Study on the controlled growth of lanthanum hydroxide and
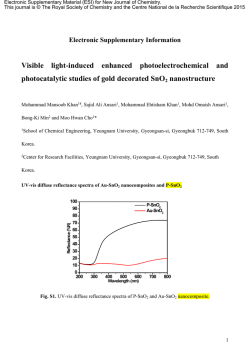
Advances in Materials 2015; 4(1): 11-15 Published online January 28, 2015 (http://www.sciencepublishinggroup.com/j/am) doi: 10.11648/j.am.20150401.13 ISSN: 2327-2503 (Print); ISSN: 2327-252X (Online) Study on the controlled growth of lanthanum hydroxide and manganese oxide nano composite under the presence of cationic surfactant Neeraj Kumar Verma Centre of Excellence, Material Science & Engineering, Department of Metallurgy, OP Jindal Institute of Technology, Raigarh Chhattisgarh, India Email address: [email protected] To cite this article: Neeraj Kumar Verma. Study on the Controlled Growth of Lanthanum Hydroxide and Manganese Oxide Nano Composite under the Presence of Cationic Surfactant. Advances in Materials. Vol. 4, No. 1, 2015, pp. 11-15. doi: 10.11648/j.am.20150401.13 Abstract: Lanthanum hydroxide and manganese oxide nano composite are synthesized by chemical routes. Physical characterization is done by TEM to look at the size and dispersion of the nano particles in the composite. Chemical characterization is done by X ray diffraction technique and FTIR to ascertain the attachment of the functionalities and bond stretching. Further thermal analysis is done by thermo gravimetric analysis to find the tendency of the thermal decomposition in the elevated temperatures range of 0-1000°C. Proper analysis and correlation of the various results obtained suggested the controlled growth of crystalline without agglomeration and good stability in the various temperature ranges of the composite. Keywords: Nanoparticles, XRD, TEM 1. Introduction Nano-materials being unique in their morphology when compared from their bulk form impart them with tremendous range of properties not ordinarily expected. Depending on the morphological configuration and size distribution various manufacturing routes for such nano materials have been established in laboratories [1].The synthesis of nanoparticles is based on the preparation of hydroxide colloidal precipitates and hydrothermal treatment at particular temperature. The particle morphology has been altered by changing the process [2] and various nanoparticles of manganese oxide. These manganese oxide nanoparticles owing to their excellent electric, magnetic, and catalytic properties[3],rechargeable lithium ion batteries[4],magnetic[5],molecular adsorption, low price, and environmental compatibility play a vital role as a building block for the nanocomposite. Especially manganese oxide is known as an effective catalyst for deNOx systems [6]. The synthetic procedures are used for these materials in order to study the influence of particle size on their properties, including conventional solid state reaction, co -precipitation [7],Sol–gel [8]and molten salts reactions [9]. Hydrophilic and hydrophobic methods for the synthesis of Mn2O4 nanoparticles [10] and Sono-synthesis of Mn3O4 nanoparticles in different media are done without any additives [11]. These Mn3O4 nanoparticles find their application in the investigation of High resolution electron energy loss spectroscopy [12]. Mn3O4 nanocubes at room temperature when undergoes appropriate thermal recycling behave as a catalyst [13]. In some cases of the Mild aqueous synthesis of octahedral Mn3O4 ,nanocrystals are showing varied oxidation states [14].Effect of organic solvents on particle size of Mn3O4 nanoparticles synthesized by a solvothermal method have been studied in detail[15]. At room temperature these Mn3O4 nanoparticles undergoes hydrothermal transformation to g-MnO2 nanorods [16]. It is generally accepted that the preparation of lanthanum hydroxide nanoparticles has been used in the synthesis of oxides or sulfides through dehydration due to straightforward approach [17] Further it has been reported that La2O3 and La (OH)3 are very susceptible to surrounding condition with CO2 . Under such appropriate condition of temperature and pressure the process of carbonation occurs which leads the formation of surface carbonates or hydroxycorbonates [18] &[19].Furthermore, the synthesis and Characterization of Lanthanum Oxide nanoparticles could be done from the thermolysis of a nano-sized Lanthanum (III) Supra molecule as a novel precursor [20]. The objective of this paper is to study on the controlled growth of Lanthanum Hydroxide and Manganese Oxide Nano Composite under the presence of 12 Neeraj Kumar Verma: Study on the Controlled Growth of Lanthanum Hydroxide and Manganese Oxide Nano Composite under the Presence of Cationic Surfactant cationic surfactant trimethylammonium bromide and tetra butylammonium bromide. The synthesized particles of lanthanum hydroxide and Manganese Oxide nanocomposties are characterized by Powder X-ray diffraction (XRD), Thermogravimetric Analysis (TGA), Fourier Transform infrared Spectroscope (FTIR) and Transmission electron microscopy (TEM) .The characterizations of the composite confirm nano rods which could help in the conductivity of biofluids proteins by suitable attachment of these nanoparticles. 2. Experimental 2.1. Synthesis of Lanthanum hydroxide- Manganese Oxide Nanocomposite For synthesis of nanocomposite, 5.6 mmole of KMnO4 (0.89020gm) with varying concentrations of La(NO3)3. 6H2O(1.4651gm), CTAB(1.7223 gm) and TBAB (1.81119 gm) are dissolved into 100 ml of distilled water. The mixture becomes homogenous under slow stirring for few minutes (40min) in order to avoid bubble formation and then monohydrate hydrazine are added rapidly to the salt/surfactant solution under vigorous magnetic stirring. varying concentration of KMnO4,La(NO3)3,CTAB, TBAB and N2H4.H2O used for synthesis with appropriate amount. The color of the solution immediately changes from dark purple to black/brown then to orange/brown. The system is heated at 70ºC for 1 hr and cooled down to room temperature by removing the heat source. An orange/brown material is precipitated and separated out by centrifugation. The solid solution washed four times with ethanol and dried under vacuum rotavapour at 45ºC at 170 pressures. The variation in concentration of lanthanum nitrate used for the synthesis of nanocomposite from 0.05 mmole to 6 mmole resulted in the change in the color (from light brown to dark brown) of nanocomposite. 3. Result & Discussion synthesis route. The particle size measurement has been carried out using XRD and a Fourier Transformation infrared (FTIR) recorded in the range from 500 to 4000 cm-1. The thermal behavior has been characterized by TGA Instrument in the temperature range 0 to 10000C and the morphological study has been carried out by TEM. 3.1. XRD Analysis X-ray diffraction pattern of synthesized sample of lanthanum manganese oxide composite after annealing is shown in Figure 1(a). This pattern clearly indicates about pure crystalline nature of composite. Figure1 ( b ) shows room temperature x-ray diffraction pattern of synthesized lanthanum manganese oxide composite before annealing .This pattern shows diffraction peaks for Mn3O4 plane which are marked(*).The peaks are found to be broadened. This indicates that Mn3O4 and La(OH) 3 in the composite material are in crystalline form. The use of higher concentration of lanthanum oxide shows up with the increased intensity of the (101) planes than corresponding to (211) diffraction line of manganese oxide in patterns. This resembles with the pattern of La0.93MnO3 ceramic mixture after annealing at 1000 0C for 1 hour .The XRD pattern of nanocomposite after annealing consist of diffraction by (012), (110), (104), (202) (006), (024), (211), (122), (116), (214), (018), (220), (208), (306) planes of rhombohedral La0.93MnO3 along with diffraction by (112), (211), (220), (224) and (400) planes of tetragonal Mn3O4.The average crystallite size d of the nanoparticles is calculated using the modified Scherrer formula t = 0.9 λ / Cos θB (BM - BS) Here λ = wavelength of x-ray used, θB = Bragg’s angle, BM = Full width at half maximum of the peak, B S = Full width at half maximum of the same peak from a standard material. Using the modified Scherrer formula the average crystallite size turns out to be 21 nm and 12 nm. The broadening of peaks shown in the fine crystal size is due to the growth of nanocrystals with respect to surfactant. The Nanocomposite has been synthesized using the chemical Figure 1(a). XRD Patterns of nano composite [La(OH)3 /Mn3O4 with aspect ratio (5/3)] after annealing. Advances in Materials 2015; 4(1): 11-15 13 Figure 1(b). XRDPatterns of nano composite [La (OH)3 /Mn3O4 with aspect ratio (5/3)] before annealing. 3.2. Fourier Transformation Infrared (FTIR) Study Figure 2. Fourier Transform Infrared Spectra of nano composite. The FTIR spectrum of nanocomposite is depicted in the above Fig.2. Analysis of the FTIR spectra clearly indicates that a peak appeared at 504.4 cm-1 which is attributed to the distortion vibration of Mn-O in octahedral site. The La-OH deformation and Mn-O stretching mode at tetrahedral sites appeared as a combined overtone at 623.9 cm-1. The broad band at 3410.7 cm-1 is due to combined vibration of physioabsorbed molecules of water. The peak at 2923.2 cm-1 is due to C-H asymmetrical stretching. The peak at 1629.1 is ascribed to physioabsorbed water molecules. The bend appeared at 1473.4cm-1 is attributed to the C-H asymmetrical bending [21]. The band at 1383.2 cm-1 can be attributed to CH3 streching of tertiary butyl of surfactant. The C-C skeletal vibration appeared at 1120.0 cm-1. The presence of C-H stretching, C-H bending and C-C skeletal vibrations confirmed the capping of tertiary butyl of surfactant and ammonium bromide surfactants at the surface of nanoparticles. 14 Neeraj Kumar Verma: Study on the Controlled Growth of Lanthanum Hydroxide and Manganese Oxide Nano Composite under the Presence of Cationic Surfactant 3.3. Thermogravimetric Analysis Figure 3. Weight loss as function for nano composite. In Thermogravimetric analysis the mass of a given material were measured as a function of temperature by heating the material at a constant rate. The prepared sample of composite was heated at a rate of 1 0 0 C per minute for this analysis. The variation of weight loss of the sample as a function of temperature is shown in Figure 3. This figure shows three step weight loss transitions. The first step weight loss transition (2.02%) in the temperature range 25- 1200C is ascribed to expulsion of physically adsorbed water molecules at the surface of nano-Mn3O4. The second step weight loss transition (5.83%) in the temperature range 120-4000C is ascribed to loss of surfactants molecules that were capped the nano-particles. In other words the decomposition of the composite is almost complete at about 6000C and above the 6000C third step weight loss transition (9.23%) is attributed to the loss of O2 due to conversion of Mn3O4/La(OH)3 nano composites to La0.93MnO3 which is almost equal to theoretical weight loss value according to following reaction: 2Mn3O4 + 5.58 La(OH)3 → 6La0.93MnO3 + 6.74 H2O + 1.63 H2 It is clear from the analysis that the conversion of Mn3O4/ La(OH)3 nano composite to La0.93MnO3 is confirmed using diffraction pattern of XRD. 3.4. Transmission Electron Microscope Analysis Figure 4. Transmission electron microscope of nanoparticles (a) Images of nanocomposite (Mn3O4/La(OH)) (b) measurements of composite in rod with 9-12 nm in width and 30-40 nm in length. It is clear from transmission electron microscope image (Fig 4.a, b) that the nanoparticles are uniform size and pebble like in shape. The diameter of nano-particles varies in the range of 12-15 nm. The electron diffraction pattern for nanoparticles is also in agreement with XRD results. The clearly uniform size of nano-particles are attributed to the surfactant Advances in Materials 2015; 4(1): 11-15 15 capping which is also confirmed from TGA and FTIR analysis The micrographs of Composites represents the formation of randomly dispersed La(OH)3 nanorods. The highmagnification image of a typical individual La(OH)3 nanorod, in which the width and length of the lanthanum hydroxide nanorod are around 10 nm and 36 nm respectively. The size of the La(OH)3 nanorod was controlled by surfactant nanorods. The driving force for the anisotropic growth of La(OH)3 nanorod is derived from the inherent crystal structure of La(OH)3 materials and their chemical potential in solution [22,23] and also shows that Mn3O4/La(OH)3 nanocomposite, the composite is in the rod like shaped. The above results indicates the presence globular Mn3O4 nanoparticles in the close vicinity of nanorods La(OH)3 which are well attached and are around 26 nm and 200 nm in width and length respectively confirm the synthesis of Mn3O4/La(OH)3 nanocomposite. The study of all the samples shows that in the case of nanocomposite rods of La2O3 have grown in size and particles are attached at their edges. [5] D. K. Kim, P. Muralidharan, H. W. Lee, R. Ruffo, Y. Yang, C. K. Chan, H. Peng, R. A. Huggins and Y. Cui, Nano Lett., 2008, 8, 3948–3952 [6] W. S. Seo, H. H. Jo, K. Lee, B. Kim, S. J. Oh and J. T. Park, Angew. Chem.,Int. Ed.,2004, 43,1115–1117; [7] Y. F. Shen, R. P. Zerger, R. N. De Guzman, S. L. Suib, L. Mc Curdy, D. I. Potter and, C. L. O'Young, Science, 1993,260, 511–515 [8] Kang,M.;Park,E.D.;Kim,J.M.;Yie,J.E.Appl.Catal.aGen.2007,3 27,261 [9] I. Maurin, P. Barboux, Y. Lassailly, J. P. Boilot, Chem. Mater.10 (1998)1727. 4. Conclusion [13] T. Rohani Bastami, M. H. Entezari, Chemical Engineering Journal Volume 164, Issue1, 15 October 2010, Pages 261–266 Lanthanum hydroxide and manganese oxide nanocompoiste were successfully prepared through chemical route. .X-ray diffraction confirms the pure crystalline nature of the nanocomposte. `Bragg’s diffraction peaks corresponding to both Lanthanum and manganese. The intensity corresponding to lanthanum (101) was found to be higher than that of Manganese (201) diffraction at 2θ .Thermogravimetric analysis on the sample showed that the nanocomposite underwent complete decomposition at about 6000C.The concentration of samples were confirmed with Lanthanum/Manganese aspect ratio as 5:3 with the anlysis of FTIR. Further more it was confirmed that the LaOH deformation and Mn-O stretching mode at tetrahedral sites were present due to cationic surfactants with monohydrate hydrazine . Hence the findings of the present work gives an detail account of the growth pattern of Lanthanum and Manganese nano particles in the presence of cationic surfactant. These results suggest the potential application of Lanthanum and Manganese nanoparticles towards fabrication of temperature sensitive sensors. References [1] M. S. Yazdan, Parast, A. Morsali, J. Inorg. Organomet. Polym. 21, 365-368(2011) [2] X. Wang, X. Sun, D. Yu, B. Zou and Y. Li, Adv. Mater ;15 (2003), p.1442 [3] T. Yamashita and A Vannice, Appl. Catal., B,1997,13,141–155 [4] M. M. Thackeray, Prog. Solid State Chem.,1997,25, 1–71; [10] C. Azquez-Vazquez, M. C. Blanco, M. A. Lopez-Quintela, R.D, Sanchez, J. Rivas, S. B. Oseroff, J.Mater.Chem.8 (1998) 991. [11] C. Ciaravino, R. Lyonnet, J. P. Scharff, B. Durand, J.P. Deloume, J. High Temp. Mater. Proc.3 (1999) 269. [12] P. Gibot, L. Laffont, Journal of Solid State Chemistry Volume 180, Issue 2, February 2007, Pages 695–701 [14] L. Laffont, P. Gibot, Materials Characterization, Volume 61, Issue 11, November 2010, Pages 1268–1273 [15] Junhao Zhang, Jin Du,Hongliang Wang, Jiao long Wang Zhaokun Qu, Liang Jia,Materials Letters Volume 65, Issues 17–18, September 2011, Pages 2565–2567. [16] X.Hao,J.Zhao,Y.LiYanZhaoDechong Ma,Linzhi Li,Colloids and Surfaces A Physicochemical and Engineering Aspects Volume, 20 January 2011, Pages 42–47 [17] Rui Songa, Shouhua Fengb, Hongjun Wangc, Changmin Houb, Chemistry Volume, June 2013, Pages 57–60 [18] Subhash Thota, Bhagwati Prasad, Jitendra Kumar, Materials Science and Engineering: B Volume 167, Issue 3, 25 March 2010, Pages 153–160 . [19] X.Z hang, Peng Yu, D. Zhang, H. Zhang, X. Sun, Y. Ma, Materials Letters 92 (2013) 401–404. [20] S. Daniele, L. G. Hubert –Pfalzgraf, J. Sol. Sci. Technol.35 (2005) P.57. [21] T. L. Van, M. Che, J. M. Tatibouent and M. Kermarec, J. Catal 142(1993), p.18. [22] S. Bernal, F. J. Botana, R. Garcia and J. m. Rodriguez Izaquirerdo, React. Solids 4(1987), p.23 [23] Maryam Ranjbar, Mostafa Yousefi, J. Inorg. Organomet. Polym. (2014) 24-652-655. [24] P. S. Kohli, Manish Kumar, K. K. Raina, J. Mater Sci: Mater Electron (2012)23:2257-2263. [25] A. Negi, V. Mahanjan, K. C. Singh, D. V. S. Jain, Appl. Catal. A-Gen.323, 51(2007)
© Copyright 2026