Prosthetic Devices, Wigs - UnitedHealthcareOnline.com
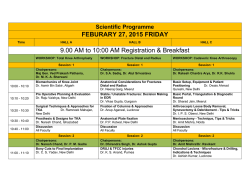
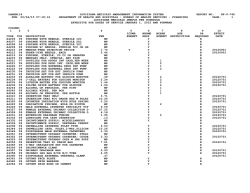
COVERAGE DETERMINATION GUIDELINE PROSTHETIC DEVICES, WIGS, SPECIALIZED, MICROPROCESSOR OR MYOELECTRIC LIMBS Guideline Number: Effective Date: CDG.018.03 February 1, 2015 Table of Contents COVERAGE RATIONALE........................................ DEFINITIONS…………………………………………… APPLICABLE CODES.............................................. HISTORY/REVISION INFORMATION...................... Page 1 5 6 22 Related Policies: DME, Orthotics, Ostomy Supplies, Medical Supplies and Repairs/Replacements INSTRUCTIONS FOR USE This Coverage Determination Guideline provides assistance in interpreting certain standard UnitedHealthcare benefit plans. When deciding coverage, the enrollee specific document must be referenced. The terms of an enrollee’s document (e.g., Certificates of Coverage (COCs), Schedules of Benefits (SOBs), or Summary Plan Descriptions (SPDs), and Medicaid State Contracts) may differ greatly from the standard benefit plans upon which this guideline is based. In the event of a conflict, the enrollee's specific benefit document supersedes these guidelines. All reviewers must first identify enrollee eligibility, any federal or state regulatory requirements and the plan benefit coverage prior to use of this guideline. Other coverage determination guidelines and medical policies may apply. UnitedHealthcare reserves the right, in its sole discretion, to modify its coverage determination guidelines and medical policies as necessary. This Coverage Determination Guideline does not constitute medical advice. UnitedHealthcare may also use tools developed by third parties, such as the MCG™ Care Guidelines, to assist us in administering health benefits. The MCG™ Care Guidelines are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice. COVERAGE RATIONALE Benefit Document Language Before using this guideline, please check enrollee’s specific benefit document and any federal or state mandates, if applicable. Essential Health Benefits for Individual and Small Group: For plan years beginning on or after January 1, 2014, the Affordable Care Act of 2010 (ACA) requires fully insured non-grandfathered individual and small group plans (inside and outside of Exchanges) to provide coverage for ten categories of Essential Health Benefits (“EHBs”). Large group plans (both self-funded and fully insured), and small group ASO plans, are not subject to the requirement to offer coverage for EHBs. However, if such plans choose to provide coverage for benefits which are deemed EHBs (such as maternity benefits), the ACA requires all dollar limits on those benefits to be removed on all Grandfathered and Non-Grandfathered plans. The determination of which benefits constitute EHBs is made on a state by state basis. As such, when using this guideline, it is important to refer to the enrollee’s specific benefit document to determine benefit coverage. Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 1 Indications for Coverage I. Prosthetic Devices and Wigs A determination of coverage for the prosthesis is based on the enrollee’s potential functional abilities. Potential functional ability is based on the reasonable expectations of the prosthetist, and treating physician, considering factors including, but not limited to: • The enrollee’s past history (including prior prosthetic use if applicable); and • The enrollee’s current condition including the status of the residual limb and the nature of other medical problems. 1. Prosthetic device coverage is limited to those prosthetic devices that replace a limb or external body part that are listed below: • Artificial arms, legs, feet and hands • Artificial eyes, ears and nose • Breast prosthesis as required by the Women’s Health and Cancer Rights Act of 1998. Benefits include mastectomy bras and lymphedema stockings for the arm. • Speech aid prosthetics and tracheo-esophageal voice prosthesis. Although these are typically external devices replacing the vocal cords, there may be an intra-oral component. These devices are covered as either DME or Prosthetics. Please check enrollee specific benefit document for coverage. 2. Prosthetic devices when covered, regardless of the setting or vendor from whom the prosthetic device is dispensed, are covered under the Prosthetic Devices section of the benefit document. 3. Prosthetic devices must be ordered by or under the direction of a physician. 4. The prosthetic device must be approved by the Food and Drug Administration (FDA) and otherwise generally considered to be safe and effective for the purposes intended and the item must be reasonable and necessary for the individual patient. 5. Breast prosthetics which include the breast prosthesis, mastectomy bra, and lymphedema arm stockings, are always covered on an unlimited basis as to number of items and dollar amounts covered as required by the Women’s Health and Cancer Act of 1998. 6. Implantable devices/prostheses, such as artificial heart valves, are not prosthetics. If covered, these devices would be covered as a surgical service. 7. Coverage is available for repair and replacement, when it is not due to misuse, malicious damage or gross neglect. 8. Several states mandate coverage for prosthetics. Please check the enrollee specific benefit document for coverage. II. Specialized, Microprocessor or Myoelectic Limbs Computerized, bionic, microprocessor or myoelectric terms are considered the same for the purpose of this policy. Some states may require coverage of prosthetics that UnitedHealthcare may not otherwise consider covered. Computerized or microprocessor limbs are based on a patient’s current functional capabilities and his/her expected functional rehabilitation potential. If more than one prosthetic limb meets a patient’s prosthetic rehabilitation needs, the least costly prosthetic will be approved. Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 2 Evidence is insufficient to permit conclusions regarding the effect of a microprocessorcontrolled prosthesis on health outcomes in limited community ambulators. Evidence is also insufficient to permit conclusions regarding the effect of a next-generation microprocessorcontrolled prosthesis on health outcomes. Therefore, these are considered investigational. 1. Computerized Prosthetic limbs are a covered health service when criteria are met: a) Ordered by a physician; and b) Patient is evaluated for his/her individual needs by a healthcare professional with the qualifications and training and under the supervision of the ordering physician to make an evaluation (documentation should accompany the order); and c) Ordering physician signs the final prosthetic proposal; and d) The records must document the patient’s current functional capabilities and his/her expected functional rehabilitation potential, including an explanation for the difference, if that is the case. (It is recognized within the functional classification hierarchy that bilateral amputees often cannot be strictly bound by functional level classifications); and e) Prosthetic replaces all or part of a missing limb; and f) Prosthetic will help patient regain or maintain function; and g) Patient is willing and able to participate in the training for the use of the prosthetic (especially important in use of a computerized upper limb); and h) Patient is able to physically function at a level necessary for a computerized prosthetic or microprocessor, e.g. hand, leg or foot 2. Coverage of computerized and specialized lower limb prostheses is based on maximum prosthetic function level of the patient (see Lower Limb Rehabilitation Classification Levels 1-4 under Definition section below.) a) Patient meets criteria in #1 (one) above; and b) Patient has or is able to gain Lower Limb Rehabilitation Classification Levels 3 or 4 for prosthetic ambulation (see Definition section below) A. Microprocessor or specialized foot or feet; i. Microprocessor controlled ankle foot system (L5973), energy storing foot (L5976), multi-axial ankle/foot (L5978), dynamic response foot with multiaxial ankle (L5979), flex foot system (L5980), flex-walk system or equal (L5981), or shank foot system with vertical loading pylon (L5987) is indicated for patients whose functional level is 3 or above. (A user adjustable heel height feature (L5990) will be denied as not meeting criteria for coverage. B. Knees: Basic lower extremity prostheses include a single axis, constant friction knee. Other prosthetic knees are indicated based upon functional classification. i. A high activity knee control frame (L5930) (e.g. i Ottobock C-Leg® Microprocessor Knee System) is covered for patients whose function level is 4. ii. A fluid, pneumatic, or electronic knee (L5610, L5613, L5614, L5722-L5780, L5814, L5822-L5840, L5848, L5856, L5857, and L5858) is indicated for patients whose functional level is 3 or above. iii. L5859 is only covered when the enrollee meets all of the criteria below: • Has a microprocessor (swing and stance phase type (L5856)) controlled (electronic) knee • K3 functional level only • Weight greater than 110 lbs and less than 275 lbs • Has a documented comorbity of the spine and/or sound limb affecting hip extention and/or quadriceps function that impairs K-3 level function with the use of a microprocessor-controlled knee alone • Is able to make use of a product that requires daily charging Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 3 • Is able to understand and respond to error alerts and alarms indicating problems with the function of the unit C. Ankles: i. An axial rotation unit (L5982-L5986) is indicated for patients whose Lower Limb Rehabilitation Classification is 2 or above. ii. A microprocessor controlled ankle foot system (L5973), energy storing foot (L5976), dynamic response foot with multi-axial ankle (L5979), flex foot system (L5980), flex-walk system or equal (L5981), or shank foot system with vertical loading pylon (L5987) is covered for beneficiaries whose functional level is 3 or above. D. Sockets: i. More than 2 test (diagnostic) sockets (L5618-L5628) for an individual prosthesis are not indicated unless there is documentation in the medical record which justifies the need. Exception: a test socket is not indicated for an immediate prosthesis (L5400-L5460) ii. No more than two of the same socket inserts (L5654-L5665, L5673, L5679, L5681, and L5683) are allowed per individual prosthesis at the same time. iii. Socket replacements are indicated if there is adequate documentation of functional and/or physiological need. It is recognized that there are situations where the explanation includes but is not limited to: changes in the residual limb; functional need changes; or irreparable damage or wear/tear due to excessive patient weight or prosthetic demands of very active amputees. 3. Myoelectric Upper Limbs (arms, joints and hands) are covered when criteria are met: a) Patient meets all the criteria in #1 (one) above; and b) Patient has a congenital missing or dysfunctional arm and/or hand; or c) Patient has a traumatic or surgical amputation of the arm (above or below the elbow); and d) The remaining musculature of the arm(s) contains the minimum microvolt threshold to allow operation of a myoelectric prosthetic device (usually 3-5 muscle groups must be activated to use a computerized arm/hand); and e) A standard body-powered prosthetic device cannot be used or is insufficient to meet the functional needs of the individual in performing activities of daily living. Coverage Limitations and Exclusions 1. Coverage for wigs/scalp hair prosthesis is excluded unless specifically listed as a covered health service. Some states mandate coverage. Check the enrollee specific benefit document for coverage. When wigs are covered, the benefit does not include coverage for hair implants or hair plugs. 2. Coverage is not available for prosthetics if the patient is eligible through a governmental program for a prosthetic due to military service related injuries and/or primary insurance coverage, e.g., VA, Medicare or TriCare. 3. Replacement of prosthetic devices due to misuse, malicious damage or gross neglect or to replace lost or stolen items. (Check enrollee specific benefit document) 4. Repairs to prosthetic devices due to misuse, malicious damage or gross neglect (Check enrollee specific benefit document) 5. If more than one prosthetic device can meet the enrollees functional needs, benefits are only available for the prosthetic device that meets the minimum specifications for the enrollees needs. Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 4 6. Coverage beyond any dollar or frequency limits specified in the enrollees specific benefit documents. DEFINITIONS Lower Limb Rehabilitation Classification Levels • For Lower Limb Determinations: A clinical assessments of patient rehabilitation potential must be based on the following classification levels: K-Level 0: Does not have the ability or potential to ambulate or transfer safely with or without assistance and prosthesis does not enhance their quality of life or mobility. K-Level 1: Has the ability or potential to use prosthesis for transfers or ambulation on level surfaces at fixed cadence. Typical of the limited and unlimited household ambulator. K-Level 2: Has the ability or potential for ambulation with the ability to traverse low level environmental barriers such as curbs, stairs or uneven surfaces. Typical of the limited community ambulator. K-Level 3: Has the ability or potential for ambulation with variable cadence. Typical of the community ambulator who has the ability to traverse most environmental barriers and may have vocational, therapeutic, or exercise activity that demands prosthetic utilization beyond simple locomotion. K-Level 4: Has the ability or potential for prosthetic ambulation that exceeds basic ambulation skills, exhibiting high impact, stress, or energy levels. Typical of the prosthetic demands of the child, active adult, or athlete. Microprocessor Controlled Ankle Foot Prosthesis: (e.g., Proprio Foot) is able to actively change the ankle angle and to identify sloping gradients and ascent or descent of stairs as the result of microprocessor-control and sensor technology. Microprocessor Controlled Lower Limb Prostheses: Microprocessor controlled knees offer dynamic control through sensors in the shin. Microprocessor controlled knees attempt to simulate normal biological knee function by offering variable resistance control to the swing or stance phases of the gait cycle. This allows the user to safely perform ramp and stair descent in a stepover-step manner. The swing-rate adjustments allow the knee to respond to rapid changes in cadence. Microprocessor controlled knee flexion enhances the stumble recovery capability of the patient by preventing unexpected knee buckling. Prosthetic knees such as the microprocessor controlled knee that focus on better control of flexion abilities without reducing stability have the potential to improve gait pattern, wearer confidence, and safety of ambulation. The microprocessor knee is more beneficial at higher ambulation speed in physically fit patients. ® Available devices include but are not limited to Otto-Bock C-Leg device , the Ossur ® ® RheoKnee or the Endolite Intelligent Prosthesis A microprocessor controlled ankle foot prosthesis (e.g., Proprio Foot) is able to actively change the ankle angle and to identify sloping gradients and ascent or descent of stairs as the result of microprocessor-control and sensor technology. Myoelectric Prosthetic: A myoelectric prosthesis uses electromyography signals or potentials from voluntarily contracted muscles within a person’s residual limb via the surface of the skin to control the movements of the prosthesis, such as elbow flexion/extension, wrist supination/pronation or hand opening/closing of the fingers. Prosthesis of this type utilizes the residual neuro-muscular system of the human body to control the functions of an electric powered prosthetic hand, wrist or elbow. This is as opposed to a traditional electric switch prosthesis, which requires straps and/or cables actuated by body movements to actuate or operate switches that control the movements of a prosthesis or one that is totally mechanical. It has a selfProsthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 5 suspending socket with pick up electrodes placed over flexors and extensors for the movement of flexion and extension respectively. Prosthetist: A person, who measures, designs, fabricates, fits, or services a prosthesis as prescribed by a licensed physician, and who assists in the formulation of the prosthesis prescription for the replacement of external parts of the human body lost due to amputation or congenital deformities or absences. A prosthetist is a person that has been certified to fit prostheses to residual limbs of the upper and lower extremities. Prosthetic Device: An external device that replaces all or part of a missing body part. Upper Limb Prosthetic Categories (Upper limb prostheses are classified into 3 categories depending on the means of generating movement at the joints: passive, body-powered, and electrically powered movement): • The passive prosthesis is the lightest of the three types and is described as the most comfortable. Since the passive prosthesis must be repositioned manually, typically by moving it with the opposite arm, it cannot restore function. • The body-powered prosthesis utilizes a body harness and cable system to provide functional manipulation of the elbow and hand. Voluntary movement of the shoulder and/or limb stump extends the cable and transmits the force to the terminal device. Prosthetic hand attachments, which may be claw-like devices that allow good grip strength and visual control of objects or latex-gloved devices that provide a more natural appearance at the expense of control, can be opened and closed by the cable system. Patient complaints with body-powered prostheses include harness discomfort, particularly the wear temperature, wire failure, and the unattractive appearance. • Myoelectric prostheses use muscle activity from the remaining limb for the control of joint movement. Electromyographic (EMG) signals from the limb stump are detected by surface electrodes, amplified, and then processed by a controller to drive batterypowered motors that move the hand, wrist, or elbow. Although upper arm movement may be slow and limited to one joint at a time, myoelectric control of movement may be considered the most physiologically natural. Myoelectric hand attachments are similar in form to those offered with the body-powered prosthesis, but are battery powered. An example of recently available technology is the SensorHand™ by Advanced Arm Dynamics, which is described as having an AutoGrasp feature, an opening/closing speed of up to 300 mm/second, and advanced EMG signal processing. Patient dissatisfaction with myoelectric prostheses includes the increased cost, maintenance (particularly for the glove), and weight. • A hybrid system, a combination of body-powered and myoelectric components, may be used for high-level amputations (at or above the elbow). Hybrid systems allow control of two joints at once (i.e., one body-powered and one myoelectric) and are generally lighter and less expensive than a prosthesis composed entirely of myoelectric components APPLICABLE CODES ® The Current Procedural Terminology (CPT ), Healthcare Common Procedure Coding System ® (HCPCS) and Current Dental Terminology (CDT ) codes listed in this guideline are for reference purposes only. Listing of a service code in this guideline does not imply that the service described by this code is a covered or non-covered health service. Coverage is determined by the enrollee specific benefit document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claims payment. Other policies and coverage determination guidelines may apply. ® CPT is a registered trademark of the American Medical Association. Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 6 ® CDT is a registered trademark of the American Dental Association. Limited to specific procedure codes? YES NO HCPCS Description Procedure Code SPEECH AID PROSTHETICS AND TRACHEO-ESOPHAGEAL VOICE PROSTHETICS Please note: • For groups on the 2001 COC, the following items are covered as durable medical equipment. For groups on the 2007 COC, the following items are covered as prosthetic devices. • For groups on the 2007 COC, a business decision was made to remove these items from prosthetics and cover as DME on the amendment and refilling of the 2007 COC due to the federal mandates. However, the filings need to be approved by the state, so the state specific filing must be reviewed to determine if coverage is provided under DME or prosthetics. • For groups on the 2011 COC, these are covered as DME. D5952 Speech aid prosthesis, pediatric D5953 Speech aid prosthesis, adult D5960 Speech aid prosthesis, modification L8500 Artificial larynx, anytype Tracheo-esophageal voice prosthesis, L8507 Patient inserted, any type, each Tracheo-esophageal voice prosthesis, L8509 Inserted by a licensed health care provider, any type BREAST PROSTHESIS Please note: The codes listed under "breast prosthesis" are always covered even when exclusion for prosthetic devices exists. Coverage is required for these codes per the Women's Health and Cancer Rights Act of 1998. Adhesive skin support attachment for use with external breast A4280 prosthesis, each Breast prosthesis, mastectomy bra,without integrated breast L8000 prosthesis form, any size, any type Breast prosthesis mastectomy bra, with integrated breast prosthesis L8001 form, unilateral, any size, any type Breast prosthesis mastectomy bra, with integrated breast prosthesis L8002 form, bilateral, any size, any type L8010 Breast prosthesis mastectomy sleeve L8015 Ext brst prosthesis garment w/ form post mastectomy L8020 Breast prosthesis mastectomy form L8030 Breast prosthesis silicone or equal L8031 Breast prostheiis, silcone or equal, with integral adhesive L8032 Nipple prosthesis, reusable, and type each L8035 Cstm breast prosthesis molded to pt post mastectmy L8039 Breast prosthesis, nos S8420 Gradient pressure aid sleeve & glove custom made S8421 Gradient pressure aid sleeve & glove ready made S8422 Gradient pressure aid sleeve cstm made med wt S8423 Gradient pressure aid sleeve cstm made heavy wt S8424 Gradient pressure aid sleeve ready made S8425 Gradient pressure aid glove cstm made med wt S8426 Gradient pressure aid glove cstm made heavy wt S8427 Gradient pressure aid glove ready made S8428 Gradient pressure aid gauntlet ready made Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 7 HCPCS Procedure Code S8429 S8460 EYE PROSTHESIS D5915 D5916 D5923 D5928 L8042 L8610 V2623 V2624 V2625 V2626 V2627 V2628 V2629 NOSE PROSTHESIS D5913 D5922 D5926 L8040 L8047 FACIAL PROSTHESIS D5911 D5912 D5919 D5929 L8041 L8043 L8044 L8046 L8048 L8049 Description Gradient pressure exterior wrap Camisole postmastectomy Orbital prosthesis Ocular prosthesis Ocular prosthesis, interim Orbital prosthesis, replacement Orbital prosthesis provided by nonphysician Ocular implant Prosthetic eye plastic cstm Polish/resurfacing of ocular prosthesis Enlargement of ocular prosthesis Reduction of ocular prosthesis Scleral cover shell Fabrication/fitting of ocular conformer Prosthetic eye, other type Nasal prosthesis Nasal septal prosthesis Nasal prosthesis, replacement Nasal prosthesis provided by nonphysician Nasal septal prosthesis prov by nonphysician Facial moulage (sectional) Facial moulage (complete) Facial prosthesis Facial prosthesis, replacement Midfacial prosthesis, provided by a non-physician Upper facial prosthesis, provided by a non-physician Hemi-facial prosthesis, provided by a non-physician Partial facial prosthesis, provided by a non-physician Unspecified maxillofacial prosthesis, by report, provided by a nonphysician Repair or modification of maxillofacial prosthesis, labor component, 15 minute increments, provided by a non-physician EAR PROSTHESIS D5914 Auricular prosthesis D5927 Auricular prosthesis, replacement L8045 Auricular prosthesis provided by nonphysician LOWER LIMB PROSTHETICS L5000 Partial foot, shoe insert with longitudinal arch, toe filler L5010 Partial foot, molded socket, ankle height, with toe filler L5020 Partial foot, molded socket, tibial tubercle height, with toe filler L5050 Ankle, symes, molded socket, sach foot Ankle, symes, metal frame, molded leather socket, articulated L5060 ankle/foot L5100 Below knee, molded socket, shin, sach foot L5105 Below knee, plastic socket, joints and thigh lacer, sach foot Knee disarticulation (or through knee), molded socket, external knee L5150 joints, shin, sach foot Knee disarticulation (or through knee), molded socket, bent knee L5160 configuration, external knee joints, shin, sach foot Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 8 HCPCS Procedure Code L5200 L5210 L5220 L5230 L5250 L5270 L5280 L5301 L5312 L5321 L5331 L5341 L5400 L5410 L5420 L5430 L5450 L5460 L5500 L5505 L5510 L5520 L5530 L5535 Description Above knee, molded socket, single axis constant friction knee, shin, sach foot Above knee, short prosthesis, no knee joint (stubbies), with foot blocks, no ankle joints, each Above knee, short prosthesis, no knee joint (stubbies), with articulated ankle/foot, dynamically aligned, each Above knee, for proximal femoral focal deficiency, constant friction knee, shin, sach foot Hip disarticulation, canadian type; molded socket, hip joint, single axis constant friction knee, shin, sach foot Hip disarticulation, tilt table type; molded socket, locking hip joint, single axis constant friction knee, shin, sach foot Hemipelvectomy, canadian type; molded socket, hip joint, single axis constant friction knee, shin, sach foot Below knee, molded socket, shin, sach foot, endoskeletal system Knee disarticulation (or through knee), molded socket, single axis knee, pylon, Above knee, molded socket, open end, sach foot, endoskeletal system, single axis knee Hip disarticulation, canadian type, molded socket, endoskeletal system, hip joint, single axis knee, sach foot Hemipelvectomy, canadian type, molded socket, endoskeletal system, hip joint, single axis knee, sach foot Immediate postsurgical or early fitting, application of initial rigid dressing, including fitting, alignment, suspension, and one cast change, below knee Immediate postsurgical or early fitting, application of initial rigid dressing, including fitting, alignment and suspension, below knee, each additional cast change and realignment Immediate postsurgical or early fitting, application of initial rigid dressing, including fitting, alignment and suspension and one cast change ak or knee disarticulation Immediate postsurgical or early fitting, application of initial rigid dressing, including fitting, alignment and suspension, ak or knee disarticulation, each additional cast change and realignment Immediate postsurgical or early fitting, application of nonweight bearing rigid dressing, below knee Immediate postsurgical or early fitting, application of nonweight bearing rigid dressing, above knee Initial, below knee ptb type socket, nonalignable system, pylon, no cover, sach foot, plaster socket, direct formed Initial, above knee, knee disarticulation, ischial level socket, nonalignable system, pylon, no cover, sach foot, plaster socket, direct formed Preparatory, below knee ptb type socket, nonalignable system, pylon, no cover, sach foot, plaster socket, molded to model Preparatory, below knee ptb type socket, nonalignable system, pylon, no cover, sach foot, thermoplastic or equal, direct formed Preparatory, below knee ptb type socket, nonalignable system, pylon, no cover, sach foot, thermoplastic or equal, molded to model Preparatory, below knee ptb type socket, nonalignable system, no cover, sach foot, prefabricated, adjustable open end socket Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 9 HCPCS Procedure Code L5540 L5560 L5570 L5580 L5585 L5590 L5595 L5600 L5610 L5611 L5613 L5614 L5616 L5617 L5618 L5620 L5622 L5624 L5626 L5628 L5629 L5630 L5631 L5632 L5634 L5636 L5637 L5638 L5639 L5640 L5642 Description Preparatory, below knee ptb type socket, nonalignable system, pylon, no cover, sach foot, laminated socket, molded to model Preparatory, above knee, knee disarticulation, ischial level socket, nonalignable system, pylon, no cover, sach foot, plaster socket, molded to model Preparatory, above knee - knee disarticulation, ischial level socket, nonalignable system, pylon, no cover, sach foot, thermoplastic or equal, direct formed Preparatory, above knee, knee disarticulation, ischial level socket, nonalignable system, pylon, no cover, sach foot, thermoplastic or equal, molded to model Preparatory, above knee - knee disarticulation, ischial level socket, nonalignable system, pylon, no cover, sach foot, prefabricated adjustable open end socket Preparatory, above knee, knee disarticulation, ischial level socket, nonalignable system, pylon, no cover, sach foot, laminated socket, molded to model Preparatory, hip disarticulation/hemipelvectomy, pylon, no cover, sach foot, thermoplastic or equal, molded to patient model Preparatory, hip disarticulation/hemipelvectomy, pylon, no cover, sach foot, laminated socket, molded to patient model Addition to lower extremity, endoskeletal system, above knee, hydracadence system Addition to lower extremity, endoskeletal system, above knee, knee disarticulation, 4-bar linkage, with friction swing phase control Addition to lower extremity, endoskeletal system, above knee, knee disarticulation, 4-bar linkage, with hydraulic swing phase control Addition to lower extremity, exoskeletal system, above knee-knee disarticulation, 4 bar linkage, with pneumatic swing phase control Addition to lower extremity, endoskeletal system, above knee, universal multiplex system, friction swing phase control Addition to lower extremity, quick change self-aligning unit, above knee or below knee, each Addition to lower extremity, test socket, symes Addition to lower extremity, test socket, below knee Addition to lower extremity, test socket, knee disarticulation Addition to lower extremity, test socket, above knee Addition to lower extremity, test socket, hip disarticulation Addition to lower extremity, test socket, hemipelvectomy Addition to lower extremity, below knee, acrylic socket Addition to lower extremity, symes type, expandable wall socket Addition to lower extremity, above knee or knee disarticulation, acrylic socket Addition to lower extremity, symes type, ptb brim design socket Addition to lower extremity, symes type, posterior opening (canadian) socket Addition to lower extremity, symes type, medial opening socket Addition to lower extremity, below knee, total contact Addition to lower extremity, below knee, leather socket Addition to lower extremity, below knee, wood socket Addition to lower extremity, knee disarticulation, leather socket Addition to lower extremity, above knee, leather socket Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 10 HCPCS Procedure Code L5643 L5644 L5645 L5646 L5647 L5648 L5649 L5650 L5651 L5652 L5653 L5654 L5655 L5656 L5658 L5661 L5665 L5666 L5668 L5670 L5671 L5672 L5673 L5676 L5677 L5678 L5679 L5680 L5681 Description Addition to lower extremity, hip disarticulation, flexible inner socket, external frame Addition to lower extremity, above knee, wood socket Addition to lower extremity, below knee, flexible inner socket, external frame Addition to lower extremity, below knee, air, fluid, gel or equal, cushion socket Addition to lower extremity, below knee, suction socket Addition to lower extremity, above knee, air, fluid, gel or equal, cushion socket Addition to lower extremity, ischial containment/narrow m-l socket Additions to lower extremity, total contact, above knee or knee disarticulation socket Addition to lower extremity, above knee, flexible inner socket, external frame Addition to lower extremity, suction suspension, above knee or knee disarticulation socket Addition to lower extremity, knee disarticulation, expandable wall socket Addition to lower extremity, socket insert, symes, (kemblo, pelite, aliplast, plastazote or equal) Addition to lower extremity, socket insert, below knee (kemblo, pelite, aliplast, plastazote or equal) Addition to lower extremity, socket insert, knee disarticulation (kemblo, pelite, aliplast, plastazote or equal) Addition to lower extremity, socket insert, above knee (kemblo, pelite, aliplast, plastazote or equal) Addition to lower extremity, socket insert, multidurometer symes Addition to lower extremity, socket insert, multidurometer, below knee Addition to lower extremity, below knee, cuff suspension Addition to lower extremity, below knee, molded distal cushion Addition to lower extremity, below knee, molded supracondylar suspension (pts or similar) Addition to lower extremity, below knee / above knee suspension locking mechanism (shuttle, lanyard, or equal), excludes socket insert Addition to lower extremity, below knee, removable medial brim suspension Addition to lower extremity, below knee/above knee, custom fabricated from existing mold or prefabricated, socket insert, silicone gel, elastomeric or equal, for use with locking mechanism Additions to lower extremity, below knee, knee joints, single axis, pair Additions to lower extremity, below knee, knee joints, polycentric, pair Additions to lower extremity, below knee, joint covers, pair Addition to lower extremity, below knee/above knee, custom fabricated from existing mold or prefabricated, socket insert, silicone gel, elastomeric or equal, not for use with locking mechanism Addition to lower extremity, below knee, thigh lacer, nonmolded Addition to lower extremity, below knee/above knee, custom fabricated socket insert for congenital or atypical traumatic amputee, silicone gel, elastomeric or equal, for use with or without locking mechanism, initial only (for other than initial, use code l5673 or l5679) Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 11 HCPCS Procedure Code L5682 L5683 L5684 L5685 L5686 L5688 L5690 L5692 L5694 L5695 L5696 L5697 L5698 L5699 L5700 L5701 L5702 L5703 L5704 L5705 L5706 L5707 L5710 L5711 L5712 L5714 L5716 L5718 L5722 L5724 Description Addition to lower extremity, below knee, thigh lacer, gluteal/ischial, molded Addition to lower extremity, below knee/above knee, custom fabricated socket insert for other than congenital or atypical traumatic amputee, silicone gel, elastomeric or equal, for use with or without locking mechanism, initial only (for other than initial, use code l5673 or l5679) Addition to lower extremity, below knee, fork strap Addition to lower extremity prosthesis, below knee, suspension/sealing sleeve, with or without valve, any material, each Addition to lower extremity, below knee, back check (extension control) Addition to lower extremity, below knee, waist belt, webbing Addition to lower extremity, below knee, waist belt, padded and lined Addition to lower extremity, above knee, pelvic control belt, light Addition to lower extremity, above knee, pelvic control belt, padded and lined Addition to lower extremity, above knee, pelvic control, sleeve suspension, neoprene or equal, each Addition to lower extremity, above knee or knee disarticulation, pelvic joint Addition to lower extremity, above knee or knee disarticulation, pelvic band Addition to lower extremity, above knee or knee disarticulation, silesian bandage All lower extremity prostheses, shoulder harness Replacement, socket, below knee, molded to patient model Replacement, socket, above knee/knee disarticulation, including attachment plate, molded to patient model Replacement, socket, hip disarticulation, including hip joint, molded to patient model Ankle, symes, molded to patient model, socket without solid ankle cushion heel (sach) foot, replacement only Custom shaped protective cover, below knee Custom shaped protective cover, above knee Custom shaped protective cover, knee disarticulation Custom shaped protective cover, hip disarticulation Addition, exoskeletal knee-shin system, single axis, manual lock Additions exoskeletal knee-shin system, single axis, manual lock, ultra-light material Addition, exoskeletal knee-shin system, single axis, friction swing and stance phase control (safety knee) Addition, exoskeletal knee-shin system, single axis, variable friction swing phase control Addition, exoskeletal knee-shin system, polycentric, mechanical stance phase lock Addition, exoskeletal knee-shin system, polycentric, friction swing and stance phase control Addition, exoskeletal knee-shin system, single axis, pneumatic swing, friction stance phase control Addition, exoskeletal knee-shin system, single axis, fluid swing phase control Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 12 HCPCS Procedure Code L5726 L5728 L5780 L5781 L5782 L5785 L5790 L5795 L5810 L5811 L5812 L5814 L5816 L5818 L5822 L5824 L5826 L5828 L5830 L5840 L5845 L5848 L5850 L5855 L5856 L5857 Description Addition, exoskeletal knee/shin system, single axis, external joints, fluid swing phase control Addition, exoskeletal knee-shin system, single axis, fluid swing and stance phase control Addition, exoskeletal knee-shin system, single axis, pneumatic/hydra pneumatic swing phase control Addition to lower limb prosthesis, vacuum pump, residual limb volume management and moisture evacuation system Addition to lower limb prosthesis, vacuum pump, residual limb volume management and moisture evacuation system, heavy-duty Addition, exoskeletal system, below knee, ultra-light material (titanium, carbon fiber or equal) Addition, exoskeletal system, above knee, ultra-light material (titanium, carbon fiber or equal) Addition, exoskeletal system, hip disarticulation, ultra-light material (titanium, carbon fiber or equal) Addition, endoskeletal knee-shin system, single axis, manual lock Addition, endoskeletal knee-shin system, single axis, manual lock, ultra-light material Addition, endoskeletal knee-shin system, single axis, friction swing and stance phase control (safety knee) Addition, endoskeletal knee-shin system, polycentric, hydraulic swing phase control, mechanical stance phase lock Addition, endoskeletal knee-shin system, polycentric, mechanical stance phase lock Addition, endoskeletal knee/shin system, polycentric, friction swing and stance phase control Addition, endoskeletal knee-shin system, single axis, pneumatic swing, friction stance phase control Addition, endoskeletal knee-shin system, single axis, fluid swing phase control Addition, endoskeletal knee-shin system, single axis, hydraulic swing phase control, with miniature high activity frame Addition, endoskeletal knee-shin system, single axis, fluid swing and stance phase control Addition, endoskeletal knee/shin system, single axis, pneumatic/swing phase control Addition, endoskeletal knee/shin system, 4-bar linkage or multiaxial, pneumatic swing phase control Addition, endoskeletal knee/shin system, stance flexion feature, adjustable Addition to endoskeletal knee-shin system, fluid stance extension, dampening feature, with or without adjustability Addition, endoskeletal system, above knee or hip disarticulation, knee extension assist Addition, endoskeletal system, hip disarticulation, mechanical hip extension assist Addition to lower extremity prosthesis, endoskeletal knee-shin system, microprocessor control feature, swing and stance phase, includes electronic sensor(s), any type Addition to lower extremity prosthesis, endoskeletal knee-shin system, microprocessor control feature, swing phase only, includes electronic sensor(s), any type Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 13 HCPCS Procedure Code L5858 L5859 L5910 L5920 L5925 L5930 L5940 L5950 L5960 L5961 L5962 L5964 L5966 L5968 L5969 L5970 L5971 L5972 L5973 L5974 L5975 L5976 L5978 L5979 L5980 L5981 L5982 L5984 L5985 L5986 Description Addition to lower extremity prosthesis, endoskeletal knee shin system, microprocessor control feature, stance phase only, includes electronic sensor(s), any type Addition to lower extremity prosthesis, endoskeletal knee-shin system, powered and programmable flexion/extension assist control, includes any type motor(s) Addition, endoskeletal system, below knee, alignable system Addition, endoskeletal system, above knee or hip disarticulation, alignable system Addition, endoskeletal system, above knee, knee disarticulation or hip disarticulation, manual lock Addition, endoskeletal system, high activity knee control frame Addition, endoskeletal system, below knee, ultra-light material (titanium, carbon fiber or equal) Addition, endoskeletal system, above knee, ultra-light material (titanium, carbon fiber or equal) Addition, endoskeletal system, hip disarticulation, ultra-light material (titanium, carbon fiber or equal) Addition, endoskeletal system, polycentric hip joint, pneumatic or hydraulic control, rotation control, with or without flexion and/or extension control Addition, endoskeletal system, below knee, flexible protective outer surface covering system Addition, endoskeletal system, above knee, flexible protective outer surface covering system Addition, endoskeletal system, hip disarticulation, flexible protective outer surface covering system Addition to lower limb prosthesis, multiaxial ankle with swing phase active dorsiflexion feature Addition, endoskeletal ankle-foot or ankle system, power assist, includes any type motor(s) All lower extremity prostheses, foot, external keel, sach foot All lower extremity prosthesis, solid ankle cushion heel (sach) foot, replacement only All lower extremity prostheses, flexible keel Endoskeletal ankle foot system, microprocessor controlled feature, dorsiflexion and/or plantar flexion control, includes power source All lower extremity prostheses, foot, single axis ankle/foot All lower extremity prosthesis, combination single axis ankle and flexible keel foot All lower extremity prostheses, energy storing foot (seattle carbon copy ii or equal) All lower extremity prostheses, foot, multiaxial ankle/foot All lower extremity prostheses, multiaxial ankle, dynamic response foot, one piece system All lower extremity prostheses, flex-foot system All lower extremity prostheses, flex-walk system or equal All exoskeletal lower extremity prostheses, axial rotation unit All endoskeletal lower extremity prosthesis, axial rotation unit, with or without adjustability All endoskeletal lower extremity prostheses, dynamic prosthetic pylon All lower extremity prostheses, multiaxial rotation unit (mcp or equal) Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 14 HCPCS Procedure Code Description All lower extremity prosthesis, shank foot system with vertical loading pylon Addition to lower limb prosthesis, vertical shock reducing pylon L5988 feature L5990 Addition to lower extremity prosthesis, user adjustable heel height L5999 Lower extremity prosthesis, not otherwise specified UPPER LIMB PROSTHETICS L6000 Partial hand, robin-aids, thumb remaining (or equal) L6010 Partial hand, robin-aids, little and/or ring finger remaining (or equal) L6020 Partial hand, robin-aids, no finger remaining (or equal) L5987 L6026 L6050 L6055 L6100 L6110 L6120 L6130 L6200 L6205 L6250 L6300 L6310 L6320 L6350 L6360 L6370 L6380 L6382 L6384 L6386 L6388 Transcarpal/metacarpal or partial hand disarticulation prosthesis, external power, self-suspended, inner socket with removable forearm section, electrodes and cables, two batteries, charger, myoelectric control of terminal device, excludes terminal device(s) Wrist disarticulation, molded socket, flexible elbow hinges, triceps pad Wrist disarticulation, molded socket with expandable interface, flexible elbow hinges, triceps pad Below elbow, molded socket, flexible elbow hinge, triceps pad Below elbow, molded socket (muenster or northwestern suspension types) Below elbow, molded double wall split socket, step-up hinges, half cuff Below elbow, molded double wall split socket, stump activated locking hinge, half cuff Elbow disarticulation, molded socket, outside locking hinge, forearm Elbow disarticulation, molded socket with expandable interface, outside locking hinges, forearm Above elbow, molded double wall socket, internal locking elbow, forearm Shoulder disarticulation, molded socket, shoulder bulkhead, humeral section, internal locking elbow, forearm Shoulder disarticulation, passive restoration (complete prosthesis) Shoulder disarticulation, passive restoration (shoulder cap only) Interscapular thoracic, molded socket, shoulder bulkhead, humeral section, internal locking elbow, forearm Interscapular thoracic, passive restoration (complete prosthesis) Interscapular thoracic, passive restoration (shoulder cap only) Immediate postsurgical or early fitting, application of initial rigid dressing, including fitting alignment and suspension of components, and one cast change, wrist disarticulation or below elbow Immediate postsurgical or early fitting, application of initial rigid dressing including fitting alignment and suspension of components, and one cast change, elbow disarticulation or above elbow Immediate postsurgical or early fitting, application of initial rigid dressing including fitting alignment and suspension of components, and one cast change, shoulder disarticulation or interscapular thoracic Immediate postsurgical or early fitting, each additional cast change and realignment Immediate postsurgical or early fitting, application of rigid dressing only Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 15 HCPCS Procedure Code L6400 L6450 L6500 L6550 L6570 L6580 L6582 L6584 L6586 L6588 L6590 L6600 L6605 L6610 L6611 L6615 L6616 L6620 L6621 L6623 L6624 L6625 L6628 L6629 L6630 L6632 Description Below elbow, molded socket, endoskeletal system, including soft prosthetic tissue shaping Elbow disarticulation, molded socket, endoskeletal system, including soft prosthetic tissue shaping Above elbow, molded socket, endoskeletal system, including soft prosthetic tissue shaping Shoulder disarticulation, molded socket, endoskeletal system, including soft prosthetic tissue shaping Interscapular thoracic, molded socket, endoskeletal system, including soft prosthetic tissue shaping Preparatory, wrist disarticulation or below elbow, single wall plastic socket, friction wrist, flexible elbow hinges, figure of eight harness, humeral cuff, bowden cable control, usmc or equal pylon, no cover, molded to patient model Preparatory, wrist disarticulation or below elbow, single wall socket, friction wrist, flexible elbow hinges, figure of eight harness, humeral cuff, bowden cable control, usmc or equal pylon, no cover, direct formed Preparatory, elbow disarticulation or above elbow, single wall plastic socket, friction wrist, locking elbow, figure of eight harness, fair lead cable control, usmc or equal pylon, no cover, molded to patient model Preparatory, elbow disarticulation or above elbow, single wall socket, friction wrist, locking elbow, figure of eight harness, fair lead cable control, usmc or equal pylon, no cover, direct formed Preparatory, shoulder disarticulation or interscapular thoracic, single wall plastic socket, shoulder joint, locking elbow, friction wrist, chest strap, fair lead cable control, usmc or equal pylon, no cover, molded to patient model Preparatory, shoulder disarticulation or interscapular thoracic, single wall socket, shoulder joint, locking elbow, friction wrist, chest strap, fair lead cable control, usmc or equal pylon, no cover, direct formed Upper extremity additions, polycentric hinge, pair Upper extremity additions, single pivot hinge, pair Upper extremity additions, flexible metal hinge, pair Addition to upper extremity prosthesis, external powered, additional switch, any type Upper extremity addition, disconnect locking wrist unit Upper extremity addition, additional disconnect insert for locking wrist unit, each Upper extremity addition, flexion/extension wrist unit, with or without friction Upper extremity prosthesis addition, flexion/extension wrist with or without friction, for use with external powered terminal device Upper extremity addition, spring assisted rotational wrist unit with latch release Upper extremity addition, flexion/extension and rotation wrist unit Upper extremity addition, rotation wrist unit with cable lock Upper extremity addition, quick disconnect hook adapter, otto bock or equal Upper extremity addition, quick disconnect lamination collar with coupling piece, otto bock or equal Upper extremity addition, stainless steel, any wrist Upper extremity addition, latex suspension sleeve, each Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 16 HCPCS Procedure Code L6635 L6637 L6638 L6640 L6641 L6642 L6645 L6646 L6647 L6648 L6650 L6655 L6660 L6665 L6670 L6672 L6675 L6676 L6677 L6680 L6682 L6684 L6686 L6687 L6688 L6689 L6690 L6691 L6692 L6693 L6694 L6695 Description Upper extremity addition, lift assist for elbow Upper extremity addition, nudge control elbow lock Upper extremity addition to prosthesis, electric locking feature, only for use with manually powered elbow Upper extremity additions, shoulder abduction joint, pair Upper extremity addition, excursion amplifier, pulley type Upper extremity addition, excursion amplifier, lever type Upper extremity addition, shoulder flexion-abduction joint, each Upper extremity addition, shoulder joint, multipositional locking, flexion, adjustable abduction friction control, for use with body powered or external powered system Upper extremity addition, shoulder lock mechanism, body powered actuator Upper extremity addition, shoulder lock mechanism, external powered actuator Upper extremity addition, shoulder universal joint, each Upper extremity addition, standard control cable, extra Upper extremity addition, heavy-duty control cable Upper extremity addition, teflon, or equal, cable lining Upper extremity addition, hook to hand, cable adapter Upper extremity addition, harness, chest or shoulder, saddle type Upper extremity addition, harness, (e.g., figure of eight type), single cable design Upper extremity addition, harness, (e.g., figure of eight type), dual cable design Upper extremity addition, harness, triple control, simultaneous operation of terminal device and elbow Upper extremity addition, test socket, wrist disarticulation or below elbow Upper extremity addition, test socket, elbow disarticulation or above elbow Upper extremity addition, test socket, shoulder disarticulation or interscapular thoracic Upper extremity addition, suction socket Upper extremity addition, frame type socket, below elbow or wrist disarticulation Upper extremity addition, frame type socket, above elbow or elbow disarticulation Upper extremity addition, frame type socket, shoulder disarticulation Upper extremity addition, frame type socket, interscapular-thoracic Upper extremity addition, removable insert, each Upper extremity addition, silicone gel insert or equal, each Upper extremity addition, locking elbow, forearm counterbalance Addition to upper extremity prosthesis, below elbow/above elbow, custom fabricated from existing mold or prefabricated, socket insert, silicone gel, elastomeric or equal, for use with locking mechanism Addition to upper extremity prosthesis, below elbow/above elbow, custom fabricated from existing mold or prefabricated, socket insert, silicone gel, elastomeric or equal, not for use with locking mechanism Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 17 HCPCS Procedure Code L6696 L6697 L6698 L6703 L6704 L6706 L6707 L6708 L6709 L6711 L6712 L6713 L6714 L6715 L6721 L6722 L6805 L6810 L6880 L6881 L6882 L6883 L6884 L6885 L6890 Description Addition to upper extremity prosthesis, below elbow/above elbow, custom fabricated socket insert for congenital or atypical traumatic amputee, silicone gel, elastomeric or equal, for use with or without locking mechanism, initial only (for other than initial, use code l6694 or l6695) Addition to upper extremity prosthesis, below elbow/above elbow, custom fabricated socket insert for other than congenital or atypical traumatic amputee, silicone gel, elastomeric or equal, for use with or without locking mechanism, initial only (for other than initial, use code l6694 or l6695) Addition to upper extremity prosthesis, below elbow/above elbow, lock mechanism, excludes socket insert Terminal device, passive hand/mitt, any material, any size Terminal device, sport/recreational/work attachment, any material, any size Terminal device, hook, mechanical, voluntary opening, any material, any size, lined or unlined Terminal device, hook, mechanical, voluntary closing, any material, any size, lined or unlined Terminal device, hand, mechanical, voluntary opening, any material, any size Terminal device, hand, mechanical, voluntary closing, any material, any size Terminal device, hook, mechanical, voluntary opening, any material, any size, lined or unlined, pediatric Terminal device, hook, mechanical, voluntary closing, any material, any size, lined or unlined, pediatric Terminal device, hand, mechanical, voluntary opening, any material, any size, pediatric Terminal device, hand, mechanical, voluntary closing, any material, any size, pediatric Terminal device, multiple articulating digit, includes motor(s), initial issue Terminal device, hook or hand, heavy-duty, mechanical, voluntary opening, any material, any size, lined or unlined Terminal device, hook or hand, heavy-duty, mechanical, voluntary closing, any material, any size, lined or unlined Addition to terminal device, modifier wrist unit Addition to terminal device, precision pinch device Electric hand, switch or myolelectric controlled, independently articulating Automatic grasp feature, addition to upper limb electric prosthetic terminal device Microprocessor control feature, addition to upper limb prosthetic terminal device Replacement socket, below elbow/wrist disarticulation, molded to patient model, for use with or without external power Replacement socket, above elbow/elbow disarticulation, molded to patient model, for use with or without external power Replacement socket, shoulder disarticulation/interscapular thoracic, molded to patient model, for use with or without external power Addition to upper extremity prosthesis, glove for terminal device, any material, prefabricated, includes fitting and adjustment Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 18 HCPCS Procedure Code Description Addition to upper extremity prosthesis, glove for terminal device, any material, custom fabricated Hand restoration (casts, shading and measurements included), partial L6900 hand, with glove, thumb or one finger remaining Hand restoration (casts, shading and measurements included), partial L6905 hand, with glove, multiple fingers remaining Hand restoration (casts, shading and measurements included), partial L6910 hand, with glove, no fingers remaining Hand restoration (shading and measurements included), replacement L6915 glove for above EXTERNAL POWER: UPPER LIMB PROSTHETICS Wrist disarticulation, external power, self-suspended inner socket, L6920 removable forearm shell, otto bock or equal switch, cables, 2 batteries and 1 charger, switch control of terminal device Wrist disarticulation, external power, self-suspended inner socket, L6925 removable forearm shell, otto bock or equal electrodes, cables, 2 batteries and one charger, myoelectronic control of terminal device Below elbow, external power, self-suspended inner socket, L6930 removable forearm shell, otto bock or equal switch, cables, 2 batteries and one charger, switch control of terminal device Below elbow, external power, self-suspended inner socket, L6935 removable forearm shell, otto bock or equal electrodes, cables, 2 batteries and one charger, myoelectronic control of terminal device Elbow disarticulation, external power, molded inner socket, removable humeral shell, outside locking hinges, forearm, otto bock or equal L6940 switch, cables, 2 batteries and one charger, switch control of terminal device Elbow disarticulation, external power, molded inner socket, removable humeral shell, outside locking hinges, forearm, otto bock or equal L6945 electrodes, cables, 2 batteries and one charger, myoelectronic control of terminal device Above elbow, external power, molded inner socket, removable humeral shell, internal locking elbow, forearm, otto bock or equal L6950 switch, cables, 2 batteries and one charger, switch control of terminal device Above elbow, external power, molded inner socket, removable humeral shell, internal locking elbow, forearm, otto bock or equal L6955 electrodes, cables, 2 batteries and one charger, myoelectronic control of terminal device Shoulder disarticulation, external power, molded inner socket, removable shoulder shell, shoulder bulkhead, humeral section, L6960 mechanical elbow, forearm, otto bock or equal switch, cables, 2 batteries and one charger, switch control of terminal device Shoulder disarticulation, external power, molded inner socket, removable shoulder shell, shoulder bulkhead, humeral section, L6965 mechanical elbow, forearm, otto bock or equal electrodes, cables, 2 batteries and one charger, myoelectronic control of terminal device Interscapular-thoracic, external power, molded inner socket, removable shoulder shell, shoulder bulkhead, humeral section, L6970 mechanical elbow, forearm, otto bock or equal switch, cables, 2 batteries and one charger, switch control of terminal device L6895 Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 19 HCPCS Procedure Code Description Interscapular-thoracic, external power, molded inner socket, removable shoulder shell, shoulder bulkhead, humeral section, L6975 mechanical elbow, forearm, otto bock or equal electrodes, cables, 2 batteries and one charger, myoelectronic control of terminal device L7007 Electric hand, switch or myoelectric controlled, adult L7008 Electric hand, switch or myoelectric, controlled, pediatric L7009 Electric hook, switch or myoelectric controlled, adult L7040 Prehensile actuator, switch controlled L7045 Electric hook, switch or myoelectric controlled, pediatric L7170 Electronic elbow, hosmer or equal, switch controlled Electronic elbow, microprocessor sequential control of elbow and L7180 terminal device Electronic elbow, microprocessor simultaneous control of elbow and L7181 terminal device Electronic elbow, adolescent, variety village or equal, switch L7185 controlled L7186 Electronic elbow, child, variety village or equal, switch controlled Electronic elbow, adolescent, variety village or equal, L7190 myoelectronically controlled Electronic elbow, child, variety village or equal, myoelectronically L7191 controlled L7259 Electronic wrist rotator, any type ADDITIONS TO UPPER EXTREMITY Addition to upper extremity prosthesis, below elbow/wrist L7400 disarticulation, ultralight material (titanium, carbon fiber or equal) Addition to upper extremity prosthesis, above elbow disarticulation, L7401 ultralight material (titanium, carbon fiber or equal) Addition to upper extremity prosthesis, shoulder L7402 disarticulation/interscapular thoracic, ultralight material (titanium, carbon fiber or equal) Addition to upper extremity prosthesis, below elbow/wrist L7403 disarticulation, acrylic material Addition to upper extremity prosthesis, above elbow disarticulation, L7404 acrylic material Addition to upper extremity prosthesis, shoulder L7405 disarticulation/interscapular thoracic, acrylic material L7499 Upper extremity prosthesis, not otherwise specified PROSTHETIC SOCKS L7600 Prosthetic donning sleeve, any material, each L8400 Prosthetic sheath, below knee, each L8410 Prosthetic sheath, above knee, each L8415 Prosthetic sheath, upper limb, each Prosthetic sheath/sock, including a gel cushion layer, below knee or L8417 above knee, each L8420 Prosthetic sock, multiple ply, below knee, each L8430 Prosthetic sock, multiple ply, above knee, each L8435 Prosthetic sock, multiple ply, upper limb, each L8440 Prosthetic shrinker, below knee, each L8460 Prosthetic shrinker, above knee, each L8465 Prosthetic shrinker, upper limb, each L8470 Prosthetic sock, single ply, fitting, below knee, each L8480 Prosthetic sock, single ply, fitting, above knee, each Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 20 HCPCS Procedure Code L8485 L8499 Description Prosthetic sock, single ply, fitting, upper limb, each Unlisted procedure for miscellaneous prosthetic services Orthotic and prosthetic supply, accessory, and/or service component of another HCPCS l code sales tax, orthotic/prosthetic/other office L9900 visits with two or more modalities to the same area, initial 30 minutes, each visit REPAIR AND REPLACEMENT L7510 Repair of prosthetic device, repair or replace minor parts L7520 Repair prosthetic device, labor component, per 15 minutes MISCELLANEOUS L8510 Voice amplifier WIGS Please note: This is exclusion for 2001, but an optional buy up for 2007 and 2011. A9282 Wig, any type, each Limited to specific diagnosis codes? YES NO Limited to place of service (POS)? YES NO Limited to specific provider type? YES NO Limited to specific revenue codes? YES NO REFERENCES 1. BCBS of Alabama, Medical Policy #083-Microprocessor-Controlled Lower Limb Prosthesis, Effective February 2010; Revised August 2013@ https://www.bcbsal.org/providers/policies/Accessed February 2, 2014 2. CGS Administrator, Lower Limb Prosthesis, L11442, Effective 01/01/2013 3. Noridian Jurisdiction D- DMERC LCD Lower Limb Prosthetics http://www.cms.gov/medicare-coverage-database/overview-and-quick-search.aspx 4. Össur [Website] Proprio Foot. Available at: http://www.ossur.com/?PageID=13460 Accessed February 2, 2013 GUIDELINE HISTORY/REVISION INFORMATION Date • • 02/01/2015 Action/Description Reorganized and renamed policy; combined content previously outlined in the CDGs titled: o Prosthetic Devices and Wigs o Specialized, Microprocessor or Myoelectric Limbs Revised coverage rationale/indications for coverage for prosthetic devices and wigs; added language to indicate: o A determination of coverage for the prosthesis is based on the enrollee’s potential functional abilities o Potential functional ability is based on the reasonable expectations of the prosthetist and treating physician, considering factors including, but not limited to: Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 21 Date Action/Description The enrollee’s past history (including prior prosthetic use if applicable); and The enrollee’s current condition including the status of the residual limb and the nature of other medical problems Revised definitions; updated “Lower Limb Rehabilitation Classification Levels” o Added applicable “K-Level” headers/descriptors o Updated description for “K-Level 0” o Removed “VA requirements for computerized limbs” Updated list of applicable HCPCS codes to reflect annual code edits (effective 01/01/2015): o Upper Limb Prosthetics: Added L6026 Removed L6025 o External Power Upper Limb Prosthetics: Added L7259 Removed L7260 and L7261 Archived previous policy version CDG.018.02 • • • Prosthetic Devices, Wigs, Specialized, Microprocessor or Myoelectric Limbs (Effective 02/01/2015) Proprietary Information of UnitedHealthcare. Copyright 2015United HealthCare Services, Inc. 22
© Copyright 2026