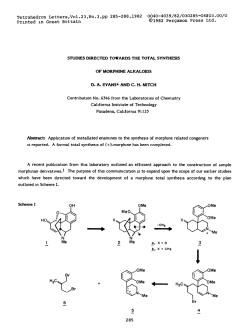
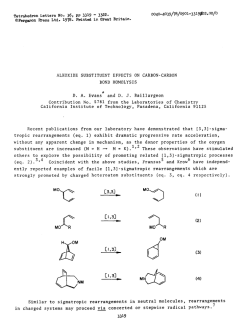
Full CV - The University of Tennessee Health Science Center
Gabor Joseph Tigyi, M.D., Ph.D. Harriet Van Vleet Professor and Department Chair Department of Physiology, University of Tennessee Health Science Center, Memphis Professor of Chemistry, University of Memphis (adjunct) Professor of Graduate Studies, Hokkaido University, Japan (visiting) Foreign Member, Hungarian Academy of Science, Budapest Member, European Academy of Arts, Letters and Sciences, Paris Chief Scientific Officer, RxBio Inc. PERSONAL Date and Place of Birth: February 20, 1958; Pécs, Hungary Citizenship: USA by naturalization Address: Department of Physiology University of Tennessee Health Sciences Center 894 Union Avenue Memphis, TN 38163 Tel.: (901) 448-4793 Fax: (901) 448-7126 Cell: (901) 233-4404 Email: [email protected] http://physio1.utmem.edu/~tigyig/index.html EDUCATION M.D. (summa cum laude), University Medical School of Pécs, Hungary, 1976-1982 Ph.D. Cellular and Molecular Neurobiology, Budapest (with honors) Thesis: Lysophosphatidate in the regulation of cell proliferation and neuronal differentiation Postgraduate Training: Massachusetts Institute of Technology, Department of Laser Spectroscopy Mentors: Lawrence R. Ryan and Geza J. Jako. 1979-1980. Biomedical Center, University of Uppsala, Department of Biochemistry Mentors: Stellan Hjerten and Paul Roos. 1983. Max-Planck Institute for Biophysical Chemistry, Laboratory of Molecular Biology, Gottingen, Germany. Mentor: Tom Jovin. 1984. University of California, Irvine. Laboratory of Cellular and Molecular Neurobiology, Department of Psychobiology. Mentor: Ricardo Miledi, Fellow of the Royal Society and Member of the National Academy of Science, USA. 19861990. ACADEMIC APPOINTMENTS 2007-present 2006-present 2005-present 2003-present 2002-present 2001-present 2000 2000 1999-present Chair, Department of Physiology, University of Tennessee Health Science Center, ,Memphis Harriet Van Vleet Chair in Oncology Research, University of Tennessee Health Science Center, Memphis Professor of Graduate Studies (visiting, on contract 2006-2011), Faculty of Pharmacology, Hokkaido University, Sapporo, Japan Professor of Chemistry (adjunct), Department of Chemistry, University of Memphis Associate Director for Basic Science, University of Tennessee Cancer Institute Founder and Chief Scientific Officer, RxBio Inc. - an emerging biotechnology company Visiting Professor, Faculty of Pharmaceutical Sciences, Hokkaido University, Japan. Visiting Professor, Ochanomizu University, Tokyo, Japan Professor, Department of Physiology, University of Tennessee Health Science Center, Memphis. Gábor Tigyi — Curriculum Vitae 1997-2005 1995-present 1995-1999 1994-present 1992-1995 1990-1992 Page 2 Visiting Professor, City University of New York, Queens College Department of Chemistry and Biochemistry Member, Cardiovascular-Renal Center of Excellence, Department of Physiology, University of Tennessee, Memphis. Associate Professor – with tenure (1996); Department of Physiology, University of Tennessee, Memphis Member, Center of Excellence in the Neurosciences, University of Tennessee, Memphis Assistant Professor, Department of Physiology and Biophysics, University of Tennessee, Memphis Assistant Professional Researcher, Laboratory of Cellular and Molecular Neurobiology, Department of Psychobiology, University of California, Irvine HONORS 2013 PR 2013 2011 Plenary lecture, Bioactive Lipid Mediates in Cancer, Inflammation and Other Diseases, St. Juan, Keynote speaker, FASEB Summer Conference on Lysophospholipids, Niseko, Japan Outstanding Research Advisor Award – College of Graduate Health Science, University of Tennessee Health Science Center 2011 Journal of Lipid Research Award – FASEB Summer Conference, Lucca, Italy 2010 NAITO Foundation Lecturer, Frontiers in Lipid Research, Sapporo, Japan 2008 RxBio developed a product that was selected by the 2008 Better World Project as one of the top 100 examples from across the globe of how innovation from academic research makes its way to the market. 2007 Member of the Board, IRIS Chamber Orchestra, Germantown, Tennessee 2007 Member, European Academy of Arts, Letters and Sciences, Paris 2006 Nominee, Wheeley Prize, University of Tennessee 2005 Keynote Speaker, University of Tokyo 21st Century COE symposium “Drug discovery and chemical biology” 2005 Keynote Speaker: FASEB Summer Conference on Lysophospholipids in Biology and Diseases 2005 President, Tennessee Road Warrirors, International Plastic Modelers Society Chapter, Memphis 2004 Foreign Member of the Hungarian Academy of Sciences 2002 Guest Editor – Biochimica et Biophysica Acta Special Issue on Lysophospholipids 2001 Takeda Foundation Lecturer, Symposium on Lipids in Signaling and Related Diseases, Tokyo 1997-2000 Established Investigator of the American Heart Association 1998 President of the Faculty Organization of the College of Medicine, University of Tennessee, Memphis. 1996 Nagayoshi Nagai Prize, Tokushima University, Tokushima, Japan 1996 Keynote Speaker, Lipid Session, Japanese Biochemical Society Annual Meeting, Kanazawa 1984 International Cell Research Organization Fellowship 1983 Merit Medal for Higher Education, Ministry of Education of Hungary for 4.0 GPA through higher education 1981 First Place Award, National Research Conference for Medical Students. Debrecen, Hungary. 1980 Special Award, 1st International Research Conference for Medical Students. Hradec Kralove, Czechoslovakia. 1979 First Place Award, National Research Conference for Medical Students. Pécs, Hungary. EDITORIAL BOARDS Progress in Lipid Research – Executive Editor 2013. Biochimica Biophysica Acta – 2000-present. Guest Editor, Special Issue on Lysophospholipids, 2002 and 2008 Journal of Biological Chemistry – 2003-2014 Journal of Cellular Biochemistry – 2003, 2008, 2012. Guest Editor, special volume on lysophospholipids GRADUATE LEVEL TEACHING Gábor Tigyi — Curriculum Vitae Page 3 Master Course in Biomedical Sciences, 2013 Interdisciplinary Graduate Course - Kidney physiology, 2005Kidney Physiology to Dentistry and Pharmacy Students, 2002Scientific Writing – through the reviewer’s eyes, 2006, 2007 Course Director, Physiology to Dentistry and Pharmacy Students, 2001 Medical Physiology – Osmoregulation, fluid balance, pH regulation, 1993-present Respiratory Physiology Lectures to Dental Students — D1 Course, UT-Memphis; 1993-1999 Graduate Electrophysiology, UT-Memphis; 1994, 1997, 2001, 2003 Human Physiology Review — to Advanced Placement Biology Students at White Station High School, Memphis City Schools; 1995, 1996 Biology '80: "Brain and behavior,” Neurobiology Lecture Course; 1991, University of California, Irvine Biology '79: "How the brain works,” Neuroscience Lecture Course; 1990, 1991, University of California, Irvine. Biology 105, Psychobiology Laboratory Course for Undergraduates; 1988, 1989, University of California, Irvine Biochemical Separation Methods Postgraduate Course, 1984. Budpaest Biophysics Laboratory Course, University Medical School; Pécs, Hungary; 1977-1982 UNDERGRADUATE/GRADUATE STUDENTS TRAINED Le-Thiet Thanh Postdoctoral UNESCO fellow, Institute of Biophysics, Biological Research Center of the Hungarian Academy of Sciences David Dyer Graduate student, Department of Psychobiology, University of California, Irvine Cindy J. Asselin Howard Hughes Undergraduate Excellence in Research Institute Sandra Win Howard Hughes Undergraduate Excellence in Research Institute David Fischer Graduate student, Department of Developmental and Cell Biology, University of California, Irvine Corey Wallace Young Memphis Scholars Program James Travis Undergraduate at Christian Brothers University and recipient of REU award from NSF. He completed his graduation thesis work in his lab, presented it at the Annual Meeting of the West Tennessee Academy of Sciences in Union City, and won third place. Vaughn Massey Recipient of REU award from NSF; medical student Frakeeta Marshall Minority student of Central High School, participant in Young Memphis Scholars Program; she won third place in the City Science Fair. Danielle Townsend Undergraduate participants in the Graduate Summer Enrichment Program Faith Marshall Young Memphis Scholars Summer Science Institute Antoni Jarret Young Memphis Scholars Summer Science Institute Brandon Sharp Young Memphis Scholars Summer Science Institute Amelia Zellander Young Memphis Scholars Summer Science Institute Steven Dirmeyer Young Memphis Scholars Summer Science Institute Rebecca West, Young Memphis Scholars Summer Science Institute Nico West Undergraduate student David Smith Undergraduate student Nilay Gandhi Young Memphis Scholars Summer Science Institute Sergio Klimkowski Young Memphis Scholars Summer Science Institute Tamás Virág Graduate student; graduated in January, 2004 Rebecca West Undergraduate student, University of Memphis Yuko Fujiwara Graduate student; graduated in March, 2004 Sana Mujahid Undergraduate, CHristion Brothers University Takemitsu Sano Graduate student; graduated in March, 2006 Gangadhar Durgam Graduate student; graduated in May, 2005 Renuka Gupte Graduate student; graduated in 2011 Shuyu E Graduate student; graduated in 2010 Alyssa Bolen Graduate student; graduated in 2011 Ryoko Tsukahara Graduate student; graduated in 2012 Gábor Tigyi — Curriculum Vitae Page 4 POSTDOCTORAL FELLOWS TRAINED 1994-1996 1994-2001 1995-1996 1996-1998 1996-1998 1997-1998 1997-2001 1998-1999 1998-2002 1998-2002 1998-2003 1999 1999-2000 1999-2000 1999-2001, 2000-2001 2000-2001 2000-2003 2001-2002 2001-2002 2002 -2003 2002-2004 2002-present 2003-2006 2003-2006 2003-present 2004-present 2005 2006-2011 2007-2010 2007-2010 2008-2012 2008-2013 2009-present 2012 2012-2013 2012-2013 2012-2013 2012-present 2012-present Michael Ferrebee, M.D. Critical Care Medicine Fellow, Le Bonheur Children's Hospital. Currently Faculty at University of West Virginia. David Fischer, Ph.D. Currently Senior Scientist, Group Leader, SERONO Inc., Boston. Thanh Le-Thiet, Ph.D. Postdoctoral fellow. Currently Assistant Professor. Zhong Guo, M.D. Károly Liliom, Ph.D. Postdoctoral fellow. Currently Senior Scientist, Group Leader, Institute of Enzymology Hungarian Academy of Sciences. Guoping Sun, Ph.D. Currenlty Patent Examiner at US Patent Office. Nora Nusser, M.D. Currently Assistant Professor. Balazs Debreceni, M.D. Currently Assistant Professor. Junming Yue, Ph.D. Currently Assistant Professor. De-an Wang, Ph.D. Currently Assistant Professor. Daniel Baker, Ph.D. Currently Assistant Professor. Agnes Sebök, M.D., Ph.D. Currently Associate Professor. Muhammad Emaduddin, Ph.D. Don Elrod, Ph.D. Currently Senior Scientist. Károly Liliom, Ph.D. Visiting Scientist. Vineet Sardar, Ph.D. Currently Senior Scientist, Pfizer. Sanford Mendonca, Ph.D. Kazuaki Yokoyama, Ph.D. Currently Associate Professor, Teikyo University. Zsolt Lorincz, Ph.D. Currently Scientific Director of Targetx Inc. Dagmar Meyer zu Heringdorf, M.D. Currently Associate Professor, University of Essen, Germany. Sandor Cseh, Ph.D. Currently Chief Scientist, Targetx Inc. Satoshi Yasuda, Ph.D. Currently Assistant Professor, Medical University of Hokkaido. Yuko Fujiwara, PhD. Research Associate. Natalia Makarova, M.D., Ph.D. Instructor. Currently Senior Scientist, Georgia Institute of Technology. Michelle Walker, Ph.D. Post-doctoral fellow. Tamotsu Tsukahara, Ph.D. Post-doctoral fellow. William J. Valentine, Ph.D. Post-doctoral fellow. Rosalie Allen, Ph.D. Post-doctoral fellow. Daniel Osbone, Ph.D. Post-doctoral fellow. Huazhang Guo, M.D., Ph.D. Research Associate. Juanxiong Liu, Ph.D. Research Associate. Gyongyi Kiss. Post-doctoral fellow. James I. Fells. Post-doctoral fellow. SueChin Lee. Post-doctoral fellow. Barbara Serrano-Flores. UNAM, Mexico. Zsolt Lorincz, Visiting Professor. Erzsebet Szabo. Post-doctoral fellow. Andrea Balogh. Post-doctoral fellow. Souvik Banerjee. Post-doctoral fellow. Yoshibumi Shimizu. Post-doctoral fellow. Dissertation Committee Member Ji-Wei Wang, Department of Physiology, Ph.D. awarded in 1995 Jianyi Zhang, Department of Physiology, Ph.D. awarded in 1996 Glen Pyle, Ph.D. Department of Physiology, awarded in 1998 Quin Ching, Department of Physiology, Ph.D. awarded in 2000 Keven Williams, Department of Physiology, Ph.D. awarded in 2000 Veronika Zsiros, Department of Anatomy and Neuroscience, 2000-2002 Matthew Fabian, Department of Physiology, Ph.D. awarded in 2001 Steven Lloyd, Department of Anatomy and Neuroscience, Ph.D. awarded in 2003 Guan Yao, Department of Biochemistry, Ph.D. awarded in 2003 Gábor Tigyi — Curriculum Vitae Page 5 Chunying Li, Department of Physiology, Ph.D. awarded in 2005 Mitzi Dunagan, Department of , Ph.D. awarded in 2011 James Fells, Department of Chemistry, University of Memphis Shi Jin, Department of Physiology, University of Tennessee Health Science Center Donna Perygin, Department of Chemistry, University of Memphis Jesica Williams, Department of Chemistry, University of Memphis ADMINISTRATIVE APPOINTMENTS National and International Conference co-chair, FASEB Summer Conference on Lysophospholipids, Il Ciocco, Italy, YEAR? Program Committee, International Eicosanoids and Lipid Mediator Conference 2011, Seattle, Washington Conference Chair, Program Committee, International Eicosanoids and Lipid Mediator Conference 2009, Cancun, Mexico Program Committee, International Eicosanoids and Lipid Mediator Conference 2007, Montreal, Canada Program Committee, International Conference on Phospholipase A2 and Lipid Mediators, Berlin, 2005 Outside Review Team, RIKEN Tokyo, Supramolecular Systems Program, 2003 Chair, FASEB Summer Conference on Lysolipid Mediators, 2003 Site Committee, RIKEN Supramolecular Biology Group, Tokyo, 2003 Co-Chair, FASEB Summer Conference on Lysolipid Mediators, 2001 National Cancer Institute Subcommittee C, Program Project Review Panel member, 2002 Member of the Organizing Committee, International Congress on Platelet Activating Factor and Lipid Mediators, Japan, 2001. Steering Committee, New York Academy of Sciences, Symposium on Lysolipid Mediators in Health and Disease, 1999. Fogarty International Collaborative Programs, NIH Study Section, Regular member, 1998-2002. Ad hoc member 2003-2005. Conference Chair, 33rd Southeastern Lipid Conference, Cashiers, North Carolina, November 4-6, 1998 Member of ad hoc grant review committee for NSF Integrative Biology Neuroscience program, 1997 Member, Scientific Advisory Council to the Biological Research Center of the Hungarian Academy of Sciences, 1991-present Organizing Committee for the Summer Science Institute, 1988 Ad hoc reviewer for the Spinal Cord Research Foundation of the Paralyzed Veterans of America, 1996 University Committees: Vice Chancellor for Research Search Committee, Co-chair, 2014 Pharmacology Chair Search Committee, Chair, 2013 Vice Chancellor for Research Search Committee, Co-chair, 2011 Research and Licensing Strategies Task Force, UTHSC, member, 2006 Execution and Implementation. Strategic Planning Working Group .Chair, 2006 UTHSC Large Equipment Committee, 2006 UT State-Wide Cancer Center Planning Task Force, 2006 UTCI Director Search Committee, 2003 UTCI Community Advisory Committee, 2002-present UT Cancer Center Internal Advisory Committee, 2002-present UT Cancer Center External Advisory Committee, 2002-present Bioinformatics/Genomics Search Committee, 2001 Anatomy and Neuroscience Chair Search Committee, 2000 President of the Dean’s Faculty Advisory Committee, College of Medicine, UT-Memphis, 1998-1999. Research Advisory Committee to the Dean-elect for the College of Medicine, UT-Memphis, 1998 Transgenesis Research Task Force, UT Memphis, 1998-present President-elect of the Faculty Organization for the College of Medicine, UT-Memphis, 1997-1998 Faculty Recruitment Committee, Department of Physiology, UT Memphis, 1997-present Coordinator for the Molecular and Cellular Neuroscience Seminar Program with the Center for Neuroscience, UT-Memphis, 1997-present Gábor Tigyi — Curriculum Vitae Page 6 Scientific Advisory Committee of the Brain Injury Research Institute, 1995. Executive Committee of the Faculty Organization for the College of Medicine, UT-Memphis, 1994-1999 Program Director on an NSF grant proposal entitled "Young Memphis Scholars — Summer Enrichment Program for High School Students,” 1993-1998 Graduate Training Committee, Department of Physiology and Biophysics, UT-Memphis,1992-1998 RESEARCH AND OTHER EXTERNAL SUPPORT Current Support 06/30/13-06/29/18. 1 U01 AI107331-01. "IND-Enabling Preclinical Development of a New Radiomitigator." This project is aimed at the preclinical development of LPA2 agonists with radiomitigator action. ROLE: PI. 3.6 calendar months. 04/01/12-03/31/17. NIH R01 2R01CA092160-11. “Anticancer Strategies Targeting the Autotaxin-LPA Receptor Axis." $1,250,000. ROLE: PI. 2.4 calendar months. VA Merit Award 1101BX001187-01, Grant 10690312. “Novel Radiomitigators Targeting LPA Receptors.” VA Merit Review Grant. Van Vleet Oncology Support fund. Continuous. $100,000 per year. This endowment supports the PI’s oncology research and provides cost sharing for the CA092160 grant. 0.1 calendar months/ Completed Support during the Last 3 Years 09/01/12-09/30/12. W911QY-12-1-0006 (PI: Strobos). “Rx100: In Vitro Mechanism of Action and Preclinical Pharmacokinetic/Pharmacodynamic Testing in an NHP, GI-ARS Model.” $394,000. The major objective of this subcontract is to provide pharmacological characterization of a Rx100 for an IND filing to the FDA. ROLE: Subcontract, PI. 2.4 calendar months. 09/01/08-07/31/13. 5 R01 AI080405-02. "Analysis of Radiomitigative Cell Signaling." $1,250,000 + $100,000 administrative supplement. The major goal of this project was to elucidate the radiomitigating cell signaling of octadecenyl thiophosphate. ROLE: PI. 3.96 calendar months. 09/01/08-07/31/13. AI-R01 080405-01. "Analysis of Radiomitigative Cell Signaling." $1,250,000 direct cost. The major goal of this project was to elucidate the radiomitigating cell signaling of octadecenyl thiophosphate. ROLE: PI. 08/01/06-05/31/11. 5 R01 HL084007-04 (PI: Parrill). "Computational Approach to Ligand Discovery for LPA GPCR and PPAR." ROLE: Co-PI. Past Support 2009-2012 1 RC2 AI087550-01. NIH/NIAID. "Preclinical Development of a GI Radiation Countermeasure." ROLE: PI. AI 078514. NIH/NIAID. "Development of a Novel Gastrointestinal Radiomitigator." $1,449,000. The main objective of this grant was to determine the therapeutic efficacy of octadecenyl thiophosphate, a novel radiomitigators, using mouse and non-human primate models of radiation-induced mucositis. ROLE: PI. 2007-2009 1 RC1 AI078514-01. "Development of a Novel Gastrointestinal Radiomitigator." ROLE: PI 2007-2009 1 RO-3 AI0170942-01 (PI: A.P. Naren). 04/1/07 to 06/30/09 NIH/NIAID. "V. Cholerae as a Bioterror Agent: New Means of Treating a Potential Pandemic." ROLE: Collaborator. 2006-2011 NIH R01 (PI: A.P. Naren). “CFTR-Dependent Protein Interactions Regulate Diarrhea.” Coinvestigator 2006-2010 NIH R01 (PI: A. Parrill). "Computational Approach to Ligand Discovery for LPA GPCR and PPAR." ROLE: Co-PI. 2006-2009 DOD, University of Tennessee Cancer Institute Enhancement Grant. “Novel Methods for Imaging PET Biomarkers and Gene Therapy of Cancer.” Gábor Tigyi — Curriculum Vitae 2005-2010 Page 7 NIH R01. “LPA Signaling through the GPCR-PPARg Axis.” $1,250000 direct cost. ROLE: PI. 2003-2007 NIH R01. “Therapeutically Promising Phospholipid Growth Factors." $1,250,000 direct cost. ROLE: PI. 2002-2003 Vascular Biology Center of Excellence Pilot Grant. "Atherogenic effects of LPA." $40,000. ROLE: PI. 2001-2011 5 R01 CA092160-09. "Ligand Recognition by Phospholipid Growth Factor Receptors." ROLE: PI. 2000-2003 NSF. US-Japan Collaborative Programs. $25,000. ROLE: PI. 2000 MCRR Shared Instrumentation Grant Proposal for a BIACORE Spectrometer (PI: L. Jennings). ROLE: Participant user. 1999-2003 NIH RO1 (PI: M. Watsky). “Influence of Ion Channels on Corneal Wound Healing.” ROLE: Co-PI. 10% effort. 1999-2002 American Heart Association National Chapter (PI: A. Parrill). "Computational Approach to S1P Receptor Function." Subcontract $32,000/year. 1999 MCRR Shared Instrumentation Grant Proposal for a Liquid Chromatography-Triple Quadrupole Mass Spectrometer (PI: D. Desiderio). ROLE: Participant user. 1998-2002 NSF. "Modulation of Neuronal Differentiationby Growth Factor-Like Phospholipids" (2nd Renewal). $495,855 direct cost. ROLE: PI. 1998-2002 NIH R01. "Therapeutically Promising Phospholipid Growth Factors." $687,159 direct cost. ROLE: PI. 1998-2002 NIH RO1 (PI: L. Johnson). “Role of Polyamines in Early GI Mucosal Restitution.” ROLE: Co-PI. 5% effort. 1997-2000 Industrial Research Grant for lipid mediator receptor research from LXR Biotechnology, Inc. $70,000/annually. 1997-2000 Established Investigator Award — application to the American Heart Association. "Cardioprotective Sphingolipids and Their Receptors." $75,000 annually. 1997 NSF. Shared Equipment Grant for a Phosphorimager. $59,651. ROLE: Co-PI with T. Patel, UT-Memphis Pharmacology Department. 1996-1998 NSF. "Young Scholars at the University of Tennessee, Memphis." $251,000. ROLE: PI/Program Director. 1995-1998 NATO. Collaborative grant with Dr. Lutz Pott of Ruhr University, Bochum, Germany. $12,000. 1994-1998 National Science Foundation, Washington (renewal). "Study of a Serum Factor That Activates the Phosphatidylinositol System." $308,000. ROLE: PI. 1994-1997 Grant-in-Aid, American Heart Association, National Center. “Molecular Analysis of the Lysophosphatidate Receptor." $120,000. ROLE: PI. 1994-1996 Spinal Cord Research Foundation of the Paralyzed Veterans of America. "Lysophosphatidic Acid — an Endogenous Inhibitor of Neurite Outgrowth — Role in Acute Spinal Cord Injury." $64,600. ROLE: PI. 1993-1995 Le Bonheur Children Hospital Research Grant. "Production of Growth Factor-Like Phospholipid Mediators during Blood Coagulation". $9,800. ROLE: Co-PI. 1993-1994 University Medical Group Corp. "Do Growth Factor-Like Phospholipid Mediators Promote Wound Healing?" $12,500. ROLE: PI. 1992-1994 American Paralysis Association. "Lysophosphatidic Acid — an Endogenous Inhibitor of Neurite Outgrowth — Role in Acute CNS Injury.". $30,000. ROLE: PI. 1990-1994 NSF. "Study of a Serum Factor That Activates the Phosphatidylinositol System." $188,000. ROLE: PI. 1984-1985 Richter Gedeon Pharmaceutical Works, Budapest. "The Effect of Vinpocetine on Acute Allergic Encephalomyelitis." $25,000. ROLE: PI. 1983-1984 Hungarian Academy of Sciences Young Investigator Award. "Changes in the Synaptic Membrane Architecture during Depolarization." Amount equivalent to U.S. $50,000. ROLE: PI. 1983 UNESCO-International Cell Research Organization Training Fellowship to attend Graduate School at the University of Uppsala, Sweden. Awarded But Not Accepted by Principal Investigator Gábor Tigyi — Curriculum Vitae 1994-1996 Page 8 American Heart Association, Tennessee Chapter. "A Study of Growth Factor-Like Lipid Mediators on Neuronal Survival and Differentiation." $50,000. ROLE: PI. Awarded, but not accepted due to funding of other projects. SOCIETY MEMBERSHIPS 2013-present 2007-present 2005-present 2004-present 2001-present 1999-present 1995-present 1992-present 1992-present 1992-present 1985-present 1985-present 1984-present 1984-present 1983-present American Pharmacological Society European Academy of Arts, Letters and Sciences, Paris North American Vascular Biology Organization Hungarian Academy of Sciences, Foreign Member American Society of Biochemistry and Molecular Biology New York Academy of Sciences American Physiological Society ΣΧ Research Society American Society for the Advancement of Science Society for Neuroscience European Association for Neurochemists Hungarian Biochemical Society Hungarian Biophysical Society IBRO Society for Basic and Clinical Immunologists COLLABORATIONS National and International Dr. Olivier Peyruchaud, INSERM, Lyon, France Dr. Akira Tokumura, Tokushima University, Japan Dr. Robert Bittman, Queens College, New York Dr. Wouter Moolenaar, Cellular Biochemistry, The Netherlands Cancer Institute, Amsterdam Dr. Lutz Pott, Head of Muscle Physiology Group, Department of Physiology, Ruhr University, Bochum, Germany Dr. Kimiko Murakami, Department of Biology, Ochanomizu University, Tokyo, Japan Dr. Hiromu Murofushi, Department of Biochemistry, Tokyo University Dr. Ian Bathurst, LXR Biotechnology, Inc., Richmond, California Dr. Ala Bielawska, Department of Medicine, Duke University, Durham, North Carolina Dr. Sarah Spiegel, Department of Biochemistry, Georgetown University, Washington, D.C. Dr. Takao Shimizu, Department of Biochemistry, University of Tokyo, Japan. Dr. Wolfgang Seiss, Cardiovascular Research Institute, University of Munich, Germany Dr. Judy Berliner, Department of Pathology, UCLA Dr. Yasuyuki Igarashi, Faculty of Pharmaceutical Science, Hokkaido University Dr. Tom Stossel, Harvard University Dr. David Brindley, Signal Transduction Group, The Heritage Foundation Research Centre, Edmonton, Canada Dr. John Castracane, Exalpha Biologicals, Boston, Massachusetts Dr. Tom McIntyre, Cleveland Clinic Foundation Dr. Nigel Pyne, Strahclyde Institute, Glasgow Dr. Fang-Tsyr Lin, University of Alabama, Birmingham Dr. Larry DeLucas, University of Alabama, Birmingham Dr. Abby Parrill, Department of Chemistry, Computational Research Institute on Materials at the University of Memphis Dr. Gordon Mills, Division of Experimental Therapeutics, MD Anderson Cancer Center Dr. Glenn Prestwich, Department of Chemistry, University of Utah Dr. Richard J. Kirby, Sanford-Burnham Institute, Florida Local Gábor Tigyi — Curriculum Vitae Page 9 Dr. Daniel Baker, University of Memphis Dr. Abby Parrill, University of Memphis Dr. Dominic Desiderio, Department of Neurology, Stout Mass Spectroscopy Laboratory, UT-Memphis Dr. Leslie Ballou, Department of Medicine, Connective Tissue Research Group, VA Medical Center, Memphis Dr. Duane Miller, Department of Pharmaceutical Sciences, UT-Memphis Dr. Ram Gollamudi, Department of Medicinal Chemistry, UT-Memphis Dr. Russell Chesney, Department of Pediatrics, UT-Memphis Dr. Yi Zheng, Department of Biochemistry, UT-Memphis Dr. Kafait U. Malik, Department of Pharmacology, UT-Memphis Dr. Leonard R. Johnson, Department of Physiology, UT-Memphis Dr. Mitchell Watsky, Department of Physiology, UT-Memphis Dr. Jonathan Jaggar, Department of Physiology, UT-Memphis Dr. A.P. Naren, Department of Physiology, UT-Memphis Dr. Christopher Waters, Department of Physiology, UT-Memphis PATENTS AND DISCLOSURES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. Procedure for separation and detection of antigen-specific cells. Najbauer, J., G. Tigyi, P. Nemeth, and F. Kilar. Registry No. 196 511. Date of validity: July 10, 1984. Method and pharmaceutical composition for treating demyelinization of autoimmune origin, particularly multiple sclerosis. Tigyi, G., B. Bozoky, K. Szegvari, and T. Frank. U.S. Patent Ser. No. 07/233,076. Date of validity: August 17, 1988. A serum factor that activates the phosphatidylinositol second messenger system. Tigyi, G., and Miledi, R. University of California Patent Disclosure, 1988. Lysophosphatidate-induced neurotrophism and neurite outgrowth. Tigyi, G. Patent disclosure, University of Tennessee Research Corporation. 1993. Lysophosphatidate-induced regeneration of corneal cells. Tigyi, G., and M. Watsky. 1995. Molecular cloning strategy for the lysophosphatidate receptors using degenerate oligonucleotides. Tigyi, G. Patent disclosure, University of Tennessee Research Corporation. 1995. sold licensing rights to LXR Biotechnology, Richmond, California. Accelerated wound healing after lysophosphatidate treatment in the skin. Tigyi, G., L. Balazs, J. Okolicany, and M. Ferrebee. Patent disclosure, University of Tennessee Research Corporation. 1995. Nucleotide sequence of a high affinity receptor for lysophosphatidic acid from Xenopus. Tigyi, G., Z. Guo, Liliom, K., and D. Fischer. 1996. Compositions containing lysophosphatidic acids which inhibit apoptosis, methods of making compositions and uses thereof. Erickson, J., D. Fischer, G. Goddard, Liliom, K., D. Miller, G. Sun, and G. Tigyi. US Patent Application, 1997. Development of new and novel phospholipid growth factor receptor ligands for cancer treatment. Miller, D., G. Tigyi, G. Sun, and D. Baker. US Patent Application, 2000. Recombinant baculoviruses expressing the lysophosphatidate receptors EDG2, 4, and 7. Patent disclosure, University of Tennessee Research Corporation. 1995. Licensed to Exalpha Biologicals, Inc., Boston, MA. Miller, D.D., G. Tigyi, D.L. Baker, J.T. Dalton, V.M. Saradar, D.B. Elrod, H. Xu, Liliom, K., D.J. Fischer, T. Virag, and N. Nusser. LPA receptor agonists and antagonists and methods of use. 6,875,757, 2001. Inhibition of neointima formation by PPARg antagonist GW9662. Baker, D.L., C. Zhang, and Tigyi, G. PD03007, 2003. Acetal phosphatidic acids as LPA receptor ligands. Miller, D., G. Tigyi, et al. 2004. Use of lysophosphatidic acid (LPA) mimics or derivatives to modulate Ppar-gamma receptor activity and inhibit autotaxin (Atx) activity. Tigyi, G., D.D. Miller, V. Gududuru, G. Durgam, D.L. Baker, and Y. Fujiwara. May 6, 2005. New synthesized glycerol acetal phosphates and thiophosphates (GAPs) as selective LPA agonists and antagonists and PPARgamma ligands. Tigyi, G., W. Deng, S. E, R. Tsukahara, G.G. Durgam, V. Gududuru, andA.L. Parrill-Baker. October 21, 2006. Gábor Tigyi — Curriculum Vitae 17. 18. 19. 20. 21. Page 10 LPA receptor agonists and antagonists and methods of use. Miller, G. Tigyi, T. Virag, G. Durgam, M.D. Walker, and R. Tsukahara. 7,217,704. 2007. Theoretical models of the LPA1, LPA2, LPA3, S1P1, S1P2, S1P3, S1P4, S1P5, and LPA4 G protein-coupled receptors. Tigyi, G., A.L. Parrill-Baker, D. Perygin, and J. Fells. May 25, 2007. Lysophosphatidic acid analogs and inhibition of neointima formation. Tigyi, G., D.L. Baker, and C. Zhang. 2008. Inhibitors of autotaxin. Gupte, R., R. Patil, G. Tigyi, and D.D. Miller. January 20, 2010. INVITED PRESENTATIONS 1983 1984 1984 1986 1992 1995 1995 1996 1996 1996 1996 1996 1996 1996 1996 1997 1997 1997 1997 1997 1997 1997 1997 1997 1997 1997 1998 1998 1998 1998 1998 1998 Monoclonal antibodies to myelin basic protein and their cross-reactivities in mapping epitope homologies with non-MBP antigens. International Symposium of Demyelinating Diseases, American Multiple Sclerosis Society. Airlie House, Virginia. Cross-reactivities of myelin basic protein specific monoclonal antibodies. Symposium on Neurotransmitter Function in Health and Disease. European Neurochemical Society Congress. Budapest, Hungary. Monoclonal antibodies and their applications in neuroimmunological disorders. XIII Annual Meeting of the Hungarian Clinical Immunological Society. Szekesfehervar, Hungary. University of California Riverside, Department of Biochemistry. Department of Neurology, University of California Irvine Medical Center. Keystone Symposium on The Role of Lipid Messengers in Signal Transduction Pathways, Cellular Regulation and Disease. LXR Biotechnology, Inc. Richmond, CA. The Netherlands Cancer Institute, Amsterdam. Department of Pharmacology, The University of Essen, Germany. Department of Physiology, The Ruhr-University. Bochum, Germany. Paralyzed Veterans of America, Scientific Review and Advisory Panel Meeting, Memphis, Tennessee. Department of Pharmacology and Toxicology, University of Freiburg, Germany. VA Medical Center, Memphis, Tennessee. Center for Excellence in Neuroscience, UT-Memphis. Department of Developmental Neuroscience, St. Jude Children’s Research Hospital. Memphis, Tennessee. International Symposium on Messengers of Life and Death in the Nervous System. The University of Kentucky, Lexington. Lipid Mediators in the Nervous System Satellite Symposium to International Society for Neurochemistry Congress. New Orleans, Louisiana. Department of Biophysics, University Medical School. Pécs, Hungary. 5th International Symposium on Eicosanoids and Other Bioactive Lipids in Cancer; Inflammation and Related Diseases. San Diego, California. Keynote speaker, Lipid Symposium, Japanese Biochemical Society Annual Meeting. Kanazawa, Japan. September 22-25, 1997. Department of Pharmacology, Tokushima University. Japan. Department of Pharmacology, University of Kyoto. Japan. Department of Biochemistry and Molecular Biology. Tokyo, Japan. Department of Biology, Ochanomizu University. Tokyo, Japan. Department of Biochemistry, University of Tokyo. Japan. Visiting Professor, Department of Chemistry and Biochemistry, Queens College, City University of New York. Department of Pharmacology, UT-Memphis. The Hormel Institute. Austin, Minnesota. Department of Organphysiologie, Ruhr Universitat. Bochum, Germany. 6th International Conference on Platelet Activating Factor and Related Lipid Mediators. New Orleans, Louisiana. 10th International Conference on Phosphoproteins and Signal Transduction. Jerusalem. Cardiovascular Research Institute, University of Munich. Germany. Gábor Tigyi — Curriculum Vitae 1998 1998 1999. 1999 1999 2000 2000 2000 2000 2000 2000 2000 2000 2000 2000 2001 2001 2001 2001 2002 2002 2002 2002 2003 2003 2003 2003 2003 2004 2004 2004 2004 2004 2004 2004 2005 2005 2005 2005 2005 2006 2006 2006 2006 2006 2006 2006 2007 2007 2007 2007 2007 Page 11 Dept. of Physiology, Ruhr Universitat. Bochum, Germany. Center for Excellence in Neuroscience, Lousina State University, School of Medicine. New Orleans, Louisiana. Lipid Signaling Group, University of Alberta. Edmonton, Canada. New York Academy of Sciences Symposium on Lysolipids. Visiting Professor, Department of Chemistry and Biochemistry, Queens College, City University of New York. Tokyo Metropolitan Institute of Gerontology. Japan. RIKEN, Supramolecular Interaction Programme. Japan. Hokkaido University, Faculty of Pharmaceutical Science. Japan. Department of Biochemistry, Medical College of Hokkaido. Japan. Mitsubishi-Kasei Institute of Life Sciences. Tokyo, Japan. Department of Biochemistry, Kanazawa Medical School. Japan. Congress of the International Society for Study of Fatty Acids and Lipids. Tsukuba, Japan. Department of Biochemistry, Eotvos Lorand University. Budapest, Hungary. 100 Years after Luigi Galvani – Symposium on Neural Transmission. University of Rome. Italy. Ceretek Incorporated, South San Francisco. California. Department of Cell Biology, University of Georgia. Athens, Georgia. 7th Congress on PAF and Lipid Mediators. Chair and plenary speaker. Tokyo, Japan. 1st Takeda Symposium on Pharmascience. Tokyo, Japan. Hokudai Symposium on Sphingolipid Biology. Sapporo, Japan. Women’s and Brigham Hospital, Harvard Medical School. Boston, Massachusetts. Department of Biochemistry, Kyoto University, School of Graduate Sciences. Japan. Department of Neuroscience, Osaka University school of Medicine. Japan. Faculty of Pharmaceutical Science, Hokkaido University. Japan. Reeves Center for Neuronal Regeneration, University of California Irvine. Faculty of Pharmaceutical Science, Hokkaido University. Japan. Ochanomizu University. Tokyo, Japan. University of Memphis, Department of Chemistry. Memphis, Tennessee. Grand Rounds, Department of Pathology, UTHCS, Memphis. Vessel Club. Washington, D.C. FEBS Lecture Course and 4th Dubrovnik Signaling Conference. Croatia. Center for Biophysics and Engineering, University of Alabama Birmingham. Department of Biochemistry, Hokkaido School of Medicine. Japan. First International Sapporo Sphingolipid Symposium, Hokkaido University. Japan. Sphingolipid Gordon Conference. Harima, Japan. 8th PAF and Lipid Mediator Conference. Berlin, Germany. Ochanomizu University. Tokyo, Japan. LPA - from ligand to mediator to medicine – Experimental Biology Symposium, Society for Pharmacology and Experimental Therapeutics. Keynote Speaker, FASEB Summer Conference on Lysophospholipids in Biology and Medicine. Symposium on Chemical Biology, University of Tokyo Center of Excellence, Faculty of Pharmacy. Japan. City University of New York, School of Graduate Studies. University of Arkansas Cancer Center. University of Kentucky, Department of Biochemistry. University of Munich, Cardiovascular Research Institute. Germany. International Symposium on Atherosclerosis. Rome, Italy Angelini Research Laboratories. Palomba, Italy. Graduate School, Hokkaido University. Japan Plenary Speaker, International Conference on Bioscience of Lipids. Pecs, Hungary. Invited seminar: University of Memphis, Bioengineering Program, January 17, 2007. Plenary Speaker, Sphingolipid Symposium in Honor of Senitchiro Hakomori. Tokushima, Japan. Invited Speaker, 3d International Symposium on Phospholipases and Lipid Mediators. Sorrento, Italy. May 24-29, 2007. Invited Speaker, FASEB Summer Conference on Lysophospholipids in Biology and Disease. Tucson, Arizona. Centenary Institute. Sydney, Australia. Gábor Tigyi — Curriculum Vitae 2007 2008 2008 2008 2008 2008 2008 2008 2008 2009 2009 2009 2009 2010 2010 2010 2010 2010 2011 2011 2011 2011 2011 2011 2011 2012 2012 2012 2012 2012 2012 2012 2012 2013 2013 2013 2013 2013 Page 12 University of Adelaide. Adelaide, Australia. University of Michigan. Ann Arbor, Michigan. Igarashi Symposium, Hokkaido University. Japan. Japanese Biochemical Society Keynote Address. Tokushima, Japan. Otsuka Pharmaceuticals Discovery Center, Tokushima, Japan. Vessel Club. San Diego, Califonia. Hungarian Biochemical Society Annual Meeting, Keynote Speaker. Szeged, Hungary. British Pharmacological Society Symposium of Lysophospholipids. West-Essex, UK. Southeastern Lipid Society Annual Meeting. Georgia. Department of Pharmacology, University of Pittsburgh. Pittsburgh, Pennsylvania. Invited Speaker - Phospholipase A2 and Lipid Mediators. Tokyo, Japan. Invited Speaker - FASEB Summer Conference. Carefree, Arizona. Department of Cell Biology, University of Alabama at Birmingham. University of California Davis. University of Tennessee, College of Veterinary Medicine. Knoxville, Tennessee. Niigata University, Department of Physiology, Center for Nephrology. Japan. City University of New York. Invited Speaker - NAITO Conference. Sapporo, Japan. University of Arkansas Cancer Center. Little Rock, Arkansas. Invited Speaker - International Biochemical Society Meeting. Merida, Mexico. Invited Speaker - FASEB Summer Conference on Lysophospholipids. Il Ciocco, Italy Invited Speaker - LIPID MAPS Annual Meeting. San Diego, California. Invited Speaker - Eicosanoids and Lipid Mediators in Cancer, Inflammation and Other Diseases. Seattle, Washingtn. Strathclyde University. Glasgow. British Biochemical Society Centennial Meeting on Cell Signaling, Edinburgh Invited Speaker - FEBS Trauining Course on Lipid Signaling in Cancer. Vico Equense, Italy. University of Kentucky, Cardiovascular Research Center. Lexington, Kentucky. Department of Chemistry and Biochemistry, Queens College, City University of New York. Invited Speaker - FASEB Summer Conference on Phospholipid Metabolism. Saxtons River. Department of Nuclear Engineering, University of Tennessee Knoxville. Veterans Medical Center. Memphis, Tennessee. Department of Physiology, Semmelweiss University. Budapest, Hungary. Keynote Speaker - Postdoctoral Research Day, University of Tennessee Health Science Center. Memphis, Tennessee. Murofushi Symposium, Ochanomizu University. Tokyo, Japan. Tokushima University. Japan, Nagasaki University. Japan, Keynote Lecture - FASEB Summer Conference. Plenary Lecture - Bioactive Lipids in Cancer, Inflammation and Related Diseases. PUBLICATIONS Book Chapters 1. Tigyi, G. Antibody production with hybridomas. In: Molecular Methods in Immunology, edited by F. Antoni and P. Zavodszky. Budapest: Medicina Press, 1987. 2. Dyer, D., Tigyi, G., and Miledi, R. Neurite retraction and suppression of early responses to nerve growth factor in PC12 cells by a serum protein. In: Proceedings of the Miami Bio/Technology Winter Symposium, edited by R.L. Rotundo et al. Oxford/Washington: IRL Press, 1989, p. 81. 3. Tigyi, G., and Miledi, R. Isolation and characterization of a novel serum factor that stimulates the phosphoinositide-Ca2+ signaling system. In: Proceedings of the Third International Regeneration Symposium, Neurology and Neurobiology Series, edited by F. J. Seil. New York: Alan R. Liss, 1989. 4. Dyer, D., Tigyi, G., and Miledi, R. Antimitogenic and neurite retraction effects caused by a serum factor that stimulates the phosphoinositide- Ca2+ signaling system. In: Proceedings of the Third International Regeneration Symposium, Neurology and Neurobiology Series, edited by F. J. Seil. New York: Alan R. Liss, 1989. Gábor Tigyi — Curriculum Vitae Page 13 5. Tigyi, G., Fischer, D.J. Liliom, K., Guo, Z., Virág, T., Sun, G, Miller, D.D., Murakami-Murofushi, K. Kobayashi, S., and Erickson, J.R. Determinants of receptor subtype specificity in the LPA-like lipid mediator family. In: Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation and Related Diseases, edited by K.V. Honn, S. Nigam, L.J. Marnett, and E. Dennis. New York/London/Washington, DC/Boston, 1998. 6. Tigyi, G., Liliom, K., Fischer, D.J., and Guo, Z. Phospholipid growth factors: identification and mechanism of action. In: Lipid Second Messengers, edited by R. Rubin and S. Laychock. CRC Press, pp. 51-81, 1998. 7. Goetzl, E.J., Lee, H., and Tigyi, G. Lysophosholipid growth factors. Cytokine reference.:/ Academic Press. New York/London, pp. 1407-1418, 2000. 8. Tigyi, G. Lysophosphatidate and sphingosine 1-phosphate receptors. In: Encyclopedia of Biological Chemistry, edited by W.J. Lennarz and M.D. Lane. Elsevier, pp. 602-604, 2005. 9. Dopico, A., and Tigyi, G. Lipid species and membrane composition., edited by A. Dopico. Humana Press, 2007. 10. Valentine, W.J., and Tigyi, G. High throughput assays to measure intracellular Ca2+ mobilization in cells that express recombinant S1P receptor subtypes. Meth. Mol. Biol. 874: 77-87, 2012. PMID: 22528441. Refereed Journal Articles 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. Hegedus, J., Tigyi, G., and Hegedus, I. (1983) Optoacoustic spectroscopy and its application in structural studies of biomaterials. Meth. Exp. Med. (Budapest) 35: 201-208. Tigyi, G., Hegedus, J. and Hegedus, I. (1983) Optoacoustic spectroscopy as a new tool for structural studies of striated muscle. Studia Biophysica (Berlin) 95: 151-160. Tigyi, G. Modern biochemical separation methods — a review. Biochemistry (Budapest) 7: 41-43, 1983. Kilar, F., Najbauer, J., and Tigyi, G. (1983) Presence of myelin basic protein in cerebrospinal fluid and sera of patients with multiple sclerosis. Acta Biochim. Biophys. 19: 108. Tigyi, G., Balazs, L., Monostori, E., and Ando, I. (1984) Isolation of the human myelin basic protein by immunoaffinity chromatography with a monoclonal antibody. Mol. Immunol. 21: 889-894. Tigyi, G., and Kovacs, K. (1985) Production and characterization of monoclonal antibodies to the hydrogenase of Thiocapsa roseopersicina. Curr. Microbiol. 11: 329-324. Tigyi, G., Juntti, N., and Johansson, D.-E. (1985) Screening for monoclonal antibodies against a mycoplasmal membrane protein by protein electroblotting. Protides in Biol. Fluids 33: 575-578. Kovacs, K., Tigyi, G., and Alfons, H. (1985) Purification by hydrogenase by fast protein liquid chromatography (FPLC) and by conventional techniques: a comparative study. Prep. Biochem. 15: 321-324. Tigyi, G., Bagyinka, Cs., and Kovacs, K. (1986) Protein structural changes associated with active and inactive states of hydrogenase from Thiocapsa roseopersicina. Biochemie 66: 6974. Najbauer, J., Tigyi, G., and Nemeth, P. (1986) ASCAA-antigen specific cell adherence assay — a simple method for separation of antigen specific hybridoma cells. Hybridoma 5: 361379. Najbauer, J., Kosaras, B., and Tigyi, G. (1987) Adherence of cells to myelin basic protein. Part 1. Adherence of red and white blood cells from patients with multiple sclerosis to myelin basic protein. Acta Neurol. Scand. 76: 172-175. Najbauer, J., Bozoky, B., and Tigyi, G. 1987 () Adherence of cells to myelin basic protein. Part 2. Adherence of red blood cells of SJL mice with chronic relapsing EAE. Acta Neurol. Scand. 76: 176-182. Kilar, F., Mod, A., and Tigyi, G. (1987) Detection of myelin basic protein and anti-myelin basic protein antibodies in the serum of patients with multiple sclerosis. Arch Neurol. (Budapest) 40: 573-576. Kovesi, Gy., Mohai, K., and Tigyi, G. (1987) Immunoscintigraphic detection of transplanted murine tumors using I125-labeled anti-myelin basic protein antibodies. Hung. Oncol. 31: 244249. Gábor Tigyi — Curriculum Vitae 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. Page 14 Kovacs, K., Bagyinka, Cs., and Tigyi, G. (1988) Proteolytic resistance and its utilization in purification of hydrogenase from Thiocapsa roseopersicina. Biochim. Biophys. Acta 935: 166-172. Najbauer, J., Szekeres-Bartho, J., and Tigyi, G. (1989) Modulation of cell-cell and cellantigen interaction by 1,25-dihydroxy vitamin D3 and D3 sulfate in vitro: a study on pregnancy lymphocytes and hybridoma cells. Immunol. Lett. 317-322. Kovacs, K.L., Seefeldt, L.C., Tigyi, G., Doyle, C.M., Mortenson, L.E., and Arp, D. (1989). Immunological relationship among hydrogenases. J. Bacteriol. 171: 430-435. Czysewska, H., Bagyinka, C. Kovacs, K.m and Tigyi, G. (1989) Purification and characterization of the high potential iron sulphur protein from Thiocapsa roseopersicina. Acta Biochim. Biophys. 266: 361-375. Tigyi, G., Matute, C., and Miledi, R. (1990) Monoclonal antibodies to cerebellar pinceau terminals obtained after immunization with brain mRNA injected Xenopus oocytes. Proc. Natl. Acad. Sci. USA 87: 528-532. Tigyi, G., Dyer, D., Matute, C., and Miledi, R. (1990) A serum factor that activates phosphoinositide signaling in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 87: 1521-1525. Kovacs, K., Tigyi, G., Le Thiet, T., and Bagyinka, Cs. (1991) Structural rearrangements in active and inactive forms of hydrogenase from Thiocapsa roseopersicina. J. Biol. Chem. 266: 947-951. Tigyi, G., and Parker, I. (1991) Microinjection into Xenopus oocytes: a precise semiautomatic instrument and optimal parameters for injection of mRNAs. J. Biochem. Biophys. Meth. 22: 243-252. Matute, C., Tigyi, G., and Miledi, R. (1991) Monoclonal antibodies against rat brain mRNA injected Xenopus oocytes — the oocyte as an immunological expression vector. J. Neurosci. Res. 29: 77-86. Tigyi, G., Henschen, A., and Miledi, R. (1991) A factor that activates oscillatory chloride currents in Xenopus oocytes copurifies with a subfraction of serum albumin. J. Biol. Chem. 266: 20602-20609. Dyer, D., Tigyi, G., and Miledi, R. (1992) The effect of serum albumin on PC12 cells. I. Neurite retraction and activation of the phosphoinositide second messenger system. Mol. Brain Res. 14: 293-301. Dyer, D., Tigyi, G., and Miledi, R. (1992) The effect of serum albumin on PC12 cells. II. Intracellular calcium transients and their role in neurite retraction. Mol. Brain Res. 14: 302309. Tigyi, G., and Miledi, R. (1992) Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J. Biol. Chem. 267: 21360-21367, 1992. Tigyi, G., Dyer, D., and Miledi, R. (1994) Lysophosphatidic acid possesses dual action in cell proliferation. Proc. Natl. Acad. Sci. USA 1: 1908-1912. Buneman, M, Ferrebee, M., Tigyi, G., and Pott, L. (1994) Characterization of an albuminassociated phospholipid factor with muscarinic activity in cardiac cells. J. Physiol. (London) 480: 90-92. Marsal, J., Tigyi, G., and Miledi, R. (1995) Incorporation of Torpedo acetylcholine receptors and Cl- channels into Xenopus oocytes following injection of Torpedo electroplaque membranes. Proc. Natl. Acad. Sci. USA. 92: 5224-5228,\. Tigyi, G., Hong, L., Shibata, M., Parfenova, H., Yakubu, M., and Leffler, C. (1995) Lysophosphatidic acid alters cerebrovascular reactivity in piglets. Am. J. Physiol. (Heart Circ. Physiol.) 37: H2048-H2055. Tigyi, G., Fischer, D., Yang, C., Dyer, D,, Sebök, A., and Miledi, R. Lysophosphatidic acidinduced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem. 66: 537-548, Tigyi, G., Fischer, D., Dyer, D,, A. Sebök, and Miledi, R. (1996) Lysophosphatidic acidinduced neurite retraction in PC12 cells: neurite protective effects of cyclic AMP signaling. J. Neurochem. 66: 549-558, 1996. Liliom, K., Murakami-Murofushi, K., Kobayashi, H., Murofushi, H., and Tigyi, G. (1996) Xenopus oocytes express multiple receptors for LPA-like lipid mediators. Am. J. Physiol. (Cell Physiol.) 39: C772-C778. Gábor Tigyi — Curriculum Vitae 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. Page 15 Bittman, R., Swords, B., Liliom, K., and Tigyi, G. (1996) A short synthesis of lipid phosphoric acids analogues inhibiting the lysophosphatidate receptor. J. Lipid Res. 37: 391398. Liliom, K., Bittman, R., Swords, B., and Tigyi, G. (1996) N-palmitoyl-serine- and Npalmitoyl-phosphoric acids are selective competitive antagonists of the lysophosphatidic acid receptors. Mol. Pharmacol. 50: 616-623. Zheng, Y., Fischer, D., Tigyi, G., Gorsky, J.Y., and Xu, Y. (1996) The faciogenital dysplasia gene product FGD1 functions as a CDC42Hs specific guanine nucleotide exchange factor. J. Biol. Chem. 271: 33169-33172. Buenemann, M., Brandts, B.K., Pott, L., Liliom, K., J.-L. Tseng, D. M. Desiderio, G. Sun, D. Miller, and Tigyi, G. Lysosphingomyelin and sphingosine 1-phosphate activate IKACh through the same high affinity receptor in guinea pig atrial myocytes. EMBO J. 15: 55245537, 1996. Guo, Z., Liliom, K., D. J. Fischer, I. C. Bathurst, D. J. Tomei, M. C. Kiefer, and Tigyi, G. Molecular cloning of a high affinity receptor for the growth factor-like phospholipid mediator lysophosphatidic acid. Proc. Natl. Acad. Sci. USA 93: 14367-14372, 1996. Tigyi, J., Tigyi, G., Liliom, K., and Miledi, R. Local anaesthetics inhibit receptors coupled to phosphoinositide signaling in Xenopus oocytes. Pflügers Archiv.-Eur. J. Physiol. 433: 478487, 1997. Santos, M. F., S. A. McCormack, J. Okolicany, Y. Zheng, L. R. Johnson, and Tigyi, G. The small molecular weight GTPase Rho is important for mucosal wound healing in vitro. J. Clin. Invest. 100: 216-225, 1997. Yakubu, M., Liliom, K., Tigyi, G., and C. Leffler. Intrathecal injection of endothelin-1 (ET1) mimics subarachnoid hematoma (SAH)-induced changes in cerebral microvascular reactivity: possible role for lysophosphatidic acid (LPA). Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 273: R703-R709, 1997. Erickson, J. R., J. J. Wu, J. G. Goddard, Tigyi, G., K. Kawanishi, L. D. Tomei, and M. C. Kiefer. The putative lysophosphatidic acid receptor Edg-2/Vzg-1 functionally couples to the yeast pheromone response pathway. J. Biol. Chem. 273: 1506-1510, 1998. Liliom, K, Z. Guan, J. L. Tseng, D. M. Desiderio, Tigyi, G., and M. A. Watsky. Growth factor-like phospholipids generated following corneal injury. Am. J. Physiol. 274 (Cell Physiol.) 43: C1065-C1074, 1998 Liliom, K., D. J. Fischer, T. Virág, G. Sun, D. D. Miller, J.-L. Tseng, D. M. Desiderio, M. Seidel, J. R. Erickson, and Tigyi, G. Identification of a novel growth factor-like lipid, 1-Ocis-alk-1’-enyl-2-lyso-sn-glycero-3-phosphate (Alkenyl-GP) that is present in commercial sphingolipid preparations. J. Biol. Chem. 273: 13461-13468, 1998. Fischer, D. J., Liliom, K., Z. Guo, T. Virág, G. Sun, D. D. Miller, KMurakami-Murofushi, K.S. Kobayashi, J. R. Erickson, and Tigyi, G. Naturally occurring analogs of lysophosphatidic acid elicit different cellular responses through selective activation of multiple receptor subtypes. Mol. Pharmacol. 54: 979-988, 1998. Siess, W., K. J. Zangl, M. Essler, M. Bauer, R. Brandl, C. Corrinth, R. Bittman, Tigyi, G., and M. Aepfelbacher, M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly-oxidized LDL and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 96: 6931-6936, 1999. Sebök, A., M. F. Santos, Z. Guo, N. Nusser, B. Debreceni, and Tigyi, G. RhoA regulates neuronal commitment and neurite outgrowth in differentiating PC12 cells. J. Neurochem. 73: 949-960,1999. Rizza, C., N. Leitinger, , M. Giese, T. Tyner, J., Yue, D.,Wang, D.J., Fischer, Tigyi, G. and J. A. Berliner. LPA as a regulator of endothelial/leukocyte interaction. Lab. Invest. 79: 12271235, 1999. Lehman, M., A. Fournier, I. Selles-Navarro, P. Dergham, N. Leclerc, Tigyi, G., and L. McKerracher. Inactivation of Rho signaling pathway promotes CNS axon regeneration J. Neurosci. 19: 7537-7547, 1999. Li, R., B. Debreceni, K. Zhu, F. Tigyi, and Y. Zheng. Localization of effector-specifiying regions of the small GTPase Cdc42. J. Biol. Chem. 274: 29648-29654, 1999. Leitinger, N., T. R. Tyner, L. Oslund, C. Rizza, G. Subbanagounder, H. Lee, P. T. Shih, N. Mackman, Tigyi, G., M. C. Territo, J. A. Berliner, and D. K. Vora. Structurally similar Gábor Tigyi — Curriculum Vitae 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. Page 16 oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc. Natl. Acad. Sci, USA 96:12010-12015, 1999. Tigyi, G., D. J. Fischer, D. Baker, D. A. Wang, J. Yue, N. Nusser, T. Virag, V. Zsiros, Liliom, K., D. Miller, and A. Parrill. Pharmacological characterization of phospholipid growth factor receptors. Ann. New York Acd.Sci. 905: 34-53, 2000. Balazs, L., J. Okolicany, M. Ferrebee, and Tigyi, G. Topical application of LPA accelerates wound healing. Ann. New York Acad. Sci. 905: 270-273., 2000 Baker, D. L., E. S. Umstot, D. M. Desiderio, and Tigyi, G. Quantitative analysis of lysophosphatidic acid in human blood fractions. Ann. New York Acad. Sci. 905: 267-269, 2000. Parrill, A. L., D. L.Baker, D.-A. Wang, D. J. Fischer, J. van Brocklyn, S. Spiegel, and Tigyi, G. Structural features of EDG1 receptor-ligand complexes revealed by computational modeling and mutagenesis. Ann. New York Acad. Sci. 905: 330-339, 2000. Liliom, K., M. Bueneman, G. Sun, D. Miller, D. M. Desiderio, B. Brandts, K. Bender, Pott, L., N. Nusser, and Tigyi, G. Sphingosylphosphorylcholine is a bona fide mediator regukating heart rate. Ann. New York Acad. Sci. 905: 308-310, 2000. Fischer, D. J., KMurakami-Murofushi, K.and Tigyi, G. Characterization of endogenous and heterologously expressed LPA receptor subtypes. Ann. New York Acad. Sci. 905: 287-289, 2000. Murakami-Murofushi, K., M. Mukai, S. Kobayashi, T. Kobayashi, Tigyi, G., and Murofushi, H. A lipid mediator, cyclic phosphatidic acid (cPA), and its biological functions. Ann. New York Acad. Sci. 905: 319-321, 2000. Essler, M., K. J. Zangl, M. Bauer, M. Retzer, Tigyi, G., M. Apfelbacher, and W. Siess. Stimulation of platelets and endothelial cells by mildly oxidized LDL proceeds through activation of lysophosphatidic acid receptors and the Rho/Rho kinase pathway. Ann. New York Acad. Sci. 905: 282-286, 2000. Watsky, M.A., M. Griffith, D. A. Wang, and Tigyi, G. Phospholipid growth factors and corneal wound healing. Ann. New York Acd.Sci. 905:142-158, 2000. Xu, J., L. M. Love, I. Singh, Q.-X. Zhang, J. Dewald, D.-A. Wang, Tigyi, G., L. G. Berthiaume, D. W. Waggoner, and D. N. Brindley. Lipid phosphate phosphatase-1 and Ca2+ control lysophosphatidate signalling through EDG-2 receptors. J. Biol. Chem. 275, 2752027530, 2000. Yatomi, Y., T. Ohmori, G. Rile, F. Kazama, H. OkamotoT., Sano, K. Satoh, S. Kume, Tigyi, G., Y. Igarashi, and Y. Ozaki. Sphingosine 1-phosphate as a major bioactive lysophospholipid which is released from platelets and interacts with endothelial cells. Blood 96: 3431-3438, 2000. Bautista,, D. L., D. L. Baker, D.-A. Wang, Fischer, D., J. van Brocklyn,, S.Spiegel, Tigyi, G., and A. Parrill. Dynamic Modeling of EDG1 Receptor Structural Changes Induced by SiteDirected Mutations. J. Mol. Struct. - Theochem. 529: 219-224, 2000. Balazs L., J. Okolicany, M. Ferrebee, B. Tolley, and Tigyi, G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 280: R466-R472, 2000. Parrill, A. L., D.-A.Wang, D. L. Bautista, J. R. van Brocklyn, Z. Lorincz, D. J. Fischer, D. L. Baker, Liliom, K., S. Spiegel, and Tigyi, G. Identification of Edg1 receptor residues that recognize sphingosine 1-phosphate, J. Biol. Chem. 275: 39379-3934, 2000. Liliom, K., G. Sun, M. Buenemann, N. Nusser, B. Brandts, K. Bender, M. J. Fabian, K. U. Malik, D. D. Miller, D. M. Desiderio, Tigyi, G., and Pott, L. Sphingosylphosphorylcholine is a naturally occurring lipid mediator in plasma: A possible role in regulating the myocardium. Biochem. J. 355:189-197, 2001. Tigyi, G. Physiological responses to lysophosphatidic acid and related glycerophospholipids. Review. Prostaglandins and Lipid Mediators. 64: 47-62, 2001. Baker, D. L., D. Desiderio, D. D. Miller, B. Tolley, and Tigyi, G. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution D. ionization liquid chromatography-mass spectrometry. Anal. Biochem. 292: 287-295, 2001. Bittman, R., D. Qin, D.-A. Wang, Tigyi, G., P. Samadder, and G. Arthur, G. Synthesis an antitumor propeties of a plasmalogen methyl ether analogue. Tetrahedron. 57: 4277-4282, 2001. Gábor Tigyi — Curriculum Vitae 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. Page 17 Fischer, D.J, Nusser, N., Virag, T., Wang, D.-A., Baker, D.L., Parrill, A.L., and Tigyi, G. (2001) Short-chain phosphatidates are subtype-selective antagonists of the lysophosphatidic acid receptors. Mol.Pharm. 60:776-784. Goetzl, E.J., Tigyi, G., and Hla, T. (2001) First International Conference on Lysolipids and Related Bioactive Lipids in Biology and Disease. The Scientific World.July -issue. Wang,D.A., Lorincz, Zs., Bautista, D., Parrill, A., Tigyi, G. (2001) A single residue determines ligand specificity for lysophosphatidic acid or sphingosine-1-phosphate in the S1P1 receptor. J. Biol. Chem. 276:49213-49920. Tigyi, G. (2001) Selective ligands for lysophosphatidic acid subtypes. Mol. Pharm. 60: 11611164. Ray, R., Patel, A., Viar, M.J., McCormack, A.S., Zheng, Y., Tigyi, G., and Johnson, L.R. (2001) Polyamines are essential regulators of events upstream and downstream of RhoA in cell migration. Gastroenterology 122:196-205. Deng, W., Balazs,L., Van Middlesworth, L.,Johnson, L.R., Tigyi, G. (2002) Lysophosphatidic acid prevents apoptosis in intestinal epithelial cells through the inhibition of Caspase-3/CPP32 activation and expression. Gastroenterology 122:.206-216 Sardar,V.M.,. Bautista, D. L., Fischer, D. J., Yokoyama, K., Nusser, N., Virag, T., Wang, DA., Baker, D. L., Tigyi, G. and Parrill, A. L. (2002) Molecular Basis for Lysophosphatidic Acid Receptor Antagonist Selectivity. Biochim. Biophys. Acta Mol.Cell. Biol. Of Lipids 1582: 310-318. Murakami-Murofushi,K., Uchiyama, A., Kobayashi, T., Kobayashi, S., Mukai, M.,Murofushi, H., and Tigyi,G. (2002) A novel lipid mediator, cyclic phosphatidic acid (cPA), and its biological functions. Biochim. Biophys. Acta. Mol.Cell. Biol. of Lipids. 1582:17. Tigyi. G. and Goetzl, E. (2002) Lysolipid mediators in cell signaling and disease. (editorial). Biochim. Biophys. Acta Mol.Cell. Biol. of Lipids 1582: 1. Yokoyama, K., Baker, D.L., Virag, T., Liliom, K., Byun, H.S., Tigyi, G. and Bittman, R. (2002) Stereochemical Properties of Lysophosphatidic Acid Signaling and Metabolism. review. Biochim. Biophys. Acta. Mol.Cell. Biol. of Lipids. 1582: 295-308. Sano,S., Baker, D., Wada, A. Yatomi, Y., Kobayashi, T., Igarashi, Y., Tigyi, G. (2002) Multiple Mechanisms Linked to Platelet Activation Result in Lysophosphatidic Acid and Sphingosine-1-Phosphate Generation in Blood. J. Biol. Chem 277:21197-21206. Baker, D., Morrison, P., Miller, B., Tolley, B., Postma, F., Moolenaar, W., Tigyi, G. (2002) Plasma Lysophosphatidic acid level is not a useful early marker of ovarian cancer. JAMA 287:3081-3082. Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. (2002) International Union of Pharmacology. XXXIV. Lysophospholipid Receptor Nomenclature. Pharmacol Rev. 54:265-269. Yokoyama, K., Baker, D.L., Virag, T., Liliom, K., Byun, H., Tigyi, G., and Bittman , R. (2002) Stereochemical properties of lysophosphatidic acid signaling and metabolism. Biochim. Biophys. Acta 1582: 296-309. Nusser, N, Gosmanova, E., Zheng, Y. and Tigyi, G. (2002) NGF signals through TrkA, PI3 kinase and Rac1 to inactivate RhoA during the initiation of neuronal differentiation of PC12 cells. J.Biol.Chem. 277: 35840-35846. Fueller, M., Wang, D.-A., Tigyi, G., Siess, W. (2002) Activation of Human Monocytic Cells by Lysophosphatidic Acid and Sphingosine-1-phosphate. Cell Signal. 5:367-75. Deng, W., Wang, D.-A.,Gosmanova, E., Johnson, L.R., Tigyi, G. (2003) Lysophosphatidic Acid Protects Intestinal Epithelial Cells from Camptothecin-Induced Apoptosis by Inhibiting the Mitochondrial Pathway. Am. J. Physiol - Liver, Gastrointest Physiol. 284(5):G821-9 Wang, D-a.,Du, H., Jaggar, J.H., Brindley, D.N., Tigyi, G., Watsky, M.A. (2003) Expression and Characterization of S1P and LPA Receptors and LPP1 Following Rabbit Corneal Wound Healing Am. J. Physiol. - Cell Physiol. 283: C1646-C1654. Virag, T., Elrod, D., Liliom, K., Sardar, V., Yokoyama, K., Parrill, A., Miller, D., Tigyi, G. (2003) Fatty alcohol phosphates are receptor subtype-specific mimetics and antagonists of the LPA receptors. Mol.Pharm. 63:1032-1042. Meyer zu Heringdorf, D., Vincent, M. E. M., Lipinski, M., Danneberg, K., Stropp, U., Wang, D. A., Tigyi, G., Jakobs, K. H. Inhibition of calcium signaling by the sphingosine-1phosphate receptor S1P1. (2003) Cell. Signal. 15(4):367-75. Gábor Tigyi — Curriculum Vitae 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. 103. 104. 105. 106. 107. 108. Page 18 Rother, E., Brandl, R., Baker, D.L., Tigyi, G., Siess. W. (2003) Subtype-selective antagonists of lysophosphatidic acid-receptors inhibit platelet shape change induced by mildly-oxidized LDL and plaque lipid core. Circulation 108:741-747. Lu, X, Cseh, S., Byun, H., Tigyi, G., Robert Bittman (2003) Total Synthesis of Two PhotoaffinityAnalogues of the Growth Factor-like Mediator Sphingosine 1-Phosphate: Differential Interaction with Protein Targets – J. Org.Chem. 68:7046-50. Tigyi, G., Parrill, A. (2003) Molecular Mechanisms of Lysophosphatidic Acid Action. Prog. Lipid Res.42:498-526. Fujiwara, Y., Sebok, A., Meakin, S., Kobayashi, T.,Murakami-Murofushi, K., Tigyi, G. (2003) The endogenous lipid mediator cyclic-phosphatidic acid mimics the actions of neurotrophins in embryonic hippocampal neurons. J. Neurochem. 87:1272-83. Yue, J., Yokoyama, K., Balazs, L., Baker, D.L., Pilquil, C., Brindley, D.N., Tigyi, G. (2003) Mice with Transgenic Over-expression of Lipid Phosphate Phosphatase-1 Display Reproductive and Hair Growth Deficits without Alteration in Circulating Lysophosphatidate Level. Cellular Signaling 16: 385-399. Haserück, N., Erl, Tigyi, G., Ohlmann, P., Ravanat, K., Gachet, C., Siess, W. (2003) The plaque lipid lysophosphatidic acid stimulates platelet aggregation and platelet-monocyte aggregate formation in whole blood Involvement of P2Y1 and P2Y12 receptors. Blood 103:2585-92. Tigyi, G. (2003) Computational approaches to phospholipid receptor structure and function. Mol. Cell. Proteomics. 2:803. Siess, W. and Tigyi, G. (2004) Thrombogenic and atherogenic activities of lysophosphatidic acid. J. Cell. Biochem. 92:1086-94. Meyer zu Heringdorf, D.,Liliom, K., Schäfer, M., Danneberg, Jaggar, J., Tigyi, G., and Karl H. Jakobs. (2003) Photolysis of intracellular caged sphingosine-1-phosphate causes Ca2+ mobilization independently of G protein-coupled receptors. FEBS Lett. 543:443-449. Zhang, C., Baker, D.L., Yasuda, S., Makarova, N., Balazs, L., Johnson, L.R., Marathe, G.K., McIntyre, T.M., Xu, Y., Prestwich, G.D., Byun, H.-S., Bittman R., and Tigyi, G. (2004) Lysophosphatidic Acid Induces Neointima Formation Through PPARγ Activation J.Exp. Med. 199:763-774. Goetzl EJ, Tigyi G. (2004) Lysophospholipids and their G protein-coupled receptors in biology and diseases. J Cell Biochem. 92:867-8. Deng, W., Poppleton, H., Yasuda, S., Makarova, N., Shinozuka, Y., Wang, D-A., Johnson, L.R., Patel T.B., and Tigyi, G. Optimal LPA-induced DNA Synthesis and Cell Migration but Not Survival Require Intact Autophosphorylation Sites of the EGF Receptor. (2004) J. Biol. Chem. 279: 47871-47880. Krump-Konvalinkova, V., Yasuda, S., Rubic, T, Makarova, N. Mages, J., Erl,W., Vosseler, C., Kirkpatrick, C.J., Tigyi, G., Siess, W. (2005) Stable knock-down of the sphingosine-1phosphate receptor S1P1 influences multiple functions of human endothelial cells. Atheroscler.Vasc. Biol. 25:546-52. Inagaki, Y., Kohno, T., Pham, T-C, T, Fujiwara, Y., Igarashi, Y., Tigyi, G., and Parrill, A.L. (2005) Distinct and Conserved Residues Mediate Sphingosine-1-Phosphate Recognition in S1P4 within the Endothelial Differentiation Gene Family of Receptors. Biochem. J. 89:18795. Jo, E, Sanna, M.G., Gonzalez-Caberra, P.J., Thangada, S., Tigyi, G., Osborne, D.A., Hla, T., Parrill, A.L., Rosen, H. (2005) S1P1-selective in vivo-active agonists from high throughput screening: Off the shelf chemical probes of receptor interactions, signaling and fate. Chem. Biol. Cell Press. 12:703-15. Li, C. Dandridge, K.S., Harris, E.L., Roy, K., Jackson, J.S., Fujiwara, Y., Makarova, N.V., Farrar, P.L., Tigyi, G. and Naren, A.P. Lysophophatidic inhibition of secretory diarrhea through CFTR-dependent protein interactions. (2005) J.Exp. Med. 202:975-86 . Nusser, N., Gosmanova, E., Fujiwara, Y., Makarova, N., Guo, F., Luo, Y., Zheng,Y., and Tigyi, G. (2006) Phosphorylation-regulated Target Selection of RhoA Is Required for NGFInduced Neurite Outgrowth Cellular Signaling 18: 704-714. Durgam, G.G.,Virag, T., Walker,M.D.,Tsukahara,R., Yasuda, S., Liliom,K., van Meeteren, L.A., Moolenaar,W.H., Wilke, N., Siess,W., Tigyi, G., and Miller, D.D. (2005) Synthesis, Gábor Tigyi — Curriculum Vitae 109. 110. 111. 112. 113. 114. 115. 116. 117. 118. 119. 120. 121. 122. 123. 124. Page 19 Structure-Activity Relationships, and Biological Evaluation of Fatty Alcohol Phosphates as Lysophosphatidic Acid Receptor Ligands, Activators of PPARγ and Inhibitors of Autotaxin. J.Med.Chem. 48(15):4919-30. Waters, C.M., Connell, M., Gorchkova, I., Long, J., Fujiwara, Y., Belmonte, K., Tigyi, G., Natarajan,W., Pyne, S., and Pyne, N.J. (2006) The sphingosine 1-phosphate receptor-1 inverse agonist SB649146 reduces platelet-derived growth factor-induced cell migration. FASEB J 20(3):509-11. Fujiwara, Y, Sardar, V., Tokumura, A., Baker, D.L., Murakami-Murofushi, K., Parrill, A., and Tigyi, G. (2006) Identification of residues responsible for ligand recognition and regioisomeric selectivity of LPA receptors expressed in mammalian cells – J. Biol. Chem. 280:35038-50. Xu, Y, Tsukahara, R., Fujiwara, R, Tigyi, G., Prestwich, G. (2006) Phosphonothioate and Fluoromethylene Phosphonate Analogues of Cyclic Phosphatidic Acid: Novel pKa Matched LPA Receptor Antagonists. J.Med. Chem. 49:5309-15 Gududuru, V., Tsukahara, R., Makarova, N., Fujiwara, Y., Pigg, K.R., Baker, D.L., Tigyi, G., Miller, D.D. (2006) Identification of Darmstoff analogs as selective agonists and antagonists of lysophosphatidic acid receptors. Biomed. Chem.Lett. 5;16(2):451-6. Durgam, G., Tsukahara, R., Makarova, N., Fujiwara, Y., Pigg, K.R., Baker, D.L., Parrill, A., Tigyi, G., Miller, D.D. (2006) Synthesis and pharmacological evaluation of secondgeneration phosphatidic acid derivatives as lysophosphatidic acid receptor ligands. Biomed. Chem. Lett. 6(3):633-40. Li, Z, Baker, D.L., Tigyi, G., Bittman R. (2006) Synthesis of Photoactivatable Analogues of Lysophosphatidic Acid and Covalent Labeling of Plasma Proteins. J. Org.Chem. 20;71:62935 Qian, L., Xu, Y., Simper, T., Aoki, J., Tsukahara, R., Makarova, N., Yu, S., Mills, G.B., Tigyi, G., Prestwich, G.D. (2006) Phosphorothioate and fluoromethylene phosphonate analogues of cyclic phosphatidic Acid: novel antagonists of lysophosphatidic Acid receptors. Chem. Med. Chem. 1:376-383. Zhang, H., Tsukahra, R., Tigyi, G., and Prestwich, G. (2006) Synthesis of Cyclic Phosphonate Analogues of (Lyso)phosphatidic Acid Using a Ring-Closing Metathesis Reaction. J. Org. Chem. 71(16):6061-6. Tsukahara, T., Tsukahara, R., Yasuda, S., Makarova, N., Valentine,W.J., Allison, P., Yuan, H., Baker,D.L., Li, Z., Bittman, R., Parrill, A., Tigyi, G. (2006) Different Residues Mediate Recognition of 1-O-Oleyl-Lysophosphatidic Acid and Rosiglitazone in the Ligand Binding Domain of PPARγ1. J. Biol. Chem. 281(6):3398-407. Long,J.S., Darroch, P,. Tate, R.J., Tolan, D.J., Tigyi, G., Pyne, N.J., Pyne, S. (2006) Lipid phosphate phosphatases are functionally active as dimers. Biochem. J. 394:495-500. Baker, D.L., Fujiwara, Y., Pigg, K.R., Tsukahara, R., Kobayashi, S., Uchiyama, A., Murakami-Murofushi, K., Murofushi, H., Koh, E., Bandle, R., Byun, H.-S., Bittman, R., Murph, M., Fan, D., Mills, G.B., Tigyi, G. (2006) Carba Analogs of Cyclic Phosphatidic Acid Are Selective Inhibitors of Autotaxin and Cancer Invasion. J. Biol. Chem. 281:2278693. Waters, C.M., Saatian, B., Moughal, N.A., Zhao, Y., Tigyi, G., Natarahan, V., Pyne, N.J. Integrin signaling regulatels the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA 1) in mammalian cells. (2005) Biochem. J. 398: 55-62. Moughal, N.A., Waters, C.M., Valentine, W.J., Connell, M., Crook, B., Harrison, S., Richardson, J., Tigyi, G., Pyne, S., Pyne, N.J. (2006) Cross talk regulation between TrkA and lysophosphatidic acid receptor signaling systems in mammalian cell. J. Neurochem –10: 1471-81. Yue, J. and Tigyi, G. (2006) MicroRNA trafficking and Human cancer. Cancer Biol Ther. 5:573-78. Liliom, K., Tsukahara, T., Tsukahara, R., Zelman-Femiak, M., Swiezewska, E., and Tigyi, G. (2006) Farnesyl phosphates are endogenous ligands of lysophosphatidic acid receptors: inhibition of LPA GPCR and activation of PPARg. Biochim.Biophys.Acta 1761(12):1506-14. Uchiyama, A., Mukai, M., Kobayashi, S., Kawai, N., Enoki, S., Tanaka, S., Murofushi, H., Niki, T., Kobayashi, T., Tigyi, G., Murakami-Murofushi, K., (2007) Inhibition of tumor cell Gábor Tigyi — Curriculum Vitae 125. 126. 127. 128. 129. 130. 131. 132. 133. 134. 135. 136. 137. 138. 139. 140. 141. Page 20 invasion and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim.Biophys. Acta 1771(1):103-12. Naor, M, Walker, D.M., Tigyi, G., Vanbrocklyn, J., Parrill, A. (2007) Sphingosine 1Phosphate pKa and Binding Constants: Intramolecular and Intermolecular Influences. J. Mol.Graph.Mod. –Sep;26(2):519-28. Pilquil, C., Dewald, J., Cherney, A., Gorshkova, I., Tigyi, G., English, D., Natarajan, W., and Brindley, D.N. (2006) Lipid Phosphate Phosphatase-1 Regulates Lysophosphatidate-induced Fibroblasts Migration by Controlling Phospholipase D2-dependent Phosphatidate Generation. J. Biol. Chem. 281(50):38418-29 Gajewiak, J., Yu, S., Tsukahara, R., Tigyi, G., Mills, G.B., Prestwich, G.D. (2007) Alkoxymethylenephosphonate Analogues of (Lyso)phosphatidic acid: Synthesis and Biological Activity. J. Org. Chem. 2(5):679-690. Jiang, G., Xu, Y., Fujiwara, Y., Tsukahara, R., Tsukahara, R., Tigyi, G., and Prestwich, G. D. (2007) α-Substituted Phosphonate Analogues of Lysophosphatidic Acid (LPA) Selectively Inhibit Production and Action of LPA. ChemMedChem.2:679-690. Deng, W., E, S., Tsukahara, R., Valentine, W., Durgam, G., Gududuru,V., Balazs, L., VanMiddlesworth, L., Johnson, L.R., Manickam, V., Arsura, M., Parrill, A.L., Miller, D.D. and Tigyi, G. (2007) The LPA2 Receptor is Required for Protection Against RadiationInduced Intestinal Injury. Gastroenterology. 132(5):1834-51. Fujiwara, Y., Osborne, D.A., Walker, M.D., Wang, D-A., Bautista,D.D., Liliom, K., Van Brocklyn, J.R., Parrill, A.L., Tigyi, G. (2007) Identification of the hydrophobic ligand binding pocket of the S1P1 receptor. J Biol. Chem. 282(4):2374-85. Pyne NJ, Waters CM, Long JS, Moughal NA, Tigyi G, Pyne S. (2007) Receptor tyrosine kinase-G-protein coupled receptor complex signaling in mammalian cells. Adv Enzyme Regul. 47:271-80. Hao, F., Tan, M., Xu, X., Han, J., Miller, D.D., Tigyi, G., Cui, M.Z. (2007) Lysophosphatidic acid induces prostate cancer PC3 cell migration via activation of LPA(1), p42and p38alpha. Biochim. Biophys. Acta 1771(7):883-892. Khurana, S., Tomar, A., George, S.P., Guo, H., Tigyi, G., Wang, Y., Siddiqui, M.R. (2008) Autotaxin and lysophosphatidic acid stimulate intestinal cell motility through LPA2 receptor activation and redistribution of the actin modifying protein villin to the developing lamellipodia. Exp. Cell. Res. 314(3):530-42. Epub 2007 Nov 12. Valentine,W.V, Fujiwara, Y., Tsukahara R., and Tigyi, G. (2008) Lysophospholipid Signaling: Beyond the EDGs. Biochem. Biophys. Acta 2008 Mar;1780(3):597-605. Epub 2007 Aug 25. Lin, F-T. , Lai, J-Y., Makarova, N., Tigyi, G., and Lin W.C. (2007) The LPA2 Receptor Mediates Down-Regulation Of Siva-1 To Promotes Cell Survival J. Biol. Chem. [Epub ahead of print, 10-26-07] 282, 37759-69. Valentine, W.V. Fells, J.I., Perygin,D.H.,, Mujahid, S., Yokoyama, K., Fujiwara, Y., Tsukahara,R., Van Brocklyn,J.R., Parrill,A.L., Tigyi, G. (2008) Subtype-Specific Residues Involved In Ligand Activation Of The Edg-Family Lysophosphatidic Acid Receptors. J.Biol.Chem. J Biol Chem. 283(18):12175-87. Epub 2008 Mar 3. Fells JI, Tsukahara R, Fujiwara Y, Liu J, Perygin DH, Osborne DA, Tigyi G, Parrill AL. (2008) Identification of non-lipid LPA(3) antagonists by virtual screening. Bioorg Med Chem. Apr 18. [Epub ahead of print], 1;16(11):6207-17 Gajewiak J, Tsukahara R, Fujiwara Y, Tigyi G, Prestwich GD. (2008) Synthesis, pharmacology, and cell biology of sn-2-aminooxy analogues of lysophosphatidic acid.Org Lett. Epub 2008 Feb 20;10(6):1111-4. Gajewiak J, Tsukahara R, Tsukahara T, Fujiwara Y, Yu S, Lu Y, Murph M, Mills GB, Tigyi G, Prestwich GD. (2008) Alkoxymethylenephosphonate Analogues of (Lyso)phosphatidic Acid Stimulate Signaling Networks Coupled to the LPA(2) Receptor. ChemMedChem. 3(2):200. Guo, H., Makarova,N., Cheng,Y., E,S., Ji,R.-R., Zhang, C., Farrar,P., Tigyi, G. (2008) Modulation of Vascular Smooth Muscle Cell Phenotype: The Role of Lysophosphatidic Acid Biochim.Biophys. Acta: Cell Mol. Biol of Lipids, 781(9):571-81. Khandoga, A.L Fujiwara, Y. Goyal, P. Tsukahara, R. Bolen, A. Guo, H. Pandey, D. Wilke, N. Liu, J. Valentine, W.J. Durgam, G. Miller, D.D. Jiang, G. Prestwich, G. Tigyi, G.and Siess Gábor Tigyi — Curriculum Vitae 142. 143. 144. 145. 146. 147. 148. 149. 150. 151. 152. 153. 154. 155. 156. 157. 158. Page 21 W. (2008) Human Platelet Responses to Lysophosphatidic Acid Revealed Through Receptorselective Probes and Albumin. Platelets 19(6):415-27. Tigyi G. (2008) Lysophospholipids in Health and Disease. Biochim.Biophys. Acta: Cell Mol. Biol of Lipids – 1781(9):423. Hasegawa Y, Murph M, Yu S, Tigyi G, Mills GB. (2008) Lysophosphatidic acid (LPA)induced vasodilator-stimulated phosphoprotein mediates lamellipodia formation to initiate motility in PC-3 prostate cancer cells. Mol Oncol. 2008 Jun;2(1):54-69. Lu, X., Sun, C., Valentine, W.J., E, ,S., Liu, J., Tigyi, G., and Robert Bittman (2009) Chiral Phosphonate Analogues of the Immunosuppressive Agent FTY720. J. Org. Chem. . J. Org. Chem. 74(8):3192-5. E, S., Lai, J.S., Tsukahara, R., Chen, C._S., Fujiwara, Y., Yue, J., Yu, J.-H., Guo, H., Kihara, A., Tigyi, G., and Lin, F.-T. (2009) The LPA2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem. 2009 May 22;284(21):14558-71. Epub 2009 Mar 17. Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, Parrill AL, Tigyi G, Fujiwara Y. (2009) Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 284(25):17304-19. Epub 2009 Apr 14. Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, Fells JI, Perygin D, Parrill AL, Tigyi G, Prestwich GD. (2009) Dual activity lysophosphatidic acid receptor panantagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009 Jul 1;69(13):5441-9. Epub 2009 Jun 9. Cheng, Y., Makarova, N., Tsukahara, R., Guo, H., E, S., Farrar, P., Balazs, L., Zhang, C., Tigyi. G. (2009) Lysophosphatidic Acid-induced Arterial Wall Remodeling: Requirement of PPARγ but Not LPA1 or LPA2 GPCR. Cell Signal. 12:1874-84. Epub 2009 Aug 23. PMID: 19709640 Fells, J.I., Tsukahara, R., Liu, J., Tigyi, G., and Parrill, A.L. (2010) Structure-based drug design identifies novel LPA3 antagonists. Bioorg. Med. Chem. 17(21):7457-64. Epub 2009 Sep 18. PMID: 19800804 Fells, J.I., Tsukahara, R., Liu, J., Tigyi, G., and Parrill, A.L. (2009) 2D Binary QSAR Modeling of LPA3 Receptor Antagonism. J. Comp. Modeling. Vidot, S., Witham, J., Agarwal,R., Greenhough, S., Bamrah, H.S., Tigyi, G.J., Kaye, S.B., Richardson, A., (2010) Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell. Signaling. 22:926–935. PMID: 20100569. Yue, J., and Tigyi, G. (2010) Conservation of miR-15a/16-1 and miR015b/16-2 clusters. Mamm. Genome 21(1-2): 88-94. [Epub ahead of print] PMID: 20013340. Fells JI, Tsukahara R, Liu J, Tigyi G, Parrill AL. (2010) 2D binary QSAR modeling of LPA3 receptor antagonism. J. Mol. Graph. Model. 2010 Jun;28(8):828-33. Epub 2010 Mar 7. Zhang Q, Wang D, Kundumani-Sridharan V, Gadiparthi L, Johnson DA, Tigyi G. J, Rao GN. PLD1-dependent PKCgamma activation downstream to Src is essential for the development of pathological retinal neovascularization. Blood 2010 Apr 26. [Epub ahead of print]. 116(8): 1377-85. PMID: 20421451. Tigyi, G. Aiming Drug Discovery at Lysophosphatidic Acid Targets. 2010. Brit. J. Pharmacol. 161(2):241-70. Tsukahara, T., Tsukahara, T., Cheng, Y., Zhang, C., Balazs, L., Du, G., Frohman, M.A., Baker, D.L., Parrill, A.L., Uchiyama, A., Kobayashi, T., Murakami-Murofushi, K., and Tigyi,G. (2010) The Novel Second Messenger Cyclic Phosphatidic Acid Negatively Regulates The Nuclear Hormone Receptor PPARγ – Mol. Cell. 39, 421-432. Tonelli, F.; Lim, K. G.; Loveridge, C.; Long, J.; Pitson, S. M.; Tigyi, G.; Bittman, R.; Pyne, S.; Pyne, N. J. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal 2010, 22, 1536-42. Valentine, W. J.; Kiss, G. N.; Liu, J.; E, S.; Gotoh, M.; Murakami-Murofushi, K.; Pham, T. C.; Baker, D. L.; Parrill, A. L.; Lu, X.; Sun, C.; Bittman, R.; Pyne, N. J.; Tigyi, G. (S)FTY720-vinylphosphonate, an analogue of the immunosuppressive agent FTY720, is a panantagonist of sphingosine 1-phosphate GPCR signaling and inhibits autotaxin activity. Cell Signal 2010, 22, 1543-53. Gábor Tigyi — Curriculum Vitae 159. 160. 161. 162. 163. 164. 165. 166. 167. 168. 169. 170. 171. 172. 173. 174. Page 22 Kosanam H, Ma F, He H, Ramagiri S, Gududuru V, Tigyi GJ, Van Rompay K, Miller DD, Yates CR. Development of an LC-MS/MS assay to determine plasma pharmacokinetics of the radioprotectant octadecyl thiophosphate (OTP) in monkeys. J. Chromatogr. 2010. 878(26):2379-83. Epub 2010 Jul 18. Yin, Z., Carbone, L.D, Gotoh, M., Postlethwaite, A., Bolen, A., Tigyi, G.J., MurakamiMurofushi, K., Watsky, M.A. Lysophosphatidic acid-activated Cl− current activity in human systemic sclerosis skin fibroblasts. Rheumatology. 2010. 49(12):2290-7. Sept. 7 Epub ahead of print]. PMID 20823096 Long, J.S., Fujiwara, Y., Tigyi, G.J., Pyne. S., and Pyne, N.J. Sphingosine 1-phosphate 4 Receptor uses HER2 (ErbB2) to regulate extracellular signal regulated kinase-1/2 in MDAMB-453 breast cancer cells. J. Biol. Chem. 2010. 2;285(46):35957-66. Epub 2010 Sep 13. PMID: 20837468 Uchiyama, A., Tigyi, G. and Murakami-Murofushi, K. (2010) Cdc42/N-WASP and Rac1/WAVE2 Required for LPA-induced Migration of Rat Ascites Hepatoma Cells. Cytologia 75: 195-201. Uchiyama, A.; Arai, Y., Kobayashi, T.; Tigyi, G.; Murakami-Murofushi, K. (2010) Transcellular Invasion of MM1 Rat Ascites Hepatoma Cells Requires Matrix Metalloproteinases Derived from Host Mesothelium. Cytologia 75, 267-272. Gupte R, Siddam A, Lu Y, Li W, Fujiwara Y, Panupinthu N, Pham TC, Baker DL, Parrill AL, Gotoh M, Murakami-Murofushi K, Kobayashi S, Mills GB, Tigyi G, Miller DD. Bioorg Med Chem Lett. 2010 Dec 15;20(24):7525-8. Epub 2010 Oct 8.PMID: 21051230 Tabchy, A., Tigyi, G., Mills, G. B. (2011) Lipid Signaling and Cancer: Towards a Crystal Clear View of the Autotaxin-LPA Story. Nat. Struct. Mol. Biol. Nat Struct Mol Biol. 2011 Feb;18(2):117-8. PMID: 21289650 Bolen, A. L.; Fells, J.; Naren, A. P.; Yarlagadda, S.; Beranova-Giorgianni, S.; Chen, L.; Norman, D.; Baker, D. L.; Best, M. D.; Rowland, M. M.; Sano, T.; Tsukahara, T.; Liliom, K.; Igarashi, Y.; Tigyi, G. The Phospholipase A1 Activity of Lysophospholipase A-I Links Platelet Activation to LPA Production During Blood Coagulation. J. Lipid. Res. 2011, May;52(5):958-70. Epub 2011 Mar 9. PMID: 21393252 Gupte, A., Patil, A., Liu, J., Wang, Y., Lee,S. L., Fujiwara, Y., Fells, J.,Bolen, A.L.,EmmonsThompson, K., Yates, C.Y.,Siddam, A.,Panupinthu, N., Pham, T-C. T., Baker, D.L., Parrill, A.L., Mills, G.B., Tigyi, G.J., and Miller, D.D. (2011) Benzyl and Naphthalene-Methyl Phosphonic Acid Inhibitors of Autotaxin with Anti-invasive and Anti-metastatic Actions. Bioorg. Med. Chem. 2011 6(5):922-35. PMID:21465666. Pan Y, Balazs L, Tigyi G, Yue J. (2011) Conditional deletion of Dicer in vascular smooth muscle cells leads to the developmental delay and embryonic mortality. Biochem. Biophys. Res. Commun. 2011 Feb 28. [Epub ahead of print] PMID: 21371421 Valentine WJ, Godwin VI, Osborne DA, Liu J, Fujiwara Y, Van Brocklyn J, Bittman R, Parrill AL, Tigyi G. (2011) FTY720 (Gilenya) phosphate selectivity of sphingosine 1phosphate receptor subtype 1 (S1P1) G protein-coupled receptor requires motifs in intracellular loop 1 and transmembrane domain 2. J Biol Chem. 2011 Jun 30. [Epub ahead of print] PMID: 21719706 Tigyi, G. LPA activates TRPV1-and it hurts. Nat Chem Biol. 2011 Dec 15;8(1):22-3. PMID: 22173354 Gotoh, M., Fujiwara, Y., Yue, J., Liu, J., Lee, S., Fells, J., Uchiyama, A., MurakamiMurofushi, K., Kennel, S., Wall, J., Patil, R., Gupte, R., Balazs, L., Miller, D.D., and Tigyi, G.J., Controlling cancer through the autotaxin-lysophosphatidic acid receptor axis. Biochem Soc Transact, 2012. 40: p. 31-6. PMID:22260662. Chen Z, Wu J, Yang C, Fan P, Balazs L, Jiao Y, Lu M, Gu W, Li C, Pfeffer LM, Tigyi G, Yue J. DiGeorge syndrome critical region 8(DGCR8) -mediated miRNA biogenesis is essential for vascular smooth muscle cell development in mice. J Biol Chem. 2012 287(23):19018-28. Apr 17. [Epub ahead of print] PMID: 22511778. Valentine WJ, Tigyi G. High-Throughput Assays to Measure Intracellular Ca(2+) Mobilization in Cells that Express Recombinant S1P Receptor Subtypes. Methods Mol Biol. 2012;874:77-87. PMID: 22528441. Kiss GN, Fells JI, Gupte R, Lee SC, Liu J, Nusser N, Lim KG, Ray RM, Lin FT, Parrill AL, Sumegi B, Miller DD, Tigyi G.J. Virtual Screening for LPA2-Specific Agonists Reveals A Gábor Tigyi — Curriculum Vitae 175. 176. 177. 178. 179. 180. 181. 182. 183. 184. 185. 186. 187. 188. 189. Page 23 Nonlipid Compound with Antiapoptotic Actions. Mol. Pharmacol. 2012;82(6):1162-73. Epub 2012 Sep 11. PMID:22968304. Kiss, Gy.N., Lee, S-C., Fells, J.I., Liu, J, Valentine, W.J., Fujiwara, Y., Emmons-Thompson, K., Yates, C.R., Sümegi, B., Miller, D.D., Tigyi, G. Mitigation of Radiation Injury by Selective Stimulation of the LPA2 Receptor. Biochim. Biophys.Acta Biochim Biophys Acta. 2013 Jan;1831(1):117-25. PMID:23127512. Brindley DN, Lin FT, Tigyi G.J. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim Biophys Acta. 2013 Jan;1831(1):7485. Epub 2012 Aug 29.PMID 22954454. Parrill AL, Tigyi G. Integrating the puzzle pieces: The current atomistic picture of phospholipid-G protein coupled receptor interactions. Biochim Biophys Acta. 2013 Jan;1831(1):2-12. Epub 2012 Sep 12. PMID: 22982815. Tigyi, G. New Trends in Lysophospholipid Research. Biochim Biophys Acta. 2013 Jan;1831(1):1. Epub 2012 Sep 23. PMID: 23010474. Schwingshackl A, Teng B, Ghosh MC, Lim KG, Tigyi GJ, Narayanan D, Jaggar JH, Waters CM (2012) Regulation of inerleukin-6 secretion by the two-ore-dinaub oitassuyn *J2P) channel Trek-1 in alveolar epithelial calls. Am. J. Phsiol. Lung Cell. Mol. Phsiol. 2012 Dec 28 [Epub ahead of print]. James I. Fell, Sue Chin Lee, Yuko Fujiwara, Derek D. Norman, Keng G.Lim, Ryoko Tsukahara, Jianxiong Liu, Jason Kirby, Sandra Nelson, William Seibel, Ruben Papoian, Abby L. Parrill, Daniel L. Baker and Gabor Tigyi. (2013) Blockade of the Hydrophobic Pocket of Autotaxin Prevents Tumor Invasion and Metastasis. Mol. Pharmacol. 84(3):415424. Epub 2013 Jun 21. Kotla, S., Singh, N.K., Heckle, M.K., Tigyi, G.J. and Gadiparthi N. Rao. (2013) The Transcription Factor CREB Enhances Interleukin-17A Production and Inflammation in a Mouse Model of Atherosclerosis. Science –Signaling. 6: ra83. [DOI: 10.1126/ scisignal.2004214 James I. Fells, Sue Chin Lee, Derek D. Norman, Ryoko Tsukahara, R. Jason Kirby, Sandra Nelson, William Seibel, Ruben Papoian, Renukadevi Patil, Duane D. Miller, Abby L. Parrill, Truc-Chi Pham, Daniel L. Baker, Robert Bittman and Gabor Tigyi (2013) Targeting the Hydrophobic Pocket of Autotaxin with Ligand-based Virtual Screening. FEBS J. – FEBS J. 2013 /febs.12674. [Epub ahead of print].PMID: 24314137. Oda SK, Strauch P, Fujiwara Y, Al-Shami A, Oravecz T, Tigyi G, Pelanda R, Torres RM. Lysophosphatidic acid inhibits CD8 T cell activation and control of tumor progression. Cancer Immunol. Res. 2013 Oct;1(4):245-55. PMID: 24455753 Hu J, Oda SK, Shotts K, Donovan EE, Strauch P, Pujanauski LM, Victorino F, Al-Shami A, Fujiwara Y, Tigyi G, Oravecz T, Pelanda R, Torres RM. Lysophosphatidic acid receptor 5 inhibits B cell antigen receptor signaling and antibody response. J. Immunol. 2014 Jun 2. PMID: 24890721. Ruisanchez É, Dancs P, Kerék M, Németh T, Faragó B, Balogh A, Patil R, Jennings BL, Liliom K, Malik KU, Smrcka AV, Tigyi G, Benyó Z. Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase. FASEB J. 2014 Feb;28(2):880-90. Epub 2013 Nov 18. PMID:24249637. Chen Z, Wang Y, Liu W, Zhao G, Lee S, Balogh A, Zou Y, Guo Y, Zhang Z, Gu W, Li C, Tigyi G, Yue J. Doxycycline inducible Krüppel-like factor 4 lentiviral vector mediates mesenchymal to epithelial transition in ovarian cancer cells. PLoS One. 2014. 9(8):e105331. PMID 25463482. Morales-Lázaro, S.L., Serrano-Flores, S.L., Llorente, I., Hernández-García, E., GonzálezRamírez, R., Banerjee, S., Miller, D.D., Gududuru, V., Fells, J.I., Norman, D.B., Tigyi, G.J., Escalante-Alcalde, D., and Rosenbaum, T. Structural Determinants Of Trpv1 Activation By Phospholipid Analogs. J. Biol. Chem. 2014 289(49):33876. PMID 25480965. Lee, S-L, Fujiwara, Y., Liu, J., Yue, J., Wang, Y., Tsukahara, R., Szabo, E., Patil, R., Banerjee, S., Miller, D.D., Balazs, L., Ghosh, M.C., Waters, C.M., Oravecz, T., and Tigyi, G.J. Autotaxin, Lysophosphatidic Acid Receptors 1 and 5 Exert Different Functions in Tumor Cells Versus the Host tissue Microenvironment in Melanoma Invasion and Metastasis. Mol. Cancer Res. 2015 :174-85. PMID: 25158955. Patil, R., Szabó, E., Fells, J.I., Balogh, A., Lim, K.G., Norman, D.B., Patil, S., Strobos, J., Miller, D.D., Tigyi, G.J. Design and Synthesis of Sulfamoyl Benzoic Acid Analogs with Gábor Tigyi — Curriculum Vitae 190. 191. 192. 193. 194. 195. 196. Page 24 Subnanomolar Agonist Activity Specific to the LPA2 Receptor. J. Med. Chem. (2014) J. Med. Chem 8;57(16):7136-40. PMID 25100502 Leblanc, R., Lee, S-C., David, M., Bordet, J-C., Norman, DB., Patil, R., Miller, D.D., Sahay, D., Ribeiro, J.,lzardin, P.C., Tigyi, G.J., and Peyruchaud, O. Interaction of Platelet-derived Autotaxin with Tumor Integrin αVβ3 Controls Breast Cancer Metastasis. Blood 2014. Blood. 2014. 124(20):3141-50. PMID 25277122. Ren, A., Moon, C., Zhang, W., Sinha, C., Yarlagadda, S., Arora, K., Wang, X., Yue, J., Parthasarathi, K., Heil-Chapdelaine, R., Tigyi, G.J., and Naren, A.P. Asymmetrical macromolecular complex formation of LPA2 mediates gradient sensing in fibroblasts. J. Biol. Chem. 2014. J Biol Chem. 289(52):35757-69. PMID:25542932 Ho Y, Yao C, Lin K, Hou F, Chen W, Chiang C, Lin Y, Li M, Lin S, Yang Y, Lin C, Lu J, Tigyi G, Lee H. Opposing regulation of megakaryopoiesis by LPA receptors 2 and 3 in K562 human erythroleukemia cells. Biochim Biophys Acta. 2014. 1851(2):172-183. PMID 25463482. Patil, R., Szabó, E., Fells, J.I., Balogh, A., Lim, K.G., Fujiwara, Y., Norman, D.B., Lee, S-C, Balazs, L., Thomas, F., Patil, S., Emmons-Thompson, K., Boler, A., Strobos, J., McCool, S.W., Yates, C.R., Stabenow, J., Byrne, G.I., Miller, D.D., Tigyi, G.J. Protection Against the Gastrointestinal and Hematopoietic Acute Radiation Syndromes by a Novel LPA2 Receptorspecific Non-lipid Agonist. Chem. Biol. (Cell Press) 2015. 22:1–11. PMID:25619933 Chen Z, Wang Y, Liu W, Zhao G, Lee S, Balogh A, Zou Y, Guo Y, Zhang Z, Gu W, Li C, Tigyi G, Yue J. Doxycycline inducible Krüppel-like factor 4 lentiviral vector mediates mesenchymal to epithelial transition in ovarian cancer cells. PLoS One. 2014 Aug 19;9(8):e105331. PMID: 25137052 Deng, W. Kimura, Y., Gududuru, V.,Wu, W., Balogh, A., Szabo, E., Emmons-Thompson, K., Yates, C.R., Balazs, L., Johnson, L.R., Miller, D.D., Strobos, J., McCool. W.S., and Tigyi, G.J. Mitigation of the Hematopoietic and Gastrointestinal Acute Radiation Syndrome by Octadecylthiophosphate a Small Molecule Mimic of Lysophosphatidic Acid. 2015. Radiation Res. – in press. Parham, K.A., Dobbins, J.R., Tooley, K.L., Sun, W.Y., Moretti, P.A., Fells, J.I., Tigyi, G.J., Pitson, S.M. and Bonder, C.S. Sphingosine 1-phosphate is a ligand for PPARγ regulating vascular function. FASEB J - submitted.
© Copyright 2026