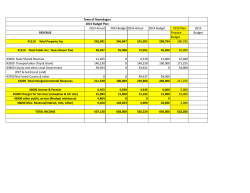
F SUNNYVALE, quarter of 20 of $113.3 mi quarter net lo Excluding
CONTACTS S: For Media Inquiiries: For Invesstor Inquiries: Jared Tipton Cepheid Corporatte Communications Tel: (408) 400 8377 communications@ @cepheid.com Jacquie Rooss, CFA Cepheid Innvestor Relations Tel: (408)) 400 8329 [email protected] m Cepheid 904 Caribbean Drivee Sunnyvale, CA 9408 89 Telephone: (408) 54 41 4191 Fax: (408) 541 4192 2 CEPH HEID REPORTSS FOURTH QU UARTER AND D FULL YEAR 22014 RESULTSS Fourth Quarte F er Commercia al Clinical Up 26% Driven bby 29% Grow wth in Xpert® Reagents SUNNYVALE, California, Jaanuary 29, 20 015 – Cepheid d (Nasdaq: CPPHD) today reeported reven nue for the fo ourth quarter of 20 014 of $131.5 5 million. Nett loss was $23 3.8 million, orr $(0.34) per sshare, which compares to revenue of $113.3 million and net loss of $10.3 million, or $((0.15) per shaare, in the fou urth quarter o of 2013. 2014 4 fourth quarter net lo oss included a legal contin ngency charge e of $20 millioon, or $(0.28)) per share. Excluding sto ock compensaation expense es, a legal con ntingency chaarge, amortizaation of debt discount and d transaction ccosts, and amortization of purchased intangible asseets, non‐GAAPP net income for the fourtth quarter of 20 014 was $7.8 million, or $0 0.11 per share e. This comp ares to non‐G GAAP net inco ome of $2.3 m million, or $0.03 per share, for the e fourth quarter of 2013, w which excludeed stock compensation exxpenses, a restructuringg charge (inclu uding impairm ment of certain assets), annd amortizatio on of purchassed intangiblee assets. Fiscal 2014 O Overview For the year ended Decem mber 31, 2014 4, Cepheid reported revennue of $470.1 million which compares tto revenue of $401.3 million in 2013. Nett loss for the year was $500.1 million, orr $(0.72) per sshare, which compares to net loss of $1 18.0 million, o or $(0.27) perr share, in 20013. 2014 fulll year net losss included a legal contingency charge of $20 0 million, or $ $(0.29) per sh hare. Excluding sto ock compensaation expense es, a legal con ntingency chaarge, amortizaation of debt discount and d transaction ccosts, and amortization of purchased intangible asseets, non‐GAAPP net income for the full year 2014 was $14.2 million, or $0.1 19 per share. This compare es to a non‐G GAAP net inco ome of $17.9 million, or $0 0.26 per e full year 201 13, which exccluded stock ccompensationn expenses, aa restructurin ng charge (inccluding share, for the impairment o of certain assets), and amo ortization of p purchased inttangible assetts. “2014 was a very strong yyear for Cephe eid, marked b by record rev enue, record GeneXpert® System placeements, and a record number of additions to ou ur Xpert test menu, in adddition to solidd progress in ffurther impro oving our gross margin,” said John B Bishop, Cephe eid’s Chairmaan and Chief EExecutive Officer. “While we are targetting Commercial C Clinical growtth of 20% or h higher in 2015, we continuue to invest s trategically and aggressiveely in further test m menu expansion, geograph hic expansion n, and markett segment exp pansion, whicch we believee sets the stage for a prrolonged periiod of growth h ahead for Ce epheid.” Operational Overview - Fourth quarter of 2014 total Clinical sales of $122.2 million grew 21% from $101.0 million in the fourth quarter of 2013. For the year ended December 31, 2014, total Clinical sales of $441.1 million grew 23% from $359.9 million in 2013. - By industry, sales were, in millions: Three Months Ended December 31, 2014 2013 Change Clinical Systems Clinical Reagents Total Clinical Non‐Clinical Total Sales $ 22.1 100.1 122.2 9.3 $ 131.5 $ 20.2 80.8 101.0 12.3 $ 113.3 9% 24% 21% ‐24% 16% 2014 Full Year Ended December 31, 2013 Change $ 84.7 356.4 441.1 29.0 $ 470.1 $ 67.0 292.9 359.9 41.4 $ 401.3 26% 22% 23% ‐30% 17% By geography, sales were, in millions: Three Months Ended December 31, 2014 2013 Change 2014 Full Year Ended December 31, 2013 Change North America Clinical Other Total North America $ 71.2 8.5 79.7 $ 58.6 11.1 69.7 21% ‐23% 14% $ 247.1 24.9 272.0 $ 212.4 37.0 249.4 16% ‐33% 9% International Clinical Other Total International 51.0 0.8 51.8 42.4 1.2 43.6 20% ‐33% 19% 194.0 4.1 198.1 147.5 4.4 151.9 31% ‐6% 30% Total Sales $ 131.5 $ 113.3 16% $ 470.1 $ 401.3 17% - Commercial Clinical sales were $104.8 million in the fourth quarter of 2014 and $356.0 million for the full year 2014. Sales to High Burden Developing Countries (HBDC) in the fourth quarter of 2014 were $17.4 million and $85.1 million for the full year 2014. - During the fourth quarter of 2014, Cepheid placed a total of 261 GeneXpert systems in its commercial Clinical business. Additionally, Cepheid placed a total of 211 GeneXpert systems as part of its High Burden Developing Country (HBDC) program. For the year ended December 31, 2014, Cepheid placed a total of 773 GeneXpert systems in its commercial Clinical business and an additional 1,743 GeneXpert systems as part of its HBDC program. As of December 31, 2014, a cumulative total of 8,025 GeneXpert systems have been placed worldwide. - GAAP gross margin on sales was 54% and non‐GAAP gross margin on sales was 56% in the fourth quarter of 2014, which compares to 47% and 50%, respectively, in the fourth quarter of 2013. GAAP gross margin on sales was 51% and non‐GAAP gross margin on sales was 52% for the full year 2014, which compares to 48% and 50%, respectively, for the full year 2013. - Cash, cash equivalents and investments were $373.1 million as of December 31, 2014. - DSO was 48 days. Business Outlook For the fiscal year ending December 31, 2015, the Company expects: - Total revenue to be in the range of $538 to $553 million; - Net loss in the range of $(0.55) to $(0.51) per share; - Non‐GAAP net income in the range of $0.19 to $0.23 per share. Expected non‐GAAP net income excludes approximately $37 million related to stock compensation expense, approximately $10 million related to the amortization of debt discount and transaction costs, and approximately $6 million related to the amortization of purchased intangible assets. The fully diluted share count for the year is expected to be approximately 71 million in the case of a net loss, and approximately 74 million shares in the case of net income. The following table reconciles net income (loss) per share to the non‐GAAP net income per share range: Guidance Range for Year Ending December 31, 2015 Low High Net Income (Loss) Per Share $ (0.55) $ (0.51) Stock‐Based Compensation Expense 0.52 0.52 Amortization of Debt Discount and Transaction Costs 0.14 0.14 Amortization of Purchased Intangible Assets 0.08 0.08 Non‐GAAP Measure of Net Income Per Share $ 0.19 $ 0.23 Accessing Cepheid’s Fourth Quarter and Full Year 2014 Results Conference Call The Company will host a management presentation at 2 p.m. Pacific Time on Thursday, January 29, 2015, to discuss the results. To access the live webcast, please visit Cepheid’s website at http://ir.cepheid.com at least 15 minutes before the scheduled start time to download any necessary audio or plug‐in software. A replay of the webcast will be available shortly following the call and will remain available for at least 90 days. About Cepheid Based in Sunnyvale, Calif., Cepheid (Nasdaq: CPHD) is a leading molecular diagnostics company that is dedicated to improving healthcare by developing, manufacturing, and marketing accurate yet easy‐to‐use molecular systems and tests. By automating highly complex and time‐consuming manual procedures, the Company’s solutions deliver a better way for institutions of any size to perform sophisticated genetic testing for organisms and genetic‐based diseases. Through its strong molecular biology capabilities, the Company is focusing on those applications where accurate, rapid, and actionable test results are needed most, such as managing infectious diseases and cancer. For more information, visit http://www.cepheid.com. Use of Non‐GAAP Measures The Company has supplemented its reported GAAP financial information with non‐GAAP measures that do not include stock‐based compensation expense, a legal contingency charge, amortization of purchased intangible assets, amortization of debt discount and transaction costs, and a restructuring charge in the quarter ended December 31, 2013. The presentation of this additional information is not meant to be considered in isolation or as a substitute for results prepared in accordance with U.S. GAAP. The Company’s management uses the non‐GAAP information internally to evaluate its ongoing business, continuing operational performance and cash requirements, and believes these non‐GAAP measures are useful to investors as they provide a basis for evaluating the Company’s cash requirements and additional insight into the underlying operating results and the Company’s ongoing performance in the ordinary course of its operations. These non‐GAAP measures may be different from non‐GAAP measures used by other companies. In addition, these non‐GAAP measures are not based on any comprehensive set of accounting rules or principles. The Company believes that non‐GAAP measures have limitations in that they do not reflect all of the amounts associated with its results of operations as determined in accordance with U.S. GAAP and that these measures should only be used to evaluate the Company’s results of operations in conjunction with the corresponding GAAP measures. As described above, the Company excludes the following items from one or more of its non‐GAAP measures when applicable: Stock‐based Compensation Expense. This consists primarily of expenses for stock options and restricted stock under ASC 718 (formerly SFAS 123(R)). The Company excludes stock‐based compensation expense from its non‐GAAP measures primarily because it is a non‐cash expense that the Company does not believe is reflective of ongoing operating results in the period incurred. Further, as the Company applies ASC 718, it believes that it is useful to investors to understand the impact of the application of ASC 718 on its results of operations. Legal Contingency. In the fourth quarter of 2014, the Company recorded a charge for an estimated loss related to ongoing legal proceedings. The Company excluded this item as it believes it is not reflective of ongoing operating results in the period incurred. Amortization of Debt Discount and Transaction Costs. The Company incurs amortization of debt discount and transaction costs in connection with the Convertible Senior Notes issued in February 2014. The Company excludes these amounts because these expenses are not reflective of ongoing operating results in the period incurred. These amounts arise from the Company’s issuance of debt and have no direct correlation to the operation of the Company’s business. Amortization of Purchased Intangible Assets. The Company incurs amortization of purchased intangible assets in connection with acquisitions. The Company excludes these amounts because these expenses are not reflective of ongoing operating results in the period incurred. These amounts arise from the Company’s prior acquisitions and have no direct correlation to the operation of the Company’s business. Restructuring. The Company excluded a restructuring charge and impairment of certain assets totaling $4.4 million in the fourth quarter of 2013. In connection with the Company’s preparation of its annual operating plan, whereby the Company routinely assesses its investments to align its operations with its highest potential opportunities, the Company terminated a small international research team, eliminated a non‐GeneXpert clinical product line acquired in a 2007 acquisition, including the impairment of an intangible asset of approximately $1.1 million and the write‐down of approximately $0.3 million of inventory, and wrote‐off certain manufacturing capital assets totaling approximately $1.3 million that management concluded will not be utilized and therefore have no future realizable value. The Company excluded this item as it believes it was non‐recurring in nature, and does not have a direct impact on the operation of the Company’s core business. Forward‐Looking Statements This press release contains forward‐looking statements that are not purely historical regarding Cepheid's or its management's intentions, beliefs, expectations and strategies for the future, including those relating to the scale and sustainability of future growth, future revenues and future net loss/income and profitability, including on a non‐GAAP basis, strategic investments, the breadth and speed of test menu expansion, geographic expansion and market segment expansion. Because such statements deal with future events, they are subject to various risks and uncertainties, and actual results could differ materially from the Company's current expectations. Factors that could cause actual results to differ materially include risks and uncertainties such as those relating to: long sales cycles and variability in systems placements and reagent pull‐through in the Company’s HBDC program; our success in increasing commercial and HBDC sales and the effectiveness of our sales personnel; the relative mix of commercial and HBDC sales; the performance and market acceptance of new products; sufficient customer demand, customer confidence in product availability and available customer budgets for our customers; our ability to develop new products and complete clinical trials successfully in a timely manner for new products; uncertainties related to the FDA regulatory and international regulatory processes; the level of testing at clinical customer sites, including for Healthcare Associated Infections (HAIs); the Company’s ability to successfully introduce and sell products in clinical markets other than HAIs; the rate of environmental biothreat testing conducted by the USPS, which will affect the amount of consumable products sold to the USPS; unforeseen supply, development and manufacturing problems; our ability to manage our inventory levels; our ability to successfully complete and bring on additional manufacturing lines; the potential need for additional intellectual property licenses for tests and other products and the terms of such licenses; the Company's reliance on distributors in some regions to market, sell and support its products; the occurrence of unforeseen expenditures, acquisitions or other transactions; costs associated with litigation; the impact of competitive products and pricing; the Company’s ability to manage geographically‐dispersed operations; and underlying market conditions worldwide. Readers should also refer to the section entitled "Risk Factors" in Cepheid's Annual Report on Form 10‐K, its most recent Quarterly Report on Form 10‐Q, and its other reports filed with the Securities and Exchange Commission. All forward‐looking statements and reasons why results might differ included in this release are made as of the date of this press release, based on information currently available to Cepheid, and Cepheid assumes no obligation to update any such forward‐looking statement or reasons why results might differ. FINANCIAL TABLES FOLLOW CEPHEID CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (in thousands, except per share data) (unaudited) Three Months Ended December 31, 2014 2013 Revenues: System and other sales Reagent and disposable sales $ Total sales Costs and operating expenses: Cost of sales Collaboration profit sharing Research and development Sales and marketing General and administrative Legal contingencies and settlements Total costs and operating expenses Loss from operations Other expense, net Loss before income taxes Provision for income taxes 24,177 107,345 131,522 $ 22,263 90,998 113,261 Years Ended December 31, 2014 2013 $ 90,849 379,292 470,141 $ 76,763 324,529 401,292 59,885 1,923 27,572 26,975 13,971 20,000 150,326 (18,804) (4,272) (23,076) 60,483 2,567 25,340 21,922 12,854 123,166 (9,905) (264) (10,169) 229,327 5,154 96,851 97,848 55,047 20,000 504,227 (34,086) (13,506) (47,592) 207,933 7,512 80,197 79,941 41,719 417,302 (16,010) (807) (16,817) (692) (148) (2,557) (1,148) $ (50,149) $ (17,965) Net loss $ (23,768) $ (10,317) Basic net loss per share $ (0.34) $ (0.15) $ (0.72) $ (0.27) Diluted net loss per share $ (0.34) $ (0.15) $ (0.72) $ (0.27) Shares used in computing basic net loss per share 70,689 68,230 70,069 67,485 Shares used in computing diluted net loss per share 70,689 68,230 70,069 67,485 CEPHEID CONDENSED CONSOLIDATED BALANCE SHEETS (in thousands) (unaudited) December 31, 2014 December 31, 2013 $ 96,663 196,729 68,809 132,635 24,274 519,110 115,765 79,731 7,847 31,440 39,681 793,574 $ 50,435 33,760 5,443 34,761 13,447 137,846 4,532 278,213 18,768 439,359 $ ASSETS Current assets: Cash and cash equivalents Short-term investments Accounts receivable, net Inventory Prepaid expenses and other current assets Total current assets Property and equipment, net Investments Other non-current assets Intangible assets, net Goodwill Total assets LIABILITIES AND SHAREHOLDERS’ EQUITY Current liabilities: Accounts payable Accrued compensation Accrued royalties Accrued and other liabilities Current portion of deferred revenue Total current liabilities Long-term portion of deferred revenue Convertible senior notes, net Other liabilities Total liabilities Shareholders’ equity: Common stock Additional paid-in capital Accumulated other comprehensive income (loss) Accumulated deficit Total shareholders’ equity Total liabilities and shareholders’ equity $ $ $ 422,151 225,529 247 (293,712) 354,215 793,574 $ $ 66,072 8,837 52,202 103,866 13,037 244,014 84,886 9,820 958 15,245 39,681 394,604 52,609 22,009 5,245 7,440 8,183 95,486 3,424 10,454 109,364 383,379 145,900 (476) (243,563) 285,240 394,604 CEPHEID CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (in thousands) (unaudited) Years Ended December 31, 2014 2013 Cash flows from operating activities: Net loss Adjustments to reconcile net loss to net cash provided by operating activities: Depreciation and amortization of property and equipment Amortization of intangible assets Unrealized foreign exchange differences Amortization of debt discount and transaction costs Impairment of acquired intangible assets, licenses, property and equipment Stock-based compensation expense Excess tax benefits from stock-based compensation Changes in operating assets and liabilities: Accounts receivable Inventory Prepaid expenses and other current assets Other non-current assets Accounts payable and other current and non-current liabilities Accrued expense for estimated legal contingency Accrued compensation Deferred revenue $ Net cash provided by operating activities Cash flows from investing activities: Capital expenditures Payments for technology licenses Cost of acquisitions, net Proceeds from sales of marketable securities and investments Proceeds from maturities of marketable securities and investments Purchases of marketable securities and investments Transfer to restricted cash Net cash used in investing activities Cash flows from financing activities: Net proceeds from the issuance of common shares and exercise of stock options Excess tax benefits from stock-based compensation Proceeds from borrowings of convertible senior notes, net of issuance costs Purchase of convertible note capped call hedge Principal payments of notes payable Net cash provided by financing activities Effect of foreign exchange rate change on cash and cash equivalents Net increase (decrease) in cash and cash equivalents Cash and cash equivalents at beginning of period Cash and cash equivalents at end of period $ (50,149) $ (17,965) 21,604 4,739 1,836 8,600 32,207 (66) 17,769 5,418 419 2,855 27,635 (16,606) (29,346) (5,184) (2) 3,438 20,000 11,751 6,372 (6,960) (32,638) (5,263) 150 17,334 5,421 837 9,194 15,012 (46,979) (18,000) 115,881 102,733 (477,485) (1,875) (325,725) (47,526) (1,125) (3,669) 2,503 1,347 (22,511) (70,981) 38,615 66 335,789 (25,082) (180) 349,208 27,512 (874) 26,638 (2,086) 30,591 66,072 (376) (29,707) 95,779 96,663 $ 66,072 CEPHEID RECONCILIATION OF GAAP TO NON-GAAP MEASURES (in thousands, except per share data) (unaudited) Cost of sales Stock-based compensation expense Restructuring charge including impairment of intangibles Amortization of purchased intangible assets Non-GAAP measure of cost of sales Three Months Ended December 31, 2014 2013 $ 59,885 $ 60,483 (796) (1,084) (2,651) (1,157) (345) $ 57,932 $ 56,403 Gross margin on sales per GAAP Gross margin on sales per Non-GAAP 54% 56% 88,518 (6,756) (20,000) (435) $ 61,327 Loss from operations Stock-based compensation expense Restructuring charge including impairment of intangibles Legal contingencies and settlements Amortization of purchased intangible assets Non-GAAP measure of income from operations $ (18,804) 7,552 20,000 1,592 $ 10,340 $ Net loss Stock-based compensation expense Restructuring charge including impairment of intangibles Legal contingencies and settlements Amortization of debt discount and transaction cost Amortization of purchased intangible assets Non-GAAP measure of net income $ (23,768) 7,552 20,000 2,453 1,592 $ 7,829 $ $ Basic net loss per share Stock-based compensation expense Restructuring charge including impairment of intangibles Legal contingencies and settlements Amortization of debt discount and transaction cost Amortization of purchased intangible assets Non-GAAP measure of net income per share $ $ $ $ $ (0.34) 0.11 0.28 0.04 0.02 0.11 (0.34) 0.11 0.28 0.04 0.02 0.11 $ 47% 50% Operating expenses Stock-based compensation expense Restructuring charge including impairment of intangibles Legal contingencies and settlements Amortization of purchased intangible assets Non-GAAP measure of operating expenses Diluted net loss per share Stock-based compensation expense Restructuring charge including impairment of intangibles Legal contingencies and settlements Amortization of debt discount and transaction cost Amortization of purchased intangible assets Non-GAAP measure of net income per share $ $ $ $ $ $ $ $ 60,116 (6,534) (1,783) (458) 51,341 (9,905) 7,618 4,434 803 2,950 (10,317) 7,618 4,201 803 2,305 (0.15) 0.11 0.06 0.01 0.03 (0.15) 0.11 0.06 0.01 0.03 Years Ended December 31, 2014 2013 $ 207,933 229,327 (4,087) (2,927) (2,651) (2,349) (1,826) 223,414 $ 200,006 51% 52% $ $ $ $ $ $ $ $ $ $ 269,746 (28,120) (20,000) (1,731) 219,895 (34,086) 32,207 20,000 3,557 21,678 (50,149) 32,207 20,000 8,600 3,557 14,215 (0.72) 0.46 0.29 0.12 0.05 0.20 (0.72) 0.45 0.28 0.13 0.05 0.19 48% 50% $ $ $ $ $ $ $ $ $ $ 201,857 (24,708) (1,783) (1,715) 173,651 (16,010) 27,635 4,434 4,064 20,123 (17,965) 27,635 4,201 4,064 17,935 (0.27) 0.41 0.07 0.06 0.27 (0.27) 0.41 0.06 0.06 0.26 Shares used in computing basic net income (loss) per share 70,689 68,230 70,069 67,485 Shares used in computing diluted net income (loss) per share 73,469 70,644 72,901 69,928
© Copyright 2026