Hidalgo Veracruz Puebla Guerrero Oaxaca Oaxaca Chiapas Chiapas Chiapas Chiapas Chiapas Chiapas N Santander Lat. 28 21 23 26 23 29 21 35 20 42 20 43 20 27 19 24 19 22 19 27 19 26 19 26 20 32 19 14 18 50 19 03 20 38 19 21 18 36 17 44 18 01 16 37 16 02 16 28 16 45 16 42 16 41 15 19 08 10 Long. 109 15 105 50 104 26 104 56 104 53 104 53 105 17 103 51 103 51 103 43 103 28 103 21 101 53 100 47 100 53 100 01 98 59 96 51 97 55 98 35 97 20 96 54 93 35 93 13 92 56 92 54 92 51 92 19 73 18 Altitude(m a.s.l.) 430 1205 1652 758 1506 1393 425 1710 1436 1146 922 1181 1917 650 710 1840 1735 667 1606 1020 1967 1525 843 1030 1086 1017 1320 1200 1230 102 n 3 3 3 3 3 3 5 4 3 3 5 5 3 5 10 3 3 3 3 3 3 3 3 3 3 3 3 12 Specie Haplotype O. acuminata F O. acuminata F O. acuminata F O. acuminata F O. fimbriata F O. fimbriata O. acuminata F O. fimbriata F O. acuminata A O. acuminata A O. acuminata F O. fimbriata F O. acuminata F O. acuminata F O. acuminata G O. fimbriata E-F O. acuminata D O. acuminata C O. acuminata F O. acuminata F O. acuminata F O. fimbriata B O. sp. nov. Chiapas I-J O. fimbriata B O. fimbriata B O. acuminata B O. fimbriata B O. glauca H O. fimbriata B ITS 2 1 1 1 3 1 4 3 3 1 3 1 1 1 4 2 3 1 1 1 3 3 1 1 3 1 2 8 Table 2. Nineteen climate variables used in GARP and MAXENT analysis and PCA loadings for the four principal components. Values in bold indicate higher loadings. Climate variable PC1 Annual Mean Temperature Mean Diurnal Range Isothermality Temperature Seasonality Max Temperature of Warmest Month Min Temperature of Coldest Month Temperature Annual Range Mean Temperature of Wettest Quarter Mean Temperature of Driest Quarter Mean Temperature of Warmest Quarter Mean Temperature of Coldest Quarter Annual Precipitation Precipitation of Wettest Month Precipitation of Driest Month Precipitation Seasonality Precipitation of Wettest Quarter Precipitation of Driest Quarter Precipitation of Warmest Quarter Precipitation of Coldest Quarter -0.975320 -0.112774 -0.023475 0.027975 -0.889275 0.769070 -0.070883 -0.906086 -0.957026 -0.946860 -0.882372 -0.002388 -0.051603 0.324830 -0.431499 -0.118377 0.373867 0.152462 0.191493 PC2 -0.143425 0.753569 -0.685331 0.728738 0.337008 -0.563384 0.860841 0.119898 -0.130334 0.124293 -0.393891 -0.906328 -0.764931 -0.423210 0.301014 -0.807392 -0.435252 -0.362475 -0.322610 103 PC3 0.032460 0.194854 -0.514544 0.623226 0.241534 -0.199986 0.418231 0.314140 0.071173 0.236632 -0.197232 0.282775 0.397377 0.511135 -0.062085 0.305036 0.549025 0.571041 0.468286 PC4 -0.145066 0.180583 0.002727 -0.033811 -0.078595 -0.190892 0.113930 -0.133796 -0.029564 -0.149047 -0.114134 0.167807 0.464326 -0.579483 0.746604 0.446147 -0.538394 0.423750 0.021680 Appendix 1: Morphological characters Culms 1. Habit: 0 = erect; 1 = apically arching/pendulous. 2. Culm internodes: 0 = all solid (at least when young); 1 = all hollow; 2 = some proximal internodes (including the basalmost ones) solid, distal internodes hollow. 3. Wall thickness (ratio of 2X wall thickness: culm diameter): 0 = walls very thin (ratio up to 0.15); 1 = walls thin (ratio 0.16-0.30); state 2 = walls moderately thick (ratio 0.31-0.45); 3 = walls thick (ratio 0.46-0.60); 4 = walls very thick (ratio 0.61-0.99). 4. Lacuna size: 0 = lacuna large, > 1/3 the diameter of the culm; 1 = lacuna small, < 1/3 the diameter of the culm. 5. Nodal line position: 0 = horizontal; 1 = dipping slightly below the bud. Buds and Branching 6. Branching pattern: 0 = intravaginal; 1 = extravaginal. 7. Branch complement (Londoño and Clark, 2002): 0 = 1 divergent branch; 1 = 1 or 2 divergent branches; 2 = 3 subequal, ascending branches Culm Leaves 8. Abaxial sheath surface: 0 = stiff, dark, irritating hairs present; 1 = glabrous, no hairs present. 9. Relative size of the culm leaf with regard to sheat: 0 = same size; 1 = a half of the sheat; 2 = a third of the sheath. 10. Relative size of the culm leaf with regard to internode: 0 = same size; 1 = larger than internode; 2 = a half of the sheat; 3 = a third of the sheat. 11. Sheath apex: 0 = more or less horizontal; 1 = symmetrically convex; 2 = symmetrically concave. 12. Sheath apex (or summit or shoulders) indument: 0 = glabrous; 1 = ciliate; 2 = fimbriate 13. Sheath summit extension: 0 = absent; 1 = present on one or both sides. 14. Oral setae: 0 = absent; 1 = present, whether adnate to the inner ligule or not. 15. Oral setae indument: 0 = glabrous; 1 = all long setae scabrous; 2 = only at base scabrous. 16. Oral setae color when live: 0 = brown; 1 = green; 2 = white; 3 = yellow; 4 = purple. 17. Oral setae length in mm: 0 = more than 13 mm; 1 = 4.5-12 mm. 18. Culm leaf blade position: 0 = erect to slightly spreading; 1 = reflexed. 19. Culm leaf blade shape: 0 = broadly triangular; 1 = more or less narrowly triangular. 20. Culm leaf blade midrib abaxial development: 0 = indistinguishable; 1 = visible or even prominent toward the apex. 21. Culm leaf sheath base indument: 0 = glabrous; 1 = ciliate; 2 = fimbriate Foliage Leaves 22. Sheath summit extension: 0 = absent; 1 = present on one or both sides. 23. Sheath summit indument: 0 = glabrous; 1 = fimbriate. 24. Sheath: 0 = rounded on the back; 1 = strongly keeled at least near the summit. 25. Sheath indument: 0 = glabrous; 1 = hispidous. 26. Outer ligule (contraligule): 0 = absent; 1 = present continuously along the width of the sheath summit; 2 = bilobed. 27. Outer ligule (contraligule), maximum length in mm of the lobes: 0 = 5 mm; 1 = 1 mm. 28. Oral setae: 0 = absent; 1 = present whether adnate to the inner ligule or not. 29. Oral setae connation: 0 = free; 1 = connate at base; 2 = connate 1/3 or more 30. Oral setae consistency: 0 = coriaceous; 1 = papyraceous. 104 31. Oral setae color when live; 0 = purple; 1 = yellow; 2 = white; 3 = brown; 4 = green. 32. Oral setae indument: 0 = glabrous; 1 = all long setae scabrous. 33. Oral setae length in mm: 0 = more than 10 mm; 1 = 6 mm. 34. Fimbria growth: 0 =curly; 1 = straight. 35. Foliage leaf blade wide: 0 = less than 1.5 cm; 1 = more than 1.6 cm 36. Midrib placement: 0 = centric; 1 = excentric (wider side of blade 1.3 times or more as wide as the narrower side). 37. Patch of cilia on the base of the blade abaxial side: 0 = absent; 1 = present. 38. Patch of cilia on the base of the blade abaxial side position: 0 = at one side of the central nerve; 1 = on both sides of the central nerve. 39. Patch of cilia on the base of the abaxial side density: 0 = very dense; 1 = dispersed. 40. Patch of cilia on the base of the abaxial side color: 0 = brown; 1 = yellow; 2 = white. Synflorescences and spikelets 41. Maximum synflorescence length in cm: 0 = 9; 1 = 10; 2 = 12; 3 = 15. 42. Maximum number of spikelets: 0 = 7; 1 = 8; 2 = 15; 3 = 30. 43. Spikelets length in cm: 0 = 2; 1 = 3; 2 = 3.5; 3 = 4. 44. Rachilla joint length in mm: 0 = 4; 1 = 5; 2 = 6; 3 = 7. 45. Glume abaxial surface: 0 = scabrous; 1 = glabrous. 46. Lemma abaxial surface: 0 = pubescent; 1 = scabrous; 2 = scabrid; 3 = glabrous. Foliar Micromorphology 47. Papillae on the long cells in the stomatal zone (abaxial): 0 = absent; 1 = present 48. Papillae on the long cells in the interstomatal zone (abaxial): 0 = absent; 1 = present 49. Papillae on the adaxial surface position: 0 = present on the long cells only; 1 = present on both bulliform and long cells 50. Distribution of stomates on foliage leaf blades: 0 = present and common on the abaxial surface only; 1 = present and common on both surfaces. 51. Vertically tall and narrow silica bodies (abaxial, intercostal): 0 = present; 1 = absent. 52. Vertically tall and narrow silica bodies (abaxial, costal): 0 = present; 1 = absent. 53. Saddle-shaped silica bodies (abaxial, costal): 0 = present; 1 = absent. 54. Horizontal dumbbell-shaped silica bodies (abaxial, costal): 0 = present; 1 = absent. 105 Appendix 2: Morphological character matrix Inapplicable data = “-“, not seen = “?”, A = 0-1 Olrecta Oa4 Oa6 Oa10 Oa9 Oa17 Oa2 Oa1 Oa3 Oa16 Of26 Of23 Of24 Of28 Oa25 Oa20 Oa18 Of5 Of5(a) Of21 Og27 Ospnov22 Oa19 Oa12 Oa18 Of15 Of11 Of7 Oa14 Oa13 000000000111111111122222222223333333333444444444455555 123456789012345678901234567890123456789012345678901234 11100000120201030010001100-100100000101-21341300001011 11300020100200---010101000-0-----0001112??????00100101 11300020100200---010101000-0-----0001112??????00110100 1231002010020100-000101000-0-----000111212220000100101 1231002010020100-000101000-0-----000111212220000100100 10--0021101110---010111000-0-----0000---12220000110101 10--0020200200---000101000-0-----0000---12220011100110 10--0020200200---000101000-0-----0000---??????11100110 00--0021221200---000001000-0-----0001112??????00100101 00--0021221200---000001000-0-----0000---??????00100100 01201020010201000010101101-110300011100033001200000100 01201020010201000010101101-110300011100033001200000101 01201020010201000010101101-110300011100033001200000100 112010200102010000A0101101-1103000111000??????00000110 00--0021121200---000101000-0-----0001112??????11100101 00--0020200200---000101000-0-----0001112??????11100101 00--0020200200---000101000-0-----0001012??????11100101 024100111121110200110001121101200-111102??????11100000 024100111121110200110001121101200-111102??????11000000 02410021222211141110010000-100001-011102??????00000101 11100020202201110110001000-100411001111200311100001011 11400020012211110111211000-1004001001012??????00101011 12310021200200---000001000-0-----0010---??????11100101 12310020100201200000101010-0-----000111212220011100100 12310020100201200000101000-0-----000111212220000100100 022001101120010101100000020120000-111101??????00100001 022001101120010101100000020120000-111101??????00100001 022001101120010101100000020120000-111101??????00100001 10--0020100201200000101010-100101001111212220011100101 10--0020100201200000101010-100101001111212220011100101 106 Appendix 3: Vouchers and specimens examined Olmeca recta Soderstr. MEXICO, Veracruz: E. Ruiz-Sánchez 132 (XAL). Otatea acuminata (Munro) C. Calderón & Soderstr. MEXICO, Colima: E. Ruiz-Sánchez et al. 101, 176 (XAL). Chiapas: E. Ruiz-Sánchez and J.L. Martínez 119 (XAL). Durango: E. RuizSánchez and P. Carrillo-Reyes 113 (XAL). Guanajuato: E. Ruiz-Sánchez and F. RodriguezGomez 173 (XAL). Guerrero: P. Carrillo-Reyes 4863 (XAL). Hidalgo: E. Ruiz-Sánchez and P. Carrillo-Reyes 114 (XAL). Jalisco: E. Ruiz-Sánchez et al. 97, 102 (XAL). Michoacán: E. RuizSánchez et-al. 181, 182 (XAL). Nayarit: E. Ruiz-Sánchez et al. 96 (XAL). Oaxaca: E. RuizSánchez and J.L. Martínez 125 (XAL). Puebla: E. Ruiz-Sánchez and J.L. Martínez 126 (XAL). Sinaloa: E. Ruiz-Sánchez et al. 105 (XAL). Sonora: E. Ruiz-Sánchez et al. 112 (XAL). Veracruz: E. Ruiz-Sánchez and F. Rodriguez-Gomez 103 (XAL). Otatea fimbriata Soderstr. COLOMBIA, Norte de Santander: X. Londoño and E. Ruiz-Sánchez 987 (CUCV). MEXICO, Colima: E. Ruiz-Sánchez et al. 183 (XAL). Chiapas: E. Ruiz-Sánchez and J.L. Martínez 118 (XAL); E. Ruiz-Sánchez 136, 155 (XAL). Estado de Mexico: E. RuizSánchez et al. 179 (XAL). Jalisco: E. Ruiz-Sánchez et al. 130, 186, 189 (XAL). Oaxaca: P. Carrillo-Reyes 4986 (XAL); E. Ruiz-Sánchez et al 217 (XAL). Otatea glauca L.G. Clark and G. Cortés MEXICO, Chiapas: E. Ruiz-Sánchez 144 (XAL). Otatea sp. nov. Chiapas MEXICO, Chiapas: P. Carrillo-Reyes 5144 (XAL); E. Ruiz-Sánchez 147 (XAL). 107 Figure Legends Figure 1. Distribution and sampling localities of the previously recognized species of Otatea. Numbers correspond to localities in Table 1. A and B show details of localities in central Mexico and Chiapas respectively. Figure 2. MPT on chloroplast DNA haplotypes for Otatea (left). Population numbers and haplotypes in parenthesis correspond to Table 1. Number below branches indicates Bootstrap values. New species retrieved by the morphological tree are indicated. Strict consensus from parsimony analysis of the ITS (center) and combined cpDNA-ITS (right). Populations numbers correspond to Table 1. Number below branches indicates Bootstrap values. New species retrieved by the morphological tree are indicated. Figure 3. Statistical parsimony network compound cpDNA-ITS genotypes. Figure 4. The single most parsimonious tree retrieved from the morphological data set (L = 179; CI = 47; RI = 76). Population numbers and haplotypes in parenthesis correspond to Table 1. Black circles indicate synapomorphies, numbers above and below the circles indicate character number and character state respectively. Numbers below the branches indicate Bootstrap and Bremer support. Ol.recta = Olmeca recta; Oa = Otatea acuminata; Of = Otatea fimbriata; Og = Otatea glauca. 108 Figure 5. GARP and Maxent niche-based distribution models for: a. and b. O. acuminata maps, c. and d. O. fimbriata, e and f. O. sp. nov. Transvolcanic maps, g and g. O. sp. nov. Jalisco. 109 110 111 112 113 114 115 CAPÍTULO IV. CUATRO ESPECIES NUEVAS EN OTATEA (POACEAE: BAMBUSOIDEAE) Y REVISIÓN TAXONÓMICA. Preparado para enviar a: Systematic Botany. 2009 116 RUIZ-SÁNCHEZ ET AL.: NEW SPECIES IN OTATEA Four new species in Otatea (Poaceae: Bambusoideae) and a taxonomic revision of the genus EDUARDO RUIZ-SÁNCHEZ 1, 4, VICTORIA SOSA1, M. TERESA MEJÍA-SAULES1, X. LONDOÑO2 AND L. G. CLARK3. 1 Biologia Evolutiva Instituto de Ecologia, A. C. Apartado Postal 63 91070 Xalapa, Veracruz, Mexico 2 3 Department of Ecology, Evolution and Organismal Biology, 253 Bessey Hall, Iowa State University, Ames, Iowa 50011-1020 U. S. A. 4 Autor for correspondence: (ruizSá[email protected]) 117 ABSTRACT. Previous molecular and morphological phylogenetic analyses, character based analysis and ecological niche modeling identified seven monophyletic groups formed by populations of Otatea (Poaceae: Bambusoideae: Bambuseae), a neotropical woody bamboo genus belonging to subtribe Guaduinae. Therefore, the genus comprises at present seven species, four of them new to science. These four new species, all from Mexico, are here described and illustrated: Otatea carrilloreyesii E. Ruiz-Sanchez, Sosa and T.M. Mejia-Saulés from Chiapas, O. mixtecana E. Ruiz-Sanchez and Londoño from Oaxaca, O. reynosoana E. Ruiz-Sanchez and L.G. Clark from the Pacific slopes of Guerrero, Jalisco and Nayarit and O. transvolcanica E. Ruiz-Sanchez, Londoño and L.G. Clark from the Transmexican Volcanic Belt in Colima, Mexico State and Jalisco. A comprehensive taxonomic revision of the genus Otatea is presented based on collections for this project and on herbarium specimens. KEYWORDS, Culm and foliar leaves fimbriae, disjunct distribution, Guaduinae, Mexican endemics, oral setae, otate. 118 Otatea (McClure & E. W. Sm) C. Calderón & Soderstr. (Poaceae: Bambusoideae: Bambuseae) is a woody bamboo genus endemic to the Neotropics and is one of the five genera currently recognized in subtribe Guaduinae (Londoño and Clark 2002; Dávila et al. 2006; RuizSánchez et al. 2008). Otatea was retrieved as a monophyletic group supported by molecular characters and by two morphological synapomorphic character states: stems with a branch complement with three subequal and ascending branches and spikelets with pubescent lemmas (Ruiz-Sánchez et al. 2008). McClure (1973) originally Otatea as a subgenus within Yushania K. H. Keng, comprising two New World species, Y. aztecorum McClure & E. W. Sm. and Y. acuminata (Munro) McClure The lack of culm proliferation form subterranean buds, synflorescences with numerous spikelets, awned lemmas and apically obtuse paleas were cited as diagnostic for the subgenus (McClure 1973). The type of Y. aztecorum was collected in El Rosario, Sinaloa, Mexico and Yushania acuminata was a combination based on Arundianria acuminata Munro with its type collected in Jalcomulco, Veracruz, Mexico. Later, Calderón and Soderstrom (1980) later elevated subgenus Otatea to generic status, based on unpublished notes of F. A. McClure and using the same characters used to give the subgenus status. But Guzmán et al. (1984) differed in opinion and recognized O. aztecorum as a subespecies O. acuminata (O. acuminata subsp. aztecorum [McClure & E. W. Sm.] R. Guzmán, Anaya & Santana) based on culms up to 3 cm in diameter, culm leaves persistent and densely hispid in the upper third and evanescent, oral setae growing from the back of the ligule and an indeterminate synflorescence in subsp. aztecorum. Two additional species were subsequently described, O. fimbriata Soderstr. and O. glauca L.G. Clark & G. Cortés, were subsequently described (McVaugh 1983; Guzmán et al. 1984; Judziewicz et al. 1999; Clark and Cortés 2004). A fourth species, still undescribed, was 119 discovered during field work for the phylogenetic study of subtribe Guaduinae (Ruiz-Sánchez et al. 2008). Otatea includes monocarpic plants with mass flowering in cycles that, according to herbarium records, last 17-30 years (Guzmán et al. 1984; Judziewicz et al. 1999) populations usually flower for two or three years, and then the plants die. Plants are medium sized, up to 3 m tall, branching is usually characterized by three subequal branches arising from a single bud, rhizomes are pachymorphic and solid or hollow culms have a short neck (Guzmán et al. 1984; Judziewicz et al. 1999). One of the most distinctive vegetative character in Guaduinae, is the occurrence of thickened appendages that originate from the culm and foliar sheaths, called oral setae (Judziewicz et al. 1999). In Otatea oral setae are papyraceous or coriaceous. In a previous study, molecular, morphological and ecological data were utilized to delimit the species comprising Otatea (Ruiz-Sánchez & Sosa, in prep.). Phylogenetic analyses including populations of all recognized species in Otatea, from their entire range of distribution were conducted. Molecular and morphological characters retrieved two contrasting phylogenetic hypotheses, in which the molecular dataset did not retreive the same groups of populations as the morphological dataset. Populations of O. acuminata and O. fimbriata were paraphyletic in molecular trees. Several arguments were proposed to explain the disagreement between the two analyses, like an incomplete lineage sorting with the retention of ancestral polymorphisms or a past gene-flow (Ruiz-Sánchez & Sosa, in prep.). In contrast, morphological trees and characterbased analyses, identified seven monophyletic well supported groups of Otatea which can be identified by diagnostic characters and which have coherent geographical distributions. As a result, four new species are described and illustrated based on (Ruiz-Sánchez & Sosa,in prep). In 120 addition, the previous three known species in Otatea are characterized and an identification key is provided for the seven species to complete a taxonomic revision of the genus. MATERIALS AND METHODS Plant material was collected in the field and voucher specimens were deposited at XAL. In adition, more than 350 specimens from the following herbaria were examined: CIIDIR, CUCV, ENCB, F, FCME, IBUG, IEB, ISC, MEXU, MO, NY, TULV, UAS, US, USON, XAL. Digital photographs were also examined from the following herbaria: CIIDIR, ENCB, IBUG, IEB, MEXU and US. Micromorphogical features of the lemma and palea were observed using scanning electron microscopy (SEM; JEOL JSM–5600). For SEM samples were glued to aluminium stubs and coated with gold–palladium (1:1). Micromorphological characters of the lemma were observed in the epidermis of the middle section, which has previously been demostrated to display a number of variable characters (Acedo and Llamas 2001). Lemma micromorphological terminology followed Metcalfe (1960) and Ellis (1979). RESULTS Morphology―CULMS― Culms in Otatea are solid or hollow. In some species culms are solid when young and they become hollow later. Otatea acuminata has both solid and hollow culms. Otatea mixtecana has solid culms and O. carrilloi, O. fimbriata, O. glauca, O. reynosoana and O. transvolcanica have hollow culms. In the (O. carrilloi, O. fimbriata, O. glauca, O. reynosoana and O. transvolcanica) the lacuna is > 50% diameter of the internode such that walls are thin. In O. transvolcanica the lacuna is <50% of the internode diameter and the walls are considered to be thick. Culms are erect or they arch at the apex; O. acuminata and O 121 fimbriata are polymorphic in this respect. Culms of O. mixtecana, O. reynosoana and O. transvolcanica are erect and culms in O. carrilloi and O. glauca are arched. CULM LEAVES―An abaxial sheath surface with irritating stiff dark hairs is observed in O. acuminata, O. carrilloi, O. fimbriata, O. glauca and O. transvolcanica whereas this surface is glabrous in O. mixtecana, O. reynosoana and in some populations of O. acuminata. Culm leaves are either longer or shorter than the internode in O. acuminata, O. carrilloi, O. fimbriata, O. transvolcanica, but in O. reynosoana they are always longer than the internode and overlapping to each other. In O. glauca, O. mixtecana and some populations of O. acuminata culm leaves are shorter than the internode and they do not overlap. The culm leaf blade is erect or reflexed. Otatea acuminata, O. fimbriata and O. reynosoana have erect blades and in O. carrilloi, O. glauca, O. mixtecana and O. transvolcanica they are reflexed. All species have persistent oral setae in the culm leaves, but only in some populations of Otatea acuminata these are absent. In O. transvolcanica these are deciduos. In living plants the color of oral setae, is brown in O. acuminata and O. fimbriata, green in O. carrilloi, O. glauca and O. transvolcanica, whitish in O. reynosoana and purple in O. mixtecana. When fimbriae are present they are free, and curly or straight. Fimbriae in Otatea transvolcanica are absent, they are barely developed in O. reynosoana and in one population of O. acuminata. In O. carrilloi the fimbriae are the largest (10-19 mm) and erect. Otatea acuminata, O. fimbriata, O. glauca and O. mixtecana have smaller (2-7 mm) curly f imbriae. BRANCHING PATTERN― There are two types of branching pattern in Otatea: O. transvolcanica is the only species with an extravaginal branching (branches emerge through and break the sheath); the rest of the species have an intravaginal branching (branches grow on the inside of the sheath). 122 BRANCH COMPLEMENT― Otatea species are characterized by having three subequal branches that arise from a single bud per node, although in O. reynosoana and O. transvolcanica one or two branches per node are sometimes found. FOLIAGE LEAF BLADES―The foliage leaf blades in Otatea, are 0.3-6 cm wide and 15-60 cm large. Otatea acuminata, O. carrilloi, O. glauca and O. mixtecana have narrow and short leaves (0.3-1.2 mm; 15-27 cm). Otatea fimbriata, O. reynosoana and O. transvolcanica have wide and large leaves (1.5-6 mm; 27-60 cm). The abaxial surface in O. fimbriata, O. mixtecana, O. reynosoana and O. transvolcanica is glaucous, while in the rest of the species the abaxial surface is green. The midrib is centric in O. acuminata, O. carrilloreyesii and O. glauca and excentric in O. fimbriata, O. mixtecana, O. reynosoana and O. transvolcanica. In some Otatea species the outer ligule (contraligule) overgrows on both sides of the apex of the foliage sheath forming two lobes. In O. reynosoana these lobes are small and poorly developed (0.6-1.5 mm) while in O. transvolcanica they are large and well developed (3-8.5mm). In the rest of the species the outer ligules are absent. In most species, when culm leaves have oral setae or fimbriae they are also present on foliage leaves, but in O. acuminata the oral setae are absent on foliage leaves. Otatea carrilloi has oral setae up to 24 mm long, which are the largest in the genus. Otatea mixtecana has the smallest oral setae (6 mm) long and they are purple. Otatea transvolcanica has purple large oral setae ca. 21.5mm long and connate for the basal third. Otatea. reyosoana has oral setae ca. 11 mm long that are white and free. Lastly Otatea fimbriata has larger oral setae up to 15 mm long, brown or purple and connate only at the base. Otatea mixtecana is the only species lacking fimbriae. The rest of Otatea species have a small to medium (2.5-14 mm) curly fimbriae, but O. carrilloi has large straight fimbriae up to 21 mm long. 123 SPIKELET COLOR―Otatea mixtecana is the only species with blue-purplish spikelets. In O. acuminata, O. fimbriata, O. glauca and O. reynosoana they are green and in O. carrilloi and O. transvolcanica the spikelet are unknown. GLUMES―In Otatea acuminata, O. mixtecana, O. reynosoana and O. fimbriata glume abaxial surface is scabrous or pubescent. In O. glauca the abaxial surface is always glabrous. Otatea acuminata has the smallest glumes (2.5-6 mm) (both glumes I and II) and O. glauca has the largest glumes (6-14 mm). LEMMA― The largest lemmas are found in Otatea glauca (14.5-21 mm). The rest of species have shorter lemmas (10-14 mm). Micromorphology – LEMMA – The abaxial surface of the lemma is variable. Otatea acuminata and O. fimbriata have rectangular intercostal long-cells with sinuous outline. Otatea glauca and O. mixtecana have U-shaped cells. Prickles are frequent in O. fimbriata, distributed irregularly on both costal and intercostal zones. Otatea acuminata has also prickles while the rest of the species lack them. In O. acuminata hooks are frequently distributed in both costal and intercostal zones but in O. fimbriata hooks are found only in the intercostal zone. Microhairs can be either absent or present in O. glauca and O. mixtecana, but if present they are usually found in intercostal zones. Microhairs are two-celled with the basal cell larger than the distal cell, obtuse at the apex. Macrohairs are present only in O. acuminata and O. mixtecana. Silica bodies are frequent in both costal and intercostal zones in all species but O. fimbriata. Silica bodies are rounded in O. acuminata and in O. mixtecana, saddle-shaped in O. glauca and O. mixtecana and irregular dumb-bell shaped in O. acuminata and O. glauca. (Fig. 1, 2). PALEA –Rectangular intercostal long-cells with sinuous outline and U-shaped sinuses are present in most of Otatea species, except in O. mixtecana which has cells with a straight outline. Prickles 124 are frequent in most Otatea species sometimes but in O. fimbriata they are absent. Pricles are distributed irregularly in both costal and intercostal zones in all species, except in O. fimbriata in which they are only found in costal zones. In O. glauca prickles have a flattened base. Hooks are frequent in O. glauca distributed in both costal and intercostal zones. In O. fimbriata they are found only in the intercostal zone. Silica bodies are present in most Otatea species; some populations of O. fimbriata lack silica bodies. Silica bodies have a saddle-shape in O. glauca, O. fimbriata and O. mixtecana and they have an irregular dumb-bell shape in O. glauca (Fig. 3). DISCUSSION Wild populations of Otatea are found in Mexico, El Salvador, Honduras and Colombia. Mexico is the only country where all seven species are present and six of them are resticted to its territory. Cultivated plants, utilized as ornamental, have been reported from Honduras, Costa Rica and the United States (Judziewicz et al. 1999). Otatea acuminata is the most abundant and widespread species and is also the species with the most reported uses. It is known as “otate” or “otate dulce” which means solid cane, a name derived form the nahuatl “otatl” and sometimes forms dense thickets, known as “otatales” (McClure 1973; Beetle et al. 1995; Vázquez-López et al. 2004). The name otate, however, is also given to some other bamboos such as Rhipidocladum McClure or Guadua Kunth or even to Arundo L. In the archaeological zone of Loma Iguana in Veracruz, it was found that culms of O. acuminata were used for construction. They were mixed with mud to build walls, known as “bajereques” or “bahereques”. Some of them still remain, and with a radiocarbon dating technique their age was estimated at 800-890 AC (Juárez and Márquez 1992). Culms of this species are currently utilized to build roofs or walls of rural houses, doors, fences, baskets, 125 walking sticks and sticks utilized in agriculture (Guzmán et al. 1984; Judziewicz et al. 1999; Cortés 2000). Otatea acuminata is the most widespread species in Mexico, occurring from Sonora to Chiapas and from Jalisco to Veracruz along the Transmexican Volcanic Belt. Morphological variation is remarkable in this species. For example, the height of plants varies from 2 to 10 m, and the basal diameter of culms varies from 1 to 6 cm. Culm leaves sometimes overlap, pubescence is either glabrous or hispidous, oral setae are present or absent in some populations, and they are absent in foliage leaves. (McVaugh 1983; Guzmán et al. 1984; Judziewicz et al. 1999; Londoño and Clark 2002; Clark and Cortés 2004). Flowering cycles in Otatea acuminata go from 30-35 years and flowers are produced in patches. In other words, not all plants in a population flower simultaneously. Seedlings are fragile and highly sensitive to herbivory. Plants germinating from seeds reach their full size in 7 to 8 years. Seeds usually germinate during the first year (Vázquez-López et al. 2004). Mostly plants reproduce vegetatively, producing new shoots every year during the rainy season (Vázquez-López et al. 2004). Guzmán et al. (1984) divided Otatea acuminata into two subspecies O. acuminata subsp. acuminata and O. acuminata subsp. aztecorum based on diameter of culm and indument of the culm leaves. However, molecular and morphological phylogenetic analyses performed by RuizSánchez and Sosa (in mss) did not retrieve groups of populations which can be assigned to these entities. Moreover, the diagnostic characters which Guzmán et al. (1984) indicated to separate the two subspecies were homoplasic (Ruiz-Sánchez and Sosa in mss). Otatea fimbriata has a disjunct distribution with populations found from Mexico to Central America (El Salvador and Honduras) and then reappear in Colombia (Londoño and Clark 1998). In spite of this distributional range, morphological variation is not as remarkable as 126 in O. acuminata. Habitats of O. fimbriata in Mexico and in Colombia are similar. Plants grow in tropical dry and oak forests on calcareous soils, either on slopes or flatter terrains near rivers (Londoño and Clark 1998). In Mexico O. fimbriata is called “carrizo”, “otate” or “otate hoja ancha” and the same uses reported for O. acuminata are cited for this species (Guzmán et al. 1984; Beetle et al. 1995). Flowering periods of O. fimbriata are from 8 to 20 years, and flowering is synchronous as reported in some populations from Chiapas. Soderstrom (1983) described Otatea fimbriata based on flowering specimens from Chiapas, Mexico. However he cited additional specimens from Jalisco; all of them were identified by us as O. transvolcanica excep for one specimen, identified as O. acuminata. Three of the new species described here Otatea mixtecana, O. reynosoana and O. transvolcanica are similar to Otatea fimbriata while O. carrillorreyesii is more similiar to O. acuminata. Differentiating characters are synthesized in Table 1. TAXONOMIC TREATMENT OTATEA (McClure & E. W. Smith) Calderón & Soderstrom, Smithsonian Contr. Bot. 44: 21. 1980. Yushania K. H. Keng subgen. Otatea McClure & E. W. Smith in McClure, Smithsonian Contr. Bot. 9: 116. 1973.―TYPE: Otatea acuminata (Munro) Calderón & Soderstrom. 127 Plants woody, unarmed, at first compactly caespitose, at maturity with an open habit, erect or apically arched; rhizomes pachymorph, sympodial, the neck from 10 to 90 cm long. Culms from 2 to 10 m tall, 0.7 to 6 cm in diameter; internodes 11-30 cm long subequal in size glabrous, yellow, green or glaucous with a waxy coating below the nodes that exfoliates like dandruff; solid or hollow with walls thin or thick. Culm leaves deciduous or disintegrating in place, overlapping or not, triangular or rectangular; sheaths hispid or glabrous with or without oral setae and fimbriae; leaf blades erect or reflexed; inner ligule truncate, entire or irregular. Branching pattern intravaginal or extravaginal (one species), branching from the middle to up high on the culm; nodes with one bud borne on a promontory, the bud usually developing into three subequal branches and in two species sometimes developing into one or two branches per node, these diverging from each other and rebranching for one or two additional orders. Foliage leaves 3-10 per complement; leaf blades 15 to 60 cm long and 0.3 to 40 (-60) mm in diameter; sheaths hispid or glabrous, rounded or slightly keeled at the apex on back; outer ligule irregular or developed into two lobes; inner ligule irregular, entire or truncate; pseudopetioles pulvinate at base. Synflorescences racemose or paniculate, 2-30 spikelets per synflorescence; spikelets 2-4 cm long, laterally compressed; glumes 2, unequal, the second longer than the first, awned, glabrous, scabrous or pubescent; lemma conspicuously awned, scabrous or pubescent; palea obtuse, broadly sulcate and 2-keeled dorsally; lodicules 3, hyaline or brownish, vasculated, the anterior pair longer than the posterior one; stamens 3; stigmas 2, plumose; caryopsis fusoid KEY TO SPECIES OF OTATEA 128 1. Culms solid if hollow with thick walls and with lacunae <50% of the internode diameter; glumes (I, II) 2.5-13.7 mm long, scabrous …………………………………………………….2 1. Culms hollow, wit thin walls and with lacunae > 50% of the internode diameter; glumes (I, II) 6-14mm long, glabrous…………………………………………………………………..O. glauca 2. Oral setae on foliage leaves absent; foliage leaf blades of the primary branches 0.3-0.7 cm wide, 10-22 cm long; …………………………………………………………………O. acuminata 2. Oral setae on foliage leaves present; foliage leaf blades of the primary branches 0.6-4 (-6) cm wide; 18-60 cm long; ……………………………………………………………………………3 3. Midrib centric; foliage leaf blades of the primary branches 0.6-0.9; 18-26 cm long; abaxially greenish; fimbriae erect, well developed, 8-21.8 m long………………………O. carrilloi 3. Midrib excentric; foliage leaf blades of the primary branches 0.7-4 (-6) cm wide; 19-60 cm long; abaxially glaucous; fimbriae curly, poorly developed, 2.5-14 mm long or absent…………………………………………………………………………...………………4 4. Culm basal diameter less than 1 cm; culm leaves not overlapping and deciduous; fimbriae on foliage leaves absent………………………………………………………………….O. mixtecana 4. Culms basal diameter more than 3 cm; culm leaves overlapping and persistent; fimbriae on foliage leaves present……..……………………………………………………………………….5 5. Culm leaves blade reflexed; pseudoauricles present and well developed 3-8 mm long; oral setae on foliage leaves connate at basal third section or more……….………O. transvolcanica 5. Culm leaves blades erect; pseudoauricles absent or poorly developed, 0.6-1.5 mm long; oral setae on foliage leaves, free or connate at the base……………………………….………………6 129 6. Oral setae of culm and foliage leaves papiraceous, whitish; one or two branches per node; patch of cilia on the base of the abaxial surface of foliage blades on both sides of the midrib, white……..................................................................................................................O. reynosoana 6. Oral setae of culm and foliage leaves coriaceous, brown or purple; three subequal branches per node; patch of cilia on the base of the foliage blade abaxial surface on one side of the midrib, brown…………………………………………………………………………….…..O. fimbriata 1. OTATEA ACUMINATA (Munro) Calderón & Soderstrom, Smithson. Contr. Bot. 44: 21. 1980. Arundinaria acuminata Munro, Trans. Linn. Soc. Bot. 26: 25. 1868. TYPE: MEXICO. Veracruz, Liebman 73 (Holotype: K!; isotype: C, US-2808847!) Yushania aztecorum McClure & E. W. Smith, Smithson. Contr. Bot. 9: 116. 1973. TYPE: MEXICO. Sinaloa, La Dispensa, F. A. McClure 21204 (holotype: US!; isotypes: F!, IEB!, ISC!, MO!, NY!) Otatea aztecorum (McClure & E. W. Smith) Calderón & Soderstrom, Smithson. Contr. Bot. 44: 21. 1980. Otatea acuminata (Munro) Calderón & Soderstrom subsp. aztecorum Guzmán, Anaya & Santana. Bol. Inst. Bot. (Universidad de Guadalajara) 5: 8. 1984. Rhizomes sympodial, pachymorph, the neck in some populations reaching up to 90 cm long. Culms 2 to 10 m tall, 1-5 cm in basal diameter, erected or apically arched; internodes glabrous, green to yellow when old, pruinose; solid or hollow with thick walls. Culm leaves 10-38 cm long, longer or shorter than the internodes, when longer overlapping; sheaths 5- 30 cm long, rectangular; the abaxial surface glabrous or hispidous, deciduous or disintegrating in place; the 130 margins glabrous or ciliate; inner ligule a coriaceous rim 1-5 mm long, truncate; oral setae up to 1.5 cm long, flat, erect, retrorsely scabrous, brown or absent; fimbriae at apex of sheath on both sides of the blade 3-7 mm long, terete, free, flexuous, curly or absent; leaf blades 6-18 cm long, triangular, erect, persistent or deciduous, shorter than sheaths, strigose-glabrescent on both sides, margin ciliate, glabrous or hirsute. Branching intravaginal; three main subequal branches per node borne on a promontory, these diverging from each other and rebraching, diverging from the main culm at 30-90 o; supranodal ridge pronounced; nodal line more or less horizontal. Foliage leaves with sheaths glabrous, puberulent or pilose; outer ligule absent or sometimes membranous, ciliate; inner ligule 0.5-2 mm long, glabrous or ciliate; oral setae mostly absent; fimbriae on both blade surfaces at apex, 1-3 mm long, terete, flexuous, free; blades 4-22 cm long, 3-12 mm wide, linear to linear lanceolate, green, densely pubescent at the base on both surfaces and glabrous or slightly pilose over the rest of the blade, midrib centric. Synflorescences 6.5-10 cm long, racemose or paniculate 6-15 spikelets per synflorescence. Spikelets 2.5-4 cm long, laterally compresed, green with 3-7 florets per spikelet; rachilla joints 5-6 cm long, densely pubescent at the apex of each joint; glumes, narrowly triangular and navicular, abaxially scabrous, 1-7 nerved, awn 1-3 mm long; glume I 2.5-5 mm long; glume II 3.5-6 mm long; lemmas 8-13 mm long, awn terminal 1-3 mm long, lanceolate, pubescent or scabrous, 9-11 nerved, awn 2-5 mm long, the margins papilose to the apex; palea 8-11 mm long, 2-keeled, keels pubescent or scabrous, sulcate over its full length, the sulcus pubescent, wings glabrous, apex bifid. Lodicules glabrous on both sides, apically ciliate 0.5mm long, the two anteriors 1.2-1.5mm long, the posterior one 0.8-1mm long; anters stramineous when old, 4.5-6.5 mm long. Ovary glabrous. Caryopsis 5.7-8.5 mm long, sublinear, tapering to a narrow beak, brown to amber. Phenology. Flowering in July to October. 131 Distribution and habitat. Otatea acuminata is endemic to Mexico. Its geographical distribution goes from the northern Sonora to Chihuahua, Sinaloa, Durango and Nayarit to Chiapas and along the Transmexican Volcanic Belt. This species is sometimes cultivated in Costa Rica, Honduras and theUnited States as an ornamental plant Plants are found on slopes with tropical dry forest, xerophytic scrub, and in the ecotone between oak forest and tropical dry forest, at altitudes from 400 to 2000 m (Fig. 5). Specimens examined―COSTA RICA. Alajuela: cultivated, Estación experimental Fabio Baudrit, 3 km S.W. de Alajuela, R.W. Pohl 14083 (ISC, NY, US). Cartago: cultived, Río Reventazón Canyon, housing area: Instituto Interamericano de Ciencias Agrícolas, Turrialba. R.W. Pohl & G. Davidse 11765 (US). Cultived on grounds of IICA (Instituto Interamericano de Ciencias Agrícolas). T.R. Soderstrom 1842 (US). CATIE field. L.C. Umaña 15 (US). Campus CATIE. R.W. Pohl & M. Gabel 13576 (ISC). HONDURAS. Morazan: Cultivated, Campus of former arboretum of EAP, El Zamorano. A. Molina & A.R. Molina 34772 (MEXU). Cultivated as ornamental, Zamorano. J. Valerio Rodríguez 3030 (MEXU). Cultivated, El Zamorano, Escuela Agricola Panamericana. F.A. McClure 21484, 21605 (US). MEXICO. Chiapas: El Aguacero, canyon of the Río La Venta G. Davidse, M. Sousa, O. Téllez, E. Martínez & J. Davidse 30076 (ISC, MO). Autopista de Tuxtla a San Cristobal, puente El Federalista Km 14.5. E. Ruiz-Sánchez and J.L. Martínez 119, 150 (XAL). Chihuahua: Barranca de Batopilas, near Creel-La Bufa, road 15 km below (east of) La Bufa; south of side of the canyon. R.S. Felger & R. Russell 8104 (MEXU). Arroyo La Bufa, on S side of Barranca de Batopilas. R. Bye 7366 (MEXU). Sierra Madre Occidental. 1-2 km south of Rio Osichi and Rio 132 Basihuare junction. P.M. Peterson & P. Catalan 17638 (US). Colima: 2 km al S del crucero Manzanillo-Cihuatlán, brecha a Peña Blanca. F.J. Santana Michel & N. Cervantes 648 (IBUG, MEXU). Near top of pass, near Minatitlán on road to Manzanillo. A.A. Beetle et al 3524 (MEXU). 8 km de El Sauz camino al Terrero, 9-10 km al NE de Minatitlán. F.J. Santana Michel 4502 (IEB). Carretera nueva a Comala de Zapotitlan de Vadillo. E. Ruiz-Sánchez, N. Jimenez, De Nova & F. Rodriguez 101 (XAL). Brecha de carretera de Minatitlán a Campo Cuatro a 6 km del entronque. E. Ruiz-Sánchez, S. Ruiz. F. Rodríguez & J. Rodríguez 175 (XAL). 4 km al N de Campo Cuatro por el camino a Lagunitas. E. Ruiz-Sánchez, S. Ruiz, F. Rodríguez & J. Rodríguez 176 (XAL). Durango: Rancho El Purgatorio, por la vereda que baja al río Humaya. M. González 2589 (CIIDIR, IEB). Huasamota. J.N. Rose 3494 (US). Entre Pedro Paulo-San Blasato. J.N. Rose 3344 (US). Carretera Mezquital-Temoaya Km 5.5 del cruce a El Troncon. E. Ruiz-Sánchez & P. Carrillo-Reyes 113 (XAL). 5 km de El Troncón por el camino a Temoaya. M. González 1007 (CIIDIR). 7 km de Agua Zarca, camino a Temoaya. M González 45 (CIIDIR). Below Los Molinos (just below Topia). M. Kimnach & H. Sánchez-Mejorada 1798 (MEXU). Estado de Mexico: Sto. Tomás. Matuda et al 27493 (MEXU). Piedras Paradas, San Antonio Tlatlaya. Matuda et al 31081 (MEXU, MO, US). Cerro Los Capulines, Palmar Chico. Matuda et al 31331 (MEXU, US). Guanajuato: Barranca del Chilar, 39 km al SW de Cuerámaro. J. Rzedowski 45021 (IEB). La Barranca, Barranca El Chilar a 500 m rumbo a Cuerámaro de La Barranca. E. Ruiz-Sánchez & F. Rodríguez 173 (XAL). Guerrero: La Esperanza reserva campesina. N. Diego, D.E. Cruz & H. Ordonemum 7568 (MEXU). 4 km el E de Omitemi, camino a Xocomanatlán. G. Lozano V. 835 (MEXU). Cañon de La Mano entre los Amates y El Naranjo, 10 km al N de Iguala por el ferrocarril. C. Catalán, F. Terán & S. Vázquez 827 (IEB, MEXU). Cañon de La Mano entre los Amates y El Naranjo, 10 km al N de Iguala por el 133 ferrocarril. C. Catalán, S.D. Koch & F. Terán 521b (IEB) 522 (MEXU). Teloloapan, 6.3 km along road to El Caracol, Acatempan. L. Clark, P. Tenorio & G. Bol 486 (ISC, MEXU, MO, US). A 4.5 km al WSW de Tecoyame. I. Calzada & C. Toledo 15796 (FCME, MEXU). Teloloapán. H. Brailly s/n (MEXU). Km 60 on highway 51 between Iguala (km 1) and Arcelia (km 126); 2 km E of Teloloapán. H. Iltis, B. Benz & M. Burd 28712 (ENCB, F, MEXU, US). 6.5 km al N de Chilpancingo por la carretera a Iguala. S.D. Koch, P.A. Fryxell & T. Wendt 79102 (ENCB, IEB, ISC, NY). 11 Km al N de Iguala por la autopista a México. S.D. Koch, P.A. Fryxell & T. Wendt 7962 (CIIDIR, IEB, NY). Rte. 85, 14.5 km E of Iguala between Iguala and Cuernavaca, between km 84-85. L. Clark, P. Davila, B. Garofalo & A. Davila 331 (ISC, NY, US). Entre el Mogote y Cacahuamilpa. R. Guzmán 6060 (MEXU, US). Salto de Valadés, arriba de Chilpanchingo. E. Matuda 38407 (MEXU, US). Iguala cañon. C.G. Pringle 13924 (US). 5Km delante de Chilpancingo por la carr. federal a Acapulco. E.M. Piedra 279 (XAL). 3.8 km al O de Santa Cruz sobre el camino a Olinalá. P. Carrillo-Reyes, F:Z. Vaz de Mello & A. Abundis 4863 (XAL). Cañada Las Pozas, 2.5 km al NO de Jitotepec, cerro Xilotzin. E. Moreno Gutiérrez et al 943 (CIIDIR, FCME). 1 km por el camino a Tlalixtaquilla al este de la carretera HuamuxtitlánTlapa. J.L Contreras Jimenez 2468 (FCME). 1.5 km al N de Tecoyo. J. Calónico Soto 2175 (FCME). 3 km al SE de Amatitlan camino a Carrizalillo. M.E Garcia Granados 53C (FCME). 1.61 km al norte de Maguey. J. Calónico Soto 18121 (FCME). 4 km al S de Poliutla, camino a San Miguel Totolapan. O. Garcia, A. Monrey & G. Segura 62 (FCME). La Lucha Campo Morado. A. Ponce 684 (FCME). Hidalgo: Barranca de Tolantongo. F.G. Medrano & P. Hiriart 10272 (MEXU). La Barranca de Tolantongo, cerro de La Corona. F.G. Medrano & M. Pontet 10553 (MEXU). Barranca de Tolantongo 13.6 Km por carretera a Barranca de Tolantongo de El Cubo. E. Ruiz-Sánchez & P. Carrillo 114 (XAL). Jalisco: Lower W-facing slopes of Cerro 134 Grande at Km. marker 2, 13.4 km by new dirt rd. WSW of El Terrero, 1 km SE of Los Sauces 34 km NW of Colima. T.S. Cochrane, M.A. Wetter & R. Cuevas 11736 (IBUG). Arroyo Los Pajaritos. L.M.V. de Puga & R. McVaugh 16590 (IBUG). W-facing slopes, ca. 1km of Caseta Forestal in hairpin curve, 9.5 km due N of Casimiro Castillo (km 177). H. Iltis., S. Solheim & R. Guzmán 3114 (ENCB, IBUG, MEXU, US). Sierra de Manantlán. L.M.V. de Puga 12652 (IBUG). En la loma frente a la vía de ferrocarril al N de Tequila cerca de la preparatoria. D. de Niz & S. Fabian s/n (IBUG). Barranca del río Santiago camino a La Soledad, desviación a Ixcatán desde la carretera Guadalajara-Saltillo. E. Sahagú-Godínez, J.A. Lomelí & R. A. Leon-M 1366 (IBUG). 2 km al oeste de Tuxpan. R. Soltero & J. Perez de la Rosa s/n (IBUG). 1 km después de pto Los Mazos rumbo a Casimiro Castillo. R. de la Mora 336 (IBUG). Camino al volcán de Tequila cerca de Choloaca. J.O. Navarro & A. Navarro 219 (IBUG). Arroyo EL Tigre, 7 km distancia área al N de Casimiro Castillo en la carretera entre Autlán y la costa. J. Judziewicz, T.S. Cochrane & R. Guzmán 5153 (IBUG). La Cueva, cerro de Tamazula. I Romero 22 (IBUG, ISC). Rancho Los Guayabos al NE de Zapopán, inmediaciones del hospital Leaño. L.M.V. Puga 3168 (IBUG). Arroyo El Tigre, 4 km al NE de El Zapotillo. F.J. Santana Michel 2529 (ENCB, IBUG, XAL). Brecha El Tezcalame-La Lobera. O. Reyna, M. Chazaro & R. Delgadillo 302 (ENCB, IBUG, IEB, MEXU). 1 km después del crucero de Zuluapan, carretera a Santo Tomás. R. Guzmán, R. de la Mora y F. Santana 4162 (IBUG, MEXU). Km 42 por la brecha El Tuito-Minas Zimapán. F.J. Santana-Michel, R. Guzmán & J. Perez de la Rosa 1212 (IBUG). 3 km al N de el Zapotillo por la carretera Melaque-Autlán s/n (IBUG, MEXU). Como a 3-4 km después de Tequila rumbo a la estación de microondas, cerro de Tequila. M. Cházaro 4208 (IBUG, IEB). Cerro El Narigon al N, exposición norte. F.J. Santana Michel 398 (IBUG). Cerro El Narigón, al N del poblado El Limón F.J. Santan Michel 1182 (IBUG, MEXU) 21 Km 135 por la brecha San Sebastián-Pto. Vallarta al W de San Sebastián. F.J. Santana Michel 1007 (IBUG, MEXU). 33 Km al SE de Autlán, carretera a Barra de Navidad. F.J. Santan Michel 801 (IBUG, MEXU). Sierra de Quila, 1 km al NNE de Lagunillas casi en el arroyo Palmillas. J.J. & E. Guerrero Nuño 314 (IBUG). Rancho La Higuera. F.J. Santana Michel, L. Clark & P. Tenorio 6210 (IBUG). Barranca de Huentitán, cercano al arroyo del zoológico. R. Acevedo & M. Hernández-Galaviz 1692 (IBUG, MEXU). Arroyo de los Pajaritos, junto al balneario, 5 km al SW de Sta. Lucía. F.J. Santana Michel 1233 (IBUG). Desviación al rancho el Rodeo, en la cascada Salto del Nogal. G. Coronado 57 (IBUG). 8 km al N de Bolaños, por la brecha a Tuxpan. F.J. Santana Michel 663 (IBUG, MEXU). La Venta de Nochtiltic, a 3 km a El Saucillo, por la carretera Magdalena-Ixtlán del Río. F.J. Santana Michel 1186 (IBUG). Por San Cristobal. Navarro & Cervantes 168 (IBUG). Km 82 brecha Tecalitlán-Jilotlán de los Dolores. F.J. Santana Michel 1078 (IBUG, MEXU). Rancho El Casco, ca. 15 km al SW de Tapalpa. E.J Lott 323 (MEXU). 8 to 10 miles southwest of Autlán. H.S. Gentry 10948 (MEXU, US). Barranca of Río Verde, between Tepatitlán and Yahualica. R. McVaugh 26700 (MEXU). Río Cihuatlán below the bridge 13 miles north of Santiago. R. McVaugh 15946 (MEXU, NY, US). 9 km adelante de Tecolotlán, carretera para Juchitlán. J.I. Calzada & J. Elizondo 8395 (ENCB, MEXU). Al S de Atenquique, en el puente por la carretera a Colima. R. Guzmán 4585 (MEXU). El Rodeo, a small pueblo in a deep valley between the Sierra de Manantlán Oriental and cerro Toxin; ca. 21 km SE of El Chante (ca. 35 km ESE of Autlán. H.H. Iltis, B.F. Benz & M. Burd 28935 (IEB). Road from Autlan to Casimiro Castillo, 1 km below Puerto Los Mazos. H.H. Iltis, B.F. Benz., A. Vazquez & M. Cházaro 29454a (IEB). Barranca de Huentitán, 2 km al E de Tonalá. A. Flores & M. Cházaro 2437 (ENCB, IEB). 5 km al sur de los Mazos, por la carretera Autlán-Barra de Navidad. R. Guzmán 6122 (ISC, US). Montañas al SSE de Puerto Vallarta, al E 136 de El Tuito, 42 km por la brecha el Tuito-Cuale. R. Guzmán 6095 (MEXU, US). 10 km de Autlán hacia Barra de Navidad, orilla de carretera. G. Cortés s/n (US). 25 Km al S de Autlán, sobre la carretera a Barra de Navidad. J. Rzedowski 14725 (US). Slopes of a ravine a Los Arboles near Autlán. F.A. McClure 21202 (US). La Venta de Mochitiltic, a 3 km del Saucito por la carretera Magdalena-Ixtlán del Río. R. Guzmán 6071 (MEXU, US). Carretera Puerto Vallarta al Tuito Km 25. E. Ruiz-Sánchez, N. Jimenez, De Nova & F. Rodriguez 97 (XAL). Carretera libre a Colima de Guadalajara. E. Ruiz-Sánchez, N. Jimenez, De Nova & F. Rodriguez 102 (XAL). Michoacán: Los Chorros del Varal 30 km al NW de Los Reyes. L. Avila s/n (IBUG). A 5 km la N de La Huacana, carretera Pátzcuaro-La Huacana. E.J Lott 1874 (MEXU, US). Los Filtros Viejos, aprox. 2 km al E de Morelia. E. Pérez C. et al 2183 (IEB, MEXU). 24 km from Tiquicheo on Hwy 51 between Erendira and Tiquicheo, road Cd. Altamirano-Morelia. L. Clark, P. Tenorio & G. Bol 487 (ISC, MEXU, MO, NY, US). 15 km south of Taretán by the MoreliaLázaro Cárdenas autopista. V.W. Steinmann 2000 (IEB). Los Filtros Viejos, aprox. 2 km al E de Morelia. E. Pérez C. & H. Díaz 2192 (IEB). 13 km al norte de Nueva Italia sobre la carretera a Uruapan. S.D. Koch 77444 (CIIDIR, ENCB, IEB, ISC, NY, US). 35 Km al N de Huetamo, por la carretera a Zitácuaro. S.D. Koch & P.A. Fryxell 8390 (CIIDIR, ISC, NY, US). Monte de San Aparicio. E. Langlasse 24 (US). West-facing slopes of Cerro Carboneras above the Río Cupatitzio, ca. 22 km south of Uruapan. T.R. Soderstrom 4866a (US). Carretera de Huetamo a Tiquicheo a 7 km de Erendira a Tiquicheo. E. Ruiz-Sánchez, E. Gándara & D. Ángulo 181 (XAL). Río San Carlos a 300 m después de San Carlos y antes del Tamarindo, carretera Tiquicheo-Morelia km 38. E. Ruiz-Sánchez, E. Gándara & D. Ángulo 182 (XAL). Morelos: El Mogote. J. Vázquez 2147 (MEXU). Nayarit: Margenes del río Santiago, aprox. 2 km río abajo del arroyo El Platanar. R. Acevedo & J. Sosa s/n (MEXU). 32.5 km al NE de Jesús María, La 137 China. G. Flores & P. Tenorio 996 (IEB, MEXU). Km 2-4 de la vereda de la Mesa del Nayar al Cangrejo (Villa de Guadalupe), cruzando el barranco. O. Tellez, P. Tenorio, G. Flores, A. Cadena & C. Ramírez 12463 (IEB, MEXU, MO). Arroyo El Salitre, Río Santiago, 3 km río abajo de Paso Golondrinas. R. Acevedo & J. Sosa 1083 (IEB). Mountains 10 miles southeast of Ahuacatlán, on road to Barrancas del Oro. R. McVaugh 15160 (US). Between Tepic and San Blas, near Otates, cultived en Quail Botanical Garden. R. Haubrich 8101.652-D (ISC). Carretera libre Tepic-Mazatlan Km 13 a 200m al rancho La Godorniz. E. Ruiz-Sánchez, N. Jimenez, De Nova & F. Rodriguez 96 (XAL). Oaxaca: Puente Morelos, presa El Boqueron 3 km al NE de Tonalá, carretera a Huajuapán. J.I. Calzada 18547 (MEXU). Arroyo El Tapesco, 2 km al S del Poblado, 37 km al O de Tehuantepec entrando por Pozo Zorrillo. C. Martínez 1344 (MEXU). La Loma 5 km al W de San Jorge Nuchita, hacia Yucuyachi. J.I. Calzada, A. Ramos & R. Torres 18250 (MEXU). Hwy. 131, Tehuacán-Oaxaca, ca. 8-10 km past San Juan Bautista de Cuicatlán, Km 149. L. Clark, M. Chazaro, P. Tenorio & G. Bol 451 (ISC, MO, NY, US). 30 km de Huahuapan al oeste, 9 km de San Marcos al oeste. A.A. Beetle 4613 (US). By small tributary stream of Rio San Carlos east of road camp 75 kms. From El Temascal on road to Huetamo. H.E. Moore, Jr., E. Hernandez X., H. Porras 5671 (US). Autopista Oaxaca-Mexico Km 107.2. E. Ruiz-Sánchez and J.L. Martínez 125 (XAL). Km 159-160, carretera libre Oaxaca-Tehuacán. E. Ruiz-Sánchez, F. Rodriguez & V. Sosa 218 (XAL). Ladera Oeste de Cerro Pluma. P. Tenorio 20635 (CIIDIR). Puebla: Montañas al E de Santana Coatepec, al SSE de Atlixco. R. Guzmán 6065 (ISC, MEXU, US). Matamoros. F. Miranda 2411 (MEXU). Mitepec. Vázquez Rojas 61 (MEXU). Rancho San Antonio, 10 km al NW de Molcaxac, brecha a Huatlatlauca. P. Tenorio, C. Romero & F. Tenorio 7669 (MEXU). Past Molcaxac, ca. 3 km befote Tepeje de Rodríguez. L. Clark, P. Tenorio, M. Chazaro & G. Bol 450 (ISC, MEXU, MO, NY, US). Northwestern slope 138 of cerro de Chantepec, 2 miles south of Pilcaye. F.A. McClure 21201 (F, US). 13 km SE of Izucar de Matamoros on highway to Oaxaca. T.R. Soderstrom 2250 (US). Carretera Tepexi a Puebla Km 5.2 saliendo de Tepexi. E. Ruiz-Sánchez and J.L. Martínez 126 (XAL). Queretaro: Un km al S de Escanelilla, por la carretera Pinal de Amoles-Ahuacatlán. R. Guzmán 5990a (MEXU, US). Carretera de Jalpan a Pinal de Amoles km 163 a 1 km pasando Escanelilla por las barrancas del Río Escanelilla. E. Ruiz-Sánchez, E. Gándara & J. Alvarez 226a (XAL). Sinaloa: Between Mazatlán and Durango near La Guayanera. A.A. Beetle et al 3667 (MEXU). La Dispensa near Rosario. F.A. McClure 21204 (F, IEB, ISC, MO, NY). 32 miles from Mexico route 15 along route 40. M.L. Roberts & D. Keil 10185. (US). Carretera Durango a Mazatlan a 1.5 Km de Chirimollos entre Santa Lucia y Chirimollos. E. Ruiz-Sánchez, P. Carrillo & De Nova 105 (XAL). El Guayabito, carretera Tepuche-El Espejo a ± 50 km al N de Culiacán. R. Vega & J.A. Hernández 9171 (UAS). A 5 km al Sureste de piedras blancas, rivera de arroyo San Pablo. R. Vega & H. Aguilar 9684 (UAS). Sonora: Arroyo San Ana at SON. 12 (Tepeca-Cd. Obregon highway), 2.3 km southwest of the turnoff La Quemada, 8.5 km by air west of Guadalupe Tayopa. T.R. Van Devender et al 97-1057 (MEXU, USON). Cueva de La Borrega, arroyo Infiernillo. M. Fishbein, G. Ferguson, S. Hale & A. Porras 988 (MEXU). Rancho san Ysidrio (ruins) at mouth of Arroyo Chinalito, a side canyon on the Río Sátachi, ca 8 airline km NE of Nacori Chico. R.S. Felger, J.T. Marshall & P. Marshall 3320 (MEXU, MO, NY, USON). Sierra de Alamos, arroyo Igualama, just below and to the north of Pico de Aguila. V.W. Steinmann 1319 (IEB). Conejos, Río Mayo. H.S. Gentry 1103 (F, US). El Pilar a Ciudad Obregon a 10 Km del entronque a Yecora y a 2.5 Km de la Quemada. E. Ruiz-Sánchez, P. Carrillo & De Nova 112 (XAL). Veracruz: Cerros calizos al suroeste de Cuetzala. M. Chazaro, L. Robles & J.L. Tapia 5805 (IBUG, IEB, MEXU). Barranca sobre el río Los Pescados, a la orilla de la carretera G. 139 Cortés & M. Nee 60 (IBUG, MEXU, US). Un km al SSE de Jalcomulco. R. Guzmán, H. H. Iltis & B. Benz 6057 (MEXU, US). Ejido Tlacotalpan, Mezeta del Barro por la vereda que va de Jalcomulco al Palmar. G. Castillo & L. Tapia 1143 (MEXU). Barranca Los Pescados, 2 km before Puente Los Pescados. L. Clark, M. Chazaro, P. Tenorio & G. Bol 456 (ISC, MEXU, US). Arroyo Techacastla, Valle de Río Pescados, ca. 1 km SSE of Jalcomulco. H.H. Iltis, B.F. Benz, R. Guzmán & M. Burd 28960 (IEB, MEXU, MO, NY, US). Barranca Los Pescados. L.G. Clark, G. Cortés, I. Calzada & D. Farrar 1312 (MEXU, US). Lomas aledañas a la curva de Cerro Gordo. 2 km sobre la carretera Cerro Gordo-Veracruz. G. Cortés, G. Cooper & J. Mora 99-14 (MEXU). Barranca de Panoaya, 1.5 km al NO de EL Coyol. N.E. Medina & G. Castillo 871 (ENCB, IEB). 1 km south of Rio Pescados on Huatusco-Jalapa road. S.M. Young 203 (US). Jalcomulco. Liebmann 127 (US). Steep, rocky, calcareous canyon walls of Río de Pescados, Barranca de Xinacatlla, 3 km SW of Jalcomulco. T.R. Soderstrom 2233 (US). Carretera de Coatepec a Cordoba Km 24 rancho El Campanario. E. Ruiz-Sánchez, & F. Rodriguez 103 (XAL). Zacatecas: San Juan Capistrano, límite de los estados de Zacatecas y Durango. J.J. Balleza 2366 (IEB). UNITED STATES. California: Cultivated, Santa Barbara, 2696 Dorking Place. C.R. Annable & R. Crombie 4034. (NY). Cultived, San Diego Zoo. J.B. Walker 1727 (NY). Hawaii: Cultived, Kauia: Koloa district, Kalhaeo. T. Flynn 5820 (US). Harris county: Bamboo garden. S.M. Young 589 (US). 2. Otatea carrilloi E. Ruiz-Sánchez, Sosa and T.M. Mejia-Saulés, sp. nov. TYPE: MEXICO. Chiapas: municipio de Tonalá, Ejido Raymundo Flores, vereda que va a El Filo, 843 m, 25 Sep. 140 2006, E. Ruiz-Sánchez, & R. Córdoba 147 (holotype: XAL; isotypes: IBUG, ISC, MEXU, MO, NY, US). Fig. 6. Otatea acuminatae similis sed folii caulinaris lamina reflexa et vagina foliari majore, differens. Vaginae caulinares fimbrias bene evolutas erectas ad 19 mm longas deciduas ferentes. Folia ramique fimbrias erectas persistentes ad 21 mm longas ferentia. Vaginarum caulinarium setae orales ad 30 mm longae, foliorum frondosarum setae ad 24 mm longae. In juventute fimbriae setaeque orales virides, deinde luteae et stramineae. Rhizomes sympodial, pachymorph, the necks 5-12 cm long. Culms 3-5 m tall, 1- 3.5 cm in basal diameter, erect and apically arching when old; internodes 11-19 cm long, terete, glabrous, pruinose between internodes, green when young and yellow when old, hollow, the walls 3.5-6.9 mm thick, the lacuna occupying > 50% of the total diameter.Culm leaves 38-54 cm long overlapping and deciduous, sheaths 19-28 cm long, 3-7 cm wide at the base, the leaf blades 1-1.4 times as long as the sheaths or sometimes the sheaths larger than blades, ± rectangular, glabrous to abaxially hispid for the upper 1/2 to 2/3, the shoulders rounded and sometimes with a small extension at apex about 2-8mm long, the margins ciliate; inner ligule 2.5-6.3 mm long, irregular; oral setae 15-30.6 mm long, 0.2-0.9 mm wide, green-yellow, free for the inner ligule, flattened, retrorsely scabrous, green in living specimens; fimbriae along the apex of the sheath on both surfaces of the blade, 10.3-19.3 mm long, 0.2 mm wide, terete, free, straight, retrorsely scabrous; blades 10-31 cm long, triangular, reflexed, adaxially and abaxially glabrous, the margins fimbriate at base and the rest glabrous, these frimbriae 8.8 to 15 mm long 0.2 mm wide, terete, retrorsely scabrous, apex attenuate-subulate. Branching intravaginal; three main, subequal 141 branches per node originating on a promontory, these diverging from each other and rebranching, 27-40 cm long, diverging from the main culm at 35-75 o; supranodal ridge pronounced; nodal line horizontal. Foliage leaves 3-5 per complement; sheaths glabrous, rounded on the back, prolonged apex of sheath present; outer ligule with an irregular glabrous or ciliolate rim up to 0.1 mm long without lobes; inner ligule 0.6-1.4 mm long, truncate; oral setae 6.6-24.1 mm long, 0.2-0.3 mm wide, green-yellow, slightly flattened, scabrid, fused with the inner ligule; fimbriae on both surfaces of the blade at the large apex, 8.4-21.8 mm long, 0.1 mm in diameter, more or less terete, scabrid, free; blades 18-26 cm long, 0.6-0.9 cm wide, linear to linear-lanceolate, adaxially glabrous, green-glaucous, abaxially glabrous, green, patch of cilia at the base of the leaf blade along one side of the midrib, 5-8 mm long, white; midrib centric, the base attenuate, the apex attenuate-subulate, the margins weakly serrulate; pseudopetioles ca. 2 mm long, greenish, pulvinate at the base. Synflorescences not seen. Paratypes―MEXICO. Chiapas: municipio de Tonalá, Ejido Raymundo Flores, 2.75 km al E. de Raymundo Flores, 860 m, 2 March 2006, P. Carrillo-Reyes, D. Cabrera-Toledo y M. A. PerezFarrera 5144 (XAL); aproximadamente 6-8 km al Norte del Ejido Raymundo Flores, al interior de la reserva La Sepultura, 900 m, 10 Sep. 2007, F. Nicolalde-Morejón & J. González-Astorga 1584 (XAL). Etymology. The specific epithet honors Pablo Carrillo-Reyes, a Crassulaceae taxonomist and an enthusiast collector, who was the first to gather plants of this species. Phenology. Flowers of this species have never been collected. 142 Distribution and habitat This species has been found only in Chiapas, from a single population. It grows on slopes in tropical dry forests from 850 to 1000 m (Fig. 7). Disscussion. Otatea carrilloi is similar to O. acuminata and O. glauca but it differs in having erect and larger fimbriae. 3. OTATEA FIMBRIATA Soderstr., Fl. Novo-Galiciana: Gramineae, 14:280. 1983. TYPE: MEXICO. Chiapas: municipio de San Fernando, Cañón El Sumidero, ca. 20 km N of Tuxtla Gutiérrez, T. R. Soderstrom 2245 (holotype: MEXU!; isotypes: CHAPA, DD, K, LE, MICH, MO!, P, PRE, US!) Rhizome neck 20-30 cm long. Culms 2.5-6 (-8) m tall, 1-6 cm in basal diameter, smooth, pruinose below nodes, solid or hollow, with very thick walls and small lacunae. Culm leaves deciduous as the branches develop, sheaths stramineous with appressed spicules, deciduous from the main culm, overlapping and persistent on the branches; oral setae at apex of the sheath 1-1.5 cm long, seeming like a ligule or fused into a broad lacerate scale, bright purple or brown when new, fading to brown or black; leaf blades triangular, erect, persistent. Branching intravaginal; three main, subequal branches per node borne on a promontory, divergent and branching again, 50 to 100 cm long; supranodal ridge pronounced; nodal line dipping slightly below the bud. Foliage leaves 5-7 per complement, sheaths glabrous; orale setae 10-15 mm long, brown or purple connate at the base, frimbria, free or absent; blades 20-33 cm long, 1-3.6 cm wide, glabrous, or commonly pilose (specially when young) in a patch near base on the abaxial surface, yellowish to brownish, extending along one side of the midrib; midrib excentric; cross-veins obscure, or prominent abaxial surface; leaf blades of secondary or smaller branches much 143 smaller and narrower, 0.5-1.2 cm wide. Synflorescence 10-15 cm long, with more than 30 spikelets. Spikelet ca. 2 cm long, 3-4 florets per spikelet, on thin pedicels 1-2 cm long; florets closely overlapping, the apical a rudimentary floret, smaller, aborting; rachilla joints about half as long as the florets or shorter; glumes awned, keeled, 7-nerved; glume I ovate-lanceolate, 5-7 mm long including the awn; glume II oblong-lanceolate, 7-9.5 mm long including the awn; lemmas 10-13 mm long including the awn, ovate-lanceolate with 10 prominent scabrous nerves; palea about as long as the lemma, with ample broad inflexed margins, entire, apex and margins scabrid; anthers 4.5-5 mm long. Caryopsis 6.4-6.8 mm long, fusiform with a broad linear hilum extending through the length of caryopsis. Phenology. Flowering in September and in October. Distribution and habitat. Otatea fimbriata has a disjunct distribution. It has been collected in Mexico (Chiapas), Central America (El Salvador and Honduras), and Colombia. The ecological conditions of O. fimbriata are similar in all of the locations. On the southern Pacific slopes (Chiapas) in dry oak and tropical forests at altitudes from 1000 to 1300 m, while in Colombia populations are found in tropical dry forest at 1230 m (Fig. 5). Specimens examined―COLOMBIA. Norte de Santander: Vereda San Luis, aproximadamente 4 km de Ocaña por la via Abrego, margen derecha del Río Algodonal. X. Londoño, A. Amaya, J. Jacome y M.V. Forgioni 884. (CUVC, NY, TULV, US). Vía de Ocaña a Abrego 7.8 km a orillas del Río El Algodonal. X. Londoño & E. Ruiz-Sánchez 987 (CUVC, TULV, XAL). El SALVADOR. Ahuachapán: Parque Nacional El Imposible, hacienda San Benito, Peña Reventada. O. Rivera Dávila s/n (MO). North of Tacuba F.A. McClure 21623 (MO, US). Usulatán: Upper slopes of the Volcan El Tigre above Santiago de María beyond the Hacienda Las Brisas. F.A. McClure 21617 (ISC, MO, NY, US). Volcan Usulatán above Hacienda San 144 Mariano F.A. McClure 21620 (MO, US). Summit of Volcán de Usulután. S. Calderón 2534 (US). HONDURAS. El Paraiso: Río Lizapa Departamento de Pariso, entre Galeras y Lizapa Grande. A Molina s/n (US). MEXICO. Chiapas: cliff faces and limestone bluffs at El Sumidero, 22 km north of Tuxtla Gutiérrez. D.E. Breedlove 27177 (ENCB, MEXU, NY, US). 15 km southwest of Suchiapa along road to Villa Flores. D.E. Breedlove 28085 (ENCB, MEXU, NY, US). Near Tuxtla Gutiérrez, cañones El Sumidero befote Mirador La Coyota. L. Clark, P. Tenorio & G. Bol 469 (ISC, MEXU, MO, NY, US). 36 Km W of Tuxtla Gutierrez on highway 190 to San Cristobal de las Casas. G. Davidse & J. Davidse 9476 (ISC, MEXU, MO). 5-6 km from Avda. 5a in Motozintla de Mendoza, on the road to Cerro Mozotal and El Porvenir. L. Clark, P. Tenorio & G. Bol 478 (ISC, MEXU, MO, NY, US). Carretera de Cañon Sumidero, 16 km N de Tuxtla Gutiérrez. S.D. Koch 75590 (ENCB, US). Km 16 camino al cañon del Sumidero. G. Cortés & J.I. Calzada 105 (ENCB, US). Km 29 sobre la carretera Tuxtla Gtz. a San Cristobal en la desviación para Pichcalco. G. Cortés 314 (MO). Forest near the Zinacantán Paraje of Muctajoc. D.E. Breedlove 53992 (NY, US). 8 km east of Las Margaritas along road to La Soledad. D.E. Breedlove 37939 (US). Km 15-16 road N of Tuxtla Gutiérrez along El Sumidero Canyon. J. Bauml., M. Kimnach & H. Sánchez-Mejorada 577 (US). Cañon del Sumidero. G. Cortés & H. González 35 (US). On road right of way along forested mountain slopes; 37 km west of San Cristobal. F.W. Gould 12703 (US). Autopista Tuxtla a San Cristobal de las Casas Km 21.2. E. Ruiz-Sánchez & J.L. Martinez 118 (XAL). Carretera Tuxtla a San Cristobal entroque a carretera a Bochil. E. RuizSánchez, J. Pacheco & X. Galarza 136 (XAL). Km 45.7 carretera antigua o libre a San Cristobal 145 de las Casas. E. Ruiz-Sánchez 153 (XAL). Colonia Roblada Grande. E. Ruiz-Sánchez, A. Robles, H. Robles & F. Robles 155 (XAL). 4. OTATEA GLAUCA L.G. Clark & G. Cortés, J. Amer. Bamboo Soc. 18: 3-6. 2004. TYPE: Mexico. Chiapas: municipio de Motozintla, Tolimán, km 39 Huixtla-Motozintla, en cañada a la orilla del río, 600 m, 20 Jan 2003, G. Cortés & W. Sánchez 306 (holotype: MEXU; isotype: ISC, MO!, US!, XAL!). Rhizome neck at least slightly elongated. Culms up to 8 m tall, 3 cm in basal diameter, erect; internodes 27-30 cm long, terete, glabrous, glaucous especially when young, hollow with the walls 1.5-2 mm thick, the lacuna occupying > 50% of the total diameter. Culm leaves 18-30 cm long; sheaths 14-22 cm long, 8-17 cm wide at the base, 2.4-5.2 times as long as the blades, triangular, abaxially hispid on upper 1/2 to 2/3, the shoulders rounded, the margins glabrous; inner ligule 0.4-0.5 mm long, truncate, ciliolate; oral setae 2.5-11.5 mm long, 0.4-0.8 mm wide, free from the inner ligule, flattened, connate at the bases for up to 2 mm, splitting into narrower segments above, these straight to barely curved and retrorsely scabrous-hispid; fimbriae at sheath apex on both blade surfaces, 1.5-4 mm long, 0.1-0.3 mm long, more or less terete, free, curly, retrorsely scabrous; leaf blades 3.5-8.2 cm long, triangular, reflexed, deciduous, adaxially densely pubescent, abaxially glabrous, apex attenuate or subulate. Branching intravaginal; three subequal branches per node originating on a promontory, diverging and branching again, up to 80 cm long, diverging from the main culm at 45-90o; supranodal ridge pronounced; nodal line more or less horizontal. Foliage leaves 4-5 per complement; sheaths glabrous, weakly keeled at apex, sheath apex extension absent; outer ligule an irregular glabrous or ciliolate rim to 0.2 mm 146 long; inner ligule 0.2-0.5 mm long, truncate; oral setae 2.5-6 mm long, 0.1-0.2 mm wide, slightly flattened, scabrid, free from inner ligule; fimbriae on both blade surfaces at apex, 1-4 mm long, 0.05-0.1 mm in diameter, more or less terete, scabrid; blades 10-16 cm long, 0.3-1 cm wide, linear to linear-lanceolate, green, adaxially glabrous, rarely pilose, not tessellate, abaxially densely pubescent at the base, with hairs extending along the midrib at base and the rest glabrous or pilose over much of surface, weakly tessellate, midrib centric, the base attenuate, the apex attenuate-subulate, the margins serrulate, sometimes weakly so; pseudopetioles ca. 1 mm long, white, pulvinate at the base. Synflorescences 4-9 cm long, racemose; rachis flattened to angular, scabrous-pubescent; pedicels 2.5-5 mm long, angular, scabrous-pubescent. Spikelets 3-4 cm long, laterally compressed, 3-5 florets per spikelet with an additional apical rudimentary floret; rachilla joints 3.5-5 mm long, minutely pubescent, densely pubescent at the apex on each joint; glumes narrowly triangular and navicular, abaxially glabrous awned; glume I 6-9.5 mm long including the awn, 7-9-nerved, the awn 1.3-3.6 mm long; glume II 9.5-14 (-17) mm long including the awn, 9-11-nerved, the awn 2-5 mm long; lemmas 14.5-21 mm long including the awn, narrowly, triangular and navicular, abaxially scabrous-pubescent, cross-vein evident, 1115-nerved, awn 3-4.7 mm long, antrorsely scabrous; paleas 14-15.4 mm long, 6-nerved, the keels scabrous, sulcate for the full length, the sulcus pubescent on the upper half, scabrid bellow, wings glabrous, apex bifid, the teeth acute. Lodicules apically ciliate, basally slightly thickened; the anterior pair 1.5-2 mm long, the posterior one narrower, ca. 1.6 mm long. Ovary glabrous; stigmas 2, plumose. Fruit not seen. Phenology. Flowers from January to April and in September. 147 Distribution and habitat. O. glauca is endemic to Chiapas and recorded from two or three localities. This species grows in the ecotone of tropical dry forests and oak forests at approximately 1200 m. (Fig. 7). Representative specimens examined―MEXICO. Chiapas: 37 km before Huixtla, Hwy. 190 between Motozintla de Mendoza and Huixtla. L. Clark, P. Tenorio & G. Bol 481 (MEXU, NY). UNITED STATES. California: cultived, Encinitas, Quail Botanical Garden. L.G. Clark 1334. (US). Los Angeles, CA. Jardín Botánico de Quail. G. Cortés & G. Cooper 333 (US). Carretera Motozintla a Huixtla km 39. E. Ruiz-Sánchez 144 (XAL). 5. Otatea mixtecana E. Ruiz-Sánchez and Londoño, sp. nov. TYPE: MEXICO. Oaxaca: hills of Las Sedas, 6000 ft, 21 Jul. 1897, G. C. Pringle 6742 (holotype: US!, isotype: CM, ENCB!, F, MO). Fig. 8. Otatea fimbriatae similis sed culmi diametro quam 1 cm non majore, folii frondosi lamina reflexa, vaginis minoribus et ramorum foliis angustioribus, differens. Setae orales foliares purpureae ad 6 mm longae. Spiculae azureo-purpureae, glumis scabro-pubescentibus. Rhizomes sympodial, pachymorph, neck 3-9 cm long. Culms 2-3 m tall, 0.6 to 1 cm in basal diameter, erect; internodes 20.5-29 cm long, terete, glabrous, greenish to glaucous, pruinose between internodes when young and ochreous when old, solid when young and hollow when old, the walls 1.8-3.1 mm thick, the lacuna occupying < 50% of the total diameter. Culm leaves 11.517.5 cm long not overlapping and deciduous; sheaths 9.5-12.5 cm long, 3.5-4 cm wide at the base, 2-4 times as long as the blades, ± rectangular, abaxially glabrous, the shoulders rounded 148 and sometimes with a small prolonged apex about 2-3 mm long, the margins ciliate; inner ligule 1.1-1.8 mm long, truncate; oral setae 4.5-12.8 mm long, 0.1-0.3 mm wide, free in the inner ligule, flattened, retrorsely scabrous, purplish in living specimens; fimbriae at apex of sheath on both surfaces, 3.9-4.8 mm long, 0.1 mm wide, flattened, curly, retrorsely scabrous; leaf blades 1.6-7.5 cm long, triangular, reflexed, deciduos, adaxially and abaxially glabrous, the margins ciliate, apex attenuate subulate. Branching intravaginal; three subequal branches per node, these diverging from each other and rebranching, 17-42 cm long, diverging from the main culm at 4570o; supranodal ridge pronounced; nodal line horizontal. Foliage leaves 3-4 per complement; sheaths glabrous, weakly keeled at apex, with an extended apex; outer ligule an irregular glabrous rim to 0.4 mm long without lobes; inner ligule 1-1.5 mm long, rounded; oral setae 4.3-6 mm long, 0.05-0.1 mm wide, flattened, glabrous, fused with the inner ligule, purple in living specimens with green apices; fimbriae absent; blades 19-27 cm long, 0.7-1.08 cm wide, linearlanceolate, adaxially slightly pilose, glaucous, abaxially scabrous, green, patch of cilia at the base of the blade very dense, white, extending along the midrib 5-10 mm and the rest scabrous; midrib excentric, the base attenuate, the apex attenuate subulate, the margins serrulate, pseudopetioles ca. 0.5 mm long, purple, pulvinate at the base. Synflorescences 6-15 cm long, paniculate, 3-5 spikelets per synflorescence, purple-blue; rachis flattened to angular, slightly scabrous; pedicels 5-20 mm long, angular, scabrous. Spikelets 2-3.5 cm long, 3-5 florets per spikelet with an additional apical rudimentary floret; rachilla joints 4-5 mm long, pubescent and densely pubescent at the apex of each joint; glumes 2, narrowly triangular and navicular, abaxially scabrous-pubescent, awned; glume I 6-11 mm long including the awn, 5-nerved, the awn 1-5 mm long; glume II 8-12.7 mm long including the awn, 7-nerved, the awn 1.5-5 mm long; lemmas 11.5-17 mm long including the awn, narrowly triangular and navicular, abaxially scabrous 149 pubescent, cross-veins evident, awned, 9-15- nerved, the awn 2.3-7 mm long, antrorsely scabrous; paleas 8.3-11 mm long, the keels scabrous, sulcate for the full length, the sulcus pubescent on long scabrous wings, apex bifid with an acute teeth. Lodicules apically ciliate; the anterior pair 1-1.3 mm long, the posterior one narrower 0.7-0.9 mm long. Anthers 6.5-7.4 mm long. Ovary yellow-amber, glabrous, ca 1-1.5mm long. Fruit not seen. Paratypes―MEXICO. Oaxaca: Tlaxiaco, municipio de San Pedro Molinos, km 64 de la carretera Tlaxiaco-Putla, 2000 m, 3 Ago. 1994, J. Panero & I. Calzada 4441 (IEB, MEXU). Distrito Ejutla, municipio de San Martín Lachilá, 2.7 Km al Oeste de El Vado sobre el camino a San Sebastián de las Grutas, 1525 m 7 Nov. 2005, P. Carrillo-Reyes, FZVM & AAS 4986 (XAL). 2.2 Km al O. de El Vado sobre el camino a San Sebastián de las Grutas, 1458 m, 14 Jun. 2008, E. Ruiz-Sánchez, F. Rodriguez & V. Sosa 217 (XAL). Etymology. The specific epithet is in relation to its distribution in the Mixtec region of Oaxaca, Mexico. Phenology. Flowering from July to August. Distribution and habitat. Endemic to Oaxaca and recorded from three localities.This species grows on slopes in dry pine-oak-juniper and tropical forests, from 1500 to 1700 m. (Fig. 7). Discussion. Morphologically, Otatea mixtecana is similar to O. fimbriata but differs in having culms smaller in diameter and the culm leaf blades are reflexed and smaller. One of the most striking characters of O. mixtecana is the blue-purple color of its spikelets (see Table 1). 150 6. Otatea reynosoana E. Ruiz-Sánchez and L.G. Clark, sp. nov. TYPE: MEXICO. Guerrero: District Minas, Río Frio, 1500 m, 11 Nov. 1936, G.B. Hinton 9879 (holotype: US!; isotype: MO). Fig. 9. Otatea fimbriatae similis sed differens setis oralibus albis papyraceis in vaginis caulinaribus et in foliis frondosis, folii caulinare recta, ciliarum caespede albo usque 8.5 mm longo ad laminae basem in pagina adaxiale posito et ad nervi centralis latera extenso, et floribus cum glumis majoribus. Rhizome neck 10-30 cm long. Culms to to 3-6 m tall, 1-6 cm in basal diameter, erect to apically arched; internodes 11-19 cm long, terete, glabrous, pruinose, light green when young and greenyellow when old, solid at based and hollow in the first third, the walls 2-4 mm thick, the lacuna occupying < 50% of the total diameter. Culm leaves 24-54 cm long overlapping and persistent; sheaths 16-31 cm long, 4-9 cm wide at the base, 1.2-1.8 times as large as the blades, rectangular, glabrous or slightly hispid abaxially for the upper 2/3, the shoulders with a small apex, about 25mm long, the margins ciliate; inner ligule 0.5-2.5 mm long, truncate, glabrous; oral setae 7-17.3 mm long, 0.1-0.4 mm wide, free for the inner ligule, flattened, glabrous, white; fimbriae absent or sometimes present at the end sheath summit on either side of the blade, 2-7 mm long, 0.05 mm wide, flattened, curly, glabrous, free; leaf blades 8-23 cm long, triangular, erect, deciduous, adaxially and abaxially glabrous, the margins ciliate, apex subulate. Branching intravaginal; one or two main, subequal branches per node sometimes three or more, 40 to 75 cm long, diverging from the main culm at 45o; supranodal ridge pronounced; nodal line more or less horizontal. Foliage leaves 4-5 per complement; sheaths hispid, rounded on the abaxial surface, sheath 151 prolonged apex absent; outer ligule an irregular glabrous rim to 0.1 mm long with two lobes on each side of the apex, 0.6-1.5 mm long, 0.8-1.8 wide; inner ligule 0.3-0.5 mm long, truncate; oral setae 5.1-11.2 mm long, , flattened, glabrous, free from the inner ligule, whitish; fimbriae on both surfaces at apex, 2.5-5 mm long, 0.05 mm in diameter, flattened, curly, glabrous and deciduous; blades 27.5-39 cm long, 0.95-4 cm wide, lanceolate, adaxially scabrous, glaucous and scabrous abaxially, patch of cilia at the base of the blade very dense, white, extending along both sides of the midrib 5.8-8.5 mm long; midrib excentric, the base attenuate, the apex attenuatesubulate, the margins serrulate; pseudopetioles ca. 2 mm long, greenish, pulvinate at the base. Synflorescences ca. 18 cm long, paniculate, ca. 33 spikelets per synflorescence, green; rachis flattened, slightly scabrous; pedicels 6-29 mm long. Spikelets 2-2.5 cm long, 3 florets per spikelet; rachilla joints 4.5-6.2 mm long, terete, densely long-scabrous to subhispid; glumes narrowly triangular and navicular, short, scabrous abaxially, finely scabrous adaxially; glume I 7-13 mm long including the awn, 3-nerved, the awn 4-6.8 mm long; glume II 9.5-13.7 mm long including the awn, 7-nerved, the awn 1.5-5 mm long; lemmas 14.3-18 mm long including the awn, narrowly triangular and navicular, slightly dorsoventrally compressed, uniformly spaced scabrous hooks moderately dense, from 0.1—0.25 mm long over the surfaces, distinctly longer and denser, hispid, in and about the margins to 0.6 mm long, 11- nerved, the awn 2.8-5.6 mm long, scabrous adaxially; paleas 9.7-11.7 mm long, similar to texture and vestiture of lemmas, the hairs usually longer, densely hispid at margins. Lodicules 3, brown, 2.5 mm, lanceolate (apex triangular), densely ciliate in the margins, scabrous abaxially, 8-veined. Ovary glabrous, brownamber ca 2 mm long. Anthers 3 per flower, 8 mm long. Caryopsis 5.8-7 mm long, fusiform with a small 0.6 mm rostellum, glabrous, dark brown, dull, subtriagonous, slightly keeled, sulcate. 152 Paratypes―MEXICO, Jalisco: municipio de San Sebastián del Oeste, 40 Km al NW de Mascota, brecha a San Sebastián del Oeste, 1610 m, 27 Dic 1981, F.J. Santana Michel 941 (IBUG, MEXU). 40 Km al NW de Mascota, brecha a San Sebastian del Oeste, 1550 m, 2 Feb. 1983, F.J. Santana Michel, R. Guzmán & J. A. Pérez de la Rosa 1222 (IBUG). 25 Km al NE de Mascota por la brecha a San Sebastian del Oeste, 2 Feb 1983, R. Guzmán 6114 (MEXU), F.J. Santana Michel, R. Guzmán & J. A. Pérez de la Rosa 1225 (IBUG). 39-40 Km al NE de Mascota por la brecha a San Sebastian del Oeste, 2 Feb. 1983, R. Guzmán 6113 (MEXU, US). km 29.7 carretera Mascota a Puerto Vallarta a 8.3 Km de La Estancia rumbo a Mascota, 1506 m, 1 Ene. 2006, E. Ruiz-Sánchez & F. Rodriguez 130 (XAL). San Sebastian del Oeste, Km 32-33 carretera de Mascota a Puerto Vallarta, 1393 m, 10 sep 2007, E. Ruiz-Sánchez, D. Ángulo & E. Gándara 189 (XAL). Mun. Atengo, Sierra Verde, vereda que va de Tacota a Mixtlán, 1 Mar. 1992, J.A. Machuca & M. Cházaro 6804 (MEXU, NY, XAL). Etymology. The specific epithet honors Jacqueline Reynoso Dueñas, a grass taxonomist from the University of Guadalajara. Phenology. Flowering in November. Distribution and habitat. Endemic to Mexico on the Pacific slopes, in Guerrero, Jalisco and Nayarit. It grows on slopes in humid pine-oak and cloud forests at elevations of 1300 to 1650 m. (Fig. 10). Discussion. Morphologicallly, Otatea reynosoana is similar to O. fimbriata but differs from it in having oral setae of culm and foliar leaves white and brown. A morphological comparison with among all species is presented in table 1. 7. Otatea transvolcanica E. Ruiz-Sánchez, Londoño and L.G. Clark, sp. nov. TYPE: MEXICO. Estado de México: municipio de Temascaltpec, Puente Río Verde a 4.5 km de Temascaltepec 153 por la carretera rumbo a Toluca, creciendo en la cañada a orilla del río Verde, 1840 m, 4 Sep. 2007, E. Ruiz-Sánchez, D. Ángulo & E. Gándara 179 (holotype: XAL; isotype: IBUG, ISC, MEXU, MO, NY, US). Fig. 11. O. fimbriatae similis sed differens folii caulinaris lamina reflexa, ramificatione extravaginali, foliorum caulinarium setis oralibus cito deciduis in primo tertio basali connatis purpureis sed ad apices viridibus, et ciliarum caespede luteo usque 26 mm longo ad laminae basem in pagina adaxiale posito et ad nervi centralis latera extenso. Rhizome neck 30-45 cm long. Culms 3-8 m tall, 1-6 cm in basal diameter, erect to apically arched; internodes 24-27 cm long, terete, glabrous, light green when young and yellow when old, pruinose between internodes, solid at based and hollow in the first third, the walls 2.8-4 mm thick, the lacuna occupying ≥ 50% of the total diameter. Culm leaves 36-46 cm long; sheaths 2429.5 cm long overlapping and persistent, 7-11 cm wide at the base, 1.3-3.2 times as long as the blades, ± rectangular, abaxially hispid for the upper 1/2 to 2/3, the shoulders rounded and sometimes with a small extended apex about 2-3mm long, the margins glabrous; inner ligule 0.92.2 mm long, truncate, glabrous; oral setae free, present only in young shoots 10-15 mm long, green; fimbriae absent; leaf blades 9-20 cm long, triangular, reflexed, deciduous, adaxially and abaxially glabrous, apex subulate. Branching extravaginal; one or two main, unequal branches per node, if three then the central 2X wider than the lateral, 110 to 130 cm long, diverging from the main culm at 45o; supranodal ridge pronounced; nodal line more or less horizontal. Foliage leaves 6-10 per complement; sheaths glabrous, rounded on the back, sheath summit extension absent; outer ligule an irregular glabrous rim to 0.1 mm long with two lobes at apex, 3-11 mm 154 long, 1.9-4.5 wide; inner ligule 0.1-0.2 mm long, truncate; oral setae 13-21.5 mm long, connate at basal 1/3 or more, splitting into narrower segments above, flattened, glabrous, free from the inner ligule, purple in living specimens with green apices; fimbriae on both surfaces of blade at apex, 5-14.8 mm long, 0.05-0.1 mm in diameter, slightly flattened, curly, glabrous and deciduous; blades 34-60 cm long, 2.5-5 cm wide, lanceolate, adaxially glabrous, glaucous, abaxially scabrous, green, patch of cilia at the base of the blade on the abaxial surface very dense, yellow, extending along both sides of the midrib 12.5-26.1 mm long; midrib excentric, the base attenuate, the apex attenuate-subulate, the margins serrulate; pseudopetioles ca. 3 mm long, yellowish, pulvinate at the base. Synflorescences not seen. Paratypes―MEXICO. Colima: municipio de Comala, E. facing slopes of Cerro Grande in deep cañada on road from Lagunillas to Campo Cuatro and Juluapan, 1500 msnm, 15 Marz 1987, H. H. Iltis, B. F. Benz, A. Vázquez & M. Cházaro 29712 (MEXU, MO, US). 5 km al N de Campo 4, por la brecha a Lagunitas, 1400 msnm, 23 Nov. 1987, F.J. Santana Michel 2670 (ENCB, IBUG, MEXU). Campo Cuatro a 7.8 Km de Campo Cuatro por el camino a Lagunitas, 1710 m, 9 Sep. 2007, E. Ruiz-Sánchez, D. Ángulo & E. Gándara 183 (XAL). Estado de México: municipio de Temascaltpec, Barrancas cerca de Temascaltepec, 3 Apr. 1981, R. Guzmán & L. Rico 1201 (MEXU, XAL). Carr. entre Tejupilco y Temascaltepec, 4 Ago. 1981, R. Guzmán & L. Rico 1478 (MEXU, US). 4.5 km al E de Temascaltepec por la carretera a México D.F. R. Guzmán 6043, 6044, 6045, 6050, 6051, 6052, 6053 (MEXU). 4.5 km al E de Temascaltepec por la carretera a México D.F. Manrique, Guerrero, Guzmán & Jaramillo 201 (MEXU). Jalisco: municipio de Tamazula, Agua Hedionda Km 55-60 brecha a Manuel M. Díeguez, 1850 m, 27 Oct. 1973, C. Díaz Luna 4513 (IBUG). Km 80 de la brecha a Manuel M. Díeguez, 1040 m, 19 Nov. 1973. 155 L.M.V. Puga 13844A (IBUG). Mun. Ciudad Guzmán, Parte Este de la cuenca Zapotlán, 1710 m, 4 Feb. 1994, J.J. Reynoso et al., 1741 (IBUG). In canyon east of Ciudad Guzmán, 6000ft, 18 Nov. 1968, F. Boutin, 2267 (US). Mun. Tecalitlán, Puente San Pedro, carretera de Pihuamo a Tecalitlán entre el Km 140-141, 1181 m, 8 Sep. 2007, E. Ruiz-Sánchez, D. Ángulo & E. Gándara 186 (XAL). Nayarit: municipio de Nayar, 5-8 km al NW de la Mesa del Nayar, camino a La Mesa del Nayar-Villa Guadalupe (Cerro Cangrejo), 1300 m, 19 Sep. 1989, G. Flores, O. Tellez, P. Tenorio & A. Salinas 1123 (MEXU). Etymology. The specific epithet relates to its geographical distribution along the Transmexican Volcanic Belt. It harbours 13 of the highest mountains of Mexico. Phenology. Flowers not seen. Distribution and habitat. Endemic to Mexico from Colima, Jalisco to the State Mexico. This species grows in slopes with humid pine-oak and cloud forests at elevations from 1040 to 1850 m. (Fig. 10). Discusion. Otatea transvolcanica differs from O. fimbriata in its reflexed culm leaf blades in a extravaginal branching pattern with one or two branches per node and in its larger and wider foliar leaves (Table 1). 156 ACKNOWLEDGEMENTS. We are particularly grateful to Robert Soreng for kindly providing images of the type specimens of Otatea from the US herbarium. We express our gratitude to Jerzy Rzedowski for preparing the Latin diagnoses. We are grateful to Edmundo Saavedra for his excellent illustrations of the new species and to Tiburcio Láez for his help with the SEM photographs. We thank Pablo CarrilloReyes, Arturo de Nova, Flor Rodríguez-Gómez, José Luis Martínez, Nelly Jiménez-Pérez, Jaime Pacheco, Xóchitl Galarza, Diego Angulo and Etelvina Gándara for their assistance during the field work, Eva María Piedra and Fernando Nicolalde collected some Otatea specimens and we appreciate the curators of the following herbaria for access to their collections and the loan of specimens: CIIDIR, CUCV, ENCB, F, FCME, IBUG, IEB, ISC, MEXU, MO, NY, TULV, UAS, US, USON and XAL. Field work was supported by a graduate student grant of the Instituto de Ecología, A. C., by a grant of the student assistance program of Bamboos of the Americas (BOTA) by a grant provided by the Red Lationamericana de Botánica (RLB07ATP01) and also by a student grant from the International Association for Plant Taxonomists. A fellowship to ER-S by CONACYT (190069) is also acknowledged. 157 LITERATURE CITED ACEDO, C. AND F. LLAMAS. 2001. Variation of micromorphological characters of lemma and palea in the genus Bromus (Poaceae). Annales Botanici Fennici 38: 1–14. BEETLE, A. A., S. J. MIRANDA., L. V. JARAMILLO., R. A. RODRÍGUEZ., M. L. ARAGÓN., B. M. VERGARA., H. A. CHIMAL AND S. O. DOMÍNGUEZ. 1995. Las Gramíneas de México IV. COTECOCA. México, D.F. CALDERÓN, C. E. AND T. R. SODERSTROM. 1980. The genera of Bambusoideae (Poaceae) of the American Continent; keys and coments. Smithsonian Contribution to Botany 44: 1-27. CLARK, L.G. AND G. CORTÉS. 2004. A new species of Otatea from Chiapas, Mexico. Bamboo Science and Culture 18: 1-6. CORTÉS, R. G. 2000. Los bambúes nativos de México. Biodiversitas 30: 12-15. DÁVILA, P.A., M.T. MEJIA-SAULÉS, M. GOMEZ-SÁNCHEZ, J. VALDÉS-REYNA, J.J. ORTÍZ, C. MORIN, J. CASTREJON AND A. OCAMPO. 2006. Catálogo de las Gramíneas de México. Universidad Nacional Autónoma de México y Comisión Nacional para el Conocimiento y uso de la Biodiversidad. México, D.F. 158 ELLIS, R. P. 1979. A procedure for standardizing comparative leaf anatomy in Poaceae. II. The epidermis seen in surface view. Bothalia 12: 641-671. GUZMÁN, R., M. C. ANAYA, and M. SANTANA. 1984. El género Otatea (Bambusoideae), en México y Centroamérica. Boletín del Instituto de Botánica 5: 2-20. JUÁREZ, E. O. AND G. MÁRQUEZ. 1992. Posibles impresiones de otate (Otatea acuminata spp. acuminata) (Gramineae: Bambusoideae) en el bajereque arqueológico de sitio Loma Iguana, Ver. La Ciencia y El Hombre 12-13: 143-159. JUDZIEWICZ, E. J., L. G. CLARK, X. LONDOÑO, and M. J. STERN. 1999. American Bamboos. Washington D.C.: Smithsonian Institution Press. LONDOÑO, X. and L. G. CLARK. 1998. Eight new taxa and two new reports of Bambuseae (Poaceae: Bambuseae) from Colombia. Novon 8: 408-428. ———. and ———. 2002. A Revision of the Brazilian bamboo genus Eremocaulon (Poaceae: Bambuseae: Guaduinae). Systematic Botany 27: 703-721. MCCLURE, F. A. 1973. Genera of bamboos native to the New World (Gramineae: Bambuosideae). Smithsonian Contribution to Botany 9: 1-148. 159 METCALFE, C. R. 1960. Anatomy of the Monocotyledons I: Gramineae. Claredon Press, Oxford. RUIZ-SÁNCHEZ, E., V. SOSA, AND M.T. MEJÍA-SAULES. 2008. Phylogenetics of Otatea inferred from morphology and chloroplast DNA sequence data and recircumscription of Guaduinae (Poaceae: Bambusoideae). Systematic Botany 33: 277-283. SODERSTROM T.R IN MCVAUGH, R. 1983. Gramineae. Pp. 1-223. in Flora Novo-Galiciana: A descriptive account of the vascular plants of Western Mexico vol. 14, ed. W. R. Anderson. Ann Arbor: The University of Michigan Press. VÁZQUEZ-LÓPEZ, J. M., H. VIBRANS, E. GARCÍA-MOYA, J. I. VALDEZ-HERNÁNDEZ, A. ROMEROMANZANARES AND R. CUEVAS-GUZMÁN. 2004. Effects of harvesting on the structure of a Neotropical woody bamboo (Otatea: Guaduinae) populations. Interciencia 29: 207-211 160 Table 1. Diagnostic characters for the new four species of Otatea. Character/taxa O. carrilloi O. mixtecana O. reynosoana O. transvolcanica Culm habit arching apically erect erect to apically arched erect to apically arched 3-5 1-3.5 2-3 0.6-1 3-6 1-6 3-8 1-6 11-19 3.5-6.9 20.5-29 1.8-3.1 11-19 2-4 24-27 2.8-4 19-28 overlapping deciduous 9.5-12.5 No overlapping deciduous 16-31 overlapping persistent 24-29.5 No overlapping persistent 10-31 reflexed fimbriate 1.6-7.5 reflexed ciliate 8-23 erect ciliate 9-20 reflexed glabrous 15-30.6 green-yellow 4.5-12.8 purple 7-17.3 white 10-15 (deciduous) green present present present or absent absent at entire apex sheath straight 10.3-19.3 intravaginal 3 only at apex of sheath curly 3.9-4.8 intravaginal 3 only at apex of sheath curly 2-7 intravaginal 1-2 extravaginal 1-2 19-27 0.7-1.08 absent 27.5-39 0.95-4 present 34-60 2.5-6 present - 0.6-1.5 0.8-1.8 3-11 1.9-4.5 Culm size length (m) diameter (cm) Internode size length (cm) wall thickness (mm) Culm leaves sheath length (cm) overlapping duration Culm leave blades length (cm) position margin base indument Oral setae on culm leaves length (mm) color in living specimens Presence of fimbriae on culm leaves position posture length (mm) Branching Branches per node Foliage leaf blade length (cm) 18-26 width (cm) 0.6-0.9 absent Lobes on foliage leaves length (mm) width (mm) Oral setae on foliage leaves 161 length (mm) 6.6-24.1 connate at base no Color in living green-yellow specimens Fimbriae on foliage leaves length (mm) 8.4-21.8 posture straight centric Midrib on foliar leaves tropical dry Habitat forest Distribution Chiapas 4.3-6 no purple 5.1-11.2 no white 13-21.5 yes purple with green tips absent excentric 2.5-5 curly excentric 5-14.8 curly excentric mixed forest with pine-oakjuniper and tropical dry forest Oaxaca humid pine-oak and cloud forest humid pine-oak and cloud forest Jalisco, Guerrero and Nayarit Colima, Jalisco and Mexico State 162 FIG. 1. Micromorphology of the abaxial surface of lemma in Otatea. A. Microhair (m) in O. glauca (E. Ruiz-Sánchez 144, XAL) and B. O. mixtecana (J. Panero & F. Calzada 4441, XAL). C. Macrohairs (ma) in O. acuminata (F. J. Santana 2529, XAL) and D. O. mixtecana (J. Panero & F. Calzada 4441 XAL). E. Prickles with extended barb (prd) in O. acuminata (F. J. Santana 2529, XAL). F. Hooks (h) in O. glauca (E. Ruiz-Sánchez 144, XAL). FIG. 2. Micromorphology of the abaxial surface of the lemma in Otatea. A. Silica bodies with an irregular dumb-bell shape (sbd) in O. glauca (E. Ruiz-Sánchez 144, XAL). B. Silica bodies saddle-shaped (sbs) in O. glauca. C. Silica bodies rounded (sbr) in O. acuminata (F. J. Santana 2529, XAL). FIG. 3. Micromorphology of the abaxial surface of palea in Otatea. A. Intercostal long-cells with sinuous outline U-shaped (Lc) in O. glauca (E. Ruiz-Sánchez 144, XAL). B. Prickles with extended barb (prd) in O. glauca. C. Prickles with extended barb (prd) in O. acuminata (F. J. Santana 2529, XAL). D. Prickles with extended barb (prd) and prickles with barb not developed (pr) in O. mixtecana (J. Panero & F. Calzada 4441 XAL). E. Silica bodies irregular dumb-bell shaped (sbd) in O. glauca. F. Silica bodies saddle-shaped (sbs) in O. mixtecana. FIG. 4. Culm leaves. A. Otatea carrilloi. B. O. trasnvolcanica. C. O. reynosoana. D. O. mixtecana. FIG. 5. Geographical distribution of Otatea acuminata and O. fimbriata. 163 FIG. 6. Otatea carrilloi. a; rhizome. b; culm with persistent culm leaf sheath. c; branch complement. d; foliage leaf complement. e; culm leaf, abaxial apical view. f; ligular area of foliage leaf with fimbriae and oral setae. (a, d-f. based on E. Ruiz-Sánchez & R. Córdoba 147; bc. based on P. Carrillo-Reyes, D. Cabrera-Toledo y M. A. Perez-Farrera 5144) FIG. 7. Geographical distribution of Otatea carrilloi, O. glauca and O. mixtecana. Fig. 8. Otatea mixtecana. a; rhizome. b; culm with culm leaves. c; branch complement. d; culm leaf apex witn oral setae. e; Synflorescence and foliage leaf complement. f; ligular area of foliage with oral setae. g; spikelet. (a-d, f. based on E. Ruiz-Sánchez, F. Rodriguez & V. Sosa 217; e, g. based on J. Panero & I. Calzada 4441) FIG. 9. Otatea reynosoana. a; culm with overlapping culm leaves. b; apical shoot with culm leaves. c; branch complement. d; synflorescence and foliage leaf complement e; ligular area of foliage leaf with oral setae. f; spikelet with proximal floret remaining. (a-c, e. based on E. RuizSánchez & F. Rodriguez 130; d, f. based on G.B. Hinton 9879) FIG. 10. Geographical distribution of Otatea reynosoana and O. transvolcanica. FIG. 11. Otatea transvolcanica. a; branch complement from mid section to culm apex. b; culm middle section with extravaginal branching. c; apical shoot with reflexed culm leaves. d; foliage leaf complement. e; ligular area of foliage leaf with oral setae and outer ligule lobes in an early stage of development. f; ligular area of foliage leaf with connate oral setae and outer ligule lobes 164 at maturity. g; culm leaf abaxial view. h; detail of foliar leaf. (a-i. based on E. Ruiz-Sánchez, D. Angulo & E. Gándara 179). 165 166 167 168 169 170 171 172 173 174 175 176 CAPÍTULO V. CONCLUSIONES GENERALES 177 Los resultados obtenidos en los análisis filogenéticos con matrices de datos moleculares y morfológicas indican que Otatea es un grupo monofilético, soportado por dos estados de caracteres sinapomorfícos morfológicos: la presencia de tres ramas subiguales por nodo y lemas pubescentes. Sin embargo, al utilizar los tres juegos de datos combinados (dos regiones de ADN del cloroplasto (rpl16 y trnH-psbA) y los caracteres morfológicos en análisis filogenéticos no pudieron identificar cuál de los géneros dentro de Guaduinae es el grupo hermano de Otatea. Guaduinae está conformada por los géneros: Apoclada, Eremocaulon, Guadua, Olmeca y Otatea (Judziewicz et al., 1999). Es importante mencionar que nuestros resultados indican que Guaduinae es un grupo natural soportado tanto por caracteres moleculares como por caracteres morfológicos siempre y cuando se incluyan en esta subtribu a Aulonemia clarkiae y A. fulgor, previamente clasificadas dentro de la subtribu Arthrostylidiinae (Judziewicz et al., 1999). Las dos especies de Aulonemia fueron descritas dentro de éste género por compartir algunas características morfológicas diagnósticas (Soderstrom, 1988; Davidse y Pohl, 1992). Sin embargo estos autores habían considerado la posibilidad de segregar a éstas dos especies en un nuevo género, por presentar caracteres morfológicos similares a algunas especies de Olmeca, aunque debido a que no había una monografía para el género decidieron mantenerlas en Aulonemia. Más aún nuestros resultados identifican a estas dos especies dentro de Guaduinae. Sin embargo, se requieren otros estudios que incluyan especies adicionales de Aulonemia para tomar una decisión taxonómica final. En conclusión, Otatea es un género monofilético, dentro de Guaduinae, y junto con Apoclada, Eremocaulon, Guadua, Olmeca, Aulonemia clarkiae y A. fulgor. La delimitación de las especies, es una tarea que los taxónomos han realizado desde hace más de 200 años. En tiempos recientes, la inclusión de marcadores moleculares y datos 178 ecológicos proveen una mayor evidencia en la delimitación de las especies (Sites y Marshall 2003, 2004). Utilizamos una metodología que combina caracteres moleculares del cloroplasto (atpF-atpH, psbI-psbK y trnL-rpl32), nucleares (ITS), morfológicos y ecológicos (modelación del nicho ecológico) para delimitar las especies Otatea. Encontramos resultados contradictorios de los análisis filogenéticos. Los marcadores moleculares no apoyan Otatea acuminata ni a O. fimbriata como grupos monofiléticos. Solamente se recuperan dos clados con las poblaciones de Otatea glauca y las de la O. sp. nov de Chiapas. Sin embargo el árbol construido con caracteres morfológicos identificó a O. acuminata, O. fimbriata, O. glauca, O. sp nov de Chiapas y tres especies mas O. sp. nov de Jalisco, O. sp. nov de Oaxaca y O. sp. nov de la Faja Volcánica Transmexicana como grupos naturales y además con los resultados del análisis de caracteres diagnósticos. Con frecuencia los resultados morfológicos y los moleculares producen resultados contradictorios en la delimitación de especies (Wiens y Penkrot, 2002; Doan y Castoe, 2003; Říčan y Kullander, 2006). Estas discrepancias se han atribuido al flujo genético (hibridización), retención del polimorfismo ancestral (asignación incompleta de linajes) y transferencia horizontal (Pamilo y Nei, 1988; Takahata, 1989; Maddison, 1997; Nichols, 2001). Se ha encontrado que la retención del polimorfismo ancestral en coníferas está facilitada por la baja tasa de sustitución (Du et al., 2009). Así mismo, se han encontrado tasas bajas de sustitución en estudios filogenéticos recientes en bambúes (Kelchner y Clark, 1997; Hodkinson, 2000; Guo et al., 2001, 2002; Guo y Li, 2004; Sun et al., 2005; Yang et al., 2007, 2008; Bouchenak-Khelladi et al., 2008; Peng et al., 2008; Ruiz-Sánchez et al., 2008; Sungkaew et al., 2008). Por otra parte, los procesos de hibridización en plantas son muy comunes y pueden derivar en procesos de especiación. Es más fácil rechazar la hipótesis de hibridazación que confirmarla con marcadores 179 moleculares (Riesberg 1997). Así mismo, las especies híbridas probablemente se originan a través de un evento fundador híbrido, en el cual una o más generaciones tempranas híbridas colonizan una nueva localidad y así llegan a estar espacial y ecológicamente aisladas de las especies parentales (Ungerer et al. 1998). Si tres de las especies de Otatea tienen un posible origen híbrido, lo vemos reflejado en los marcadores moleculares y en su distribución geográfica, no así en la morfología. Nuestros resultados de la intercambiabilidad ecológica sugieren que pudo haber una conservación del nicho o una divergencia del mismo. Un nicho conservado en especies hermanas sugiere la falta de evolución del nicho lo que facilita la especiación produciendo distribución alopátrica en respuesta a los cambios de las condiciones ambientales (Ricklefs y Latham, 1992; Peterson et al., 1999; Wiens, 2004; Kozak y Wiens, 2006). Por otra parte, un nicho divergente en especies hermanas indica adaptación a diferentes condiciones ambientales permitiendo la especiación (Losos et al., 2003; Graham et al., 2004; Kozak y Wiens, 2006; Raxworthy et al., 2007). Aparentemente en las especies de Otatea pueden estar ocurriendo tres procesos; 1) retención del polimorfismo ancestral, 2) especiación híbrida y 3) reticulación (flujo genético pasado) causantes de la incongruencia encontrada entre los marcadores moleculares y la morfología. Sin embargo, necesitamos otro tipo de marcadores como los AFLP´s o microsatélites para discernir entre hibridación o retención del polimorfismo ancestral. Así mismo, falta un estudio cariológico de todas las especies de Otatea, porque hasta la fecha no se ha determinado el número cromosómico o tipo de cromosomas de ninguna de ellas. Por lo anterior tomamos las siguientes decisiones taxonómicas: 1)Las poblaciones correspondientes a las subespecies de Otatea acuminata no se identificaron en clados diferentes y no presentaron caracteres diagnósticos s. 2) la población disyunta de Colombia de O. fimbriata, 180 aparentemente es la población más ancestral de las especies de Otatea, resultado obtenido del análisis de haplotipos en una red de parsimonia estadística y no presenta caracteres diagnósticos que pudieran elevarla a especie nueva, por lo que se mantiene como parte de O. fimbriata. 3) Otatea glauca y O. carrilloi son posiblemente las especies donde el proceso de retención del polimorfismo ancestral pudo haber actuado y dos de las especies más divergentes morfológica y molecularmente. 4) Otatea reynosoana y O. transvolcanica probablemnte son de origen híbrido o han tenido intercambio genético con poblaciones de O. acuminata. Otatea agrupa siete especies; seis de ellas son endémicas a México y O. fimbriata se encuentra en México, Centroamérica y Sudamérica. 181 LITERATURA CITADA (CAPITULO V) 182 LITERATURA CITADA Bouchenak-Khelladi, Y., Salamin, N., Savolainen, V., Forest, F., van der Bank, M., Chase, M.W., Hodkinson, T.R. 2008. Large multi-gene phylogenetic trees of the grasses (Poaceae): progress towards complete tribal and generic level sampling. Mol. Phylogenet. Evol. 47, 488505. Davidse, G., Pohl, W.P. 1992. New taxa and nomenclatural combinations of Mesoamerican grasses (Poaceae). Novon 2: 81-110. Doan, T.M., Castoe, T.A. 2003. Using morphological and molecular evidence to infer species boundaries within Proctoporus bolivianus Werner (Squamata: Gymnophthalmidae). Herpetologica 59, 432-449. Du, F.K., Petit, R.J., Quan, L. J. 2009. More introgression with less gene flow: chloroplast vs. mitochondrial DNA in the Picea asperata complex in China, and comparison with other Conifers. Mol. Ecol. 18, 1396-1407. Graham, C.H., Ron, S.R., Santos, J.C., Schneider, C.J., Moritz, C. 2004. Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution 58, 1781-1793. 183 Guo, Z.H. Li, D.Z. 2004. Phylogenetics of the Thamnocalamus group and its allies (Gramineae: Bambusoideae) inferred from the sequences of GBSSI gene and ITS spacer. Mol. Phylogenet. Evol. 30, 1-12. Guo, Z.H., Chen, Y.Y., Li, D.Z., Yang, J.B. 2001. Genetic variation and evolution of the alpine bamboos (Poaceae: Bambusoideae) using DNA sequence data. J. Plant Res. 114, 315-322. Guo, Z.H., Chen, Y.Y., Li, D.Z. 2002. Phylogenetic studies on Thamnocalamus group and its allies (Bambusoideae: Poaceae) based on ITS sequence data. Mol. Phylogenet. Evol. 2, 20-30. Hodkinson, T.R., Renvoize, S.A., Ni Chonghaile, G., Stapleton, C.M.A., Chase, M.W. 2000. A comparison of ITS nuclear rDNA sequence data and AFLP markers for phylogenetic studies in Phyllostachys (Bambusoideae, Poaceae). J. Plant Res. 113, 259-269. Judziewicz, E.J., Clark, L.G., Londoño, X., Stern, M.J. 1999. American Bamboos. Smithsonian Institution Press. New York. Kelchner, S.A., Clark, L.G. 1997. Molecular Evolution and Phylogenetic Utility of the Chloroplast rpl16 intron in Chusquea and the Bambusoideae (Poaceae). Mol. Phylogenet. Evol. 8: 385-397. Kozak, K.H., Wiens, J.J. 2006. Does niche conservatism promote speciation? A case study in North American salamanders. Evolution 60, 2604-2621. 184 Losos, J.B., Leal, M., Glor, R.E., de Queiroz, K., Hertz, P.E. 2003. Niche lability in the evolution of a Caribbean lizard community. Nature 424, 542-550. Maddison, W.P., 1997. Gene trees in species trees. Syst. Biol. 46, 523-536. Nichols, R. 2001. Gene trees and species trees are not the same. Trends Ecol. Evol. 16, 358-364. Pamilo, P., Nei, M. 1988. Relationships between gene trees and species trees. Mol. Biol. Evol. 5, 568-583. Peng, S., Yang, H.Q., Li, D.Z. 2008. Highly heterogeneous generic delimitation within the temperate bamboo clade (Poaceae: Bambusoideae): evidence from GBSSI and ITS sequences. Taxon 57, 799-810. Peterson, A.T., Soberón, J., Sánchez-Cordero, V. 1999 Conservatism of ecological niches in evolutionary time. Science 285, 1265-1267. Raxworthy, C.J., Ingram, C.M., Pearson, R.G. 2007. Species delimitation applications for ecological niche modeling: a review and empirical evaluation using Phelsuma day gecko groups from Madagascar. Syst. Biol. 56, 907-923. 185 Říčan, O., Kullander, S.O. 2006. Character- and tree-based delimitation of species in the ‘Cichlasoma’ facetum group (Teleostei, Cichlidae), with the description of a new genus. J. Zool. Syst. Evol. Res. 44, 136-152. Ricklefs, R.E., Latham, R.E. 1992. Intercontinental correlation of geographical ranges suggests stasis in ecological traits of relict genera of temperate perennial herbs. Am. Nat. 139, 1305-1321. Riesberg, L.H. 1997. Hybrid origins of plant species. Annu. Rev. Ecol. Syst. 28: 359-389 Ruiz-Sánchez, E., Sosa, V., Mejía-Saules, M.T. 2008. Phylogenetics of Otatea inferred from morphology and chloroplast DNA sequence data and recircumscription of Guaduinae (Poaceae: Bambusoideae). Syst. Bot. 33, 277-283. Sites, J.W., Marshall, J.C. 2003. Delimiting species: a renaissance issue in systematic biology. Trends Ecol. Evol. 18, 462-470. Sites, J.W., Marshall, J.C. 2004. Operational criteria for delimiting species. Annu. Rev. Ecol. Evol. Syst. 35, 199-227. Soderstrom, T.R. 1988. Aulonemia fulgor (Poaceae: Bambusoideae), a new species from Mexico. Brittonia 40: 22-31. 186 Sun, Y., Xia, N., Lin, R. 2005. Phylogenetic analysis of Bambusa (Poaceae: Bambusoideae) based on internal transcribed spacer sequences of nuclear ribosomal DNA. Biochem Genet. 43, 603-612. Sungkaew, S., Stapleton, C.M.A., Salamin, N., Hodkinson, T.R. 2008. Non-monophyly of the woody bamboos (Bambuseae; Poaceae): a multi-gene region phylogenetic analysis of Bambusoideae s.s. J Plant Res. 122, 95-108. Takahata, N. 1989. Gene genealogy in three related populations: consistency probability between gene and population trees. Genetics 122, 957-966. Ungerer, M.C., Baird S.J.E., Pan, J., Rieseber, L.H. 1998. Rapid hybrid speciation in wild sunflowers. Proc. Natl. Acad. Sci. USA. 95: 11757-11762. Wiens, J.J. 2004. Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution 58, 193-197. Wiens, J.J. Penkrot, T.A., 2002. Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus). Syst. Biol. 51, 69-91. Yang, H.Q., Peng, S., Li, D.Z. 2007. Generic delimitations of Schizostachyum and its allies (Gramineae: Bambusoideae) inferred from GBSSI and trnL-F sequence phylogenies. Taxon 56, 45-54. 187
© Copyright 2026