Hazardous drug list
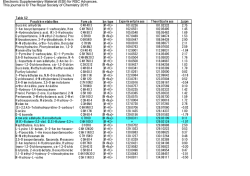
BC CANCER AGENCY HAZARDOUS DRUG LIST (Refer to: Pharmacy Directive VI-80 Hazardous Drug List) The BC Cancer Agency Hazardous Drug List (BCCA HD List) will be posted in each BCCA Regional Cancer Centre Pharmacy. BCCA workers who handle hazardous drugs (HD) must utilize safe handling practices to minimize exposure to these agents. The BCCA HD List is comprised of two parts. First, the most current version of the National Institute for Occupational Safety and Health-US (NIOSH) HD list will be adopted for use as published. Second, a BCCA addendum has been created for drugs not evaluated by NIOSH. Because the NIOSH HD list is only updated periodically, new oncology drugs that are approved for use between published NIOSH HD list updates and/or oncology drugs that are not evaluated by NIOSH will be evaluated by a BCCA Provincial Pharmacy Drug Information Pharmacist using the six NIOSH HD characteristics as per Directive VI-80. Oncology drugs which are assessed as hazardous by BCCA will be added to the BCCA Hazardous Drug List Addendum to the NIOSH List (BCCA HD List Addendum). The BCCA HD List will be comprised of both the currently published NIOSH HD List (see Appendix 1) and the BCCA HD List Addendum (see Appendix 2). An asterisk (*) after the drug name on the NIOSH HD List will signify drugs which have been designated as biohazardous (BioHD) at BCCA. Refer to appropriate BCCA safe handling directives for further information. Appendix 1 of 2 NIOSH Hazardous Drug List* * NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings (2012) Table 1. Sample List of Drugs that Should be Handled as Hazardous Acitretin Aldesleukin Alefacept Alitretinoin Altretamine Ambrisentan Amsacrine Anastrozole Arsenic trioxide Asparaginase Azacitidine Azathioprine Bacillus Calmette - Guerin (BCG)* Bendamustine HCl Bexarotene Bicalutamide Bleomycin Bortezomib Bosentan Busulfan Cabergoline Capecitabine Carbamazepine Carboplatin Carmustine Cetrorelix acetate Chlorambucil Chloramphenicol Choriogonadotropin alfa Cidofovir Cisplatin Cladribine Clofarabine Clonazepam Colchicine Cyclophosphamide Cyclosporine Cytarabine Dacarbazine Dactinomycin Dasatinib Daunorubicin HCl Decitabine Degarelix Denileukin Diethylstilbestrol Dinoprostone Docetaxel Doxorubicin Dronedarone HCl Dutasteride Entecavir Epirubicin Ergonovine/methylergonovine Estradiol Estramustine phosphate sodium Estrogen-progestin combinations Estrogens, conjugated Refer to: BC Cancer Agency Pharmacy Directive VI-80 Hazardous Drug List. 1/5 Estrogens, esterified Estrone Estropipate Etoposide Everolimus Exemestane Nilotinib Nilutamide Finasteride Floxuridine Fludarabine Fluorouracil Fluoxymesterone Flutamide Fulvestrant Paclitaxel Palifermin Paroxetine Pazopanib Pegaspargase Pemetrexed Pentamidine isethionate Pentetate calcium trisodium Pentostatin Phenoxybenzamine HCl Pipobroman Plerixafor Podofilox Podophyllum resin Pralatrexate Procarbazine Progesterone Progestins Ganciclovir Ganirelix acetate Gemcitabine Gemtuzumab ozogamicin Gonadotropin, chorionic Goserelin Hydroxyurea Idarubicin Ifosfamide Imatinib mesylate Irinotecan HCl Ixabepilone Leflunomide Lenalidomide Letrozole Leuprolide acetate Lomustine Mechlorethamine Medroxyprogesterone acetate Megestrol Melphalan Menotropins Mercaptopurine Methotrexate Methyltestosterone Mifepristone Mitomycin Mitotane Mitoxantrone HCl Mycophenolate mofetil Mycophenolic acid Nafarelin Nelarabine Oxaliplatin Oxcarbazepine Oxytocin Raloxifene Rasagiline mesylate Ribavirin Risperidone Romidepsin Sirolimus Sorafenib Streptozocin Sunitinib malate Tacrolimus Tamoxifen Televancin Temozolomide Temsirolimus Teniposide Testolactone Testosterone Tetracycline HCl Thalidomide Thioguanine Thiotepa Topotecan Toremifene citrate Tretinoin Trifluridine Refer to: BC Cancer Agency Pharmacy Directive VI-80 Hazardous Drug List. 2/5 Triptorelin Uracil mustard Valganciclovir Valproic acid/ divalproex Na Valrubicin Vidarabine Vigabatrin Vinblastine sulfate Vincristine sulfate Vinorelbine tartrate Vorinostat Zidovudine Ziprasidone HCl Zoledronic acid Zonisamide End of Appendix 1. See following page for Appendix 2: BCCA HD List Addendum to the NIOSH List. Refer to: BC Cancer Agency Pharmacy Directive VI-80 Hazardous Drug List. 3/5 Appendix 2 of 2 BCCA HD List Addendum to the NIOSH List (BCCA HD List Addendum) Because the NIOSH HD list is only updated periodically, new oncology drugs that are approved for use between published NIOSH HD list updates and/or oncology drugs that are not evaluated by NIOSH will be evaluated by a BCCA Provincial Pharmacy Drug Information Pharmacist using the six NIOSH HD characteristics as per Directive VI-80. The following list of oncology drugs have been assessed as hazardous by BCCA and BCCA workers must utilize safe handling practices to minimize exposure to these agents. An asterisk (*) after the drug name on the BCCA Addendum will signify drugs which have been designated as biohazardous (BioHD) at BCCA. Refer to appropriate BCCA safe handling directives for further information. Revised: 01 February 2015 Abiraterone Afatinib Anagrelide Axitinib Bevacizumab Brentuximab Buserelin Cabazitaxel Crizotinib Dabrafenib Doxorubicin, pegylated Enzalutamide Eribulin Ipilimumab Lanreotide Lapatinib Ofatumumab Paclitaxel, nab Pembrolizumab Pertuzumab Quinagolide Raltitrexed Ramucirumab Regorafenib Refer to: BC Cancer Agency Pharmacy Directive VI-80 Hazardous Drug List. 4/5 Reovirus Serotype 3 – Dearing Strain* Ruxolitinib Tocilizumab Trastuzumab Trastuzumab Emtansine Vemurafenib Vismodegib Refer to: BC Cancer Agency Pharmacy Directive VI-80 Hazardous Drug List. 5/5
© Copyright 2026
![1 [Billing Code: 4140-01-P] DEPARTMENT OF HEALTH AND](http://s2.esdocs.com/store/data/000481670_1-8e8a4da9fa52328752f9c73fe145ffa0-250x500.png)