A Controlled Comparison of the Efficacy of Hetastarch and
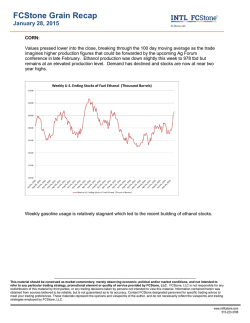
From www.bloodjournal.org by guest on February 6, 2015. For personal use only. A Controlled Comparison of the Efficacy of Hetastarch and Pentastarch in Granulocyte Collections by Centrifugal Leukapheresis By J.-H. Lee, SF. Leitman, and H.G. Klein Compared with hetastarch (HS), the low molecular weight analog pentastarch (PS) has been reported to be equally effectivefor granulocyte collectionby centrifugal leukapheresis, to result in fewer adverse donor reactions (ADR), and to have a more rapid elimination profile. We prospectively comparedthe granulocytecollection efficiency (GCE), granulocyte yield, and ADR in 72 randomly paired granulocytapheresis proceduresfrom 36 volunteer donors using the model CS-3000 Plus Blood Cell Separator(CS) and either PS or HS as the sedimenting agent. Paired collections from each donor allowed us to compare the two agents directly while controlling for intrinsic donor differences. In 33 of 36 (92%) donors, HS procedures were significantly more efficient than PS procedures ( P < .001). As anaverage, HS collections yielded 2.3 rt 0.67 x 10” granulocytes at 58% 2 8.8% GCE, whereas PS proceduresresulted in 1.4 2 0.76 x 10” granulocytes at 33% 2 15% GCE. No starch-induced ADR were seen with either agent. For granulocyte harvests using the CS, (1) in most donors,using HS as the red blood cellsedimenting agent during centrifugal leukapheresisresults in significantly higher(nearly twofold) GCE and larger granulocyte yields in comparison with using PS, (2) ADR were not observed with either agent, and (3) the potential benefit of more rapid PS elimination should be balancedagainst significantly lower granulocyte yields. This is a US government work. There are no restrictions on its use. M aged most collectors to substitute PS for HS in procuring granulocytes. However, equivalent efficacy for PS and HS has not been shown in a controlled trial. The present controlled study examines the granulocyte collection efficiency (GCE), granulocyte yield, and the ADR in randomly paired PS-HS collection procedures from the same donor and directly compares the performance characteristics of the two agents for use in centrifugal granulocytapheresis. ACROMOLECULEShave beenroutinelyadministered to volunteer blood donors during granulocyte collection to promote redbloodcell (RBC) sedimentation and increase granulocyte yield.’ During the past decade, 6% hydroxyethyl starch (hetastarch [HS]) has been the preferred sedimenting agent because it greatly enhances granulocyte yield, the transient plasma expansion is generally well tolerated, and it has few clinically significant side effects in conventional doseand administrationfrequency.’,’ However, significant HS blood levels persist for weeks andtrace amountscanbe detected years afterits Delayed HSclearanceandconsequent long-term donor safety concerns resulted in a search for alternative agents. Ten percent pentastarch (PS), a more recently developed low molecular weight HS analog, is cleared from thecirculation relatively rapidly and has been reported to be equally effective in enhancing granulocyte collection. Theability of PS to separate blood cells and induce RBC sedimentation, as assessed in vitroby the recovery of blood cellsin supernatant plasma, has been reported to be similar to that of HS.‘ The results of a subsequent multicenter clinical trialinvolving 75 volunteer donors who underwent 179 centrifugation leuPS as kapheresis procedures stronglysuggestedthatusing thesedimenting agentresultsinan adequategranulocyte yield comparableto that from using HS, and also documented almostno clinicallysignificant adversedonoreffects.’ Initial studies could not detect PS in the blood within a fewdays of its administration,’and the adversedonor reactions (ADR) profile has been inferred to be even more favorable thanthat of HS.*These initial studies have encourFrom the Department of Transfusion Medicine, Warren G. Mugnuson Clinical Center, National Institutes of Health, Bethesdu, MD. Submitted November 7, 1994; accepted July 31, 1995. Addressreprintrequeststo J.-H. Lee,MD, Bldg IO, Room IC71l . National Institutes of Health, Bethesda, MD 20892. The publication costs of this article were defrayed in part by page chargepayment. This article must thereforebehereby murked “advertisement” i n accordunce with 18 U.S.C. section 1734 solely to indicute this fuct. This is a US government work. There are no restrictions on its use. 0006-497//95/8612-0001$0.00/0 4662 MATERIALS AND METHODS We prospectively studied 72 consecutive granulocyte collections from 36 volunteer blood donors using the model CS3000 Plus Blood Cell Separator (CS; Fenwal Laboratories, Deerfield, IL). Each donor underwent paired collections separated by approximately 2 months (range, 2 weeks to 7 months) and randomly received a standard 500 mL dose of either 10%PS (McGaw, Inc, Irvine, CA) or 6% HS (NPBI, Emmer-Compascuum, The Netherlands) during the first procedure and the alternate agent during the subsequent collection. The HS preparation used was chemically and pharmacologically identical to the preparation more widely used in the United States (McGaw, Irvine, CA). Thirty milliliters of 46.7% trisodium citrate added to 500 mL of either PS or HS was evenly distributed over approximately 7 L of processed whole blood (1 :13 ratio with blood). After obtaining informed consent for the procedure for procuring granulocytes, all donors were premedicated with 8 mgof oral dexamethasone 12 hours before each procedure. The two RBC sedimenting agents are compared in Table I . The specific instrument settings used are summarized in Table 2. GCE. The GCE of each procedure was calculated by dividing the granulocyte yield by the total number of granulocytes processed. The yield was calculated as the product of the component cell count and volume. The number of granulocytes processed (GP) was calculated as follows: GP = (WBC)(%G)(L), where WBC represents the donor peripheral white blood count per liter, %G represents the granulocyte fraction (neutrophils including bands), and L represents the total volume of processed whole blood in liters. We used the average of the cell counts immediately before and after each procedure, for both WBC and %G. The accuracy of averaging the two cell counts has been previously verified.’ All cell counts were performed on the Coulter Counter Model S-PLUS V (Coulter Electronics, Inc, Hialeah, FL). PS versus HS. For each donor, we plotted the GCE (Fig 1) and the granulocyte yield (Fig 2) from the procedure using HS asa function of the donor’s corresponding values using PS. We also Blood, Vol 86, No 12 (December 15), 1995: pp 4662-4666 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 4663 HYDROXYETHYLSTARCH IN GRANULOCYTAPHERESIS Table 1. A Comparison of the Biochemical and Pharmacokinetic Characteristicsof 10% PS and 6% HS Characteristics PS 480,000 264,000 Average molecular weight (MW) 71,00063,000 No. average molecular weight (MN)* 6.0 10.0 Hydroxyethyl starch concentration (g/dL) 0.70 0.45 Hydroxyethyl groups per glucose residue 25.5 (h)* 2.5 Time to reach 50% peak blood level 7 24-h plasma distribution (% dose)' 33 70 24-h urinary excretion (% dose) 33 24-h extravascular distribution23(% dose)* 96 h Overall survival in blood* HS 3% 17-26 wk * Mishler et al.' Characteristics not taken from Mishler et all have been listed directly from thepackage insert of each agent. compared the mean and the standard deviation of the GCE and the granulocyte yield for all procedures using PS versus all procedures using HS. The GCE distribution of the 36 procedures on each sedimenting agent is compared in Fig 3. Thedonorswereverballyquestioned to detectanyADRfrom either starch preparation at completion of each procedure and were instructed to reportbyphoneanyproblemsarisingsubsequentto each procedure. All granulocyte donors in this study werealso regularplateletdonorsat our collection center,andsubsequent donor visits allowed the opportunity to confirm absence of ADR. Statistical methods. The significance of the results on GCE and granulocyte yield wasanalyzed using the sign test." In brief,the datapoints above the line of identity were labeled plus and those below the line of identity were labeled minus in determining the x' (with one degree of freedom)and P values. Theadequacy of the sample size was inferred from the resulting degree of significance. In Figs 1 and 2, we used linearregression analysis (sum of least squares method) to generate trend lines. substantially more granulocytes in 32 ofthe 36 donors (89%, P < .001). The wide interdonor variability in the GCE and the granulocyte yield using PS were minimized by using HS, as indicated by the relatively horizontal slope values less than 1 (0.24 and 0.52 for Figs 1 and 2, respectively). For all 72 procedures, using HS resulted in a significantly higher mean GCE (58% ? 8.8% v 33% 2 15%, P < .001) and a larger mean granulocyte yield (2.3 -+ 0.67 v 1.44 2 0.76 X 10'' granulocytes, P < .OO1) than using PS. The greater variability in cell yield with PS than with HS may be also appreciated from Fig 3, which shows a distribution plot of the granulocyte yield for the 36 procedures with each agent. The less efficient and more variable PS collections included 9 procedures (25%) with unacceptably low cell numbers (< 1 X 10" granulocytes) and were more likely (12 procedures [33%]) to result in a yield between 1.0 and 1.5 X 10'' granulocytes. In contrast, only one HS collection (3%) generated less than 1 X 10" granulocytes. HS collection commonly (13 procedures [36%]) generated components with a cell dose between 2.0 and 2.5 X 10" granulocytes. Using HS assured a minimum GCE of 40%, whereas using PS resulted in a collection failure with a GCE less than 10% in 3 of the 36 procedures (8.3%).Infrequent ADR 100 - Hetastarch GCE (%) .. 80 A RESULTS A Figure 1 compares the expected relationship between HS and PS if the GCE for the two agents are equal (dashed line) with the observed relationship (solid line). The increased yintercept of the observed line describes minimization, by using HS instead of PS, of unacceptably poor GCE or collection failures. Thirty-three of the 36 donors (92%) showed a higher GCE with HS than with PS ( P < .001). The actual granulocyte yields are plotted in Fig 2, in which comparable predonation leukocyte counts for the two collections from each donor resulted in a line comparable to that in Fig 1. The overall mean predonation leukocyte count of approximately 8 X 109/L with80% granulocytes showed substantial interdonor but relatively little intradonor variability. In comparison with PS, using HS resulted in a component with A . 60 ................. 0 c A A 40 . 20 - ,, 0 1,000 Whole flow blood rate Centrifuge RPM volume Blood processed Interface detector setting Program Chamber type 50 mUmin 6.5-7.0 L 33 2 Granulo ,, 0 O L Table 2. Apheresis Parameters for Granulocyte Collection onthe Model CS3000 Plus Blood Cell Separator , /'A A 5 I I 10 15 I 20 I 25 I 30 I 35 I 40 I 45 I 50 I 55 Pentastarch GCE (8) Fig 1. A comparison of the GCE for PS and HS. The GCE using HS was roughly linearly correlatedwith the GCE using PS, and a given donor with a relatively highGCE on PSwas ah0 more likelyto show a high GCE on HS. In 33 of 36 donors (92%). HS resulted in a superior GCE in cornperiwnto PS. The 3 remaining donors showed a cornparabla GCE with either PS or HS. The dashed line indicates the hypothetical plot assuming that the two agents are equally effective. From www.bloodjournal.org by guest on February 6, 2015. For personal use only. LEE, LEITMAN, AND KLEIN 4664 from dexamethasone premedication (sleep disturbance) and the apheresis procedure (mild vasovagal reactions and citrate toxicity) did not favor collections using either agent. None of the 36 donors reported ADR directly attributable to either agent. Collections 14 . .-Pentastsrch 12 ............. * - Hetastarch .....""" ................... DISCUSSION l Advances in blood component therapy and the therapeutic efficacy of RBC, platelet, and plasma components allow effective transfusion support of patients with complicated hematologic disorders. As a result, infectious complications unresponsive to optimal antibiotic therapy have become an increasing cause of morbidity and mortality in patients with prolonged cytopenias. In contrast to RBCs and platelets, granulocytes have developed slowly as a therapeutic blood component, owing in part to the difficulty in collecting sufficient numbers of cells that permit well-controlled studies to assess granulocyte transfusion efficacy. Thus, despite numerous studies that have attempted to assess the therapeutic role of granulocyte transfusions (GTs)in neutropenic patients, definitive information is not available and GTs have fallen out of favor. Seven controlled studies comparing antibiotic therapy alone versus GT in addition to antibiotic ther- . . . . . . . . . . . . * . . . . . . . . . . . .., ........................... ;i L! l .. 'i ...... .; ri . ...... 4 -:l _. . . . . . . . . . . . . . . . . . . . . . ..... 5. .l# ................................. I I-! -. -: m = Fi Ij ...................................................... l 0.5 HS Yield $1 li3 1 1.5 2 2.5 3 3.5 4 Granulocyte Yield A A I I AI I' A I . . . . . . . . . . . . . .~I" " ' . . . " " " " ' . . . . . .. / A I / / I I I / I / 0 I I I l I I 0.5 1 1.5 2 2.5 3 3.5 PS Yield Fig2. A comparisonof the granulocyte yield for PS and HS. A plot of the actual granulocyte yield rather than the GCE generated a line analogous to the line shown in Fig 1. In 32 of 36 donors (89%). HS resulted in a superior granulocyte yield in comparison to PS.The 4 remaining donors showed a comparable yield with either agent. The dashed line indicates the hypothetical plot assuming that the two agents are equally effective. Fig 3. The granulocyte yield distributions for the two sedimenting agents. The number of procedures generating a particular granulocyte yield was plotted as a function ofthat yield ( x 10" granulocytes, 0.5) for the 36 procedureswith each agent. grouped in increments of with a higher granuUsing HS resulted in more uniform components locyte yield than usingPS. apy in infected neutropenic patients have generated conflicting conclusions. Five of the seven studies report at least partial success of GT; no benefit was seen in the remaining two studies involving 47 and 22 subjects. However, the latter two studies used extremely low cell doses, approximately only 20% of currently achievable dose." Recent reports using granulocyte colony-stimulating factor to stimulate normalblood donors again raise the possibility of routinely collecting greater numbers of granulocytes in the future.'' Advances that increase cell dose may significantly improve the efficacy of GT, especially in the pediatric setting, in which a higher dose of granulocytes per kilogram of body weight is possible. It thus seems appropriate to reanalyze the technique for collecting granulocytes in optimal numbers. The granulocyte yield depends primarily on the efficiency at which the cells are extracted during an apheresis collection procedure. The similarity of RBC and granulocyte sedimentahas necessitated the use of tion rates during macromoleculesto enhance RBC sedimentation and to achieve optimal separation of these cells for a successful granulocyte colle~tion.'~.'~ The macromolecules most widely used include hydroxyethyl starch preparations, dextrans, and modified fluid gelatins; of these, HS gained initial favor because it effectively enhancedgranulocyteyield with few ADR in conventional From www.bloodjournal.org by guest on February 6, 2015. For personal use only. HYDROXYETHYL STARCH IN GRANULOCYTAPHERESIS 4665 The initial studies on PS, which included one multicenter dose and administration frequency.' However, significant HS blood levels persist for weeks, and trace amounts may be declinical trial, concluded thatPS is as effective as HSin tected years after its administrati~n.~-~ Incidence estimates of centrifugal granulo~ytapheresis.~.~ In view of the current litclinically apparent HS-induced adverse reactions range from erature on the effects of macromolecules on the physics 0.09%to 0.7%.','6,'7In doses of less than 1,500mL,HS induces of blood cell separationz3 and the biochemical differences onlytransient,mild,clinicallyinsignificantlaboratoryalterbetween PS and HS,' it would be surprising ifin fact PS ations,includingprolongedpartialthromboplastinand prowere as effective as HS. One intrinsic donor variable that thrombin times, decreased platelet counts and fibrinogen levels, predictably influences granulocyte harvest by centrifugal leuvon Willebrand-likesyndrome,and hyperamyla~emia.'.'~.'~ kapheresis is the donor erythrocyte sedimentation rate However, serious reactions, including severe pruritus, dissemi- (ESR).9 Results of studies from our laboratory have sugnatedintravascularcoagulopathy,andshock,havebeenregested that PS may be less effective than HS, based on an ported.2s22Althoughthey are exceedingly rare, these serious indirect comparison of the respective formulas that predict reactions and concern about long-term toxicity as a result of GCE from the donor ESR. The slope and the y-intercept of delayed HS clearance have prompted a search fora sedimenting the linear relationship GCE (%) = 1.3 ESR (m&) + 45 agentwith a morefavorableeliminationprofile.Theinitial when one uses HS as the cell sedimenting agent decreases studies which suggested that PS is as effective as HS while to 0.8 and 20, respectively, when PS is substituted for HS being eliminated much more rapidly encouragedmany collecin otherwise identical collection procedure^.'^ In this study, tors to choosePS for use in granulocyte harvests by centrifugal we directly compare the two agents in a controlled clinical leukapheresis.6-8 trial; our results confirm the indirect comparison study. The The separation of granulocytes from erythrocytes, which more consistent HS collections assured a 40% minimum dictate GCE, depends on the relative sedimentation rates of GCE, whereas a substantial fraction (8.3%) of the less prethe two cell types and the granulocyte sedimentation rate dictable PS procedures resulted in collection failures at less relative to the upward plasma flow resulting from downward than 10% GCE. Routine PS use will more likely result in cell displacement. Macromolecules that promote RBC roufewer granulocytes than the criteria established by the Amerleaux formation increase the effective cell mass to cell surican Association of Blood Banks Standards Committee, or face area ratio and thereby increase both the cell sedimenta1.0 X 10" granulocytes per unitin75%of tested units.z6 tion rate and the consequent upward plasma flow. RBCs, Finally, the lack of ADR from either agent in our series of with a highly negatively charged outer cell surface, are much only 36 collections in each of the two arms from 72 donors more susceptible to polar macromolecules than are neutral is not unexpected in view of the previously reported incigranulocytes, and RBC sedimentation is selectively indence of less than creased over granulocyte sedimentation through both direct We conclude the following for centrifugal granulocytaphcell and indirect plasma effects.23Although the precise moeresis using the model CS3000 Plus blood cell separator and lecular mechanism of RBC rouleaux formation is unknown, either PS or HS as the RBC sedimenting agent. (1) In most kinetic studies using nuclear magnetic resonance relaxation donors, HS results in a significantly higher (nearly twofold) methods have shown that HS rapidly induces over 20 secGCE and a larger granulocyte yield in comparison to PS. onds the formation of aggregates containing 4 RBCson (2) Sedimenting agent-induced ADR were notobserved with average at equilibrium, which effectively doubles the mass either HS or PS. (3) The potential benefit of more rapid PS to surface area ratio in comparison to a single RBC." elimination should be balanced against the significantly Two properties of macromolecules independently influlower granulocyte yield. The relative efficacy of PS and HS ence RBC sedimentation. There appears to be a critical mousing other blood cell separators and under other collection lecular weight (CMW) as well as a critical concentration conditions requires further study. (CC) below which RBC sedimentation is not enhanced. Although rigorous numbers are difficult to establish, the CMW ACKNOWLEDGMENT and the CC for hydroxyethyl starch compounds have been The authors sincerely thank David W. Alling, MD, PhD of the determined to be 300,000 and 0.3 g/dL, respectively.' HS, Biometry Branch, National Institute of Allergy and Infectious Diswith a weight-average molecular weight (MW) of 480,000 eases, for his expert comments and advice in statistically analyzing and relatively slow elimination over several days, readily the data. We also gratefully appreciate the assistance from our demeets these criteria under virtually all collection conditions partmental staff (Karen Diggs, Cynthia Martin, Sandra Bangham, used today. In contrast, the substantially lower PS MW of Phyllis Byrne, Rolande Grammont, Jeanette Rothberg, Kathleen 264,000 (below the CMW) may explain in part the decreased Swisher, and Margaret Weller) for their skilled hemapheresis operaefficacy relative to HS. Because the number-average molecution. We thank Gail Carter and Virginia Morgan for diligent donor lar weights of the two agents are fairly comparable, the recruitment, Herb Cullis and Charles Carter for insightful technical relatively few but large molecules present to a greater extent suggestions, and Tim Jacobson for his helpful technical assistance. in HS than in PS may be an important element that contributes to the more uniform, increased HS efficacy. FurtherREFERENCES more, the rapid PS plasma clearance within hours (and within 1. Mishler JM, Hester JP, Huestis DW, Rock GA, Strauss RG: the time frame of a single leukapheresis procedure) decreases Dosage and scheduling regimens for erythrocyte-sedimenting macrothe potential cumulative effect of infusing the sedimenting molecules. J Clin Apheresis 1:130, 1983 agent into recirculated donor blood already containing the 2. Rock G, McCombie N: Alternate dosage regimens for highagent in significant amounts. molecular-weight hydroxyethyl starch. Transfusion 25:417, 1985 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 4666 3. Trivedi SM, Humphrey RL, Braine HG, Frondoza CG: Hydroxyethyl starch serum levels in leukapheresis donors measured by modified periodic acid-Schiff staining technique. Transfusion 24:260, 1984 4. Maguire LC, Strauss RC, Koepke JA, Bowman RJ, Zelenski KR, Lambert RM, Hulse JD, Atnip AK: The elimination of hydroxyethyl starch from the blood of donors experiencing single or multiple intermittent-flow centrifugation leukapheresis. Transfusion 21 :347, 1981 S . Boon JC, Jesch F, Ring J, Messmer K: Intravascular persistence of hydroxyethyl starch in man. Eur Surg Res 8:497, 1976 6. Strauss RG: In vitro comparison of the erythrocyte sedimenting properties of dextran, hydroxyethyl starch and a new low-molecularweight hydroxyLthy1 starch. Vox Sang 37:268, 1979 7. Strauss Rti, Hester JP, Vogler WR, Higby DJ, Snikeris AC, Imig KM, Greazel C, Mallard G, Burnett D, Gupta S, Hulse JD: A multicenter trial to document the efficacy and safety of a rapidly excreted analog of hydroxyethyl starch for leukapheresis with a note on steroid stimulation of granulocyte donors. Transfusion 26:258, I986 8. Strauss RG, Stansfield C, Henriksen RA, Villhauer PS: Pentastarch may cause fewer effects on coagulation than hetastarch. Transfusion 28:257, 1988 9. Lee J-H, Klein HG:The effect of donor erythrocyte sedimentation rate on efficiency of granulocyte collection by centrifugal leukapheresis. Transfusion 35:384, 1995 IO. Siege1 S: Nonparametric Statistics for the Behavioral Sciences. New York, NY, McGraw-Hill, 1956, p 68 11. Strauss RG: Therapeutic granulocyte transfusions in 1993. Blood 81:1675, 1993 12. Van Wie BJ, Sofer SS: Sedimentation theory and practical considerations for the design of centrifugal blood cell processors. Int J Artif Org 7:215, 1984 13. Chien S, King RG, Skalak R, Usami S, Copley AL: Viscoelas- LEE, LEITMAN, AND KLEIN tic properties of human blood and redcell suspensions. Biorheology 12:341, 1975 14. Mishler JM: New dosage regimens for HES during intensive leukapheresis. Transfusion 18: 126, 1978 15. Strauss RG, Rhoret PA, Randels MJ, Winegarden DC: Granulocyte collection. J Clin Apheresis 6:241, 1991 16. Ring J, Messmer K: Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1:466, 1977 17. DiNubile MJ: Therapeutic role of granulocyte transfusions. Rev Infect Dis 7:232, 1985 18. Sanfelippo MJ, Suberviola PD, Geimer NF: Development of a von Willebrand-like syndrome after prolonged use of hydroxyethyl starch. Am J Clin Pathol 88:653, 1987 19. Strauss RG: Review of the effects of hydroxyethyl starch on the blood coagulation system. Transfusion 2 1 :299, 1981 20. Sz’epfalusi Z, Parth E, Jurecka W, Luger TA, Kraft D: Human monocytes and keratinocytes in culture ingest hydroxyethylstarch. Arch Dermatol Res 285:144, 1993 21. Jurecka W, Sz’epfalusi Z, Parth E, Schiemetta W, Gebhart W, Scheiner 0, Kraft D: Hydroxyethylstarch deposits inhuman skin-A model for pruritus? Arch Dermatol Res 285:13, 1993 22. Chang JC, Gross HM, Jang NS: Disseminated intravascular coagulation due to intravenous administration of hetastarch. Am J MedSci 300:301, 1990 23. Brown RI: The physics of continuous flow centrifugal cell separation. Artif Organs 13:4, 1989 24. Caines CH, Goldstein JH: NMR investigation of hydroxyethyl starch-induced aggregation ofhuman erythrocytes. J Magn Reson Imaging 5:67, 1987 25. Lee J-H, Klein HG: The efficacy of pentastarch in granulocyte collection by centrifugal leukapheresis. Transfusion 34:4s, 1994 (abstr) 26. Klein HG (ed): Standards for Blood Banks and Transfusion Services (ed 16). Bethesda, MD, American Association of Blood Banks, 1994, p 13 From www.bloodjournal.org by guest on February 6, 2015. For personal use only. 1995 86: 4662-4666 A controlled comparison of the efficacy of hetastarch and pentastarch in granulocyte collections by centrifugal leukapheresis JH Lee, SF Leitman and HG Klein Updated information and services can be found at: http://www.bloodjournal.org/content/86/12/4662.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.
© Copyright 2026