Nitric oxide synthase in Entamoeba histolytica: its
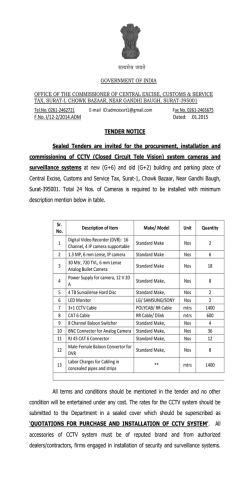
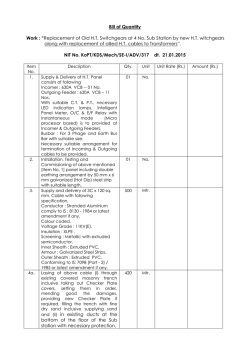
Experimental Parasitology 104 (2003) 87–95 www.elsevier.com/locate/yexpr Nitric oxide synthase in Entamoeba histolytica: its effect on rat aortic rings Marıa Elena Hern andez-Campos,a Rafael Campos-Rodrıguez,a Victor Tsutsumi,b Mineko Shibayama,b Ethel Garcıa-Latorre,c Carlos Castillo-Henkel,a and Ignacio Valencia-Hern andeza,* a b Secci on de Estudios de Posgrado e Investigaci on de la Escuela Superior de Medicina, I. P. N. Plan de San Luis y Dıaz Mir on, Col. Casco de Sto. Tom as, MEX-11340 Mexico, D.F., Mexico Centro de Investigaci on y Estudios Avanzados del I. P. N. Ticom an e Instituto Polit ecnico Nacional s/n, Col. Zacatenco, Mexico, D.F., Mexico c Departamento de Inmunologıa de la Escuela Nacional de Ciencias Biol ogicas, I. P. N. Prol. de Carpio y Plan de Ayala, Col. Casco de Sto. Tom as, MEX-11340 Mexico, D.F., Mexico Received 3 January 2002; received in revised form 20 June 2003; accepted 1 July 2003 Abstract NADPH-diaphorase activity has been considered as a nitric oxide synthase (NOS) marker. Therefore, the presence of NADPH-d activity in Entamoeba histolytica suggests that they have NOS activity. The aim of this work was to provide support for this contention. The amebic culture medium or amebic purified proteins induced relaxation of endothelium-denuded rat aortic rings precontracted with phenylephrine (10À6 M), which was inhibited when the amebas were incubated with N G -monomethyl-L -arginine or aminoguanidine (NOS inhibitors), or by pretreatment of the aortic rings with methylene blue. L -Arginine reverted the L -NAME inhibitory effect. In addition, trophozoites produce NO in culture and they have proteins which were recognized by antibodies specific to NOS and show activity of NO synthase. In conclusion, our results provide evidence about the production of NO by trophozoites. This molecule may be responsible for the relaxation elicited by the amebic culture medium and may participate in the pathogenesis of the invasive amebiasis. Ó 2003 Elsevier Inc. All rights reserved. Index Descriptors and Abbreviations: Entamoeba histolytica; NO, nitric oxide; NOS, nitric oxide synthase; iNOS, inducible nitric oxide synthase; ecNOS, endothelial nitric oxide synthase; NADPH-d, NADPH-diaphorase enzyme; b-NADPH, b-nicotinamide-adenine dinucleotide; L -NAME, Nx-nitro-L -arginine methyl ester hydrochloride; NBT, nitobluetetrazolium; PBS, phosphate-buffered saline; EDTA, ethylenediaminetetraacetic acid; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis 1. Introduction Nitric oxide (NO) regulates the vascular tone (Palmer et al., 1987), inhibits the platelet aggregation (Radomski et al., 1990) and the leukocyte adherence (Kubes et al., 1991), is a neurotransmitter (Garthwaite et al., 1988; Snyder, 1991), has antitumoral and antimicrobial activities (Nathan and Hibbs, 1991) as well as an established role in the immune system (Ghigo et al., 1995; Hibbs et al., 1988; Stuehr and Marletta, 1985). NO is produced from L -arginine in physiological and pathophysiological * Corresponding author. Fax: +52-5577-90045. E-mail address: [email protected] (I. Valencia-Hernandez). 0014-4894/$ - see front matter Ó 2003 Elsevier Inc. All rights reserved. doi:10.1016/S0014-4894(03)00133-4 conditions by constitutive and inducible isoforms of NO synthase (NOS) (Grant et al., 1998; Fan et al., 1997). The constitutive form of NOS is responsible for the production of ‘‘physiological’’ amounts of NO (Gryglewski, 1995; Sessa et al., 1992). On the other hand, the inducible NOS is responsible for generation of toxic amounts of NO involved in the pathogenesis of different diseases (Moncada et al., 1991; Stoclet et al., 1993). The ability of vertebrate animals, especially mammals, to produce NO has been clearly established. Some invertebrates, such as certain insect species (Weiske and Wiesner, 1999), mollusks (Huang et al., 1997; Moroz et al., 1996; S anchezAlvarez et al., 1994), and nematodes as Ascaris suum, Dirofilaria immitis, and Brugia species (Bascal et al., 88 M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 1995, 1996; Kaiser et al., 1998; Mupanomunda et al., 1997; Pfarr and Fuhrman, 2000), are also able to produce NO, but as far as we know there is little about this ability in protozoa. Nevertheless, it has been demonstrated that the erythrocyte stage of Plasmodium falciparum exhibits a high NOS activity (Ghigo et al., 1995). Experimental studies using several tissues (Bouwens and Kloppel, 1994; Gabbott and Bacon, 1993; Kugler et al., 1994; Liu et al., 1996; OÕBrien et al., 1995; Worl et al., 1994; Young et al., 1992) have shown that the activity of the NADPH-diaphorase (NADPH-d) is present in the same places where NOS activity is located. These observations suggest that both activities correspond to the same enzyme. Additionally, fixation with paraformaldehyde produces the loss of NADPH-d activity while maintaining the NOS activity (Grozdanovic and Gossrau, 1995; Nakos and Gossrau, 1994). During the last few years the NADPH-d activity resistant to fixers has been considered as a NOS marker. In this sense, previous studies have shown that Entamoeba histolytica extracts have NADPH-d activity which is related to the transport of electrons and this enzymeÕs location seems to be cytosolic (Bruchhaus et al., 1998; Lo and Reeves, 1980; Winbach et al., 1978). Hence, it seems reasonable to suppose that E. histolytica could produce NO. In accordance with this conclusion, it has been shown recently that E. histolytica trophozoitesinfected hamsters have serum nitrites (an indicator of NO production) and their production increases proportionally to tissue lesion (Pacheco-Yepez et al., 1997, 2001). However, nitrites might come from cells different than amebas, like macrophages (Marletta et al., 1988), neutrophiles, vascular endothelial cells (Palmer et al., 1987) or hepatocytes (Muriel, 2000). Therefore, the question about the ability of E. histolytica to produce NO remains to be answered. Hence, the purpose of this work was to establish both if the NOS is present in the ameba and if this protozoa is able to produce NO that can be detected by means of a bioassay using rat aortic rings without endothelium. 2. Materials and methods concentrations of 10À2 M. The required working solutions were made by dilution in de-ionized water immediately before use. All concentrations referred to are from the final bath. 2.2. Cultivation and harvesting of Entamoeba histolytica Trophozoites of E. histolytica strain HM1:IMSS were grown axenically in TYI-S-33 medium (Diamond et al., 1978), at 37 °C without CO2 and harvested at 72 h. 2.3. NADPH-diaphorase staining E. histolytica trophozoites fixed with 4% paraformaldehyde were used and the NADPH-d determination was done according to previous studies (Grozdanovic and Gossrau, 1995; Nakos and Gossrau, 1994). Briefly, the culture was incubated for 30 min in 0.1 M of a phosphate buffer solution (37 °C, pH 7.4) containing 1 mg/mL of b-NADPH and 0.1 mg/mL NBT. A 100 lL aliquot of a fixed-ameba solution was put onto slides and was observed using an optical microscope. A violet color indicates a positive histochemical reaction (Dawson et al., 1991; Lin et al., 1996; Scherrer-Singler et al., 1983). 2.4. Obtention of membrane and cytosolic fractions of amebas The fractions were obtained according to SerranoLuna et al. (1998). Briefly, amebas were suspended in 5 volumes of Tris–HCl buffer (50 mM, pH 7.4, containing 0.1 mM EDTA, 12 mM 2-mercaptoethanol, 1 mM leupeptin, 1 mM pepstatin A, and 1 mM phenylmethyl sulfonyl fluoride). After centrifugation at 15,000g for 30 min at 4 °C, the cytosolic and membrane fractions obtained were collected. The membrane fraction was washed with 1 M KCl to eliminate adsorbed cytosolic proteins and resuspended in a 0.01 M, pH 7.0, phosphate buffer. Finally, proteins were quantified by the method of Bradford (1976) in both fractions. 2.5. Photometric determination of NADPH-diaphorase activity 2.1. Reagents L -Phenylephrine hydrochloride, acethylcholine chloride, aminoguanidine, methylene blue, L -arginine, N -(1 Naphthyl)ethyl-enediamide dihydrochloride, sulfanilamide, sodium nitrate, sodium nitrite, hydrochloric acid and phosphoric acid, D -Lactic dehydrogenase Lactobacillus leichmanii, and pyruvic acid (each from Sigma Chemical, St. Louis, MO, USA), N-x-nitro-L arginine methyl ester hydrochloride (L -NAME, from ICN Pharmaceuticals, Costa Mesa, CA, USA). All drugs were freshly diluted in de-ionized water at initial It was performed by NBT-dependent b-NADPH reduction. Both membrane and cytosolic fractions were incubated during 10 min at 37 °C with a 0.5 mM NBT, 1 mM b-NADPH, and 0.1 M, pH 7.2, Tris–HCl buffer solution in a final volume of 100 lL. Addition of 50 lL of 0.5 M H2 SO4 and 100 lL of dimethylsulfoxide, followed by a vigorous shake stopped the reaction. Then, 200 lL was taken from each sample and transferred to wells in appropriate plates in order to measure absorbance at 595 nm. The NADPH-d activity was obtained by subtracting the absorbance found in the absence of M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 89 b-NADPH from that obtained in the presence of b-NADPH (Tracey et al., 1993). substrate kit for peroxidase (Vector Laboratories, Burlingame, CA, USA). 2.6. Enzyme purification 2.9. Assay of NO synthase NOS was purified from trophozoites extracts by using 20 ,50 -ADP affinity chromatography (F€ orstermann et al., 1991). In brief, whole extracts were obtained from (4 Â 106 ) trophozoites harvested after 72 h of culture, chilled on ice for 10 min, and collected by centrifugation at 500g for 5 min at 4 °C. Pellets containing amebic trophozoites were washed two times with PBS buffer, pH 7.4. Extracts were prepared from trophozoites disrupted by four cycles of freeze–thawing, maintained at 4 °C, and then collected by centrifugation at 500g for 5 min at 4 °C. Pellets and supernatant were separately collected. The supernatant was applied to 15 mL 20 ,50 -ADP–agarose column equilibrated with 50 mM Tris–HCl buffer, pH 7.4. The column was subsequently washed with 100 mL of the same buffer. The enzyme active fractions were then eluted with 15 mL of 10 mM Tris–HCl buffer, pH 7.4 (containing 10 mM b-NADPH, 1 mM EDTA, and 5 mM 2-mercaptoethanol) and 20 mL of 10 mM Tris–HCl buffer. Thirty fractions of 1 mL were collected and read at 280 nm. Two major protein peaks were obtained in the elution profile. The first peak has NOS and NADPH-d activities (NADPH-d/NOS fraction) and the second has only NADPH-d activity (data not shown). NO synthesis by proteins of E. histolytica was measured by the microplate assay for nitrite based on the Griess reaction (Stuehr, 1996). Aliquots (200 lL) from column fractions were transferred to wells in a 96-well plate. Each well contained 40 mM Tris–HCl buffer, pH 8, supplemented with 1 mM L -arginine, 1 mM b-NADPH, the final volume being 300 lL. The reaction was initiated by adding b-NADPH and was run at 37 °C for 180 min. Residual NADPH, which interfered with the calorimetric assay, was removed at the end of the incubation by adding pyruvic acid to a final concentration of 5 mM and 40 U/mL of lactate dehydrogenase and then incubated for 60 min at 37 °C. The nitrite which has accumulated in the wells during NO synthesis is then quantified by colorimetric method based on the Griess reaction. 2.7. Rabbit antiserum Antiserum to the first purified fraction, which containing NADPH-d and NOS activities, was obtained by intradermic immunization of a rabbit with 100 lg of the fraction emulsified in complete FreundÕs adjuvant, followed by two booster immunizations at intervals of 14 days with the same amount of protein emulsified in incomplete FreundÕs adjuvant. 2.8. Immunoblotting After electrophoresis on 10% SDS–PAGE gel with the discontinuous buffered system of Laemmli (1970), the NADPH-d/NOS fraction was transferred to nitrocellulose membrane (from Bio-Rad, Hercules, CA, USA.) for 1 h at 350 mA. The membranes were blocked by incubation with PBS buffer, pH 7.4, containing 10% nonfat dry milk for 1 h at 37 °C, followed by incubation for 16 h at 4 °C with rabbit anti-NADPH-d/NOS at 1:100 dilution, anti-mouse iNOS 2 at 1:200 dilution, anti-human ecNOS 3 at 1:200 dilution (last two from Santa Cruz Biotechnology, Santa Cruz, CA, USA) in PBS–Tween buffer, pH 7.4, containing 0.05% Tween 20. The second antibody was a peroxidase-conjugate goat anti-rabbit IgG (1:500) in PBS–Tween buffer, pH 7.4, for 2 h at 37 °C. Detection was done by using a DAB À 2.10. Measurement NOÀ 2 and NO3 production Nitrate was reduced to nitrite with cadmium granules (Hevel and Marletta, 1994) and then, nitrite concentration was measured with the Griess reagent (Green et al., 1982). Briefly, 150 lL of Griess reagent (1% sulfanilamide and 0.1% N-1 naphthylethylenediamine dihydrochoride in 5% H3 PO4 ) was added to 150 lL of sample, previously deproteinized using a solution of zinc sulfate (ZnSO4 ) at 30%. The absorbance was read at 570 nm after incubation at room temperature for 15 min. NOÀ 2 concentration was determined with reference to a standard curve by using concentrations from 0.5 to 100 lM of sodium nitrite in PBS. 2.11. Treatment of the amebic culture Two million amebas were inoculated in 120 mL of culture media (30 g peptone biosate, 10 g dextrose, 2 g sodium chloride, 0.06 g monobasic potassium phosphate, 1 g dibasic potassium phosphate, 1 g L -cisteine, 0.2 g ascorbic acid, and 0.0236 g ammonium ferric citrate). Some groups of amebas were incubated with L NAME (10À3 M) or aminoguanidine (10À3 M), which are NOS inhibitors (Fan et al., 1997; Grant et al., 1998). Later, the supernatant of the cultures was obtained at 72 h after the inoculation by centrifugation at 1000g for 5 min. Supernatant was filtered and their activity was tested on the aortic rings. 2.12. Vascular preparation Male Wistar rats (225–275 g) were anesthetized with diethyl ether and subsequently sacrificed by cervical 90 M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 dislocation. The thoracic aorta was quickly excised and placed in Krebs–Henseleit solution containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2 PO4 , 1.2 mM MgSO4 Á 7H2 O, 2.5 mM CaCl2 Á 2H2 O, 25 mM NaHCO3 , 11.7 mM dextrose, and 0.026 mM calcium disodium EDTA. This solution was aerated with 95% O2 /5% CO2 . Aorta were cleaned of surrounding tissue and cut into rings 4–5-mm long. The endothelium was removed mechanically by inserting the tip of a forceps into the lumen of the aorta and rolling the rings back and forth gently several times on a paper towel wet with Krebs solution. The aortic rings were mounted in a 10 mL tissue chamber baths filled with Krebs solution at 37 °C, pH 7.4, and continuously aerated with 95% O2 /5% CO2 . To record the semi-isometric force development, rings were suspended on two wire hooks (NUBRYTE wire) as well as being fixed to the bottom of the chamber and to a force transducer (BIOPAC TSD105) connected to a BIOPAC MP100WSW system (from BIOPAC systems, Santa Barbara, CA, USA). Under a basal tension of 4 g, responses to 10À6 M phenylephrine were obtained every 20 min until maximal reactivity was observed (usually 2 h). The absence of the endothelium was confirmed by no response after administration of 10À6 M acethylcholine on aortic rings pre-contracted with 10À6 M phenylephrine. The p values less than 0.05 were considered to be statistically significant. 2.13. Vascular studies 3.3. Immunoblotting of the fraction containing nitric oxide synthase and NADPH-diaphorase activities In a first series of experiments, the vessels were precontracted by using 10À6 M phenylephrine. In the plateau, an aliquot of 1 mL of the supernatant of amebic culture medium or the purified proteins (NADPH-d/ NOS) was added. Supernatant was obtained at 72 h in the log-phase growth. Only one aliquot was added for every test of phenylephrine. To establish the mechanism of effect, some vessels were treated with the guanylate cyclase inhibitor, methylene blue (3.1 Â 10À5 M) or the NOS inhibitor, L -NAME (10À3 M). In the second series of experiments, phenylephrine pre-contracted aortic rings were used and an aliquot of 1 mL of the amebic culture medium obtained from amebas treated with 10À3 M L -NAME or 10À3 M aminoguanidine was added. Finally, 0.1 mL of the NADPH-d/NOS fraction was used in the absence or in the presence of L -NAME or L -NAME and L -arginine on the phenylephrine precontracted aortic rings. Relaxant results were expressed as percent of the pre-contraction elicited by 10À6 M phenylephrine. 2.14. Statistics All data are expressed as the means Æ SEM from eight experiments. Significant differences were established by applying analysis of variance (ANOVA) and the differences between means by the Dunnet t test. 3. Results 3.1. NADPH-diaphorase/nitric oxide synthase activity Fig. 1 shows the photography of one of the preparations of amebas fixed with 4% paraformaldehyde in which a histochemical reaction was made to detect NADPH-d. In the majority of cases the amebas were intensely stained. Only a small number of them were weakly stained. 3.2. Photometric quantification of the NADPH-diaphorase activity in the cytosolic and membrane fractions Photometric determination of NADPH-d activity in cytosolic and membrane fractions was performed by NBT-dependent b-NADPH reduction. The reduction of b-NADPH was significantly greater (p < 0:05) in the cytosolic portion. The maxima absorbances observed were 0.8 Æ 0.1 and 1.36 Æ 0.08 for membrane and cytosolic fractions, respectively (250 lg/lL of amebic protein). To support the argument that NOS is present in E. histolytica, antibodies to NADPH-d/NOS of E. histolytica, antibodies to mouse iNOS 2, and human ecNOS 3 were used for Western blotting. Fig. 2 shows the Western blot analysis of anti-NADPH-d/NOS, anti-iNOS, and anti-ecNOS immunoreactivities against a purified amebic cytosolic fraction. Immunoblotting with the anti-NADPH-d/NOS antibody revealed several bands including a slowly migrating protein with a molecular weight of 224 kDa, and three more rapidly migrating proteins of molecular weights of 42, 36, and 26 kDa. Anti-iNOS 2 antibody revealed two bands, one of them a slowly migrating protein with a molecular weight of 195 kDa, and the other more rapidly migrating protein with a molecular weight of 42 kDa. Antibody to ecNOS 3 revealed the same proteins as anti-iNOS 2. À 3.4. In vitro NOÀ 2 /NO3 production by trophozoites and proteins of Entamoeba histolytica In order to confirm the presence of NOS activity in E. histolytica, we analyzed the in vitro production of NO by trophozoites as well as the NOS activity in the preparation of NADPH-d/NOS obtained by affinity chromatography. In the supernatant of the culture medium obtained at 72 h after the addition of E. histolytica, M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 91 Fig. 1. Histochemical detection of NADPH-diaphorase in amebas fixed with 4% paraformaldehyde (A). In (B), amebas in which the histochemical reaction was not carry out (control). Table 1 NO production by trophozoites of Entamoeba histolytica incubated in NaCl 0.9% À NOÀ 2 /NO3 (lM) # Control L -Arginine L -NAME 14.0 Æ 1.6 20.3 Æ 2.9 9.1 Æ 1.0Ã À3 L -arginine and L -NAME were used at 10 M. Results are expressed as means Æ SEM. # 4 Â 105 trophozoites/mL. * Significantly different from L -arginine (p < 0:05). Table 2 NO synthase activity in the purified protein of Entamoeba histolytica NO synthase activity (nM nitrite lgÀ1 minÀ1 ) Fig. 2. Western blot analysis of amebic purification cytosolic fraction. Experiments were performed with polyclonal antibodies anti-NAPDPH-d (lane 1), anti-iNOS 2 (lane 2), and anti-ecNOS 3 (lane 3). À the concentration of NOÀ 2 /NO3 was higher than in the supernatant of the control medium incubated 72 h without amebas (65 Æ 0.5 vs 13.4 Æ 1.5 lM; p < 0:05) This means that E. histolytica produced NO in small quantities in spite of the reduced concentrations of O2 in the culture medium. When the trophozoites were cultivated in aerobic conditions for 3 h, they produced NOÀ 2/ NOÀ even in the absence of L -arginine (Table 1). The 3 NO production was inhibited by L -NAME and did not occur in the absence of NADPH. These results suggest that E. histolytica constitutively produces NO without exogenously adding L -arginine. Control L -Arginine L -Arginine + L -NAME 0.5 Æ 0.05 5.26 Æ 0.37Ã 2.0 Æ 1.0ÃÃ The date are means Æ SEM of NO synthase activity assayed by the microplate assay for nitrite based on the Griess reaction. * Significantly different from control (p < 0:05). ** Significantly different from incubation with L -arginine. NOS activity in proteins (NADPH-d/NOS fraction) of E. histolytica was estimated by the microtiter plate assay for nitrate. The activity of the NO synthase was approximately 5 nmol/min per lg of protein (Table 2). The NO production was inhibited by L -NAME and did not occurred in the absence of NADPH. However, the exogenous addition of Ca2þ or cofactors (tetrahydrobiopterin, FAD, FMN) was not required for NO formation. 92 M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 3.5. Effect of aminoguanidine or L -NAME treatment of amebas on the relaxant effect induced by the supernatant the figure we only shown the relaxing effect induced by the supernatant obtained from 72 h culture. As can be observed in Fig. 3, the administration of the supernatant from a 72 h culture induced a reduction in the contraction elicited by phenylephrine. This effect was partially inhibited if the supernatant came from amebas treated with aminoguanidine or L -NAME. 3.7. Relaxant effect induced by NADPH-d/NOS fraction with or without L -NAME or L -NAME and arginine 3.6. Effect of methylene blue or L -NAME treatment of aortic rings on relaxing effect induced by the supernatant of the amebic culture Fig. 3 shows the effect induced by the pretreatment of the aortic rings with either methylene blue or L -NAME on relaxing effect elicited by the supernatant of the amebic culture. Methylene blue but not L -NAME inhibited the relaxing effect induced by the supernatant. In Fig. 3. Inhibitory effect induced by L -NAME (N) or aminoguanidine (A) treatment of amebas (left panel) or methylene blue (MB) or L -NAME treatment of aortic rings (right panel) on the relaxant effect produced by the supernatant from the amebic culture obtained at 72 h. Bars represent the means Æ SEM of eight experiments. *p < 0:05 versus control (C). Fig. 4. Relaxant effect induced by NADPH-d/NOS fraction in the absence (C) or in the presence of L -NAME (N) or aminoguanidine (A) treatment of amebas (left panel) or methylene blue (MB) or L -NAME and L -arginine (N + R). Bars represent the means Æ SEM of eight experiments. *p < 0:05 versus control (C). As can be observed in Fig. 4, the administration of the fraction induced a reduction in the contraction elicited by phenylephrine. This effect was inhibited if the fraction containing L -NAME (10À3 M) and such inhibitory effect was reverted with L -arginine (10À3 M). 4. Discussion The most outstanding feature of the present report is that, for the first time, we demonstrated the production of NO by trophozoites of E. histolytica. The source of NO may be an isoform of NOS that does not require the exogenous addition of Ca2þ and cofactors. In accordance with other authors (Lo and Reeves, 1980; Winbach et al., 1978) we confirm the presence of NADPH-d activity in E. histolytica by using histochemical, photometric, and Western blot procedures. In E. histolytica trophozoites fixed with paraformaldehyde, and in membrane and cytosolic fractions of amebas, NADPH-d activity was found through the determination of NBT-dependent b-NADPH reduction. The NADPH-d activity was located mainly in the cytosolic fraction where the NBT-dependent b-NADPH reduction was higher. As has been accepted, NADPH-d activity may be useful as an indicator of NOS activity. In this sense, it has been demonstrated that both enzymes co-localize and NOS possesses activity of NADPH-d (Bouwens and Kloppel, 1994; Gabbott and Bacon, 1993; Kugler et al., 1994; Liu et al., 1996; OÕBrien et al., 1995; Worl et al., 1994; Young et al., 1992). Indeed, many researchers have concluded that NOS is present in several tissues by using NADPH-d activity as an indicator (Bouwens and Kloppel, 1994; Gabbott and Bacon, 1993; Kugler et al., 1994; Liu et al., 1996; OÕBrien et al., 1995; Talavera et al., 1997; Weiske and Wiesner, 1999). However, there are biochemical and morphological evidences indicating that a NOS-independent NADPH-d activity could be present in some cells (Grozdanovic and Gossrau, 1995; Tracey et al., 1993; Worl et al., 1994). For instance, when tissues are fixed with paraformaldehyde or oxidative agents like cytochrome c, NADP, H2 O2 or permanganate a great part of the diaphorase activity is lost (Grozdanovic and Gossrau, 1995; Nakos and Gossrau, 1994). Nevertheless, in these circumstances the NADPH-d activity related with the NOS is maintained. Therefore, the NADPH-d activity resistant to fixers identified in the present work (Fig. 1) seems to correspond to NOS activity. However, considering that the diaphorase activity alone is not definitive evidence of M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 the presence of NOS, we decided to demonstrate its presence by biochemical analyses, Western blotting and provide functional evidence of the NOS–NO system in the amebas by performing a bioassay in which the effect of the amebic culture supernatant or NADPH-d/NOS fraction on rat aortic rings was assessed. Immunoblotting provided evidence for the presence of NOS and NADPH-d in trophozoites of E. histolytica. Indeed, the NADPH-d/NOS antibody detected four proteins (Fig. 2), one of them with an apparent molecular weight of 224 kDa, which until now has not been identified in trophozoites of E. histolytica. This protein has a molecular weight close to the NADPH-d (molecular weight of 170–180 kDa) from brain of rats (Kuonen et al., 1988), but was not recognized by both NOS antibodies (Fig. 2). The molecular weights of the second and third proteins (42 and 36 kDa, respectively) are very close to that of NADPH-d (40–35 kDa) of E. histolytica described by Lo and Reeves (1980) and Bruchhaus et al. (1998). The last protein had a molecular weight of 26 kDa. Up to now this protein has not been identified in E. histolytica and is not related to other NADPH-d from other species. NOS was detected in the cytosolic purified fraction of E. histolytica by using two polyclonal antibodies against iNOS2 and ecNOS3. Two proteins were recognized by both antibodies (Fig. 2). One of them corresponds to a slowly migrating protein with a molecular weight of 195 kDa. This protein has a molecular weight close to the NOS reported in mammalian and in Drosophila that have 130–160 kDa (Klatt et al., 1996; M€ uller, 1997; Nathan, 1992). The fact that NOS antibodies used do not show cross-reactivity between iNOS 2 and ecNOS 3 in mammalians suggests that this isoform is different. The last band corresponded to a rapidly migrating protein with a molecular weight of 42 kDa. NOS has also been purified from P. falciparum (Ghigo et al., 1995), Nocardia species (Chen and Rosazza, 1995), and Helix pomatia (Huang et al., 1997) with molecular weights of 97, 52, and 6 kDa, respectively. These results suggest that trophozoites of E. histolytica express a protein with epitopes recognized by antibodies anti-NOS which probably is different from NOS isoforms found in mammalian cells, bacteria, and invertebrates as well as these found in protozoa. Additionally, the 42 kDa protein was recognized by the three different antibodies (Fig. 2). These data suggest that the protein shares homology with NADPH-d and NOS sequences. Possibly, this isoform has NOS and NADPH-d activities. Entamoeba histolytica trophozoites produced NOÀ 2/ NOÀ in long-term or short-term culture (Table 1). In the 3 À short-culture, the NOÀ production required 2 /NO3 NADPH and was inhibited by L -NAME. The proteins of E. histolytica purified by affinity chromatography (NADPH-d/NOS fraction) contained activity of NOS that required L -arginine and NADPH and its activity 93 was also inhibited by L -NAME (Table 2). In virtue of that the reduced production of NO in the absence of NADPH or L -arginine and the stereo-specific inhibition by L -NAME is considered as a valid method for measuring NO synthase activity (Knowles et al., 1990), our results suggest that E. histolytica has a NOS. The activity of the ameba NOS was constitutive and did not require the addition of Ca2þ or cofactors (tetrahydrobiopterin, FAD, FMN) for NO formation. On the other hand, the supernatant of the amebic culture and the NADPH-d/NOS proteins (Figs. 3 and 4) produced a relaxant effect on endothelium-denuded rat aortic rings pre-contracted with phenylephrine. The relaxant effect was inhibited when L -NAME or aminoguanidine was added to the incubation medium of the amebic culture. These results suggest that the relaxation is mediated by the NO released from the amebas. Further support to this contention is provided by the results obtained with methylene blue, which inhibits the enzyme guanylate cyclase, the normal target of NO in smooth muscle (Fig. 3). The vasodilatation elicited by NO depends on the production of cGMP by guanylate cyclase (Arnold et al., 1977; Gruetter et al., 1981) and then it is susceptible to blocking by methylene blue. Pretreatment of the aortic rings with this dye inhibited the relaxation induced by the supernatant of the amebic culture (Fig. 3), as should be expected if NO were responsible for this effect. On the other hand, the probability that NO originates in the vascular smooth muscle cells instead of in the amebas is discarded since pretreatment of the aortic rings with L -NAME did not affect the relaxant effect of the supernatant (Fig. 3). Hence, NO released from the amebas or produced by soluble NOS in the supernatant seems to be responsible for the relaxant effect provoked by the supernatant. An additional evidence about presence of NOS activity in trophozoites of E. histolytica was the relaxant effect induced by NADPH-d/NOS fraction in aortic rings pre-contracted with phenylephrine (10À6 M), such effect was inhibited when L -NAME (10À3 M) was added to the fraction and the inhibition was reverted with the natural substrate of NOS, L -arginine. Many questions may emerge as a consequence of the finding that amebas appear to be able to produce NO. For instance, it is important to find out the kind of stimulus which is responsible for NO production by amebas, the calcium and cofactors requirements of NOS, whether or not the amebas have the capacity to produce NO under both basal and stimulated conditions. In addition, it should be elucidated whether or not NO has a role in the tissue damage associated with the amebic disease, since the production of NO by amebas might be an additional mechanism by which hepatocellular damage could be induced. In this sense, Ma et al. (1995) mention that NO is toxic for hepatic cells. 94 M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 In conclusion, our results suggest that E. histolytica produce NO through the activity of an isoform of NOS locate mainly in the cytosol. The NO is responsible for the relaxation elicited by the supernatant of the amebic culture medium and may participate in the pathogenesis of the amebiasis through direct toxic effects on cells or through changes in the vascular tone that modify the parenchymal irrigation. Acknowledgments This work was partially supported by grants from CONACYT and Coordinaci on General de Posgrado e Investigaci on del I.P.N. (Mexico). Hern andez-Campos is a recipient of scholarship from CONACYT. CamposRodrıguez, Garcıa-Latorre, and Valencia-Hernandez are fellow of COFAA-IPN and EDI. We are indebted to Mr. Alan Larsen and Dr. Rosa Adriana Jarillo-Luna for providing helpful comments and Hermilo GarcıaFarf an for technical photographical assistance. References Arnold, W.P., Mittal, C., Katsuki, S., Murad, G., 1977. Nitric oxide activates guanylate cyclase and increase guanosine 30 ,50 -monophosphate levels in various tissue preparations. Proceedings of the National Academy of Sciences USA 74, 3203–3207. Bascal, Z.A., Montgomery, A., Holden-Dye, L., Williams, R.G., Walker, R.J., 1995. Histochemical mapping of NADPH diaphorase in the nervous system of the parasitic nematode Ascaris suum. Parasitology 110, 625–637. Bascal, Z.A., Montgomery, A., Holden-Dye, L., Williams, R.G., Thorndyke, M.C., Walker, R.J., 1996. NADPH diaphorase activity in peptidergic neurones of the parasitic nematode Ascaris suum. Parasitology 112, 125–134. Bouwens, L., Kloppel, G., 1994. Cytochemical localization of NADPH-diaphorase in the four types of pancreatic islet cell. Histochemistry 101, 209–214. Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254. Bruchhaus, I., Richter, S., Tannich, E., 1998. Recombinant expression and biochemical characterization of a NADPH: flavin oxidoreductase from Entamoeba histolytica. Biochemical Journal 330, 1217–1221. Chen, Y., Rosazza, J.P.N., 1995. Purification and characterization of nitric oxide synthase (NOSNOC ) from a Nocardia species. Journal of Bacteriology 177, 5122–5128. Dawson, T.M., Bredt, D.S., Fotuhi, M., Hwang, P.M., Snyder, S.H., 1991. Nitric oxide synthase and neural NADPH-diaphorase are identical in brain peripheral tissues. Proceedings of the National Academy of Sciences USA 88, 7797–7801. Diamond, L.S., Harlow, D.R., Cunnick, C.C., 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Transactions of the Royal Society of Tropical Medicine and Hygiene 72, 431–432. Fan, B., Wang, J., Stuehr, D.J., Rousseau, D.L., 1997. NO synthase isozimes have distinct substrate binding sites. Biochemistry 36, 12660–12665. F€ orstermann, U., Schmidt, H.H.W., Pollock, J.S., Sheng, H., Mitchell, J.A., Warner, T.D., Nakane, M., Murad, F., 1991. Isoform nitric oxide synthase: characterization and purification from different cell types. Biochemical Pharmacology 42, 1849–1857. Gabbott, P.L., Bacon, S.J., 1993. Histochemical localization of NADPH-dependent diaphorase (nitric oxide synthase) activity in vascular endothelial cells in the rat brain. Neuroscience 57, 79–95. Garthwaite, J., Charles, S.L., Chess-Williams, R., 1988. Endotheliumderived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336, 385–388. Ghigo, D., Todde, R., Ginsburg, H., Costamagna, C., Gautret, P., Bussolino, F., Ulliers, D., Giribaldi, G., Deharo, E., Gabrielli, G., Pescarmona, G., Bosia, A., 1995. Erythrocyte stages of Plasmodium falciparum exhibit a high nitric oxide sinthase (NOS) activity and release an NOS-inducing soluble factor. Journal of Experimental Medicine 182, 677–688. Grant, S.K., Green, B.G., Stiffey Wilusz, J., Durette, P.L., Shah, S.K., Kozaich, J.W., 1998. Structural requirements for human inducible nitric oxide synthase substrates and substrate analogue inhibitors. Biochemistry 37, 4174–4180. Green, L.C., Wagner, D.A., Glogowski, Skipper, J.S., Wishnok, P.L., Tannenbaum, R.S., 1982. Analysis of nitrate, nitrite, and [15 N]nitrate in biological fluids. Analytical Biochemistry 126, 131–138. Grozdanovic, Z., Gossrau, R., 1995. Alfa-NADPH appears to be primarily oxidized by the NADPH-diaphorase activity of nitric oxide synthase (NOS). Acta Histochemica 97, 313–320. Gruetter, C.A., Gruetter, D.Y., Lyon, J.E., Kadowitz, P.J., Ignarro, L.J., 1981. Relationship between cyclic guanosine 30 ,50 -monophosphate formation and relaxation of coronary arterial smooth muscle by gliceryl trinitrate, nitroprusside, nitrite, and nitric oxide: effects of methylene blue. Journal of Pharmacology and Experimental Therapeutics 219, 181–186. Gryglewski, R.J., 1995. Interactions between endothelial mediators. Pharmacology and Toxicology 77, 1–9. Hevel, J.M., Marletta, M.A., 1994. Nitric-oxide synthase assays. Methods in Enzymology 233, 250–258. Hibbs Jr., J.B., Taintor, R.R., Vaurin, Z., Rachlin, E.M., 1988. A citotoxic activated macrophage effector molecule. Biochemical and Biophysical Research Communications 157, 87–94. Huang, S., Kerschbaum, H.H., Engel, E., Hermann, A., 1997. Biochemical characterization and histochemical localization of nitric oxide synthase in the nervous system of the snail, Helix pomatia. Journal of Neurochemistry 69, 2516–2528. Kaiser, L., Geary, T.G., Williams, J.F., 1998. Dirofilaria immitis and Brugia pahangi: filarial parasites make nitric oxide. Experimental Parasitology 90, 131–134. Klatt, P., Schmidt, K., Werner, E.R., Mayer, B., 1996. Determination of nitric oxide synthase cofactors: Heme, FAD, FMN, and tetrahydrobiopterin. Methods in Enzymology 268, 358–375. Knowles, R.G., Merrett, M., Salter, M., Moncada, S., 1990. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochemical Journal 270, 833–836. Kubes, P., Suzuki, M., Gronger, D.N., 1991. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proceedings of the National Academy of Sciences USA 88, 4651–4655. Kugler, P., Hofer, D., Mayer, B., Drenckhahn, D., 1994. Nitric oxide synthase and NADP-linked glucose-6-phosphate dehydrogenase are co-localized in brush cells of rat stomach and pancreas. Journal of Histochemistry and Cytochemistry 42, 1317–1321. Kuonen, D.R., Kemp, M.C., Roberts, P.J., 1988. Demonstration and biochemical characterization of rat brain NADPH-dependent diaphorase. Journal of Neurochemistry 50, 1017–1025. Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. Lin, A.Y.J., Chodobska, J.S., Rahman, M.P., Mayer, B., Monfils, P.R., Johanson, C.E., 1996. Immunohistochemical localization of M. Elena Hernandez-Campos et al. / Experimental Parasitology 104 (2003) 87–95 nitric oxide synthase in rat anterior choroidal artery, stromal blood microvessels, and choroids plexus epithelial cells. Cell and Tissue Research 285, 411–418. Liu, H.P., Tay, S.S., Leong, S.K., 1996. Nitrergic neurons in the pancreas of newborn guinea pig: their distribution and colocalization with various neuropeptides and dopamine-beta-hydroxylase. Journal of Autonomic Nervous System 61, 248–256. Lo, H.S., Reeves, R.D., 1980. Purification and properties of NADPH: flavin oxidoreductase from Entamoeba histolytica. Molecular and Biochemical Parasitology 2, 23–30. Ma, T.T., Ischiropoulos, H., Brass, C.A., 1995. Endotoxin-stimulated nitric oxide production increases injury and reduces rat liver chemiluminescence during reperfusion. Gastroenterology 108, 463– 469. Marletta, M.A., Yoon, P.S., Iyenger, R., Leaf, C.D., Wishnok, J.S., 1988. Macrophage oxidation of L -arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 27, 8706–8711. Moncada, S., Palmer, R.M., Higgs, E.A., 1991. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacology Review 43, 109–142. Moroz, L.L., Chen, D., Gillette, M.U., Gillette, R., 1996. Nitric oxide synthase activity in the molluscan CNS. Journal of Neurochemistry 66, 873–876. M€ uller, U., 1997. The nitric oxide system in insects. Progress in Neurobiology 51, 363–381. Mupanomunda, M., Williams, J.F., MacKenzie, C.D., Kaiser, L., 1997. Dirofilaria immitis: hearthworm infection alters pulmonary artery endothelial cell behavior. Journal of Applied Physiology 82, 389–398. Muriel, P., 2000. Regulation of nitric oxide synthesis in the liver. Journal of Applied Toxicology 20, 189–195. Nakos, G., Gossrau, R., 1994. When NADPH diaphorase (NADPHd) works in the presence of formaldehyde, the enzyme appears to visualize selectively cells with constitutive nitric oxide synthase (NOS). Acta Histochemica 96, 335–343. Nathan, C., 1992. Nitric oxide as a secretory product of mammalian cells. FASEB Journal 6, 3051–3064. Nathan, C., Hibbs Jr., J.B., 1991. Role of nitric oxide synthesis in macrophage antimicrobial activity. Current Opinion in Immunology 3, 65–70. OÕBrien, A.J., Young, H.M., Povey, J.M., Furness, J.B., 1995. Nitric oxide synthase is localized predominantly in the Golgi apparatus and cytoplasmic vesicles of vascular endothelial cells. Histochemistry and Cell Biology 103, 221–225. Pacheco-Yepez, J., Campos-Rodrıguez, R., Ramırez-Rosales, A., Martınez-Palomo, A., Tsutsumi, V., 1997. Role of nitric oxide in the experimental hepatic amebiasis. Archives of Medical Research, vol. 28 (Special issue, Proceedings of the XIII Seminar on Amebiasis), pp. 214–216. Pacheco-Yepez, J., Campos-Rodriguez, R., Shibayama, M., VenturaJuarez, J., Serrano-Luna, J., Tsutsumi, V., 2001. Entamoeba histolytica: production of nitric oxide and in situ activity of NADPH diaphorase in amebic liver abscess of hamsters. Parasitology Research 87, 49–56. Palmer, R.M., Ferrige, A.G., Moncada, S., 1987. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526. 95 Pfarr, K.M., Fuhrman, J.A., 2000. Brugia malayi: localization of nitric oxide synthase in a lymphatic filariid. Experimental Parasitology 94, 92–98. Radomski, M.W., Palmer, R.M., Moncada, S., 1990. An L -arginine/ nitric oxide pathway present in human platelets regulates aggregation. Proceedings of the National Academy of Sciences USA 87, 5193–5197. Sanchez-Alvarez, M., Leon-Olea, M., Talavera, F., Pellicer, F., Sanchez-Islas, E., Martinez-Lorenzana, G., 1994. Distribution of NADPH diaphorase in the perioesophageal ganglia of the snail, Helix aspersa. Neuroscience Letters 169, 51–55. Scherrer-Singler, U., Vincent, S.R., Kimura, H., McGeer, R.C., 1983. Demonstration of a unique population of neurons with NADPHdiaphorase histochemestry. Journal of Neuroscience Methods 9, 229–234. Serrano-Luna, J.J., Negrete, E., Reyes, M., de la Garza, M., 1998. Entamoeba histolytica HM1:IMSS: hemoglobin-degrading neutral cysteine proteases. Experimental Parasitology 89, 71–77. Sessa, W.C., Harrison, J.K., Barber, C.M., Zeng, D., Dureux, M.E., Angelo, D.D., Lynch, K.R., Peach, M.J., 1992. Molecular cloning and expression of cDNA encoding endothelial cells nitric oxide synthase. Journal of Biological Chemistry 267, 15274–15276. Snyder, S.H., 1991. Nitric oxide as a neural messenger. Trends in Pharmacology Sciences 12, 125–127. Stoclet, J.C., Fleming, J., Gray, G., Julou-Schaeffer, G., Schneider, F., Schott, C.A., Schott, C., Parrat, J.R., 1993. Nitric oxide and endotoxaemia. Circulation 87 (Suppl. 5), 77–80. Stuehr, D.J., Marletta, M.A., 1985. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccaride. Proceedings of the National Academy of Sciences USA 82, 7738–7742. Stuehr, D.J., 1996. Purification and properties of nitric oxide synthases. Methods in Enzymology 268, 324–333. Talavera, E., Martinez-Lorenzana, G., Cordiki, G., Leon-Olea, M., Condes-Lara, M., 1997. NADPH-diaphorase-stained neurons after experimental epilepsy in rats. Nitric Oxide 1, 484–493. Tracey, W.R., Nakane, M., Pollock, J.S., F€ orstermann, U., 1993. Nitric oxide synthases in neuronal cells, macrophages and endothelium are NADPH diaphorases, but represents only a fraction of total cellular NADPH diaphorase activity. Biochemical and Biophysical Research Communications 195, 1035–1040. Weiske, J., Wiesner, A., 1999. Stimulation of NO synthase activity in the immune-competent lepidopteran Estigmene acraea hemocyte line. Nitric Oxide 3, 123–131. Winbach, E.C., Claggett, E., Takeuchi, T., Diamond, L.S., 1978. Biological oxidation and flavoprotein catalysis in Entamoeba histolytica. Archives of Medical Research (Mex) 9 (Suppl. 1), 89– 98. Worl, J., Wiesand, M., Mayer, B., Greskotter, K.R., Neuhuber, W.L., 1994. Neuronal and endothelial nitric oxide synthase immunoreactivity and NADPH-diaphorase staining in rat and human pancreas: influence of fixation. Histochemistry 102, 353–364. Young, H.M., Furness, J.B., Shuttleworth, C.W., Bred, D.S., Snyder, S.H., 1992. Co-localization of nitric oxide synthase inmunoreactivity and NADPH diaphorase staining in neurons of guinea-pig intestine. Histochemistry 97, 375–378.
© Copyright 2026